Abstract
BACKGROUND
Adverse associations of low- and no-calorie sweetened beverages (LNCSB) with cardiometabolic outcomes in observational studies may be explained by reverse causality and residual confounding.
PURPOSE
To address these limitations we used change analyses of repeated measures of intake and substitution analyses to synthesize the association of LNCSB with cardiometabolic outcomes.
DATA SOURCES
MEDLINE, Embase, and the Cochrane Library were searched up to 10 June 2021 for prospective cohort studies with ≥1 year of follow-up duration in adults.
STUDY SELECTION
Outcomes included changes in clinical measures of adiposity, risk of overweight/obesity, metabolic syndrome, type 2 diabetes (T2D), cardiovascular disease, and total mortality.
DATA EXTRACTION
Two independent reviewers extracted data, assessed study quality, and assessed certainty of evidence using GRADE. Data were pooled with a random-effects model and expressed as mean difference (MD) or risk ratio (RR) and 95% CI.
DATA SYNTHESIS
A total of 14 cohorts (416,830 participants) met the eligibility criteria. Increase in LNCSB intake was associated with lower weight (5 cohorts, 130,020 participants; MD −0.008 kg/year [95% CI −0.014, −0.002]). Substitution of LNCSB for sugar-sweetened beverages (SSB) was associated with lower weight (three cohorts, 165,579 participants; MD, −0.12 [−0.14, −0.10,] kg/y) and lower incidence of obesity (OB) (one cohort, 15,765 participants; RR 0.88 [95% CI 0.88, 0.89]), coronary heart disease (six cohorts, 233,676 participants; 0.89 [0.81, 0.98]), cardiovascular disease mortality (one cohort, 118,363 participants; 0.95 [0.90, 0.99]), and total mortality (one cohort, 118,363 participants; 0.96 [0.94, 0.98]) with no adverse associations across other outcomes. Substitution of water for SSB showed lower weight (three cohorts, 165,579 participants; MD −0.10 kg/year [−0.13, −0.06]), lower waist circumference (one cohort, 173 participants; −2.71 cm/year [−4.27, −1.15]) and percent body fat (one cohort, 173 participants; −1.51% per year [−2.61, −0.42]), and lower incidence of OB (one cohort, 15,765 participants; RR 0.85 [0.75, 0.97]) and T2D (three cohorts, 281,855 participants; 0.96 [0.94, 0.98]). Substitution of LNCSB for water showed no adverse associations.
LIMITATIONS
The evidence was low to very low certainty owing to downgrades for imprecision, indirectness, and/or inconsistency.
CONCLUSIONS
LNCSB were not associated with cardiometabolic harm in analyses that model the exposure as change or substitutions. The available evidence provides some indication that LNCSB in their intended substitution for SSB may be associated with cardiometabolic benefit, comparable with the standard of care, water.
Introduction
Sugars have been implicated in the epidemics of obesity (OB) and type 2 diabetes (T2D) and their downstream cardiometabolic complications (1,2). Major health agencies as well as T2D and heart associations have recommended that added/free sugars be reduced to <5–10% of calories (3,4). Sugar-sweetened beverages (SSB), as the single most important food source of added/free sugars in North America (5–7) and many European countries (8), have become the dominant public health target of these recommendations. Despite safety approvals by the major international health and regulatory bodies (9–12), low- and no-calorie sweetened beverages (LNCSB) are generally not recommended as an effective replacement strategy for SSB. Although major OB and TD2 associations have supported a narrow indication for the use of LNCSB to displace calories from SSB (13–16), water remains the preferred replacement strategy for SSB, and various countries have either explicitly recommended against their use in national dietary guidelines (4,17) or imposed excise taxes on both SSB and LNCSB (18).
Much of the concern regarding LNCSB has been focused on their failure to show established benefits in large prospective cohort studies. Several highly influential systematic reviews and meta-analyses of prospective cohort studies have shown LNCSB to be associated with higher risk of weight gain (19), T2D (20), cardiovascular disease (CVD) events (19,21,22), and all-cause mortality (23). It is well recognized by prospective cohort study investigators, content experts, and guidelines committees (24–26) that these observations come at high risk of reverse causality (i.e., being high risk for OB, T2D, and CVD causes one to increase LNCSB intake as a risk reduction strategy, as opposed to the other way around) and residual confounding from an incomplete adjustment of confounders and behavior clustering (4,15,24–30). The assessment of changes in exposure rather than baseline or prevalent exposure and further modeling of the intended substitution of LNCSB for SSB appear to provide more consistent, robust, and biologically plausible associations (26,28,29,31). Whether LNCSB as a replacement strategy for SSB have the intended benefits remains an important clinical and public health question.
To address the sources of bias in the epidemiology and strengthen causal inferences for the update of the European Association for the Study of Diabetes (EASD) clinical practice guidelines for nutrition therapy (32), the Diabetes and Nutrition Study Group (DNSG) of the EASD commissioned a systematic review and meta-analysis of the available evidence from prospective cohort studies of the relation of LNCSB to cardiometabolic outcomes, restricting the analyses to cohort comparisons where investigators adjusted for initial adiposity and modeled the exposure as either change in intake or substitution of LNCSB for SSB (“intended substitution”), LNCSB for the standard of care, water (“reference substitution”), or water for SSB (“standard of care substitution”).
Methods
Data Sources and Searches
The present systematic review and meta-analysis were conducted in accord with the Cochrane Handbook for Systematic Reviews of Interventions (33), and results are reported in accord with the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) (34) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (35) guidelines.
MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) databases were searched from inception to 10 June 2021 for identification of studies that examined the change in LNCSB intake and substitution of LNCSB and cardiometabolic health, supplemented by hand searching of referenced studies of included studies. Abstracts from conferences were included, and no language restrictions were applied. An additional search of MEDLINE, Embase, and CENTRAL databases from inception to 10 June 2021 was conducted to identify studies that examined the substitution of water for SSB and cardiometabolic health. Details of search strategies can be found in Supplementary Tables 1 and 2.
Study Selection
We included prospective cohort studies of adults (age >19 years) of ≥1 year in duration assessing the association of LNCSB intake, defined as “diet” or “low- and no-calorie” beverages where dietary sugars are replaced with no-calorie (e.g., aspartame, sucralose) and/or low-calorie (e.g., stevia) sweeteners to lower the total caloric content. To mitigate residual confounding and reverse causality, we prespecified the inclusion of cohort comparisons where investigators adjusted for initial adiposity and used one of two analytical approaches: 1) change models of repeated measures capturing change in LNCSB intake over time or 2) substitution models. The substitution models were limited to the substitution of LNCSB for SSB, water for SSB, and LNCSB for water. Supplementary Text elaborates on the change and substitution models included in the study.
Outcomes included change in clinical measures of adiposity (body weight, body mass index [BMI], percent body fat [%BF], waist circumference [WC]) and incidence of overweight (OW) or OB, metabolic syndrome, T2D, CVD events and mortality (coronary heart disease [CHD], stroke, and total CVD), and total mortality. Studies were selected with subjects from all health backgrounds including people who did not have the binary outcome of interest at inception.
After the removal of duplicates by a reviewer, two independent reviewers (J.J.L. and T.A.K.) screened and assessed records for eligibility.
Data Extraction and Quality Assessment
Two independent reviewers (J.J.L. and T.A.K.) extracted relevant data of the largest covariate- adjusted models of primary results, including author information, study design, country of origin, cohort descriptions, follow-up duration, frequency of data collection, confounding variables, type of low- or no-calorie sweetener, statistical analyses, outcome and assessment method, and funding source. Change in means of body weight, %BF, and WC per year were expressed as mean difference (MD) with 95% CIs. Risk of OW/OB, T2D, metabolic syndrome, CVD events, and total mortality were expressed as risk ratios (RR) with 95% CIs. The authors were contacted for missing outcome data. If required, values were extracted from figures with use of WebPlotDigitizer (https://automeris.io/WebPlotDigitizer/).
When data were only available for the substitution of SSB or water for LNCSB, published data were inverted for estimation of the association of substitution of LNCSB for SSB or water with outcomes. When the beverage substitution was presented in relative terms (i.e., percentage of beverage substitution) (36), 100% substitution was assumed for analyses. If the results were given as a categorical analysis only, the RR change per unit serving for the study was estimated with use of the drmeta routine in Stata 16.1 (37). If several cohort comparisons provided results on the same outcome with inclusion of overlapping groups of individuals, results from studies with the longest follow-up were used to avoid double counting.
Study quality of each of the included cohort comparisons was assessed with the Newcastle-Ottawa Scale (NOS) (38) by the same two independent reviewers (J.J.L., T.A.K.). Up to 9 points were awarded based on cohort selection, ascertainment of the outcome, and comparability of outcomes. (Adjustments for confounding variables were prespecified based on the outcomes, as outlined in Supplementary Table 3.) Cohort comparisons were adjudged for high (score ≥7), moderate (score = 6), or low (score ≤5) study quality (39).
Data Synthesis and Analysis
Data were analyzed with Stata (version 16.1; StataCorp). Pooled summary estimates were calculated for each outcome by pooling of MD or log-RRs with 95% CIs with use of the generic inverse variance method with DerSimonian-Laird random-effects models (40). When ≤5 cohort comparisons were available for analysis, a fixed-effects model was used to calculate the pooled summary estimates (41). For studies with hazard ratios or odds ratios reported, values were converted to RRs (42,43). We performed separate analyses based on the prespecified study designs. For the change analysis, the associations of increasing one serving size per day (serving size = 330 mL, the standard manufacturers’ portion sizes in the U.K., as previously reported [44]) with outcomes per year were assessed. When change in outcomes was not reported per year (e.g., with reporting per 2 years or per 4 years), we assumed a linear relationship over the given time period to estimate the change per year. For the substitution analysis, the association of substituting LNCSB for SSB or water, matched by volume (1 mL:1 mL), with outcomes was assessed.
For comparison of summary estimates among outcomes on the same scale, the effect estimates of MD and RR were converted into standardized MD (SMD) (also known as Cohen d) and 95% CIs with the formula described in the Cochrane Handbook for Systematic Reviews of Interventions (33).
Heterogeneity was assessed with the Cochran Q (χ2) statistic with a significance set at PQ < 0.10 and quantified with the I2 statistic. Sources of heterogeneity were investigated by sensitivity through systematic removal of each cohort comparison and recalculation of summary estimates to assess the influence of each cohort comparison. A cohort comparison was considered influential if it changed the direction, significance of the pooled estimates, or the evidence of heterogeneity. If >10 cohort comparisons were available, then we also performed a priori subgroup analyses by follow-up duration, sex, study quality, and funding source with subgroup differences assessed with meta-regression. Results of both the change (cardiometabolic outcome assessed against the increasing beverage [1 serving] intake over time) and substitution (difference between regression coefficients of the two beverages included as continuous terms of dose-intake) analyses were assumed to represent linear dose-response associations. If enough data points were available, the shape of the dose-response association was also assessed with a one-stage mixed model using restricted cubic splines with three knots according to Harrell’s recommended percentiles (10%, 50%, and 90%) (37,45). If >10 cohort comparisons were available, we assessed publication bias through visual inspection of funnel plots for asymmetry and formal testing with the Begg and Egger tests with adjustment for funnel plot asymmetry using the Duval and Tweedie trim-and-fill method (46).
Quality Assessment
Grading of Recommendations Assessment, Development and Evaluation (GRADE) was used to assess the certainty of the evidence, with certainty of evidence ranging from “very low” to “high” (47,48). GRADE was completed by two independent reviewers (J.J.L., T.A.K.), with any disagreement resolved by a third reviewer (J.L.S.). Observational studies start at a rating of “low” certainty of evidence. Downgrades or upgrades based on established criteria are then applied. Criteria to downgrade include risk of bias (weight of studies show low study quality by NOS), inconsistency (substantial unexplained heterogeneity, I2 > 50%, PQ < 0.10), indirectness (presence or absence of factors that limit generalizability based on populations, exposures, and outcomes), imprecision (95% CIs cross minimally important difference of 5%), and publication bias (evidence of small study effects). Criteria to upgrade included a large magnitude of effect (RR <0.5 or >2 in the absence of plausible confounders), a dose-response gradient, and attenuation by plausible confounders.
At the request of the referees, certainty of evidence was also assessed with NutriGrade (49). We performed a sensitivity analysis comparing the results from the two methods.
The predefined protocol for this systematic review and meta-analysis was registered at ClinicalTrials.gov (clinical trial reg. no. NCT04245826).
Data and Resource Availability
Full data sets can be obtained from the corresponding author at john.sievenpiper@utoronto.ca.
Results
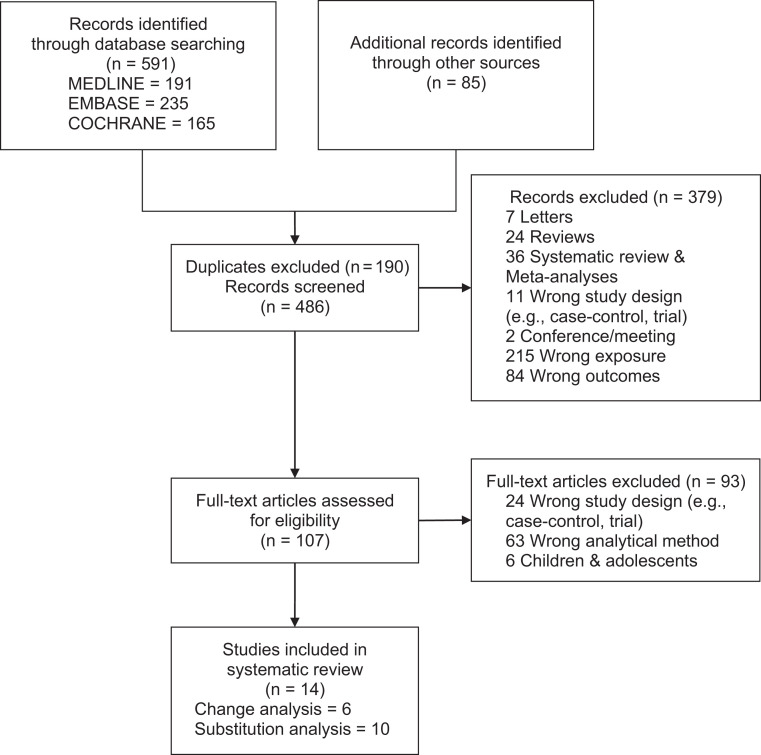
Figure 1 shows the flow of the literature with examination of the association of LNCSB using change and substitution analyses. Of 486, 14 studies (14 unique cohort comparisons, n = 416,830) met the eligibility criteria. In six studies (six unique cohort comparisons, n = 204,380), the change in LNCSB intake was assessed. In 10 (12 unique cohort comparisons, n = 409,683), 8 (6 unique cohort comparisons, n = 297,793), and 4 (5 unique cohort comparisons, n = 272,967) studies, investigators assessed the substitution of LNCSB for SSB, water for SSB, and LNCSB for water, respectively. We identified at least one cohort comparison with assessment of one or more of the prespecified cardiometabolic outcomes, not including metabolic syndrome. Three authors provided additional information (50–52). Supplementary Fig. 1 shows the flow of the literature examining the effect of substituting water for SSB and cardiometabolic health.
Figure 1.
CONSORT diagram outlining the summary of the evidence search and selection for LNCSB and cardiometabolic outcomes. Of the 486 studies screened, 379 were excluded based on title and abstract review. The remaining 107 studies were reviewed in full. A total of 14 studies met the inclusion criteria and qualified for further analysis.
Tables 1 and 2 show the characteristics of the included studies. Most of the cohort comparisons were from the U.S. (eight cohort comparisons) with one cohort comparison each from the U.K., Spain, Finland, and Mexico. The participants were predominantly middle-aged (baseline median age 50 years [range 25–75]) and female (79.3% female and 20.7% male) with varying cardiometabolic risk profiles inclusive of T2D (except for the analyses of T2D, with exclusion of people with T2D). Median follow-up was 17.5 years (range 1–34). Ascertainment of incident cases and mortality was by medical record linkage (CHD events, CHD mortality, CVD mortality, and total mortality) (20% of cohort comparisons for T2D incidence), calculated with self-reported values (OB incidence) by self-report (20% of cohort comparisons for T2D incidence), and on the basis of confirmed diagnosis according to the National Diabetes Data Group criteria (53) (60% of cohort comparisons for T2D incidence). Mean intakes of LNCSB and SSB were 0.57 and 0.40 servings/day, respectively, while mean water intake was 3.73 servings/day (n = 6). Dietary intake was assessed through semiquantitative food-frequency questionnaires (sFFQ) (78.6%), food diaries (7.1%), or 24-h recalls (14.3%). All studies reported funding from agency alone.
Table 1.
Characteristics of prospective cohort comparisons in examining the relationship between increasing LNCSB intake and cardiometabolic outcomes
| Cohort comparison (first author, year) | Country | Total follow-up duration (years) | Sex | N | Baseline age (years) | Baseline LNCSB intake (servings/day) | Baseline SSB intake (servings/day) | Dietary assessment | Outcome(s) | Incidence | Outcome assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HPFS (Drouin-Chartier, 2019 [52]) | U.S. | 26 | M | 34,224 | 40–75 | 0.52 | 0.39 | sFFQ | T2D incidence | 5,993 | Confirmed diagnosis |
| HPFS (Mozaffarian, 2011 [85])‡ | U.S. | 20 | M | 22,557 | 50.8 (7.5) | 0.54 | 0.33 | sFFQ | Body weight | Self-report | |
| HPFS (Pan, 2013 [54])‡ | U.S. | 20 | M | 21,988 | 50.6 | 0.56 | 0.40 | sFFQ | Body weight | Self-report | |
| HPFS (Smith, 2015 [31]) | U.S. | 24 | M | 21,472 | 41–63 | 0.55 | 0.33 | sFFQ | Body Weight | Self-report | |
| NHS (Drouin-Chartier, 2019 [52]) | U.S. | 26 | F | 76,531 | 30–55 | 0.58 | 0.26 | sFFQ | T2D incidence | 3,613 | Confirmed diagnosis |
| NHS (Mozaffarian, 2011 [85])‡ | U.S. | 20 | F | 50,422 | 52.2 (7.2) | 0.55 | 0.22 | sFFQ | Body weight | Self-report | |
| NHS (Pan, 2013 [54])‡ | U.S. | 20 | F | 50,013 | 51.8 | 0.56 | 0.26 | sFFQ | Body weight | Self-report | |
| NHS (Smith, 2015 [31]) | U.S. | 24 | F | 48,449 | 30–44 | 0.55 | 0.22 | sFFQ | Body weight | Self-report | |
| NHS II (Drouin-Chartier, 2019 [52]) | U.S. | 22 | F | 81,597 | 25–42 | 1.08 | 0.50 | sFFQ | T2D incidence | 2,300 | Confirmed diagnosis |
| NHS II (Mozaffarian, 2011 [85])‡ | U.S. | 12 | F | 47,898 | 37.5 (4.1) | 1.09 | 0.33 | sFFQ | Body weight | Self-report | |
| NHS II (Pan, 2013 [54])‡ | U.S. | 16 | F | 52,987 | 37.7 | 1.16 | 0.50 | sFFQ | Body weight | Self-report | |
| NHS II (Smith, 2015 [31]) | U.S. | 16 | F | 48,071 | 40–63 | 1.09 | 0.33 | sFFQ | Body weight | Self-report | |
| Mexican Teachers’ Cohort (Stern, 2017 [50]) | Mexico | 2 | F | 11,218 | 43.3 (5.2) | 0.11 | 0.44 | sFFQ | Body weight and WC | Self-report | |
| PREMIER (Chen, 2009 [86]) | U.S. | 1.5 | Both | 810 (500 female, 310 male) |
50.0 (8.9) | 1 | 0.94 | 24-h dietary recalls | Body weight | Measured |
Baseline age is represented as mean, mean (SD), or range as presented in the original article. For baseline LNCSB intake and SSB intake, data are means; serving size of LNCSB and SSB was defined as 330 mL, the standard manufacturers’ portion sizes in the U.K., as previously reported (44). F, female; M, male.
Baseline characteristics were only reported as combined values from both cohort comparisons.
Cohort comparisons that were not included in the meta-analyses for avoidance of double counting of results.
Table 2.
Characteristics of prospective cohort comparisons with examination of the relationship between substituting LNCSB, SSB, and water and cardiometabolic outcomes
| Cohort comparison (first author, year) | Country | Follow-up duration (years) | Sex | N | Baseline age (years) | Baseline LNCSB intake (servings/day) | Baseline SSB intake (servings/day) | Baseline water intake (servings/day) | Dietary assessment | Substituted beverage(s) | Outcome(s) | Incidence | Outcome assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stanford A TO Z (Stookey, 2008 [36]) | U.S. | 1 | F | 173 | 25–50 | 1.35 | 1.25 | 2.52 | 24-h dietary recalls | SSB, water | Body weight, WC, %BF | Measured | |
| ARIC, females (Keller, 2020 [51]) | U.S. | 9.2‡ | F | 5,238 | 53.9 | 0.52‖ | 0.42‖ | NR | sFFQ | SSB | CHD incidence | 123 | Verified by records |
| ARIC, males (Keller, 2020 [51]) | U.S. | 9.2‡ | M | 6,481 | 54.6 | 0.40‖ | 0.35‖ | NR | sFFQ | SSB | CHD incidence, CHD mortality | Events, 269; deaths, 52 | Verified by records |
| ATBC (Keller, 2020 [51]) | Finland | 6* | M | 21,141 | 57.3 | 0.40‖ | 0.35‖ | NR | sFFQ | SSB | CHD incidence, CHD mortality | Events, 1,339; deaths, 534 | Verified by records |
| EPIC-Norfolk (O'Connor, 2015 [44]) | UK | 10 | Both | 24,653 (F 13,485 and M 11,168) | 58.7 (9.3) | 0.52 | 0.25 | NR | 7-day food diary | SSB, water | T2D incidence | 847 | Verified by records |
| HPFS (Drouin-Chartier, 2019 [52]) | U.S. | 26 | M | 34,224 | 40–75 | 0.52 | 0.39 | NR | sFFQ | SSB, water | T2D incidence | 2,300 | Confirmed diagnosis |
| HPFS (Keller, 2020 [51]) | U.S. | 9.7‡ | M | 41,684 | 53.4 | 0.52‖ | 0.35‖ | NR | sFFQ | SSB | CHD incidence, CHD mortality | Events, 1,272; deaths, 420 | Verified by records |
| HPFS, NHS (Bernstein, 2012 [87]) | U.S. | HPFS, 22; NHS, 28 | HPFS, M; NHS, F | 127,456 (F 84,085 and M 43,371) | HPFS, 40–75; NHS, 30–55 | NR | NR | NR | sFFQ | SSB, water | Stroke incidence | 4,354 (F 2,938, M 1,416) | Verified by records |
| HPFS, NHS (Malik, 2019 [77]) | U.S. | HPFS, 28; NHS, 34 | HPFS, M; NHS, F | 118,363 (F 80,647 and M 37,716) | 40–75 | NR | NR | NR | sFFQ | SSB | Total CVD mortality, total mortality | CVD, 7,896 (F 4,139 and M 3,757); total, 36,436 (F 23,432 and M 13,004) | Verified by records |
| HPFS, NHS, NHS II (Pan, 2013 [54]) | U.S. | HPFS, 20; NHS, 20; NHS, 16 | HPFS, M; NHS, F; NHS II, F | 124,988 (F 51,500 and M 21,988) | HPFS, 50.6; NHS, 51.8; NHS II, 37.7 | HPFS, 0.51; NHS, 0.5; NHS II, 1.16 | HPFS, 0.41; NHS, 0.26; NHS II, 0.50 | HPFS, 3.00; NHS, 3.20; NHS II, 3.23 | sFFQ | SSB | Body weight | Self-report | |
| IWHS (Keller, 2020 [51]) | U.S. | 10.0‡ | F | 29,528 | 61.4 | 0.40‖ | 0.42‖ | NR | sFFQ | SSB | CHD mortality | 291 | Verified by records |
| NHS (Drouin-Chartier, 2019 [52]) | U.S. | 26 | F | 76,531 | 30–55 | 0.58 | 0.26 | NR | sFFQ | SSB | T2D incidence | 5,993 | Confirmed diagnosis |
| NHS80 (Keller, 2020 [51]) | U.S. | 6.5‡ | F | 81,412 | 46.9 | 0.52‖ | 0.42‖ | NR | sFFQ | SSB | CHD incidence, CHD mortality | Events, 397; deaths, 97 | Verified by records |
| NHS86 (Keller, 2020 [51]) | U.S. | 10.0‡ | F | 61,700 | 52.6 | 0.52‖ | 0.42‖ | NR | sFFQ | SSB | CHD incidence, CHD mortality | Events, 696; deaths, 208 | Verified by records |
| NHS II (Drouin-Chartier, 2019 [52]) | U.S. | 22 | F | 81,597 | 25–42 | 1.08 | 0.52 | NR | sFFQ | SSB, water | T2D incidence | 3,613 | Confirmed diagnosis |
| NHS II (Pan, 2012 [88])† | U.S. | 18 | F | 82,902 | 36.0 (4.7) | 1.11 | 0.50 | 3.0 | sFFQ | SSB | T2D incidence | 2,718 | Confirmed diagnosis |
| SUN (Fresán, 2016 [89]) | Spain | 2 | Both | 15,765¶ (F 9,431 and M 6,334) | 37.9 (11.7) | 0.12 | 0.20 | 4.22 | sFFQ | SSB, water | Body weight, OB incidence | 873 | Self-report |
| WHI (Huang, 2017 [55]) | U.S. | 8.4 | F | 64,850 | 50–79 | 0.36 | 0.46 | NR | sFFQ | SSB, water | T2D incidence | 4,675 | Self-report |
| WHS (Keller, 2020 [51]) | U.S. | 5.3‡ | F | 37,161 | 53.9 | 0.52‖ | 0.42‖ | NR | sFFQ | SSB | CHD incidence | Events, 152 | Verified by records |
Follow-up duration is presented as means unless otherwise indicated. Baseline age is represented as means, means (SD), or range. Baseline LNCSB, SSB, and water intake are means. Serving size of LNCSB and SSB was defined as 330 mL (44), and serving size of water was defined as 200 mL (89). ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study; EPIC, European Prospective Investigation into Cancer and Nutrition; F, female; IWHS, Iowa Women’s Health Study; M, male; NR, not reported; SUN, Seguimiento Universidad de Navarra.
Cohort comparisons that were not included in the meta-analyses for avoidance of double counting of results.
Median follow-up duration in years.
Baseline beverage intake was reported as means pooled by sex as reported in the original study (51).
Number of female and male participants included in the analysis was estimated from the proportion of all female and male participants in the cohort (89).
Supplementary Table 4 shows the statistical adjustments in the included studies. The largest covariate-adjusted models included 10 to 27 covariates. All studies included adjustment for the prespecified primary covariate (age) and at least three of seven prespecified secondary covariates (sex; markers of adiposity; smoking; energy intake; family history of metabolic syndrome, T2D, or CVD; physical activity; and alcohol intake).
Supplementary Table 5 shows the study quality assessments by NOS. The quality of all cohort comparisons was rated as high (score 7–9) to moderate (score 6), with no studies assessed to be of low quality (<6 NOS score). Sources of low quality included indirect exposure assessment, indirect outcome assessment, and no adjustment for prespecified key confounding variables.
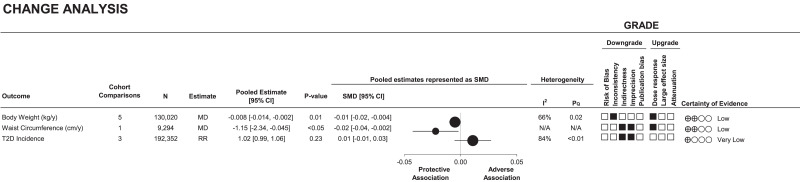
Figure 2 and Supplementary Fig. 2A–C show the association of change in LNCSB intake with cardiometabolic outcomes. A 1 serving/day increase of LNCSB was associated with lower weight (MD −0.008 kg/year [95% CI −0.014, −0.002]; evidence of interstudy heterogeneity, I2 = 66%, PQ = 0.02; n = 5) and lower WC (−1.15 cm/year [−2.34, −0.045]; n = 1), but there was no association with risk of T2D (RR 1.02 [95% CI 0.99, 1.06]; evidence of interstudy heterogeneity, I2 = 84%, PQ < 0.01; n = 3).
Figure 2.
Summary plot of the association between increasing intake of LNCSB by one serving (330 mL) per day and cardiometabolic outcomes (change analysis). For comparison of summary estimates among outcomes on the same scale, the effect estimates of MD and RR were converted into SMD and 95% CIs. SMD, ●; 95% CI, horizontal lines. Values of I2 ≥ 50% (PQ < 0.10) indicate substantial interstudy heterogeneity. Values >0 indicate an adverse association. With GRADE for prospective cohort studies, studies were by default rated to have low certainty of the evidence, with the rating downgraded by five domains and upgraded by three domains. ▪, downgrades or upgrades for each outcome. N/A, not applicable; y, year.
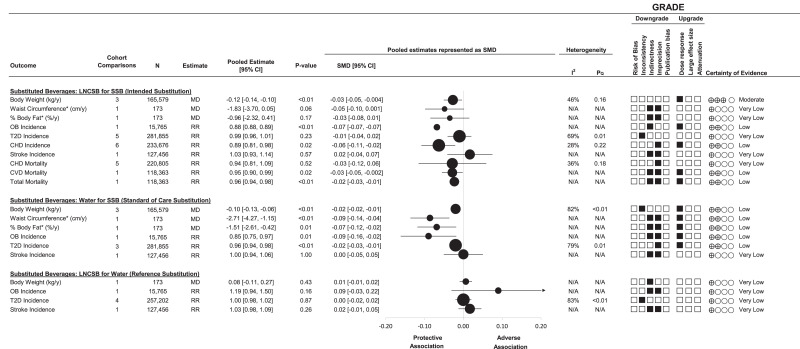
Figure 3 (top panel) and Supplementary Fig. 3A–J show the association of the substitution of LNCSB for SSB with cardiometabolic outcomes. Substitution of LNCSB for SSB was associated with lower weight (MD −0.12 kg/year [95% CI −0.14, −0.10]; no evidence of heterogeneity, I2 = 46%, PQ = 0.16; n = 3) and lower risk of OB (RR 0.88 [95% CI 0.88, 0.89]; n = 1), CHD (0.89 [0.81, 0.98]; no evidence of heterogeneity, I2 = 28%, PQ = 0.22; n = 6), total CVD mortality (0.95 [0.90, 0.99]; n = 1), and total mortality (0.96 [0.94, 0.98]; n = 1). No other associations were significant.
Figure 3.
Summary plot of the association between substituting LNCSB, SSB, and water (matched by volume) and cardiometabolic outcomes. For comparison of summary estimates among outcomes on the same scale, the effect estimates of MD and RR were converted into SMD and 95% CIs. SMD and 95% CIs are represented by ● and horizontal lines, respectively. Values of I2 ≥ 50% (PQ < 0.10) indicate substantial interstudy heterogeneity. Values >0 indicate an adverse association. With GRADE for prospective cohort studies, studies were by default rated to have low certainty of the evidence, with the rating downgraded by five domains and upgraded by three domains. ▪, downgrades or upgrades for each outcome. *We divided SMD and 95% CIs by 3 to allow the outcomes to be plotted within the available graph space. N/A, not applicable; y, year.
Figure 3 (middle panel) and Supplementary Fig. 4A–F show the substitution analysis association of the substitution of water for SSB with cardiometabolic outcomes. Substitution of water for SSB was associated with lower weight (MD −0.10 kg/year [95% CI −0.13, −0.06]; evidence of interstudy heterogeneity, I2 = 82%, PQ < 0.01; n = 3), lower WC (−2.71 cm/year [−4.27, −1.15]; n = 1) and %BF (−1.51% per year [−2.61, −0.42]; n = 1), and lower risk of OB (RR 0.85 [95% CI 0.75, 0.97]; n = 1) and T2D (0.96 [0.94, 0.98]; evidence of interstudy heterogeneity, I2 = 79%, PQ < 0.01; n = 3).
Figure 3 (bottom panel) and Supplementary Fig. 5A–D show the substitution analysis of the association of the substitution of LNCSB for water with cardiometabolic outcomes. Substitution of LNCSB for water was not associated with changes in any outcomes.
Supplementary Fig. 6A and B show the influence analyses for the change in LNCSB and cardiometabolic outcomes. Removal of several cohort comparisons explained the heterogeneity (Health Professionals Follow-Up Study [HPFS] [31] and Nurses’ Health Study [NHS] II] [31] for body weight and NHS [52] for T2D) or altered the significance of the association (HPFS [31] and NHS II [31] for body weight and NHS [52] for T2D). None of the other cohort comparisons influenced the significance, direction, or magnitude of the associations or the evidence for heterogeneity.
Supplementary Figs. 7A–D, 8A and B, and 9 show the influence analysis for the substitution of LNCSB for SSB, LNCSB for water, and water for SSB and cardiometabolic outcomes. Removal of several cohort comparisons explained the heterogeneity (HPFS/NHS/NHS II [52] for both the substitution of water for SSB and substitution of LNCSB for water and T2D) and altered the significance (pooled HPFS/NHS/NHS II [54] for the substitution of LNCSB for SSB and body weight; Atherosclerosis Risk in Communities [ARIC] study [females] [51], HPFS [51], NHS80 [51], or Women’s Health Study [WHS] [51] for the substitution of LNCSB for SSB and CHD incidence; pooled HPFS/NHS/NHS II [54] for the substitution of water for SSB and body weight; Women’s Health Initiative [WHI] [55] for the substitution of water for SSB and T2D; and NHS [52] for the substitution of LNCSB for water and T2D) or direction (NHS II [52] for the substitution of LNCSB for SSB and T2D and NHS80 [51] for the substitution of LNCSB for SSB and CHD mortality) of the association. None of the other cohort comparisons influenced the significance, direction, or magnitude of the associations or the evidence for heterogeneity.
Supplementary Fig. 10 shows the shape of the dose response for the change in LNCSB intake and T2D incidence across the whole range of intake. Neither linear nor nonlinear dose-response relationship was significant for the change in LNCSB intake with change in T2D incidence.
Prespecified subgroup analyses and publication bias could not be assessed, as <10 cohort comparisons were available for analyses.
Supplementary Table 6 shows the GRADE assessments. In the change analyses, the evidence was assessed as “low” for the association with lower body weight and WC and “very low” for the lack of association with T2D, owing to downgrades for inconsistency, indirectness, or imprecision with an upgrade for dose-response association for body weight and WC. In the substitution analyses for the LNCSB for SSB the evidence was assessed as “moderate” for lower body weight with no downgrades, “low” for incident OB and CHD and for CVD and total mortality with downgrades for indirectness, imprecision, or inconsistency and upgrade for dose-response association for all the above outcomes. The evidence was “low” for substitution of water for SSB for all outcomes except stroke incidence owing to downgrades for indirectness, imprecision, or inconsistency and upgrade for dose-response association. For all other associations the evidence was rated as “very low” owing to downgrades with no upgrades.
We performed a post hoc sensitivity analysis comparing the ratings for the certainty of evidence using GRADE against NutriGrade (Supplementary Table 6). For the three outcomes for change analysis with NutriGrade, one had the same rating as GRADE, while one was rated higher and another was rated lower compared with GRADE. In the substitution analysis with NutriGrade, 13 of 20 (65%) outcomes had the same rating as GRADE, while 7 of 20 (35%) were rated higher compared with GRADE.
Conclusions
We conducted a systematic review and meta-analysis of 14 prospective cohort studies (14 cohort comparisons) of the relation of LNCSB and cardiometabolic outcomes in 416,830 adults with varying cardiometabolic risk profiles inclusive of T2D. To mitigate the influence of reverse causality and residual confounding, we restricted our analyses to cohort comparisons with adjustment for initial adiposity and modeled the exposure as change in intake or the substitution of LNCSB for SSB (intended substitution), water for SSB (standard of care substitution), and LNCSB for water (reference substitution). An increase in LNCSB intake was associated with lower weight and borderline lower WC without any adverse association with T2D. The intended substitution of LNCSB for SSB was associated with lower weight and lower risk of incident OB, CHD, CVD morality, and total mortality without an adverse association with any other cardiometabolic outcomes including T2D. Substitution of water for SSB showed lower weight, lower WC and %BF, and lower incidence of OB and T2D. Substitution of LNCSB for water as the standard of care showed no associations with any cardiometabolic outcomes.
Although our findings are not consistent with those of other systematic reviews and meta-analyses of prospective cohort studies that have relied largely on baseline or prevalent intakes of LNCSB (19,20,23,56,57), importantly they are in agreement with those of studies with modeling that specifically accounts for the displacement of calories from SSB by LNCSB or other sugar reduction strategies in beverages (58–63). Findings of systematic reviews and meta-analyses of prospective cohort studies have shown that SSB are associated with greater energy intake and risk of weight gain, OB, T2D, metabolic syndrome, hypertension, and CVD (23,56,64–66). In an analysis of data from the Netherlands it was predicted that the displacement of SSB through the substitution of LNCSB for SSB would result in ∼80 kcal/day lower total energy intake and in turn lower BMI and prevalent OB (58). Other modeling studies from the U.K. (59), Portugal (60), Australia (61), Mexico (62), and Argentina (63) have shown that a reduction in SSB with or without low- and no-calorie sweeteners would reduce body weight and prevalent OW, OB, T2D, and/or CVD.
Our findings are also in agreement with the evidence from systematic reviews and meta-analyses of randomized controlled trials of intermediate cardiometabolic risk factors that account for the displacement of calories from SSB. Fructose-containing sugars providing excess calories especially in beverage form have been shown to lead to weight gain (67) and increase in triglycerides (68), glycemia (69), insulinemia (69), uric acid (70,71), and nonalcoholic fatty liver disease markers (72). The substitution of low- and no-calorie sweeteners for these sugars in food/beverages has resulted in the expected reductions in adiposity markers including body weight, BMI, WC, and fat mass as shown by several systematic reviews and meta-analyses in predominantly OW/OB participants (73–76). Small reductions in BMI and blood pressure were also seen with the substitution of low- and no-calorie sweeteners for sucrose in food/beverages in predominantly healthy participants (21). On the other hand, in analyses restricted to the effect of LNCSB in substitution for water or matched noncaloric comparators that did not allow for the displacement of calories from SSB (placebo, no intervention, water, or weight loss diet) (27,73–75), no differences were found in body weight in participants predominantly with OW/OB.
Interpreted together with the modeling studies and randomized controlled trials, the findings from our prespecified change and substitution analyses are consistent with the mechanism that LNCSB lead to lower weight insofar as they contribute to a reduction in net energy intake. Although both models were associated with reductions in adiposity outcomes with LNCSB, these associations did not translate into the expected reductions in T2D risk, but reductions were seen in the substitution of water for SSB. One reason may be an inability to mitigate reverse causality through incomplete adjustment for adiposity and other risk factors for T2D more so than CHD. Those with high intake of LNCSB in the available cohorts were at higher risk of T2D (44,52). Another reason may be surveillance bias owing to the increased risk of T2D for those with high intake. The three largest prospective cohort studies in the analysis showed a greater prevalence of fasting glucose screening among individuals who increased their LNCSB intake compared with those who maintained stable intake (52). A third reason could be the unexplained large heterogeneity among studies examining the substitution of SSB for LNCSB and water and change in LNCSB intake in relation to T2D risk. Post hoc analysis with alternative modeling (random effects if fixed effects were used because of only five or fewer studies) only changed the result for the substitution of SSB for water, but the direction of association still indicated benefit. This highlights the need for further high-quality cohort studies assessing substitution of water or LNCSB for SSB with T2D to increase the precision, direction, and certainty of this association.
There are several strengths of our synthesis. First, we included statistical models of exposure that minimize reverse causality and residual confounding from incomplete adjustment of confounders and behavior clustering, providing evidence that is more robust, biologically plausible, and consistent with the evidence from randomized controlled trials. Prevalent or baseline analyses of LNCSB cannot capture the intended replacement strategy of the substitution of LNCSB for SSB and are susceptible to reverse causation, resulting in an underestimation of the intended cardiometabolic benefits (13,24,28,29,31,52,77,78). Second, we used a systematic approach to identify all available prospective cohort studies including the systematic search strategy, quantitative synthesis, and assessment of the certainty of the evidence with GRADE. While GRADE has been recommended as the standard for assessing certainty of evidence for dietary recommendations from nutrition synthesis (79); our sensitivity analysis with the alternative NutriGrade showed, on average, a higher degree of confidence in the results. Third, the available prospective cohort studies provided large sample sizes, long durations of follow-up, and adjustment for multiple dietary and lifestyle factors. Finally, both the change and substitution analyses were considered as dose-response analyses where the association was significant, which strengthened the certainty of the evidence.
There were several limitations of our synthesis. First, the certainty of evidence started at low owing to the observational nature of the prospective cohort studies and the inability to exclude both unmeasured and measured residual confounding (80), make any causal relationships, or completely eliminate the effects of reverse causality. Second, there was serious inconsistency in the estimates for changes in LNCSB and body weight, the substitution of LNCSB for water and T2D, and the substitution of water for SSB and body weight. Third, there were sources of serious indirectness owing to the limited number of available cohorts with use of the two prespecified statistical models. Only single-sex cohort comparisons were available to assess the evidence of change in LNCSB intake and WC (50) and substitution of LNCSB or water for SSB in relation to adiposity measures (36), and no studies included assessment of metabolic syndrome. Finally, the small number of available prospective cohort studies resulted in serious imprecision in the pooled estimates for many outcomes. The pooled estimates and 95% CIs contained clinically important benefits and harms, and there was instability in the estimates in sensitivity analyses for several outcomes. Balancing the limitations and strengths of this analysis, the available evidence was rated as low or very low across the outcomes.
Our findings are relevant for informing guidance on the role of LNCSB as part of sugar reduction strategies. Whereas there is a universal call to reduce SSB (3,4), support for LNCSB as a replacement strategy for SSB has been mixed owing to concerns that LNCSB may increase the risk of OB, T2D, and CVD (19–23). Our prespecified models show that LNCSB were not associated with higher risk; rather, they were associated with a lower risk in important cardiometabolic outcomes in the intended substitution for SSB and may provide some benefits as the standard of care in substitution for water across cardiometabolic outcomes. We suggest that, in updates of clinical practice guidelines (32), national dietary guidelines (17,81), and the resulting food, nutrition, and public health policies and programs that target a reduction in SSB (18,82–84), recommending LNCSB be considered as an alternative replacement strategy to the standard of care water along with other currently recommend alternatives.
In conclusion, LNCSB are not associated with weight gain or an increase in adverse cardiometabolic outcomes and may be associated with some advantages in analyses that model the change in intake of LNCSB or the substitutions of LNCSBS for SSB in people with varying cardiometabolic risk profiles inclusive of T2D. The available evidence provides some indication that increased intake of LNCSB is associated with lower adiposity and LNCSB in their intended substitution for SSB are modestly associated with lower adiposity, lower risk of OB and CHD, and reductions in total mortality; these associations are comparable with those of the standard of care, water. Our confidence in the pooled estimates was reduced largely by the few available prospective cohort studies, which contributed to imprecision and indirectness. More prospective cohort studies with robust analytical approaches will be important for addressing these uncertainties and strengthening causal inferences, but there is also a need for large high-quality randomized trials of clinical outcomes. In the meantime, given the importance of targeting reductions in SSB, the evidence supports the use of LNCSB as an alternative to water as part of clinical and public health strategies to reduce SSB consumption.
Article Information
Acknowledgments. The authors thank Amelie Keller, Jean-Phillippe Drouin-Chartier, and Dahlia Stern for data sharing and performing additional analysis.
Funding. The Diabetes and Nutrition Study Group (DNSG) of the European Association of the Study of Diabetes (EASD) commissioned this systematic review and meta-analysis and provided the primary funding and logistical support for meetings as part of the development of the EASD Clinical Practice Guidelines for Nutrition Therapy. This work was also supported by the Canadian Institutes of Health Research (funding reference number, 129920) through the Canada-wide Human Nutrition Trialists’ Network (NTN). The Diet, Digestive tract, and Disease (3-D) Centre, funded through the Canada Foundation for Innovation (CFI) and the Ministry of Research and Innovation’s Ontario Research Fund (ORF), provided the infrastructure for the conduct of this project. J.J.L. was funded by a Canadian Institutes of Health Research (CIHR) Frederick Banting and Charles Best Canada Graduate Scholarship Doctoral Award and Banting and Best Diabetes Centre Novo Nordisk Studentship. T.A.K. was funded by a Toronto 3D Post-doctoral Fellowship Award. NM was funded by the CIHR Canada’s Graduate Scholarship - Master’s award. J.L.S. was funded by a PSI Graham Farquharson Knowledge Translation Fellowship, Diabetes Canada Clinician Scientist award, CIHR INMD/CNS New Investigator Partnership Prize, and Banting and Best Diabetes Centre Sun Life Financial New Investigator Award. The Clinical Practice Guidelines Committee of the DNSG of the EASD had input on all aspects of the work. No other funders had a role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, approval of the manuscript or decision to publish.
Duality of Interest. T.A.K. has received research support from the National Honey Board. N.M. was a full-time employee with Loblaw Companies Limited from November 2011 to December 2017. V.S.M. reports personal fees from Kaplan Fox & Kilsheimer, LLP, outside the submitted work. V.S.M. has been on a pro bono retainer for expert support for the Center for Science in the Public Interest in litigation related to sugar-sweetened beverages and has served as a consultant for the City of San Francisco for a case related to health warning labels on soda. V.S.M. reports personal fees from city and county of San Francisco outside the submitted work. J.O.H. is a member of the Scientific Advisory Committee for General Mills, McCormick Science Institute, and Milk Producers Educational Program. He has equity in Gelesis and Shakabuku, LLC. J.O.H. receives research funding from National Institutes of Health and from the National Cattlemen’s Beef Association. P.B.J. is running a nonprofit public-funded research project entitled: Innosweet- integrated perception, psychology, and physiology for maintaining sweetness perception via sugar replacement and reduction for value added healthy beverage applications (6150-00037A). P.B.J. is an honorary member of European Stevia Association (EUSTAS) and was until 2018 a board member of the DNSG of the EASD. D.R. is the president of Croatian Society for Diabetes and Metabolic Disorders of Croatian Medical Association. He serves as an Executive Committee member of Croatian Endocrine Society, Croatian Society for Obesity, and Croatian Society for Endocrine Oncology. He was a board member and secretary of International Diabetes Federation (IDF) Europe and currently he is the chair of the IDF Young Leaders in Diabetes Programme. He has served as an Executive Committee member of Diabetes and Nutrition Study Group of EASD and currently, he serves as an Executive Committee member of Diabetes and Cardiovascular Disease Study Group of EASD. He has received travel support, speaker fees, and honoraria from advisory board engagements and/or consulting fees from International Sweeteners Association. D.R. is director of Vuk Vrhovac University Clinic for Diabetes, Endocrinology and Metabolic Diseases at Merkur University Hospital, Zagreb, Croatia. He has served as principal investigator or co-investigator in clinical trials of AstraZeneca, Eli Lilly, Merck Sharp & Dohme (MSD), Novo Nordisk, Sanofi, Solvay, and Trophos. He has received travel support, speaker fees, and honoraria from advisory board engagements and/or consulting fees from Abbott, Amgen, AstraZeneca, Bayer, Belupo, Boehringer Ingelheim, Eli Lilly, Lifescan/Johnson & Johnson, Krka, Medtronic, Mediligo, Novartis, Novo Nordisk, MSD, Pfizer, Pliva, Roche, Salvus, Sandoz, Sanofi, and Takeda. H.K. is Director of Clinical Research at the Physicians Committee for Responsible Medicine, a nonprofit organization providing nutrition education and research, and a board member of the DNSG of the EASD. J.S.-S. reports serving on the board of and receiving grant support through his institution from Eroski Foundation. He has received research support from CIBERobn, Instituto de Salud Carlos III, Ministerio de Educación y Ciencia, Departament de Salut Pública de la Generalitat de Catalunya, European Commission, Almond Board of California, Patrimonio Comunal Olivarero, La Morella Nuts, and Borges S.A. He reports receiving consulting fees or travel expenses from Danone, Eroski Foundation, Nuts for Life, Nestlé, Abbott Laboratories, and Aguas Font Vella y Lanjarón. J.S.-S. reports serving on the board of and receiving grant support through his institution from the International Nut and Dried Fruit Council. He reports serving on the Executive Committee of Instituto Danone (Spain). He has received research reports from the National Institutes of Health, California Walnut Commission and reports receiving consulting fees or travel expenses from Instituto Danone (Spain) and Australian Nut Industry Council. He is on the Clinical Practice Guidelines Expert Committee of the EASD and served in the Scientific Committee of the Spanish Agency for Food Safety and Nutrition and the Spanish Federation of the Scientific Societies of Food, Nutrition and Dietetics. He is a member of the International Carbohydrate Quality Consortium (ICQC) and Executive Board Member of the DNSG of the EASD. C.W.C.K. has received grants or research support from NIHR, International Nut and Dried Fruit Council, and International Tree Nut Council Research & Education Foundation. In addition, he has received in-kind research support, travel support, and/or honoraria from the California Walnut Commission, International Nut and Dried Fruit Council, International Pasta Organisation, and Oldways Preservation Trust. He has served on the scientific advisory board for the International Tree Nut Council, International Pasta Organisation, and Oldways Preservation Trust. He is a member of the ICQC, Executive Board member of the DNSG of the EASD, is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the EASD, and is a director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. C.W.C.K. has received grants or research support from the Advanced Foods and Materials Network, Agriculture and Agri-Food Canada, Almond Board of California, Peanut Institute, Barilla, Canola Council of Canada, Loblaw Companies Limited, Pulse Canada, and Unilever. He has received in-kind research support from the Almond Board of California, American Peanut Council, Barilla, Kellogg Canada, Loblaw Companies Limited, Quaker (PepsiCo), Primo, Unico, Unilever, and WhiteWave Foods/Danone. He has received travel support and/or honoraria from the American Peanut Council, Barilla, Canola Council of Canada, General Mills, Loblaw Companies Limited, Nutrition Foundation of Italy, Paramount Farms, Peanut Institute, Pulse Canada, Sun-Maid, Tate & Lyle, Unilever, and WhiteWave Foods/Danone. He has served on the scientific advisory board for McCormick Science Institute. J.L.S. has received research support from the Canadian Foundation for Innovation, Ontario Research Fund, Province of Ontario Ministry of Research and Innovation and Science, CIHR, Diabetes Canada, American Society for Nutrition (A.S.N.), I.N.C. International Nut and Dried Fruit Council Foundation, National Honey Board (the U.S. Department of Agriculture [USDA] honey “Checkoff” program), Institute for the Advancement of Food and Nutrition Sciences (IAFNS), Pulse Canada, Quaker Oats Center of Excellence, The United Soybean Board (the USDA soy “Checkoff” program), The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), The Plant Protein Fund at the University of Toronto (a fund which has received contributions from I.F.F.), and The Nutrition Trialists Fund at the University of Toronto (a fund established by an inaugural donation from the Calorie Control Council). He has received food donations to support randomized controlled trials from the Almond Board of California, California Walnut Commission, Peanut Institute, Barilla, Unilever/Upfield, Unico/Primo, Loblaw Companies, Quaker, Kellogg Canada, WhiteWave Foods/Danone, Nutrartis, and Dairy Farmers of Canada. He has received travel support, speaker fees and/or honoraria from A.S.N., Danone, Dairy Farmers of Canada, FoodMinds L.L.C., Nestlé, Abbott, General Mills, Nutrition Communications, International Food Information Council (IFIC), Calorie Control Council, International Sweeteners Association, and International Glutamate Technical Committee. He has or has had ad hoc consulting arrangements with Perkins Coie L.L.P., Tate & Lyle, Phynova, and Inquis Clinical Research. He is a former member of the European Fruit Juice Association Scientific Expert Panel and former member of the Soy Nutrition Institute (S.N.I.) Scientific Advisory Committee. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes (EASD), Canadian Cardiovascular Society (C.C.S.), and Obesity Canada/Canadian Association of Bariatric Physicians and Surgeons. He serves or has served as an unpaid member of the Board of Trustees and an unpaid scientific advisor for the Carbohydrates Committee of IAFNS. He is a member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His spouse is an employee of A.B. InBev.
Author Contributions. T.A.K. and J.L.S. took responsibility for the integrity of the data and the accuracy of the data analysis. J.J.L., T.A.K., and J.L.S. had full access to all of the data. J.J.L. and T.A.K. developed and executed the search strategy, extracted data, performed the analysis and interpretation of data, and wrote the first draft of the manuscript. N.M., V.S.M., J.O.H., L.A.L., P.B.J., D.R., H.K., J.S.-S., C.W.C.K., and J.L.S. participated in the interpretation of data and critically revised the manuscript for important intellectual content. P.B.J., D.R., H.K., J.S.-S., C.W.C.K., and J.L.S. were responsible for the original concept, design, and supervision of the work. All authors read and approved the final version of the manuscript.
Prior Presentation. Parts of this study were presented in abstract form at NUTRITION 2020 LIVE ONLINE, 1-4 June 2020; Advances and Controversies in Nutrition and Diabetes, 22–23 January 2021; and the 38th International Symposium on the Diabetes and Metabolism, 21–24 June 2021.
Footnotes
Clinical trial reg. no. NCT04245826, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.19692403.
References
- 1. Lustig RH, Schmidt LA, Brindis CD. Public health: the toxic truth about sugar. Nature 2012;482:27–29 [DOI] [PubMed] [Google Scholar]
- 2. Malik VS, Hu FB. Sugar-sweetened beverages and health: where does the evidence stand? Am J Clin Nutr 2011;94:1161–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Guideline: Sugars Intake for Adults and Children. Geneva, World Health Org., 2015 [PubMed] [Google Scholar]
- 4. United States Department of Agriculture (USDA), United States Department of Health and Human Services (HHS) . Dietary Guidelines for Americans, 2020-2025. 9th ed., 2020. Accessed 30 December 2020. Available from https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf
- 5. Brisbois TD, Marsden SL, Anderson GH, Sievenpiper JL. Estimated intakes and sources of total and added sugars in the Canadian diet. Nutrients 2014;6:1899–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Welsh JA, Sharma AJ, Grellinger L, Vos MB. Consumption of added sugars is decreasing in the United States. Am J Clin Nutr 2011;94:726–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rana H, Mallet M-C, Gonzalez A, Verreault M-F, St-Pierre S. Free sugars consumption in Canada. Nutrients 2021;13:1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Azaïs-Braesco V, Sluik D, Maillot M, Kok F, Moreno LA. A review of total & added sugar intakes and dietary sources in Europe. Nutr J 2017;16:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. U.S. Food and Drug Administration . High-Intensity Sweeteners, 2020. Accessed 15 July 2020. Available from https://www.fda.gov/food/food-additives-petitions/high-intensity-sweeteners
- 10. Government of Canada . Sugar Substitutes, 2004. Accessed 15 July 2020. Available from https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/food-additives/sugar-substitutes.html
- 11. Mortensen A. Sweeteners permitted in the European Union: safety aspects. Scand J Food Nutr 2006;50:104–116 [Google Scholar]
- 12. Maki KC, Curry LL, Carakostas MC, et al. The hemodynamic effects of rebaudioside A in healthy adults with normal and low-normal blood pressure. Food Chem Toxicol 2008;46(Suppl. 7):S40–S46 [DOI] [PubMed] [Google Scholar]
- 13. Johnson RK, Lichtenstein AH, Anderson CAM, et al.; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Quality of Care and Outcomes Research; and Stroke Council . Low-calorie sweetened beverages and cardiometabolic health: a science advisory from the American Heart Association. Circulation 2018;138:e126–e140 [DOI] [PubMed] [Google Scholar]
- 14. American Diabetes Association . 5. Facilitating behavior change and well-being to improve health outcomes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S48–S65 [DOI] [PubMed] [Google Scholar]
- 15. Sievenpiper JL, Chan CB, Dworatzek PD, Freeze C; Diabetes Canada Clinical Practice Guidelines Expert Committee . Nutrition therapy. Can J Diabetes 2018;42(Suppl. 1):S64–S79 [DOI] [PubMed] [Google Scholar]
- 16. Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ 2020;192:E875–E891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Health Canada . Canada’s Dietary Guidelines for Health Professionals and Policy Makers. 2019 Accessed 22 January 2021. Available from https://food-guide.canada.ca/sites/default/files/artifact-pdf/CDG-EN-2018.pdf
- 18. European Commission. Health Promotion and Disease Prevention . Sugars and Sweeteners, 2020. Accessed 13 April 2021. Available from https://ec.europa.eu/jrc/en/health-knowledge-gateway/promotion-prevention/nutrition/sugars-sweeteners#_Frenchrepublic2017
- 19. Azad MB, Abou-Setta AM, Chauhan BF, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ 2017;189:E929–E939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Imamura F, O’Connor L, Ye Z, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 2015;351:h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Toews I, Lohner S, Küllenberg de Gaudry D, Sommer H, Meerpohl JJ. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ 2019;364:k4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Narain A, Kwok CS, Mamas MA. Soft drinks and sweetened beverages and the risk of cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Clin Pract 2016;70:791–805 [DOI] [PubMed] [Google Scholar]
- 23. Mullee A, Romaguera D, Pearson-Stuttard J, et al. Association between soft drink consumption and mortality in 10 European countries. JAMA Intern Med 2019;179:1479–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ashwell M, Gibson S, Bellisle F, et al. Expert consensus on low-calorie sweeteners: facts, research gaps and suggested actions. Nutr Res Rev 2020;33:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bright OM, Wang DD, Shams-White M, et al. Research priorities for studies linking intake of low-calorie sweeteners and potentially related health outcomes: research methodology and study design. Curr Dev Nutr 2017;1:e000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khan TA, Sievenpiper JL. Low-energy sweeteners and cardiometabolic health: is there method in the madness? Am J Clin Nutr 2020;112:917–919 [DOI] [PubMed] [Google Scholar]
- 27. Sievenpiper JL, Khan TA, Ha V, Viguiliouk E, Auyeung R. The importance of study design in the assessment of nonnutritive sweeteners and cardiometabolic health. CMAJ 2017;189:E1424–E1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khan TA, Malik VS, Sievenpiper JL. Letter by Khan et al regarding article, “artificially sweetened beverages and stroke, coronary heart disease, and all-cause mortality in the Women’s Health Initiative”. Stroke 2019;50:e167–e168 [DOI] [PubMed] [Google Scholar]
- 29. Malik VS. Non-sugar sweeteners and health. BMJ 2019;364:k5005. [DOI] [PubMed] [Google Scholar]
- 30. de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr 2011;93:1321–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith JD, Hou T, Hu FB, et al. A comparison of different methods for evaluating diet, physical activity, and long-term weight gain in 3 prospective cohort studies. J Nutr 2015;145:2527–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mann JI, De Leeuw I, Hermansen K, et al.; Diabetes and Nutrition Study Group (DNSG) of the European Association . Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr Metab Cardiovasc Dis 2004;14:373–394 [DOI] [PubMed] [Google Scholar]
- 33. Higgins JP, Thomas J, Chandler J, et al. The Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, 2019, 2nd edition, Chichester, UK. [Google Scholar]
- 34. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–2012 [DOI] [PubMed] [Google Scholar]
- 35. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stookey JD, Constant F, Popkin BM, Gardner CD. Drinking water is associated with weight loss in overweight dieting women independent of diet and activity. Obesity (Silver Spring) 2008;16:2481–2488 [DOI] [PubMed] [Google Scholar]
- 37. Crippa A, Discacciati A, Bottai M, Spiegelman D, Orsini N. One-stage dose-response meta-analysis for aggregated data. Stat Methods Med Res 2019;28:1579–1596 [DOI] [PubMed] [Google Scholar]
- 38. Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000 [Google Scholar]
- 39. Stevens RD, Dowdy DW, Michaels RK, Mendez-Tellez PA, Pronovost PJ, Needham DM. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med 2007;33:1876–1891 [DOI] [PubMed] [Google Scholar]
- 40. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188 [DOI] [PubMed] [Google Scholar]
- 41. Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid-Based Healthc 2015;13:196–207 [DOI] [PubMed] [Google Scholar]
- 42. Symons MJ, Moore DT. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol 2002;55:893–899 [DOI] [PubMed] [Google Scholar]
- 43. Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280:1690–1691 [DOI] [PubMed] [Google Scholar]
- 44. O’Connor L, Imamura F, Lentjes MA, Khaw KT, Wareham NJ, Forouhi NG. Prospective associations and population impact of sweet beverage intake and type 2 diabetes, and effects of substitutions with alternative beverages. Diabetologia 2015;58:1474–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harrell FEJ. Regression Modeling Strategies - With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY, Springer, 2001 [Google Scholar]
- 46. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–463 [DOI] [PubMed] [Google Scholar]
- 47. Schünemann HJ, Higgins JPT, Vist GE, et al. Completing ‘summary of findings’ tables and grading the certainty of the evidence. In Cochrane Handbook for Systematic Reviews of Interventions, 2019, p. 375–402 [Google Scholar]
- 48. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–394 [DOI] [PubMed] [Google Scholar]
- 49. Schwingshackl L, Knüppel S, Schwedhelm C, et al. Perspective: NutriGrade: a scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr 2016;7:994–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stern D, Middaugh N, Rice MS, et al. Changes in sugar-sweetened soda consumption, weight, and waist circumference: 2-year cohort of Mexican women. Am J Public Health 2017;107:1801–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Keller A, O’Reilly EJ, Malik V, et al. Substitution of sugar-sweetened beverages for other beverages and the risk of developing coronary heart disease: results from the Harvard Pooling Project of Diet and Coronary Disease. Prev Med 2020;131:105970. [DOI] [PubMed] [Google Scholar]
- 52. Drouin-Chartier J-P, Zheng Y, Li Y, et al. Changes in consumption of sugary beverages and artificially sweetened beverages and subsequent risk of type 2 diabetes: results from three large prospective U.S. cohorts of women and men. Diabetes Care 2019;42:2181–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. National Diabetes Data Group . Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- 54. Pan A, Malik VS, Hao T, Willett WC, Mozaffarian D, Hu FB. Changes in water and beverage intake and long-term weight changes: results from three prospective cohort studies. Int J Obes 2013;37:1378–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang M, Quddus A, Stinson L, et al. Artificially sweetened beverages, sugar-sweetened beverages, plain water, and incident diabetes mellitus in postmenopausal women: the prospective Women’s Health Initiative observational study. Am J Clin Nutr 2017;106:614–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yin J, Zhu Y, Malik V, et al. Intake of sugar-sweetened and low-calorie sweetened beverages and risk of cardiovascular disease: a meta-analysis and systematic review. Adv Nutr 2021;12:89–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Normand M, Ritz C, Mela D, Raben A. Low-energy sweeteners and body weight: a citation network analysis. BMJ Nutr Prev Health 2021;4:319–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hendriksen MA, Tijhuis MJ, Fransen HP, Verhagen H, Hoekstra J. Impact of substituting added sugar in carbonated soft drinks by intense sweeteners in young adults in the Netherlands: example of a benefit-risk approach. Eur J Nutr 2011;50:41–51 [DOI] [PubMed] [Google Scholar]
- 59. Ma Y, He FJ, Yin Y, Hashem KM, MacGregor GA. Gradual reduction of sugar in soft drinks without substitution as a strategy to reduce overweight, obesity, and type 2 diabetes: a modelling study. Lancet Diabetes Endocrinol 2016;4:105–114 [DOI] [PubMed] [Google Scholar]
- 60. Goiana-da-Silva F, Severo M, Cruz E Silva D, et al. Projected impact of the Portuguese sugar-sweetened beverage tax on obesity incidence across different age groups: a modelling study. PLoS Med 2020;17:e1003036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lal A, Peeters A, Brown V, et al. The modelled population obesity-related health benefits of reducing consumption of discretionary foods in Australia. Nutrients 2020;12:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Basto-Abreu A, Braverman-Bronstein A, Camacho-García-Formentí D, et al. Expected changes in obesity after reformulation to reduce added sugars in beverages: a modeling study. PLoS Med 2018;15:e1002664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Salgado MV, Penko J, Fernandez A, et al. Projected impact of a reduction in sugar-sweetened beverage consumption on diabetes and cardiovascular disease in Argentina: a modeling study. PLoS Med 2020;17:e1003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Te Morenga LA, Howatson AJ, Jones RM, Mann J. Dietary sugars and cardiometabolic risk: systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am J Clin Nutr 2014;100:65–79 [DOI] [PubMed] [Google Scholar]
- 65. Jayalath VH, de Souza RJ, Ha V, et al. Sugar-sweetened beverage consumption and incident hypertension: a systematic review and meta-analysis of prospective cohorts. Am J Clin Nutr 2015;102:914–921 [DOI] [PubMed] [Google Scholar]
- 66. Malik VS, Popkin BM, Bray GA, Després J-P, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr 2013;98:1084–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stanhope KL, Medici V, Bremer AA, et al. A dose-response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am J Clin Nutr 2015;101:1144–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Raben A, Møller BK, Flint A, et al. Increased postprandial glycaemia, insulinemia, and lipidemia after 10 weeks’ sucrose-rich diet compared to an artificially sweetened diet: a randomised controlled trial. Food Nutr Res 2011;55:5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ 2008;336:309–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Choi HK, Willett W, Curhan G. Fructose-rich beverages and risk of gout in women. JAMA 2010;304:2270–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Maersk M, Belza A, Stødkilde-Jørgensen H, et al. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr 2012;95:283–289 [DOI] [PubMed] [Google Scholar]
- 73. Rogers PJ, Hogenkamp PS, de Graaf C, et al. Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int J Obes 2016;40:381–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rogers PJ, Appleton KM. The effects of low-calorie sweeteners on energy intake and body weight: a systematic review and meta-analyses of sustained intervention studies. Int J Obes 2021;45:464–478 [DOI] [PubMed] [Google Scholar]
- 75. Laviada-Molina H, Molina-Segui F, Pérez-Gaxiola G, et al. Effects of nonnutritive sweeteners on body weight and BMI in diverse clinical contexts: systematic review and meta-analysis. Obes Rev 2020;21:e13020. [DOI] [PubMed] [Google Scholar]
- 76. Miller PE, Perez V. Low-calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies. Am J Clin Nutr 2014;100:765–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Malik VS, Li Y, Pan A, et al. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation 2019;139:2113–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mela DJ, McLaughlin J, Rogers PJ. Perspective: standards for research and reporting on low-energy (“artificial”) sweeteners. Adv Nutr 2020;11:484–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schwingshackl L, Schünemann HJ, Meerpohl JJ. Improving the trustworthiness of findings from nutrition evidence syntheses: assessing risk of bias and rating the certainty of evidence. Eur J Nutr 2021;60:2893–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fewell Z, Davey Smith G, Sterne JAC. The impact of residual and unmeasured confounding in epidemiologic studies: a simulation study. Am J Epidemiol 2007;166:646–655 [DOI] [PubMed] [Google Scholar]
- 81. Dietary Guidelines Advisory Committee . Scientific Report of the 2020 Dietary Guidelines Advisory Committee. Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services. Washington, DC, U.S. Department of Agriculture, Agricultural Research Service, 2020. Accessed 13 April 2021. Available from https://www.dietaryguidelines.gov/sites/default/files/2020-07/ScientificReport_of_the_2020DietaryGuidelinesAdvisoryCommittee_first-print.pdf
- 82. Center for Science in the Public Interest . Encouraging healthier choices in hospitals, 2014. Accessed 13 April 2021. Available from https://cspinet.org/resource/encouraging-healthier-choices-hospitals
- 83. National Health Service . Sugar: The Facts, 2020. Accessed 13 April 2021. Available from https://www.nhs.uk/live-well/eat-well/how-does-sugar-in-our-diet-affect-our-health/
- 84. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board . Strategies to Limit Sugar-Sweetened Beverage Consumption in Young Children: Proceedings of a Workshop. Washington, DC, National Academies Press, 2017 [PubMed] [Google Scholar]
- 85. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chen L, Appel LJ, Loria C, et al. Reduction in consumption of sugar-sweetened beverages is associated with weight loss: the PREMIER trial. Am J Clin Nutr 2009;89:1299–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bernstein AM, de Koning L, Flint AJ, Rexrode KM, Willett WC. Soda consumption and the risk of stroke in men and women. Am J Clin Nutr 2012;95:1190–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pan A, Malik VS, Schulze MB, Manson JE, Willett WC, Hu FB. Plain-water intake and risk of type 2 diabetes in young and middle-aged women. Am J Clin Nutr 2012;95:1454–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fresán U, Gea A, Bes-Rastrollo M, Ruiz-Canela M, Martínez-Gonzalez MA. Substitution models of water for other beverages, and the incidence of obesity and weight gain in the SUN cohort. Nutrients 2016;8:688. [DOI] [PMC free article] [PubMed] [Google Scholar]