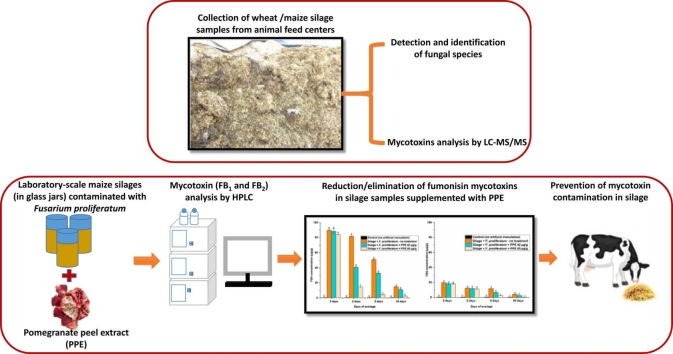
Abstract
A study was conducted on six animal feed centers in Israel where fungal and mycotoxin presence was examined in maize and wheat silages. Fumonisin mycotoxins FB1 and FB2 were present in every maize silage sample analyzed. Interestingly, no correlation was found between the occurrence of specific mycotoxins and the presence of the fungal species that might produce them in maize and wheat silages. We further investigated the effect of pomegranate peel extract (PPE) on Fusarium infection and fumonisin biosynthesis in laboratory-prepared maize silage. PPE had an inhibitory effect on FB1 and FB2 biosynthesis by Fusarium proliferatum, which resulted in up to 90 % reduction of fumonisin production in silage samples compared to untreated controls. This finding was supported by qRT-PCR analysis, showing downregulation of key genes involved in the fumonisin-biosynthesis pathway under PPE treatment. Our results present promising new options for the use of natural compounds that may help reduce fungal and mycotoxin contamination in agricultural foodstuff, and potentially replace traditionally used synthetic chemicals.
Keywords: Silage, Fungi, Mycotoxin analysis, Fumonisins, Pomegranate peel extract
Graphical Abstract
Highlights
-
•
The mycotoxins fumonisin B1 and B2 were detected in all analyzed maize silage samples.
-
•
No correlation was found between mycotoxins and their fungal sources in silages.
-
•
Treatment with PPE demonstrated strong anti-mycotoxigenic activity in silages samples.
1. Introduction
Silage, which is one of the main animal feed sources for dairy cattle, can be contaminated with mycotoxins that are produced as secondary metabolites by filamentous fungi belonging mainly to the genera Aspergillus, Penicillium and Fusarium. When ingested, mycotoxins can have severe acute and chronic toxic effects, presenting a serious risk to animal and human health. Silage is stored under anaerobic conditions and acidification of the ensiled forages by lactic acid-producing bacteria. Most mycotoxigenic fungi are unable to grow in the acidic silage environment under low oxygen levels [21], [37]. However, mycotoxins produced by mycotoxigenic fungi in the field may remain unchanged during the silage process, due to their high stability. For example, the concentration of zearalenone (ZEN) remained almost unchanged in maize silage over a 12-week period, while its main producer in the field, Fusarium culmorum, could no longer be detected in the silage after 11 days, suggesting that the ZEN had been produced before ensiling [19]. The use of various antifungal compounds is expected to prevent the growth of mycotoxigenic fungi during ensiling and limit mycotoxin contamination formed during this time. A number of studies have shown that the use of biocontrol agents can decrease aerobic spoilage and reduce or prevent fungal contamination in silage [16], [17]. Maize silage inoculated with Lactobacillus buchneri and Pediococcus pentosaceus had lower yeast and mold counts than untreated silage [38]. That study also demonstrated that treatment with potassium sorbate reduces fungal contamination and aerobic spoilage in maize silage. However, application of such additives had no effect on the concentrations of Fusarium producing toxins in maize silage [16], [18], [38]. Since the current strategies are not effective enough to eliminate or reduce mycotoxin contamination to safe threshold levels, there is a need for alternative, environmentally friendly methods to control these toxic substances during ensiling.
Plants produce a large variety of compounds that are responsible for a wide range of biological and pharmacological properties, including antimicrobial activities [41]. Some plant-derived compounds have been reported to exhibit direct antifungal activity in treated plant hosts [24]. Different parts of the pomegranate (Punica granatum L.) fruit, especially the peel, are considered a rich source of polyphenols, such as ellagitannins, mainly including α and β isomers of punicalagin, gallic acid, ellagic acid, and its glycosylated derivatives, anthocyanins, proteins, bioactive peptides and polysaccharides [2], [28], [36]. Several in-vitro and in-vivo studies have reported that pomegranate peel extracts (PPEs) had a higher content of total polyphenols, as well as strong antioxidant, antitumor, antibacterial and antifungal activities [1], [13], [26], [31], [35], [8]. Moreover, our recent work demonstrated that beyond its antifungal activity, PPE has the ability to inhibit aflatoxin production by Aspergillus flavus [33]. In that study, PPE inhibited aflatoxin B1 production without affecting the fungal growth, suggesting that the extract may affect specific genes encoding enzymes involved in the aflatoxin-biosynthesis pathway. These findings led us to explore PPE as a potential inhibitor of mycotoxin production in agricultural commodities.
Here, the mycotoxins fumonisin B1 and B2 (FB1 and FB2, respectively), which are produced by several Fusarium species, were detected in all randomly selected maize silage samples collected from six animal feed centers across Israel. This indicated that mycotoxins could persist, even in well-preserved silage. Furthermore, PPE inhibited FB1 and FB2 produced by Fusarium proliferatum during maize ensiling on a laboratory-scale, suggesting this extract’s potential to prevent mycotoxin contamination of animal feed.
2. Materials and methods
2.1. Sample collection
A total of 320 samples of silage for dairy cattle, 160 of wheat and 160 of maize, were collected from six animal feed centers located in northern, central and southern districts of Israel over a 2-year period (2018–2019). The samples were collected when the silages were approximately 6 months old. The sample aliquots (~ 500 g) were taken from silage stacks, in an area at least 1 m distant from the sides, top and bottom, using a silage drill approximately 1 m behind the cutting face of the silage stack. Upon collection into sterile plastic bags, the samples were kept cool during transport to the laboratory. Each sample was divided into two subsamples: a first subsample of 100 g was analyzed for fungal colony counts immediately after arrival at the laboratory, and the remaining sample was stored at − 20 ℃ until further mycotoxin analysis. An aqueous extract of the silage sample was prepared for pH measurement with a pH electrode.
2.2. Culturing and identification of fungal species
Silage samples (100 g wet weight) were transferred to flasks containing 400 ml peptone water (Difco; Becton Dickinson, Sparks, MD, USA). The suspension was transferred to a plastic bag and homogenized in a stomacher blender (Interscience, Saint-Nom-la-Bretèche, France) for 2 min. Ten-fold dilutions were prepared in peptone water and samples (100 µl) were plated on potato dextrose agar (PDA) plates supplemented with chloramphenicol (20 µg/ml) and dichloran (2 µg/ml) to prevent bacterial contamination and growth of Mucorales fungi, respectively. Molds were enumerated using the standard plate count method, following incubation at 28 °C for 5 days, and the results were expressed in number of colony-forming units per gram of silage sample (CFU/g). Individual colonies were transferred singly to PDA plates to obtain a pure culture for further identification of fungal species by morphological analysis and sequencing of ribosomal DNA internal transcribed spacer (ITS). Fungal DNA was extracted from lyophilized mycelium using a CTAB-based method as previously described [32]. The yield and quality of DNA were assessed using a NanoDrop One spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). The ITS rRNA gene regions in fungi were amplified by PCR and sequenced using universal primers ITS1/ITS4 (Table S1). The sequence data were analyzed and compared using BLAST against the NCBI database (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
2.3. Mycotoxin analysis
2.3.1. Preparation of mycotoxin standard solutions
Individual stock standard solutions (1 mg/ml) of aflatoxins B1, B2, G1, and G2 (AFB1, AFB2, AFG1, AFG2, respectively), ochratoxin A (OTA), patulin (PAT), gliotoxin (GLIO), zearalenone (ZEN), deoxynivalenol (DON), FB1 and FB2, T-2 toxin (T-2) and HT-2 toxin (HT-2) (Fermentek, Israel) were prepared in methanol. Mixed multi-mycotoxin standard solutions at three concentration levels were prepared by dilution of the single analyte stock standard solutions in methanol. All solutions were stored at − 20 ℃ until use.
2.3.2. Mycotoxin analysis by high-performance liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS)
Mycotoxin concentrations were analyzed in 82 randomly selected samples, consisting of 38 maize and 44 wheat silage samples. The samples were freeze-dried and ground in a laboratory grinder. Each ground sample (2.5 g) was mixed with 7.5 ml distilled water and extracted with 15 ml of extraction solvent mixture (acetonitrile/ethyl acetate/acetic acid, 10:5:0.15, v/v). After agitation on an orbital shaker for 30 min, the samples were centrifuged at 2150 g for 10 min. A 1-ml aliquot of supernatant was transferred to a 15-ml glass tube and evaporated under a stream of nitrogen gas at 50 °C. The dried residue was reconstituted with 0.3 ml of a 1:1 (v/v) methanol/water mixture, and an aliquot was filtered through a 0.22-µm PTFE filter into a glass injection vial and stored at − 20 °C prior to analysis. The samples were analyzed by LC–MS/MS as described previously [32]. LC separation of 2 µl injected sample was performed on a Nexera X2 UHPLC system (Shimadzu, Tokyo, Japan) with a 100 × 2.1 mm, 2.6 µm Kinetex C18 column, (Phenomenex, Torrance, CA, USA). The column temperature was 40 ℃. The mobile phase were (A) ammonium acetate 2.5 mM acidified with 0.1 % acetic acid, and (B) methanol. The concentration of solution B was raised gradually from 5 % to 95 % within 8 min, then brought back to the initial conditions at 9 min, and allowed to stabilize for 3 min. A flow rate of 0.4 ml/min was used. The LC system was coupled with an API 6500 hybrid triple quadrupole/linear ion trap mass spectrometer (Sciex, Concord, ON, Canada) equipped with a turbo-ion electrospray ion (ESI) source. The mass spectrometer was operated in the multiple reaction monitoring (MRM) in both positive and negative mode within a single run. Positive polarity was applied for all analytes except for DON, ZEN, PAT and GLIO. Analyte specific detection parameters are specified in Table S2 (A, B). Source temperature was set at 350 ℃, ion-spray voltages at − 4500 V (negative mode) and 5000 V (positive mode), curtain gas at 35 arbitrary units (au), nebulizer gas at 60 au, and turbo gas at 40 au.
2.3.3. Validation of analytical parameters
Method performance and validation parameters were determined according to European Commission (EC) regulation no. 401/2006 [10]. Three wheat and three maize silage samples that were not contaminated, or only slightly contaminated with the major mycotoxins were spiked with multi-mycotoxin standard solutions at three concentrations (calibration levels are specified in Table S2). Extraction and analysis were performed as described in Section 2.3.2. The spiking experiments were performed in triplicate at three different time points. Validation parameters, such as precision, accuracy, limit of detection (LOD), limit of quantification (LOQ) and specificity, were determined.
2.4. Laboratory silage studies
2.4.1. Preparation of PPE
Pomegranate (Punica granatum L.) variety Wonderful fruit were purchased from local markets. The fruit peels were freeze-dried and milled into a fine powder using a laboratory grinder. The dried powder (100 g) was extracted with 500 ml of 80 % methanol for 72 h at room temperature in the dark. The suspension was filtered through Whatman no. 1 filter paper and concentrated using a rotary evaporator (Buchi R-100, Flawil, Switzerland) at 45 oC. Then, the extract was freeze-dried and a concentrated stock solution of PPE (100 mg/ml) was prepared in sterile water, which stored at 4 °C until use.
2.4.2. Fungal strain
Fumonisin-producing Fusarium proliferatum strain YO3, isolated from red onion cv. Mata Hari, was used during the field study. The strain was refreshed from − 80 °C by subculturing on PDA plates and maintained at 28 °C before each experiment. Spores (macroconidia) were collected in sterile saline from cultures grown for 4 days and macroconidial suspension was adjusted to the required concentration by counting in a hemocytometer. Inoculum concentration was verified by plating on PDA plates for determination of CFU counts.
2.4.3. Field experiments
The field trial was held in 2019, in Kibbutz Yotvata, Israel, with the maize hybrid "Overland". The ears were inoculated with F. proliferatum at initial silk formation (4–7 days post–silk emergence) by injecting 5-ml of macroconidia suspension (105 conidia/ml) through the silk canals (inside the husk cavity and above the cob). The experiment consisted of three replicates, each with two rows. Maize ears inoculated with sterile saline water served as a non-treated control group. The field was maintained for additional 40 days until ear maturity. At this point the entire plants including the ears from the treated plots were harvested, bagged, and brought to the laboratory and transferred to the lab for ensiling.
2.4.4. Ensilage experiments
The harvested ears were stripped of their husks and shelled manually; then, husks and cobs were cut using a hand cutter into 10- to 20-mm pieces. Silages were prepared as follows: 350 g of the chopped fresh matter was packed into a sterilized glass jar (0.5-l volume) with the addition of a lactic acid bacteria (LAB) inoculant (Lactobacillus plantarum, 3 × 106 CFU/g, ECOSIL™, Port Talbot, West Glamorgan, UK) and compressed by hand. Silages were prepared with the addition of either 5 ml PPE at different concentrations (42 or 85 µg/g) or sterile saline (control) in three replications each. Glass containers of all plant materials and treatments were sealed with a rubber-lined lid and stored in a temperature-controlled room at 25 ℃. Jars were opened on days 2, 5, 30, and 90 for fungal CFU determination and mycotoxin analysis. Silage dry matter was determined by drying to a constant weight (105 ℃, 18 h). The pH value was measured each day the silage was opened in an aqueous extract of the silage sample using a pH electrode. F. proliferatum CFUs in the samples were determined before and after ensiling by the method described in Section 2.2. Briefly, 50 g chopped plant material was transferred to a plastic bag containing 450 ml peptone water and homogenized in a stomacher blender for 2 min. Ten-fold dilutions were prepared in peptone water and samples (100 µl) were plated on PDA plates supplemented with chloramphenicol (20 µg/ml) to prevent bacterial contamination.
2.4.5. Mycotoxin analysis of laboratory-ensiled samples
After opening the jars, 50 g of each sample was freeze-dried and ground. FB1 and FB2 were extracted using FumoniTest™ wide-bore immunoaffinity columns (VICAM, Milford, MA, USA) according to the manufacturer’s protocol. Briefly, 2 g of ground sample was extracted with 10 ml of extraction solvent mixture (acetonitrile/methanol/water, 25:25:50, v/v). After agitation on an orbital shaker for 30 min, the samples were centrifuged at 8580 g for 15 min. A 2-ml aliquot of the supernatant was diluted with 8 ml phosphate buffered saline (PBS). Then 10 ml of the diluted extract was passed through an immunoaffinity column, and the column was rinsed with 10 ml PBS. FB1 and FB2 were eluted by passing 1 ml methanol followed by 1 ml water through the column, and the eluate was evaporated to dryness under a nitrogen stream at 60 °C.
The analytes were derivatized by combining methanol (250 µl), 0.05 M sodium borate buffer (250 µl), sodium cyanide (125 µl), and 2,3-naphthalenedicarboxaldehyde (NDA, 125 µl). The mixture was allowed to react for 20 min at 60 °C (water bath) and then cooled down to room temperature. Then 250 µl of 0.05 M phosphate buffer (pH 7.4)/acetonitrile (40:60, v/v) was added to the mixture. The derivatized samples were filtered through a 0.22-µm PTFE filter and quantitatively analyzed by injection of 20 µl into a reversed phase HPLC/UHPLC system (Waters ACQUITY Arc, Milford, MA, USA) with an isocratic mobile phase consisting of 0.1 M sodium phosphate monobasic salt adjusted to pH 3.3 with orthophosphoric acid/methanol (230:770, v/v) at a flow rate of 1 ml/min through a Kinetex 3.5 µm XB-C18 (150 × 4.6 mm) column (Phenomenex). The column temperature was 30 °C. The non-contaminated silage samples were spiked with different concentrations of fumonisins to construct the calibration curves. The FB1 and FB2 peaks were detected with a fluorescence detector (excitation at 420 nm and emission at 500 nm) and quantified by comparing with calibration curves of the mycotoxin standard.
2.5. Mycotoxin and gene-expression assays in artificially contaminated wheat grain samples
Sterilized wheat grain samples (10 g) were placed in sterile Petri dishes and inoculated with 1 ml macroconidial suspension (106 conidia/ml) of F. proliferatum (YO3), with the addition of either 1 ml PPE at different concentrations (100, 500 or 1000 µg/g) or sterile saline (control) in three replications each; the samples were incubated at 28 ℃ for 8 days. Then, the grain samples were freeze-dried and milled to a fine powder using a laboratory grinder. The fumonisin toxins (FB1 and FB2) were extracted and analyzed by HPLC using the protocol in Section 2.4.5. For expression analysis of genes associated with fumonisin biosynthesis, total RNA was extracted from 100 mg of lyophilized wheat grain powder using the Hybrid-R RNA Isolation Kit (Gene All, Seoul, South Korea) according to the manufacturer’s instructions. The DNase and reverse-transcription reactions were performed on 1 µg of total RNA with the Maxima First-Strand cDNA Synthesis Kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. Quantitative real-time PCR (qRT-PCR) was performed using Fast SYBR Green Master Mix (Applied Biosystems, Waltham, MA, USA) in a StepOnePlus Real-Time PCR System (Applied Biosystems) with the following program: 95 °C for 20 s, followed by 40 cycles of 95 °C for 3 s and 60 °C for 20 s. The samples were normalized using the housekeeping gene β-tubulin as an endogenous control and relative expression levels were measured using the 2(−ΔΔCt) analysis method. Results were analyzed with StepOne software v2.3. Primer sequences used for qRT-PCR analysis are listed in Table S1.
2.6. Statistical analysis
All experiments described here are representative of at least three independent experiments with the same patterns of results. The statistical analysis of the data was performed using one‐way analysis of variance (ANOVA). If one‐way ANOVA reported a p value of < 0.05, further analyzes were performed using Tukey's single‐step honestly significant difference test to determine significant differences between the treatments.
3. Results
3.1. Occurrence of filamentous fungi and mycotoxins in silages
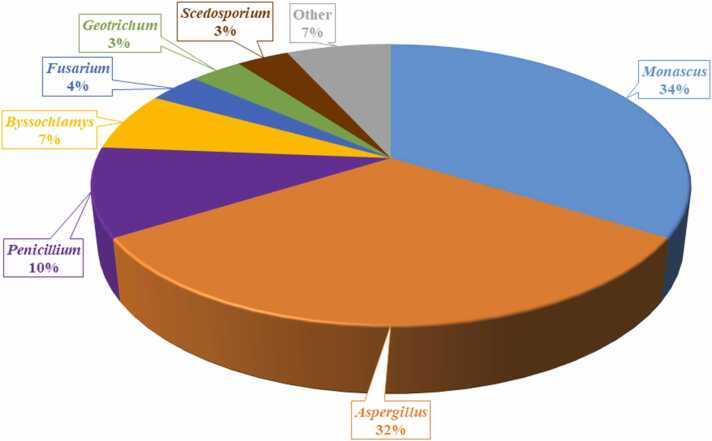
A total of 59 fungal isolates cultured from wheat and maize silage samples were identified by ITS region sequencing, and most of them were assigned to the genera Monascus (34 %), Aspergillus (32 %), Penicillium (10 %), Byssochlamys (7 %), Fusarium, Geotrichum and Scedosporium (3.4 % for each genus) (Fig. 1). Monascus ruber, Monascus purpureus, Aspergillus fumigatus, Penicillium chrysogenum and Byssochlamys nivea predominated among the isolated fungal species and were equally present in both wheat silage and maize silage samples. Other fungal species, such as Talaromyces columbinus, Sordaria fimicola, Nigrospora sphaerica and Purpureocillium lilacinum, were isolated from the silage samples at lower incidence (Table S3). Total fungal counts of all silage samples showed a moderate degree of contamination, ranging from 102 to 104 CFU/g, and did not exceed the limits (1 × 104 CFU/g) recommended by the Good Manufacturing Practice to ensure hygienic quality of animal feed [14].
Fig. 1.
Pie chart showing the relative abundance of most dominant fungal genera isolated from wheat and maize silage samples collected from six animal feed centers. The isolates were identified by sequencing the ITS region in fungi; the sequences were determined via BLAST matches to the NCBI database.
The LC–MS/MS multi-toxin method was optimized for the simultaneous detection and quantification of 13 mycotoxins in silage samples. As shown in Table 1, aflatoxins (AFB1, AFB2, AFG1, AFG2) were among the most prevalent mycotoxins, being detected in 34.2 % of the maize silage samples, followed by OTA, PAT and ZEN found in 23.7 %, 7.9 % and 2.6 % of the samples, respectively. However, only two samples exceeded the EU maximum acceptable limit of 5 µg/kg for AFB1 in maize silage (Table 1) [11]. Surprisingly, every tested maize silage sample was contaminated with FB1 and FB2 (Table 1). Although the maximum concentrations were as high as 3274 µg/kg for FB1 and 647 µg/kg for FB2, these levels were below the EU regulations of 50,000 µg/kg for FB1 and FB2 in feedstuffs for adult ruminants [11]. In contrast, a much lower incidence of mycotoxins was observed in wheat silages: out of the 44 analyzed wheat silage samples, only 12 contained one or more of the detectable analytes at concentrations above their LODs (Table 2). FB1 was detected in 18 % of the wheat silage samples, followed by AFG1 (11.3 %), FB2, PAT and ZEN (each mycotoxin in 2.3 % of the samples). Concentrations of the regulated mycotoxins detected in wheat silages (FB1, FB2, ZEN) were far below the guidance values recommended by the EC for products intended for animal feeding [11]. T-2, HT-2, GLIO and DON were not detected in either type of silage. In general, the findings clearly indicated that there was no correlation between the occurrence of Fusarium, Aspergillus or Penicillium toxins and the presence of the fungal species that could produce them.
Table 1.
Mycotoxin contamination detected in maize silage samples collected from animal feed centers across Israel.
| Animal feed center | Sample # | Mycotoxin concentration (ng/g) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AFB1 | AFB2 | AFG1 | AFG2 | DON | FB1 | FB2 | GLIO | HT-2 | OTA | PAT | T-2 | ZEN | ||
| I | 1 | – | – | – | – | – | 540 | 151 | – | – | – | – | – | – |
| 2 | – | – | – | 4.3 | – | 1183 | 191 | – | – | – | – | – | – | |
| 3 | – | – | – | – | – | 1147 | 264 | – | – | – | – | – | – | |
| 4 | 4.2 | – | – | – | 1 | 1083 | 269 | – | – | – | – | – | – | |
| 5 | – | – | – | – | – | 1539 | 342 | – | – | – | 12.4 | – | – | |
| 6 | 10.5 | – | – | – | – | 167 | 29 | – | – | 8.4 | – | – | – | |
| 7 | – | – | – | – | – | 2368 | 364 | – | – | – | – | – | – | |
| II | 8 | – | – | – | – | – | 2233 | 397 | – | – | 0.8 | – | – | 1 |
| 9 | – | – | – | – | – | 1290 | 212 | – | – | 3.2 | – | – | – | |
| 10 | – | – | 1 | – | – | 1625 | 313 | – | – | 0.1 | – | – | – | |
| 11 | – | – | – | – | – | 46 | 10 | – | – | – | – | – | – | |
| 12 | – | – | – | – | – | 199 | 38 | – | – | – | – | – | – | |
| 13 | – | – | – | – | – | 1711 | 350 | – | – | 8.9 | 41 | – | – | |
| 14 | – | – | – | – | – | 3260 | 486 | – | – | 0.4 | – | – | – | |
| 15 | – | – | – | – | – | 1649 | 263 | – | – | 1 | – | – | – | |
| III | 16 | – | – | – | – | – | 1621 | 255 | – | – | – | – | – | – |
| 17 | – | – | – | – | – | 3274 | 418 | – | – | – | – | – | – | |
| 18 | – | – | – | – | – | 2664 | 390 | – | – | – | – | – | – | |
| IV | 19 | – | – | – | 2.5 | – | 593 | 167 | – | – | – | – | – | – |
| 20 | – | – | – | – | – | 341 | 43 | – | – | – | – | – | – | |
| 21 | – | – | – | 3 | – | 712 | 156 | – | – | – | – | – | – | |
| 22 | – | 0.4 | – | – | – | 936 | 220 | – | – | – | – | – | – | |
| 23 | – | – | 0.7 | – | – | 2441 | 647 | – | – | – | – | – | – | |
| 24 | – | 0.1 | – | – | – | 1472 | 280 | – | – | – | – | – | – | |
| 25 | – | – | – | – | – | 183 | 50 | – | – | – | 27.4 | – | – | |
| 26 | – | – | – | – | – | 452 | 78 | – | – | – | 4.4 | – | – | |
| 27 | – | – | – | – | – | 88 | 15 | – | – | – | – | – | – | |
| V | 28 | 8.6 | 0.4 | 0.3 | 4.6 | – | 2162 | 359 | – | – | 3.4 | – | – | – |
| 29 | – | – | – | – | – | 806 | 104 | – | – | 9.4 | – | – | – | |
| 30 | – | – | 0.4 | – | – | 403 | 49 | – | – | 8.4 | – | – | – | |
| 31 | – | – | – | 2 | – | 52 | 10 | – | – | – | – | – | ||
| VI | 32 | – | – | 0.5 | – | – | 167 | 34 | – | – | – | – | – | – |
| 33 | – | – | – | – | – | 147 | 24 | – | – | – | – | – | 3 | |
| 34 | – | – | – | – | – | 207 | 46 | – | – | – | – | – | 7 | |
| 35 | – | – | – | – | – | 1475 | 342 | – | – | – | – | – | – | |
| 36 | – | – | – | – | – | 1781 | 369 | – | – | – | – | – | – | |
| 37 | – | – | – | 2.1 | – | 88 | 15 | – | – | – | – | – | – | |
| 38 | – | – | – | – | – | 925 | 165 | – | – | – | – | – | – | |
"–" not detected.
Table 2.
Mycotoxin contamination detected in wheat silage samples collected from animal feed centers across Israel.
| Animal feed center | Sample # | Mycotoxin concentration (ng/g) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AFB1 | AFB2 | AFG1 | AFG2 | DON | FB1 | FB2 | GLIO | HT-2 | OTA | PAT | T-2 | ZEN | ||
| I | 1–5 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| II | 6 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 7 | – | – | – | – | – | 30 | – | – | – | – | – | – | – | |
| 8 | – | – | 1.7 | – | – | 32 | – | – | – | – | – | – | – | |
| 9–11 | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| 12 | – | – | 2.2 | – | – | – | – | – | – | – | – | – | – | |
| 13–14 | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| III | 15–16 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 17 | – | – | 1.4 | – | – | 26 | – | – | – | – | – | – | – | |
| 18 | – | – | – | – | – | – | – | – | – | – | 3.2 | – | – | |
| 19 | – | – | – | – | – | 27 | – | – | – | – | – | – | – | |
| 20 | – | – | – | – | – | – | – | – | – | – | – | – | 7 | |
| 21–22 | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| 23 | – | – | – | – | – | 449 | 75 | – | – | – | – | – | – | |
| 24 | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| IV | 25 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 26 | – | – | 3.4 | – | – | – | – | – | – | – | – | – | – | |
| 27 | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| 28 | – | – | – | – | – | 32 | – | – | – | – | – | – | – | |
| 29 | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| 30 | – | – | – | – | – | 22 | – | – | – | – | – | – | – | |
| 31 | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| V | 32 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 33 | – | – | – | – | – | 26.5 | – | – | – | – | – | – | – | |
| 34–35 | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| 36 | – | – | 19.4 | – | – | – | – | – | – | – | – | – | – | |
| VI | 37–44 | – | – | – | – | – | – | – | – | – | – | – | – | – |
"–" not detected.
3.2. Effectiveness of ensilage in preventing fungal growth
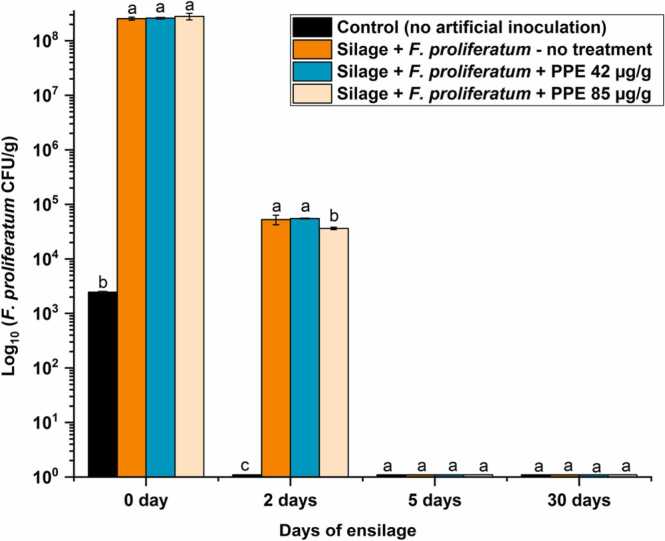
The effects of ensiling process and PPE treatment on fungal growth were assessed in the laboratory-scale silages prepared from maize ears inoculated with F. proliferatum. Fig. 2 shows the dynamics of F. proliferatum colonization of maize over the 90-day ensilage period. Quantitative analysis of F. proliferatum colonization showed more than 108 CFU/g in the infected maize samples before ensiling (at 0 h). There was evidence of natural background F. proliferatum infection at the field site; the fungus was recovered at a relatively lesser extent (~ 2.5 × 103 CFU/g) from ears inoculated with saline only (Fig. 2). Two days of ensilage had a strong effect on F. proliferatum counts, resulting in an up to 3.68 log10 CFU/g reduction in the fungal population compared to the samples at harvest (before ensiling) (Fig. 2). A similar reduction in fungal load (up to 3.88 log10 CFU/g) was observed in the silage samples treated with PPE, indicating no additional antifungal activity of the compound during ensiling. No fungal presence was found in silages after 5 and 30 days of ensiling, suggesting that F. proliferatum could not survive typical silage conditions. Maize silages revealed generally low pH values (3.75–4.05), indicating adequate preservation throughout the ensiling process.
Fig. 2.
Dynamics of F. proliferatum colonization in laboratory-scale silages. Silages were prepared as described in Section 2, with or without PPE supplementation. Data are means of at least three independent repetitions ± standard deviation. One-way ANOVA differences were considered significant when p < 0.05. Different letters above the error bars indicate statistically significant differences among treatments in each time point, as determined using the Tukey’s honest significant difference test.
3.3. Anti-mycotoxigenic activity of PPE
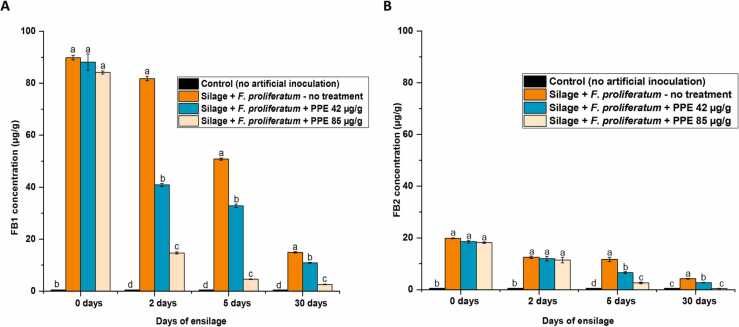
FB1 and FB2 were detected in maize inoculated with F. proliferatum in the field experiment at mean concentrations of 88 and 18 µg/g, respectively (Fig. 3). Interestingly, despite a lack of antifungal activity at the tested concentrations, PPE displayed strong inhibition of fumonisin production by F. proliferatum in maize silages during ensiling. In particular, after just 2 days of ensiling, treatment with PPE at 42 and 85 µg/g significantly inhibited FB1 production by 50 % and 82 %, respectively, compared to silages without PPE supplement (Fig. 3A). The silage process appeared to reduce mycotoxin concentrations as well. Reduction of FB1 content in maize silage with no PPE was recorded after 5 days of ensiling. However, more pronounced inhibition of mycotoxin production was detected at this time point due to the PPE treatment, resulting in an up to 91 % and 78 % decrease of FB1 and FB2 contents, respectively, in the silage samples compared to untreated controls (Fig. 3A, B). The toxins' contents continued to decline in untreated samples and were found at 15 and 4.2 µg/g for FB1 and FB2, respectively, by day 30 of the ensiling. The mycotoxins were detected at their lowest levels in maize silages opened on that same day (2.6 µg/g for FB1 and 0.46 µg/g for FB2) which had been treated with PPE at the highest concentration (85 µg/g).
Fig. 3.
Effects of ensiling process and PPE on FB1 (A) and FB2 (B) accumulation in laboratory-scale silages. Average values of three replicates (± standard deviation) are presented. Experiments were repeated three times and results of a single representative experiment are shown. One-way ANOVA differences were considered significant when p < 0.05. Different letters above the error bars indicate statistically significant differences among treatments in each time point, as determined using the Tukey’s honest significant difference test.
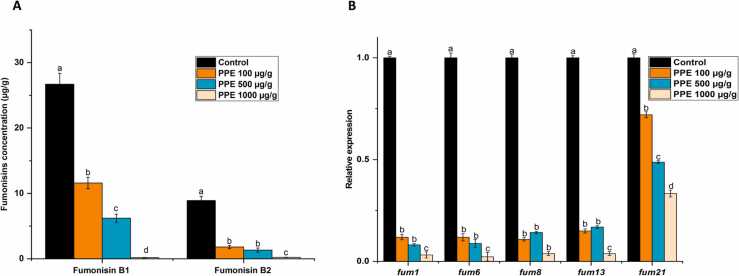
To assess PPE’s inhibitory activity on fumonisin production independent of the ensiling process, wheat grains inoculated with F. proliferatum were treated with PPE compound at different concentrations, ranging from 100 to 1000 µg/g. PPE inhibited FB1 and FB2 synthesis up to 99.4 % and 97.6 %, respectively, and this effect was dose-dependent (Fig. 4A). Furthermore, the effect of PPE on the expression level of key genes in the fumonisin-biosynthesis cluster – fum1 (polyketide synthase), fum6 (fumonisin C-14 and C-15 hydroxylation), fum8 (α-oxoamine synthase), fum13 (C-3 carbonyl reductase) and fum21 (transcription factor) – was analyzed by qRT-PCR. The expression levels of these genes were significantly downregulated under PPE treatment at all tested doses, directly depressing fumonisin production (Fig. 4B).
Fig. 4.
Anti-mycotoxigenic activity of PPE. (A) Effect of PPE on FB1 and FB2 production by F. proliferatum in wheat grains. (B) Effect of PPE on the expression of key fumonisin biosynthesis pathway genes in F. proliferatum. Relative expression was normalized using b-tubulin as an internal control. Error bars represent standard deviation of three independent biological replicates. One-way ANOVA differences were considered significant when p < 0.05. Different letters above the error bars indicate statistically significant differences among treatments in each group, as determined using the Tukey’s honest significant difference test.
4. Discussion
Fungal spoilage and mycotoxin contamination of animal feed, such as silage for dairy cattle, remain a significant threat to farmers worldwide. The presence of mycotoxigenic fungi and mycotoxins in silage adversely affects the safety and quality of the feed that can lead to poor animal performance [23]. The results of the current survey, which evaluated the fungal incidence in wheat and maize silages collected from animal feed centers in Israel, are consistent with previous studies, where the most colonizing fungal species belonging to the genera Aspergillus, Penicillium, Fusarium, Byssochlamys and Monascus have been regularly isolated from silages [29], [3], [30], [7]. Monascus spp., Aspergillus fumigatus, Penicillium spp. and Byssochlamys nivea were among the predominant species found in the current study. Our findings are in agreement with those of other research groups that have reported a high prevalence of these toxigenic fungi in different types of silage due to their ability to survive ensiling conditions [21], [9],[27], [29]. Only two species of Fusarium, F. solani and F. falciforme, were found in the maize silage samples from one feed center in the current study. Nevertheless, fumonisin toxins were detected in all tested maize silage samples and in 18 % of the wheat samples. There were no correlations between the occurrence of fumonisins and the presence of Fusarium spp. in our samples, such as F. proliferatum and F. verticillioides, which may produce FB1 and FB2. This means that toxins could also have been produced before ensiling. These findings are in alignment with those reported by Schenck et al. [34], who found no correlation between the occurrence of specific Fusarium toxins (such as DON, T-2, HT-2, ZEN) and presence of the toxin-producing Fusarium spp. in wrapped forage bales.
Among Aspergillus species isolated from silage samples in the present study, A. fumigatus was the most prevalent, detected in four animal feed centers. However, GLIO, which is produced by A. fumigatus and has a variety of adverse biological effects, was not found in any of the analyzed sample (Table 1, Table 2). In contrast, other studies have reported a significant correlation between the occurrence of GLIO and the presence of A. fumigatus in silages [15], [30]. Aflatoxins and OTA were detected mainly in maize silage samples in this survey. Yet, none of the potential aflatoxin and OTA producers among Aspergillus and Penicillium species were found in the analyzed silage samples, suggesting that the presence of these mycotoxins may be a result of the initial fungal contamination in the field before ensiling. Maize and wheat crops are usually affected by aflatoxin contamination in tropical and/or subtropical areas, although temperate regions could increase in importance due to climate change [4]. Similar to the current survey, aflatoxin contamination has been found in silage samples collected in Argentina [27], France [29] and Egypt [9], indicating that specific environmental conditions may influence mycotoxin synthesis.
One of the prevalent species found in the current study was B. nivea. This species has the ability to produce several mycotoxins, among them PAT, which was found in silage along with growth of B. nivea (Fig. 1, Table 1, Table 2). PAT, which is considered an indicator of Penicillium toxins, is produced primarily by Penicillium expansum, but this fungus was not detected in any sample examined in the present study.
Laboratory-scale silage experiments clearly demonstrated that F. proliferatum, which is one of the most common field-infecting and fumonisin-producing fungal strains, cannot survive proper ensiling conditions over time. An almost 50 % reduction in fungal load was observed after 2 days of ensiling in the glass jar maize silages; F. proliferatum could no longer be detected after 5 days of ensiling (Fig. 2). Several studies have already reported that Fusarium species are not commonly isolated from ensiled samples, because they are sensitive and cannot survive in an anaerobic and low pH environment of the silage [12], [21], [34], [39]. Nevertheless, FB1 and FB2 toxins were found in the laboratory-scale silage samples throughout the experiment, suggesting that these mycotoxins were produced in the field and were already prevalent in the maize ears before ensiling. A decrease in mycotoxin concentrations in the non-treated samples could be explained by microbial degradation or adsorption (for instance by LAB) during fermentation [22], [40], [6]. Treatment with PPE at the concentrations used in the study (42 and 85 µg/g) did not contribute to fungal inhibition beyond the ensiling process. Given that PPE has been shown to be effective in inhibiting major postharvest fungal pathogens at concentrations ranging from 1.2 to 12 g/l [20], [25], [26], the use of low concentrations of the extract in the present study explains the absence of its antifungal activity. This was in agreement with our previous study, where PPE was found to be active against several fungal isolates, including F. proliferatum, at relatively high concentrations, with MIC values between 1250 and 5000 µg/ml [33]. However, beyond the silage process, PPE treatment resulted in further inhibition of fumonisin production by F. proliferatum at concentrations considerably lower than those required for fungal growth inhibition. These results indicate that PPE’s inhibitory activities of fungal growth and mycotoxin production are independent of each other, and that the inhibition of fumonisin synthesis by the extract could involve downregulation of specific enzymes in the fumonisin biosynthetic pathway. Indeed, PPE treatment of infected wheat grains resulted in significant inhibition of FB1 and FB2 synthesis by F. proliferatum, accompanied by downregulated transcript levels of the key genes in fumonisin biosynthesis (Fig. 4B). Similar findings have been reported in our recent study [33], where combined treatment with suboptimal doses of PPE and the commercial antifungal drug prochloraz led to complete inhibition of AFB1 production by A. flavus, which correlated with the downregulation of key genes in the aflatoxin-biosynthesis cluster.
5. Conclusions
The results of this study showed that filamentous fungi and mycotoxins are commonly present in wheat and maize silages in Israel; however, there was no correlation between the occurrence of specific mycotoxins and the presence of the fungal species that might produce them in our samples. FB1 and FB2 were the most prevalent mycotoxins, both being present in every maize silage sample, but their concentrations were below the guidance values recommended by the EC for products intended for animal feeding. No viable fumonisin-producing Fusarium spp. were isolated from the silage samples in the present study. This finding was confirmed by laboratory silage experiments, where F. proliferatum could barely survive the silage environment and disappeared within a few days of ensiling. Furthermore, treatment with PPE at relatively low concentrations demonstrated strong anti-mycotoxigenic activity against mycotoxigenic fungi. This natural antimicrobial compound, which has been proven effective against a variety of plant and foodborne pathogens [5], significantly inhibited FB1 and FB2 production by F. proliferatum in a dose-dependent manner by downregulating specific enzymes in the fumonisin-biosynthesis pathway. Nevertheless, further investigation is needed to elucidate the mechanisms by which PPE influences gene clusters involved in mycotoxin biosynthesis. Although no signs of toxicity of the extract have been reported to date, further research is needed to confirm the safety of PPE for animal and human health. The potential use of PPE as a silage additive may lead to significant reductions in fungal and mycotoxin contaminations, and improved quality and safety of animal feed.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by the Chief Scientist of the Israeli Ministry of Agriculture and Rural Development, Grant no. 20-06-0045.
Handling Editor: Dr. Lawrence Lash
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2022.07.011.
Appendix A. Supplementary material
Supplementary material
Data Availability
Data will be made available on request.
References
- 1.Akhtar S., Ismail T., Fraternale D., Sestili P. Pomegranate peel and peel extracts: chemistry and food features. Food Chem. 2015;174:417–425. doi: 10.1016/j.foodchem.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 2.Alexandre E.M.C., Silva S., Santos S.A.O., Silvestre A.J.D., Duarte M.F., Saraiva J.A., Pintado M. Antimicrobial activity of pomegranate peel extracts performed by high pressure and enzymatic assisted extraction. Food Res. Int. 2019;115:167–176. doi: 10.1016/j.foodres.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 3.Alonso V.A., Pereyra C.M., Keller L.A.M., Dalcero A.M., Rosa C.A.R., Chiacchiera S.M., Cavaglieri L.R. Fungi and mycotoxins in silage: an overview. J. Appl. Microbiol. 2013;115(3):637–643. doi: 10.1111/jam.12178. [DOI] [PubMed] [Google Scholar]
- 4.Battilani P., Toscano P., Van Der Fels-Klerx H.J., Moretti A., Camardo Leggieri M., Brera C., Rortais A., Goumperis T., Robinson T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016;6:1–7. doi: 10.1038/srep24328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belgacem I., Li Destri Nicosia M.G., Pangallo S., Abdelfattah A., Benuzzi M., Agosteo G.E., Schena L. Pomegranate peel extracts as safe natural treatments to control plant diseases and increase the shelf-life and safety of fresh fruits and vegetables. Plants. 2021;10(3):453. doi: 10.3390/PLANTS10030453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boudra H., Morgavi D.P. Reduction in Fusarium toxin levels in corn silage with low dry matter and storage time. J. Agric. Food Chem. 2008;56(12):4523–4528. doi: 10.1021/jf800267k. [DOI] [PubMed] [Google Scholar]
- 7.Cheli F., Campagnoli A., Dell’Orto V. Fungal populations and mycotoxins in silages: from occurrence to analysis. Anim. Feed Sci. Technol. 2013;183(1–2):1–16. doi: 10.1016/j.anifeedsci.2013.01.013. [DOI] [Google Scholar]
- 8.Dikmen M., Ozturk N., Ozturk Y. The antioxidant potency of Punica granatum L. fruit peel reduces cell proliferation and induces apoptosis on breast cancer. J. Med. Food. 2011;14(12):1638–1646. doi: 10.1089/jmf.2011.0062. [DOI] [PubMed] [Google Scholar]
- 9.El-Shanawany A.A., Eman Mostafa M., Barakat A. Fungal populations and mycotoxins in silage in Assiut and Sohag governorates in Egypt, with a special reference to characteristic Aspergilli toxins. Mycopathologia. 2005;159(2):281–289. doi: 10.1007/s11046-004-5494-1. [DOI] [PubMed] [Google Scholar]
- 10.European Commission, Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling analysis for the official control of the levels of mycotoxins in foodstuffs, Off. J. Eur. Union, vol. 70, 2006, pp. 12–34.
- 11.European commission, Commission Recommendation (EU) 2016/1319 of 29 July 2016 amending Recommendation 2006/576/EC as regards deoxynivalenol, zearalenone and ochratoxin A in pet food, Off. J. Eur. Union, vol. 208, 2016, pp. 58–60.
- 12.J. Fink-Gremmels, Mycotoxins in forages, in: D.E. Diaz (ed.), The Mycotoxin Blue Book, Nottingham University Press, Nottingham, UK, 2005, pp. 249–68.
- 13.Foss S., Nakamura C., Ueda-Nakamura T., Cortez D., Endo E., Dias Filho B. Antifungal activity of pomegranate peel extract and isolated compound punicalagin against dermatophytes. Ann. Clin. Microbiol. Antimicrob. 2014;13(1):32. doi: 10.1186/s12941-014-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.GMP, Certification Scheme Animal Feed Sector, Version Marzo 2008. Appendix 1: Product Standards (Including Residue Standards), Productschap Diervoeder, The Hague, 2006, pp. 1–39.
- 15.Keller L.A.M., Keller K.M., Monge M.P., Pereyra C.M., Alonso V.A., Cavaglieri L.R., Chiacchiera S.M., Rosa C.A.R. Gliotoxin contamination in and pre- and postfermented corn, sorghum and wet brewer’s grains silage in Sao Paulo and Rio de Janeiro State, Brazil. J. Appl. Microbiol. 2012;112(5):865–873. doi: 10.1111/j.1365-2672.2012.05273.x. [DOI] [PubMed] [Google Scholar]
- 16.Kristensen N.B., Sloth K.H., Højberg O., Spliid N.H., Jensen C., Thøgersen R. Effects of microbial inoculants on corn silage fermentation, microbial contents, aerobic stability, and milk production under field conditions. J. Dairy Sci. 2010;93(8):3764–3774. doi: 10.3168/JDS.2010-3136. [DOI] [PubMed] [Google Scholar]
- 17.L. Jr. Kung, R.S. Martin, C.J. Lin, Silage additives, in: D.R. Buxton, R.E. Muck, J.H. Harrison (eds.), Silage Science and Technology, ASA Inc., Madison, 2003, pp. 305–60.
- 18.Latorre A., Dagnac T., Lorenzo B.F., Llompart M. Occurrence and stability of masked fumonisins in corn silage samples. Food Chem. 2015;189:38–44. doi: 10.1016/j.foodchem.2014.10.156. [DOI] [PubMed] [Google Scholar]
- 19.Lepom P., Baath H., Knabe O. Occurrence of Fusarium species and their mycotoxins in maize. 3. The influence of silaging on the zearalenone content of CCM maize. Arch. Anim. Nutr. 1988;38(9):817–823. doi: 10.1080/17450398809430909. [DOI] [PubMed] [Google Scholar]
- 20.Li M.G., Nicosia D., Pangallo S., Raphael G., Romeo F.V., Strano M.C., Rapisarda P., Droby S., Schena L. Control of postharvest fungal rots on citrus fruit and sweet cherries using a pomegranate peel extract. Postharvest Biol. Technol. 2016;114:54–61. doi: 10.1016/j.postharvbio.2015.11.012. [DOI] [Google Scholar]
- 21.Mansfield M.A., Kuldau G.A. Microbiological and molecular determination of mycobiota in fresh and ensiled maize silage. Mycologia. 2007;99(2):269–278. doi: 10.1080/15572536.2007.11832586. [DOI] [PubMed] [Google Scholar]
- 22.Niderkorn V., Boudra H., Morgavi D.P. Binding of Fusarium mycotoxins by fermentative bacteria in vitro. J. Appl. Microbiol. 2006;101(4):849–856. doi: 10.1111/j.1365-2672.2006.02958.x. [DOI] [PubMed] [Google Scholar]
- 23.Ogunade I.M., Martinez-Tuppia C., Queiroz O.C.M., Jiang Y., Drouin P., Wu F., Vyas D., Adesogan A.T. Silage review: mycotoxins in silage: occurrence, effects, prevention, and mitigation. J. Dairy Sci. 2018;101(5):4034–4059. doi: 10.3168/jds.2017-13788. [DOI] [PubMed] [Google Scholar]
- 24.Palou L., Ali A., Fallik E., Romanazzi G. GRAS, plant- and animal-derived compounds as alternatives to conventional fungicides for the control of postharvest diseases of fresh horticultural produce. Postharvest Biol. Technol. 2016;122:41–52. doi: 10.1016/j.postharvbio.2016.04.017. [DOI] [Google Scholar]
- 25.Pangallo S., Li Destri Nicosia M.G., Agosteo G.E., Schena L. Control of olive anthracnose and leaf spot disease by bloom treatments with a pomegranate peel extract. J. Saudi Soc. Agric. Sci. 2022;21:248–254. doi: 10.1016/j.jssas.2021.09.001. [DOI] [Google Scholar]
- 26.Pangallo S., Li M.G., Nicosia D., Agosteo G.E., Abdelfattah A., Romeo F.V., Cacciola S.O., Rapisarda P., Schena L. Evaluation of a pomegranate peel extract as an alternative means to control olive Anthracnose. Biol. Control. 2017;107:1462–1467. doi: 10.1094/PHYTO-04-17-0133-R. [DOI] [PubMed] [Google Scholar]
- 27.Pereyra M.L.G., Alonso V.A., Sager R., Morlaco M.B., Magnoli C.E., Astoreca A.L., Rosa C.A.R., Chiacchiera C., Dalcero A.M., Cavaglieri L.R. Fungi and selected mycotoxins from pre- and postfermented corn silage. J. Appl. Microbiol. 2008;104(4):1034–1041. doi: 10.1111/j.1365-2672.2007.03634.x. [DOI] [PubMed] [Google Scholar]
- 28.Reddy M.K., Gupta S.K., Jacob M.R., Khan S.I., Ferreira D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 2007;73:461–467. doi: 10.1055/s-2007-967167. [DOI] [PubMed] [Google Scholar]
- 29.Richard E., Heutte N., Bouchart V., Garon D. Evaluation of fungal contamination and mycotoxin production in maize silage. Anim. Feed Sci. Technol. 2009;148(2–4):309–320. doi: 10.1016/j.anifeedsci.2008.02.004. [DOI] [Google Scholar]
- 30.Richard E., Heutte N., Sage L., Pottier D., Bouchart V., Lebailly P., Garon D. Toxigenic fungi and mycotoxins in mature corn silage. Food Chem. Toxicol. 2007;45(12):2420–2425. doi: 10.1016/j.fct.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Rosas-Burgos E.C., Burgos-Hernández A., Noguera-Artiaga L., Kačániová M., Hernández-García F., Cárdenas-López J.L., Carbonell-Barrachina Á.A. Antimicrobial activity of pomegranate peel extracts as affected by cultivar. J. Sci. Food Agric. 2017;97:802–810. doi: 10.1002/jsfa.7799. [DOI] [PubMed] [Google Scholar]
- 32.Sadhasivam S., Britzi M., Zakin V., Kostyukovsky M., Trostanetsky A., Quinn E., Sionov E. Rapid detection and identification of mycotoxigenic fungi and mycotoxins in stored wheat grain. Toxins. 2017;9:302. doi: 10.3390/toxins9100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadhasivam S., Shapiro O.H., Ziv C., Barda O., Zakin V., Sionov E. Synergistic inhibition of mycotoxigenic fungi and mycotoxin production by combination of pomegranate peel extract and azole fungicide. Front. Microbiol. 2019;10:1919. doi: 10.3389/fmicb.2019.01919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schenck J., Müller C., Djurle A., Jensen D.F., O’Brien M., Johansen A., Rasmussen P.H., Sporndly R. Occurrence of filamentous fungi and mycotoxins in wrapped forages in Sweden and Norway and their relation to chemical composition and management. Grass Forage Sci. 2019;74(4):613–625. doi: 10.1111/gfs.12453. [DOI] [Google Scholar]
- 35.Singh B., Singh J.P., Kaur A., Singh N. Antimicrobial potential of pomegranate peel: a review. Int. J. Food Sci. Technol. 2019;54(4):959–965. doi: 10.1111/ijfs.13964. [DOI] [Google Scholar]
- 36.Sorrenti V., Randazzo C.L., Caggia C., Ballistreri G., Romeo F.V., Fabroni S., Timpanaro N., Raffaele M., Vanella L. Beneficial effects of pomegranate peel extract and probiotics on pre-adipocyte differentiation. Front. Microbiol. 2019;10:660. doi: 10.3389/FMICB.2019.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spadaro D., Bustos-Lopez M.P., Gullino M.L., Piano S., Tabacco E., Borreani G. Evolution of fungal populations in corn silage conserved under polyethylene or biodegradable films. J. Appl. Microbiol. 2015;119(2):510–520. doi: 10.1111/JAM.12852. [DOI] [PubMed] [Google Scholar]
- 38.Teller R.S., Schmidt R.J., Whitlow L.W., Kung L. Effect of physical damage to ears of corn before harvest and treatment with various additives on the concentration of mycotoxins, silage fermentation, and aerobic stability of corn silage. J. Dairy Sci. 2012;95(3):1428–1436. doi: 10.3168/JDS.2011-4610. [DOI] [PubMed] [Google Scholar]
- 39.Vandicke J., De Visschere K., Ameye M., Croubels S., De Saeger S., Audenaert K., Haesaert G. Multi-mycotoxin contamination of maize silages in flanders, Belgium: monitoring mycotoxin levels from seed to feed. Toxins. 2021;13(3):1–22. doi: 10.3390/toxins13030202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wambacq E., Vanhoutte I., Audenaert K., De Gelder L., Haesaert G. Occurrence, prevention and remediation of toxigenic fungi and mycotoxins in silage: a review. J. Sci. Food Agric. 2016;96(7):2284–2302. doi: 10.1002/jsfa.7565. [DOI] [PubMed] [Google Scholar]
- 41.Wink M. Modes of action of herbal medicines and plant secondary metabolites. Medicines. 2015;2(3):251–286. doi: 10.3390/medicines2030251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.