Abstract
Objective
To explore the efficacy of human papillomavirus (HPV) vaccination on the risk of HPV infection and recurrent diseases related to HPV infection in individuals undergoing local surgical treatment.
Design
Systematic review and meta-analysis
Data sources
PubMed (Medline), Scopus, Cochrane, Web of Science, and ClinicalTrials.gov were screened from inception to 31 March 2021.
Review methods
Studies reporting on the risk of HPV infection and recurrence of disease related to HPV infection after local surgical treatment of preinvasive genital disease in individuals who were vaccinated were included. The primary outcome measure was risk of recurrence of cervical intraepithelial neoplasia grade 2 or higher (CIN2+) after local surgical treatment, with follow-up as reported by individual studies. Secondary outcome measures were risk of HPV infection or other lesions related to HPV infection. Independent and in duplicate data extraction and quality assessment were performed with ROBINS-I and RoB-2 tools for observational studies and randomised controlled trials, respectively. Grading of Recommendations Assessment, Development, and Evaluation (GRADE) was implemented for the primary outcome. Observational studies and randomised controlled trials were analysed separately from post hoc analyses of randomised controlled trials. Pooled risk ratios and 95% confidence intervals were calculated with a random effects meta-analysis model. The restricted maximum likelihood was used as an estimator for heterogeneity, and the Hartung-Knapp-Sidik-Jonkman method was used to derive confidence intervals.
Results
22 articles met the inclusion criteria of the review; 18 of these studies also reported data from a non-vaccinated group and were included in the meta-analyses (12 observational studies, two randomised controlled trials, and four post hoc analyses of randomised controlled trials). The risk of recurrence of CIN2+ was reduced in individuals who were vaccinated compared with those who were not vaccinated (11 studies, 19 909 participants; risk ratio 0.43, 95% confidence interval 0.30 to 0.60; I2=58%, τ2=0.14, median follow-up 36 months, interquartile range 24-43.5). The effect estimate was even stronger when the risk of recurrence of CIN2+ was assessed for disease related to HPV subtypes HPV16 or HPV18 (six studies, 1879 participants; risk ratio 0.26, 95% confidence interval 0.16 to 0.43; I2=0%, τ2=0). Confidence in the meta-analysis for CIN2+ overall and CIN2+ related to HPV16 or HPV18, assessed by GRADE, ranged from very low to moderate, probably because of publication bias and inconsistency in the studies included in the meta-analysis. The risk of recurrence of CIN3 was also reduced in patients who were vaccinated but uncertainty was large (three studies, 17 757 participants; 0.28, 0.01 to 6.37; I2=71%, τ2=1.23). Evidence of benefit was lacking for recurrence of vulvar, vaginal, and anal intraepithelial neoplasia, genital warts, and persistent and incident HPV infections, although the number of studies and participants in each outcome was low.
Conclusion
HPV vaccination might reduce the risk of recurrence of CIN, in particular when related to HPV16 or HPV18, in women treated with local excision. GRADE assessment for the quality of evidence indicated that the data were inconclusive. Large scale, high quality randomised controlled trials are required to establish the level of effectiveness and cost of HPV vaccination in women undergoing treatment for diseases related to HPV infection.
Systematic review registration
PROSPERO CRD42021237350.
Introduction
The introduction of human papillomavirus (HPV) vaccines has revolutionised the prevention of cervical cancer.1 HPV vaccines are highly effective in preventing HPV infection and diseases related to HPV infection when given to prepubertal girls and boys, but do not clear or reduce persistence of the virus in women with ongoing infections.2
The cost effectiveness of HPV vaccination declines substantially after the age of 26.3 Women undergoing local surgical treatment for cervical intraepithelial neoplasia (CIN), however, have been identified as a target high risk population that would benefit from adjuvant vaccination to reduce the risk of cervical cancer. Women who develop high grade CIN are particularly sensitive to infection with HPV and can rapidly re-acquire infections after local surgical treatment.4 These women have an increased risk of recurrent CIN and other malignancies related to HPV infection,5 6 and repeat conisations have been associated with adverse reproductive outcomes.7 8 9 10 11 12
A previous study has shown that HPV vaccines are much more immunogenic than the infection.13 Natural immunity from induced antibodies seems to wane over time, and vaccines have been reported to provide protection from reinfection or reactivation in individuals who are seropositive with a previous cleared infection.14 15 The vaccine might have beneficial effects against new infections and reinfections from the same HPV subtype soon after treatment, although it is less likely that this effect promotes clearance of an existing infection in isolation. Whether the vaccine can boost the effect of local surgical treatment and promote viral clearance is unclear.
Evidence of the efficacy of HPV vaccination during conisation procedures is contradictory. Post hoc analyses of randomised controlled trials provided indirect evidence of a reduction in the recurrence of high grade CIN for individuals who had been vaccinated and subsequently required conisation compared with those who had been vaccinated with a placebo.16 17 Although some observational studies found a reduction in the recurrence of high grade CIN of up to 80% in women who were vaccinated at the time of treatment,18 other studies refuted these findings and suggested no benefit.19 20 Also, two randomised controlled trials suggested a reduction in the recurrence of CIN but both studies were grossly underpowered with less than 250 patients recruited in each study.21 22 In this systematic review and meta-analysis, our aim was to review the existing literature and examine the effect of HPV vaccination on the risk of HPV infection and recurrence of preinvasive disease related to HPV infection after local surgical treatment for cervical disease or other diseases related to HPV infection.
Methods
Our systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.23
Literature search
We searched PubMed (Medline), Scopus, Cochrane, Web of Science, and ClinicalTrials.gov from inception to 31 March 2021. Two of the authors (KSK and AA) developed the search strategy and screened the articles independently (supplementary material).
Eligibility criteria
The systematic review included all studies reporting on HPV infection rates and recurrence of diseases related to HPV infection after local surgical treatment for genital diseases related to HPV in individuals who were vaccinated. In the meta-analysis, we only included studies that also reported results from a cohort who were not vaccinated. Studies were included irrespective of study design, type of vaccine, timing of vaccination, and surgical technique (supplementary material).
Data extraction and risk of bias
Data were independently extracted by KSK and AA in prespecified forms. Discrepancies were discussed and resolved by other authors (IK and MK). Risk of bias was assessed by two authors independently (KSK and SJB) with the ROBINS-I tool (Risk Of Bias In Non-randomised Studies–of Interventions) for observational studies and the RoB-2 tool (Risk of Bias for randomised trials) for randomised controlled trials24 25 (supplementary material).
The GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) tool was used to assess the quality of evidence for the primary outcome. The assessment was based on five parameters: risk of bias, inconsistency (or heterogeneity) between studies, indirectness, imprecision (risk of random errors), and publication bias. The evidence for each item was rated as high, moderate, low, or very low (supplementary material).26
Definitions of outcome
Our primary outcome was recurrence of cervical intraepithelial neoplasia grade 2 or higher (CIN2+) after local surgical treatment related to all HPV types. Secondary outcomes included recurrence of CIN grade 1 or higher (CIN1+), CIN grade 3 (CIN3), CIN2, CIN1, CIN2+ related to HPV16 or HPV18 subtypes, and CIN1+ related to HPV16 or HPV18. We also examined the risk of recurrence of vulvar, vaginal, and anal intraepithelial neoplasia, genital warts, the risk of abnormal cytology (defined as atypical squamous cells of undetermined significance or worse, based on the Bethesda System, or borderline changes or worse, based on the British Association of Cytopathology), and HPV infections (persistent or incident) after treatment.
Statistical analysis
We conducted two separate meta-analyses based on the design of the studies. For our main analysis, we pooled data from observational studies and randomised controlled trials reporting on the efficacy of vaccination when given shortly before, at the time of, or up to 12 months after the local surgical treatment. Our secondary analysis included post hoc analyses of randomised controlled trials reporting on the risk of recurrence in individuals who had undergone local surgical treatment and who were vaccinated at study entry and randomisation, and then developed preinvasive disease. We also performed an analysis that included all studies, irrespective of study design (supplementary material). We used adjusted data in preference to unadjusted data, when available, for the primary outcome (risk of recurrence of CIN2+ after local surgical treatment), and sensitivity and subgroup analyses. The median of the median length of follow-up across studies, along with its interquartile range, were calculated for the primary outcome.
We combined study effect sizes using risk ratios and corresponding 95% confidence intervals with the random effects model (inverse variance method).27 In the random effects model, we estimated each summary effect size and 95% confidence interval with the Hartung-Knapp-Sidik-Jonkman method28 29 30 to deal with meta-analyses that included a small number of studies. We also calculated prediction intervals as a way of expressing heterogeneity compared with confidence intervals.
Visual inspection of the contour enhanced funnel plot and Egger’s test were used to assess the effects of small studies and publication bias when more than 10 studies were available for each outcome.31 When funnel plot asymmetry was detected, we performed the Copas selection model to look for publication bias.32 Statistical heterogeneity was assessed with the χ2 test (P<0.10 indicating significant heterogeneity), and I2 and τ2 (to quantify the degree of heterogeneity); the restricted maximum likelihood estimator was used to estimate variance (τ2) between studies.33 34 35 36 37
To determine possible sources of heterogeneity, we performed predefined sensitivity analyses for the primary outcome (risk of recurrence of CIN2+ after local surgical treatment) restricted to: peer reviewed articles, studies with a moderate or low risk of bias, studies with low attrition bias (<10% loss during follow-up), studies with histopathological confirmation of the outcome, and studies with unadjusted data. Because our meta-analysis examined rare events and publication bias was possible, we performed a fixed effects meta-analysis with the Mantel-Haenszel method; this method provides more robust estimates of the summary effect but ignores heterogeneity.38
We also conducted a series of subgroup analyses to determine sources of heterogeneity and differences in summary estimates for the primary outcome (risk of recurrence of CIN2+ after local surgical treatment) according to: continent, timing of vaccination (up to three months before v at the time of or up to 12 months after treatment), type of vaccine (Gardasil v Gardasil or Cervarix v unknown type), age (mean age >35 v mean age ≤35), median follow-up (<24 months v ≥24 months), and study design (randomised controlled trials excluding post hoc analyses of randomised controlled trials v observational studies). Analyses were performed with the R software (meta package),39 and the selection model to assess publication bias was performed in R with the rjags package.32 40
Patient and public involvement
Patients and the wider public were involved from the outset through informal interviews in the clinic and through patient advocate representative bodies. We formed a group of patient representatives that met regularly every six months and will support dissemination of the results. The research questions and outcomes were developed based on the patients’ concerns and priorities. Patients were not involved in the interpretation of results or writing of the article.
Results
We identified 10 662 articles; 22 were eligible for the systematic review (fig 1 and table S1).16 17 18 19 20 21 22 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 Eighteen studies reported on a non-vaccinated group (no vaccine or placebo) and were included in the meta-analyses. We retrieved two randomised controlled trials, 12 observational studies (six retrospective and six prospective), and four post hoc analyses of randomised controlled trials (supplementary material). The median of the median length of follow-up for the primary outcome (risk of recurrence of CIN2+ after local surgical treatment) was 36 months (interquartile range 24-43.5) for observational studies and randomised controlled trials, and 27 months (21-39) for post hoc analyses of randomised controlled trials (table S2).
Fig 1.
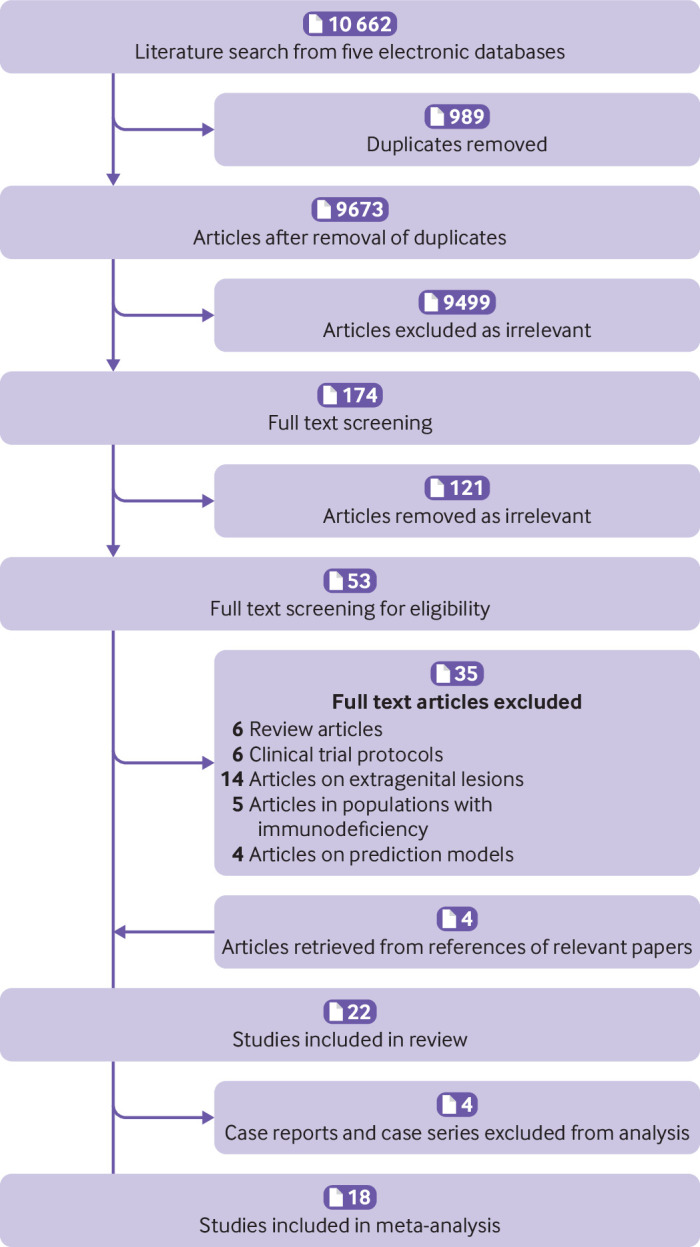
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart
Risk of bias
The two randomised controlled trials were classified as low risk of bias with adequate design (RoB-2 tool). In the observational studies and post hoc analyses, risk of bias was moderate for seven studies, serious for seven, and critical for two, based on the ROBINS-I tool (table S3). Asymmetry was not present in the funnel plot when adjusted data were used (fig S1a), but asymmetry was found with unadjusted data, indicating the possibility of publication bias (fig S1b).
CIN recurrence and risk of HPV infection after local surgical treatment for CIN
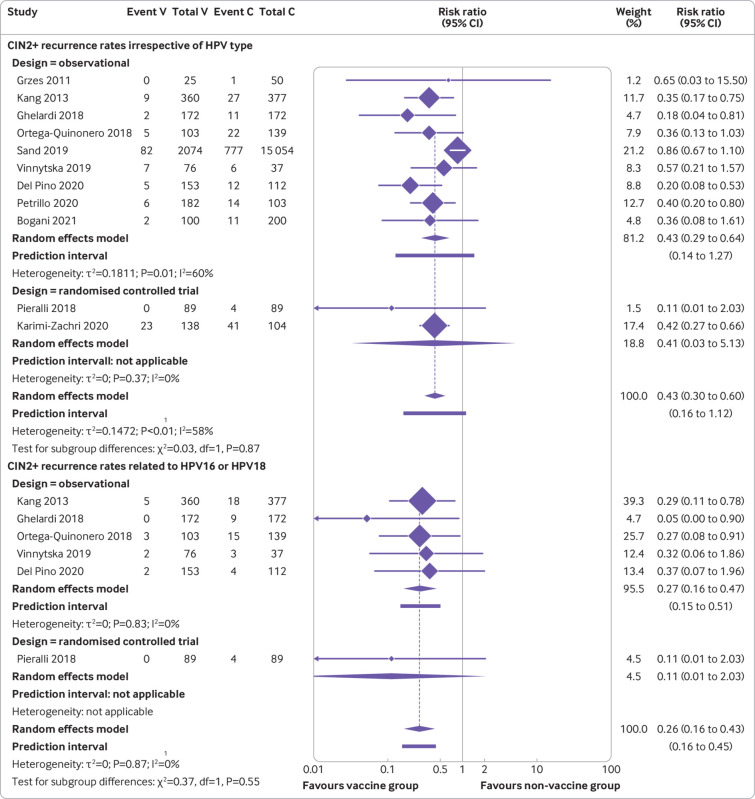
Our main analysis of observational studies and randomised controlled trials showed that women who were vaccinated at the time of treatment had a reduction in the risk of recurrence of CIN2+ compared with those who were not vaccinated (11 studies, 19 909 participants; risk ratio 0.43, 95% confidence interval 0.30 to 0.60; I2=58%, τ2=0.14; median follow-up 36 months, interquartile range 24-43.5) (fig 2 and table 1). Confidence in this meta-analysis ranged from very low to moderate according to GRADE because of the presence of small study effects indicating potential publication bias (table S4). The risk of recurrence of CIN2+ related to the HPV subtypes HPV16 or HPV18 was also reduced with vaccination compared with no vaccination (six studies, 1879 participants; 0.26, 0.16 to 0.43; I2=0%, τ2=0); a similar result was found for the risk of recurrence of CIN1+ related to HPV16 or HPV18 (one study, 178 participants; 0.25, 0.05 to 1.14) (fig 2 and table 1). Confidence in the meta-analysis of CIN2+ related to the HPV subtypes HPV16 or HPV18 ranged from very low to moderate according to GRADE because of the high risk of bias in the included studies and inconsistency (only one randomised controlled trial provided data) (table S4). The risk of recurrence of CIN3 was reduced among patients who were vaccinated but uncertainty was large (three studies, 17 757 participants; 0.28, 0.01 to 6.37; I2=71%, τ2=1.23) (table 1).
Fig 2.
Forest plots assessing risk of recurrence of cervical intraepithelial neoplasia grade 2 or higher (CIN2+) between human papillomavirus (HPV) vaccinated (V) and non-vaccinated control (C) groups after local conservative treatment for cervical intraepithelial neoplasia, irrespective of HPV type (top) and related to HPV subtypes HPV16 and HPV18 (bottom) (randomised controlled trials and observational studies)
Table 1.
Effect of human papillomavirus (HPV) vaccination on risk of recurrence of lesions related to HPV infection and HPV clearance after local surgical treatment for genital disease (randomised controlled trials and observational studies)
| Outcome | No of studies* (references) | Total No of participants | Vaccine group (events per total n/N (%)) |
Non-vaccine group (events per total n/N (%)) |
Risk ratio (95% CI) random effects model (inverse variance method) |
I2 | τ2 | PI |
|---|---|---|---|---|---|---|---|---|
| CIN2+† | 11 (18, 19, 21, 22, 42, 44-49) | 19 909 | 141/3472 (4%) | 926/16437 (5.6%) | 0.43 (0.30 to 0.60) | 58% | 0.14 | 0.16 to 1.12 |
| CIN1+ | 5 (21, 22, 44, 47, 48) | 1045 | 89/587 (15.1%) | 116/458 (25.3%) | 0.55 (0.31 to 0.96) | 63% | 0.15 | 0.13 to 2.26 |
| CIN3 | 3 (18, 19, 47) | 17 757 | 45/2428 (1.4%) | 420/15329 (2.7%) | 0.28 (0.01 to 6.37) | 71% | 1.23 | N/A |
| CIN2 | 3 (18, 19, 47) | 17 757 | 15/2428 (0.6%) | 365/15329 (2.3%) | 0.23 (0.11 to 0.48) | 0% | 0 | 0.03 to 1.99 |
| CIN1 | 4 (21, 22, 47, 48) | 970 | 55/562 (9.7%) | 44/408 (10.7%) | 0.86 (0.31 to 2.33) | 62% | 0.27 | 0.06 to 11.88 |
| CIN2+ related to HPV16 or HPV18 | 6 (18, 21, 42, 45, 46, 48) | 1879 | 12/953 (1.2%) | 53/926 (5.7%) | 0.26 (0.16 to 0.43) | 0% | 0 | 0.16 to 0.45 |
| CIN1+ related to HPV16 or HPV18 | 1 (21) | 178 | 2/89 (2.2%) | 8/89 (8.9%) | 0.25 (0.05 to 1.14) | N/A | N/A | N/A |
| Abnormal cytology | 1 (21) | 178 | 7/89 (7.8%) | 23/89 (25.8%) | 0.30 (0.14 to 0.67) | N/A | N/A | N/A |
| VIN1+ or VaIN1+ | 1 (21) | 178 | 0/89 (0%) | 3/89 (3.3%) | 0.14 (0.01 to 2.73) | N/A | N/A | N/A |
| VIN2+ or VaIN2+ | 2 (21, 50) | 296 | 8/131 (6.1%) | 27/165 (16.3%) | 0.56 (0.01 to 35.16) | 0% | 0 | N/A |
| Genital warts | 1 (41) | 171 | 45/91 (49.4%) | 35/80 (43.7%) | 0.14 (0.01 to 2.73) | N/A | N/A | N/A |
| High grade AIN | 1 (43) | 152 | 12/38 (31.5%) | 35/114 (40.7%) | 1.03 (0.60 to 1.77) | N/A | N/A | N/A |
| Persistent HPV infection‡ | 1 (18) | 344 | 26/166 (15.6%) | 32/178 (17.9%) | 0.87 (0.54 to 1.40) | N/A | N/A | N/A |
AIN=anal intraepithelial neoplasia; CIN=cervical intraepithelial neoplasia, grade 1 (CIN1), grade 1 or higher (CIN1+), grade 2 (CIN2), grade 2 or higher (CIN2+), grade 3 (CIN3); N/A=not applicable; PI=prediction interval; VIN1+ or VaIN1+=vulvar or vaginal intraepithelial neoplasia grade 1 or higher, grade 2 or higher (VIN2+ or VaIN2+).
All studies included an intervention (vaccine) and a control (non-vaccine) group.
Adjusted data were used when available
HPV type detected at baseline and six months after local surgical treatment.
The effect estimates for the primary outcome (risk of recurrence of CIN2+ after local surgical treatment) were similar in all sensitivity analyses (table S5). Heterogeneity was reduced compared with the main analysis after excluding studies with a high and serious overall risk of bias (fig S3a). In contrast, heterogeneity remained similar to the main analysis after the exclusion of grey literature, of studies with high attrition bias, and of studies that did not use histopathological confirmation of diagnosis. A sensitivity analysis with only unadjusted data showed a similar effect estimate as the main analysis with slightly reduced heterogeneity (11 studies, 19 909 participants; risk ratio 0.41, 95% confidence interval 0.30 to 0.56; I2=51%, τ2=0.12) (fig S3g).
The results for the primary outcome (risk of recurrence of CIN2+ after local surgical treatment) were largely consistent with a series of subgroup analyses and were not substantially affected by continent, study design, type of vaccine, or timing of vaccination (table S6). The risk of recurrence of CIN2+ was similar in studies that used the vaccine Gardasil (four studies, 1501 participants; risk ratio 0.38, 95% confidence interval 0.23 to 0.60; I2=0%, τ2=0) and those that used the Gardasil or Cervarix vaccine (five studies, 1205 participants; 0.36, 0.23 to 0.57; I2=0%, τ2=0) (fig S4a). Cervarix alone was not reported in any study, and two studies did not state the type of vaccine (unknown). The point estimates were similar when the vaccine was given at the time of or after treatment (10 studies, 15 546 participants; 0.41, 0.28 to 0.60; I2=62%, τ2=0.17), and when the vaccine was given before treatment (two studies, 4183 participants; 0.37, 0.02 to 6.43; I2=0%, τ2=0) (figure S4b). The effect estimate was comparable in studies with a median follow-up of >24 months (seven studies, 19 214 participants; 0.44, 0.26 to 0.73; I2=61%, τ2=0.19) versus ≤24 months (two studies, 507 participants; 0.33, 0 to 28.56; I2=47%, τ2=0.13) (fig S4f).
The second meta-analysis of post hoc analyses of randomised controlled trials showed similar point estimates (table 2). Although HPV vaccination reduced the risk of recurrence of CIN2+ compared with no vaccination, uncertainty was large (four studies, 2268 participants; risk ratio 0.45, 95% confidence interval 0.13 to 1.57; I2=14%, τ2=0.05; median follow-up 27 months, interquartile range 21-39) (fig S5a). HPV vaccination was also associated with a reduced risk of recurrence of CIN1+ (three studies, 1958 participants; 0.64, 0.45 to 0.90; I2=0%, τ2=0) (fig S5b).
Table 2.
Effect of human papillomavirus (HPV) vaccination on risk of recurrence risk of lesions related to HPV infection and HPV clearance after local surgical treatment for genital disease (post hoc analyses of randomised controlled trials)
| Outcome | No of studies* (references) | Total No of participants | Vaccine group (events per total n/N (%)) |
Placebo group (events per total n/N (%)) |
Risk ratio (95% CI) Random effect model (inverse variance method) |
I2 | τ2 | PI |
|---|---|---|---|---|---|---|---|---|
| CIN2+ | 4 (16, 17, 20, 51) | 2268 | 12/999 (1.2%) | 38/1269 (2.9%) | 0.45 (0.13 to 1.57) | 14% | 0.05 | 0.06 to 3.2 |
| CIN1+ | 3 (17, 20, 51) | 1958 | 43/857 (5%) | 89/1101 (8%) | 0.64 (0.45 to 0.90) | 0% | 0 | 0.23 to 1.78 |
| CIN3 | 2 (17, 20) | 1503 | 3/667 (0.4%) | 14/836 (1.6%) | 0.30 (0.28 to 0.32) | 0% | 0 | N/A |
| CIN2 | 2 (17, 20) | 1503 | 5/667 (0.7%) | 14/836 (1.6%) | 0.48 (0.08 to 3) | 0% | 0 | N/A |
| CIN1 | 3 (17, 20, 51) | 1957 | 34/857 (3.9%) | 53/1100 (4.8%) | 0.85 (0.44 to 1.62) | 0% | 0 | 0.12 to 5.8 |
| CIN2+ related to HPV16 or HPV18 | 4 (16, 17, 20, 51) | 2268 | 4/999 (0.4%) | 9/1269 (0.7%) | 0.63 (0.07 to 5.89) | 14% | 0.44 | 0.01 to 40.46 |
| CIN1+ related to HPV16 or HPV18 | 2 (17, 51) | 1804 | 2/777 (0.2%) | 16/1027 (1.5%) | 0.22 (0.00 to 91.80) | 0% | 0 | N/A |
| Abnormal cytology | 2 (16, 51) | 765 | 70/332 (21%) | 60/433 (13.8%) | 1.48 (1.26 to 1.73) | 0% | 0 | N/A |
| VIN1+ or VaIN1+ | 3 (17, 20, 51) | 1670 | 20/744 (2.6%) | 23/926 (2.4%) | 1.30 (0.23 to 7.43) | 29% | 0.29 | N/A |
| VIN2+ or VaIN2+ | 3 (17, 20, 51) | 1666 | 5/738 (0.6%) | 6/928 (0.6%) | 1.01 (0.24 to 4.15) | 0% | 0 | 0.02 to 65.78 |
| Genital warts | 1 (17) | 1063 | 7/474 (1.4%) | 22/589 (3.7%) | 0.40 (0.17 to 0.92) | N/A | N/A | N/A |
| Persistent HPV infection† | 1 (16) | 311 | 14/142 (9.8%) | 8/169 (4.7%) | 1.85 (0.83 to 4.15) | N/A | N/A | N/A |
| Incident HPV infection‡ | 1 (16) | 311 | 8/142 (5.6%) | 21/169 (12.4%) | 0.45 (0.21 to 1) | N/A | N/A | N/A |
AIN=anal intraepithelial neoplasia; CIN=cervical intraepithelial neoplasia, grade 1 (CIN1), grade 1 or higher (CIN1+), grade 2 (CIN2), grade 2 or higher (CIN2+), grade 3 (CIN3); N/A=not applicable; PI=prediction interval; VIN1+ or VaIN1+=vulvar or vaginal intraepithelial neoplasia grade 1 or higher, grade 2 or higher (VIN2+ or VaIN2+).
All studies included an intervention (vaccine) and a control (placebo) group.
HPV type detected at baseline and six months after local surgical treatment.
New HPV type detected at six months or later after local surgical treatment.
In the analysis of all studies irrespective of design (observational studies, randomised controlled trials, and post hoc analyses of randomised controlled trials), we found that the effect estimates and heterogeneity were similar to the main analysis for recurrence of CIN2+ (15 studies, 22 177 participants; risk ratio 0.43, 95% confidence interval 0.32 to 0.59; I2=51%, τ2=0.12; median follow-up 36 months, interquartile range 24-45) (fig S7a). To investigate the effect of timing of vaccination, we performed a subgroup analysis irrespective of study design that showed similar point estimates when the vaccine was given before (six studies, 6451 participants; 0.39, 0.24 to 0.64; I2=0%, τ2=0) versus at the time of or after treatment (10 studies, 15 726 participants; 0.41, 0.28 to 0.60; I2=62%, τ2=0.17) (fig S7b).
Recurrence of non-cervical diseases related to HPV infection after local surgical treatment of non-cervical disease
The risk of recurrence of vulvar or vaginal intraepithelial neoplasia grade 2 or higher was reduced in women who were vaccinated and treated locally for cervical, vulvar or vaginal intraepithelial neoplasia grade 2 or higher compared with women not vaccinated, but uncertainty was large (two studies, 296 participants; risk ratio 0.56, 95% confidence interval 0.01 to 35.16; I2=0%, τ2=0) (table 1 and fig S2e). Only one study assessed risk of vulvar intraepithelial neoplasia or vaginal intraepithelial neoplasia grade 1 or higher in women who were vaccinated and treated locally for CIN2+, and no differences were found. Also, one study assessed the risk of recurrence of high grade anal intraepithelial neoplasia in men treated locally for anal intraepithelial neoplasia and found no difference (table 1 and supplementary material).
The second meta-analysis of post-hoc analyses of randomised controlled trials showed no benefit of HPV vaccination on recurrence of lesions of vulvar or vaginal intraepithelial neoplasia grade 1 or higher, or vulvar or vaginal intraepithelial neoplasia grade 2 or higher, in women treated for genital HPV related disease (table 2, figs S5i and S5j, and supplementary material).
Discussion
The findings of our meta-analysis suggest that adjuvant HPV vaccination at the time of local excision for CIN might lead to a reduction in the risk of recurrence of high grade preinvasive disease (CIN2+). The effect estimate was even more pronounced for CIN2+ related to HPV16 or HPV18. Publication bias might be present, however, affecting the overall quality of evidence, indicating that the evidence is inconclusive. Evidence was lacking on the benefit of HPV vaccination for recurrence of vulvar, vaginal, or anal intraepithelial lesions, genital warts, or for incident or persistent HPV infections, although the data were scarce with small numbers of studies and participants. Analysis of the post hoc studies from randomised controlled trial data with historic vaccination at randomisation before the development of the disease reported inconsistent results. The effect size on secondary outcomes in the main analysis of our study showed possible beneficial effects, although the evidence was inconclusive because the number of included studies was small.
Four meta-analyses have attempted to combine the evidence so far.56 57 58 59 Although our findings on the summary estimate were in agreement with recent meta-analyses examining similar questions,56 57 58 59 our analysis raised concerns about the quality of the data and we concluded that the evidence is inconclusive. Some of the previous meta-analyses published in 2020 and 2021 only included a fraction of the published literature, failed to include grey literature and unpublished data, had exclusion criteria that affected the final number of studies with potentially relevant data, and the presence of publication bias was not assessed and was not associated with the overall quality of the data. These studies did not include extensive sensitivity and subgroup analyses to determine the effect of risk of bias, type of vaccine, heterogeneity between studies, and attrition rate on the primary outcome, and the tools used to assess the quality of studies and risk of bias were not optimal.
Lichter et al56 included six studies in their systematic review and meta-analysis (three post hoc analyses of randomised controlled trials, two observational studies, and one randomised controlled trial) and reported on a series of CIN and non-cervical outcomes with consistent estimates. However, the post hoc analyses of randomised controlled trials were misreported as randomised controlled trials in the analysis, ignoring the fact that randomisation in these studies was not made for treatment as intervention. The risk of bias and timing of the vaccine were also not examined, and most of the data were extracted from one to three studies, with very low to moderate quality of evidence based on GRADE.
Another systematic review and meta-analysis57 published in 2020 included 10 studies and reported similar estimates to our study for the risk of recurrence of CIN2+. A subgroup analysis based on study design was not included, however, and risk of bias for observational studies was assessed with the RoB-2 tool, commonly used for randomised controlled trials. Di Donato et al’s meta-analysis58 included 11 studies and subgroup analysis was performed based only on study design. Risk of bias was assessed with the ROBINS-I tool for all studies, although RoB-2 is more suitable for randomised controlled trials; GRADE assessment was not carried out. Bartels et al59 retrieved data from only five studies for their meta-analysis on the risk of recurrence of CIN2+ and did not include sensitivity or subgroup analyses. GRADE assessment was not performed, and the RoB tools used (MINORS and JADAD) are not recommended by the Cochrane Library.
Strengths and limitations
Our systematic review and meta-analysis reported on the role of HPV vaccination at the time of local surgical treatment for cervical and other non-cervical diseases related to HPV infection, with rigorous methodological assessment of risk of bias, heterogeneity, and appropriate data pooling from different study designs. Post hoc analyses of randomised controlled trials with historic vaccination were analysed separately from observational or randomised studies that administered the vaccine at or around the time of local treatment, removing potential inaccurate assessment of effect estimates and reducing heterogeneity. We used established risk of bias tools for observational studies and randomised controlled trials (ROBINS-I and RoB-2) to examine the quality of the studies included in the meta-analysis. We also investigated the possibility of publication bias in our meta-analysis with a selection model and performed analyses irrespective of HPV type and subgroup analyses for HPV16 and HPV18 disease. We performed a thorough assessment of the grey literature and we also assessed our findings with fixed and random effects meta-analyses, as well as with a series of subgroup and sensitivity analyses that controlled for risk of bias, timing of vaccination, type of vaccine, and attrition bias.
Our findings should be interpreted with caution, however. Most of the published literature were observational studies that are at risk of bias, and the two randomised controlled trials included only 17821 and 242 patients,22 respectively. The observational studies were of low to moderate quality based on the ROBINS-I bias assessment tool, and only five studies provided adjusted data. The low to moderate quality could be attributed, at least partially, to the presence of confounders, differences in baseline characteristics between intervention and control groups, and suboptimal selection of participants in the included studies. Specifically, the mean age of participants was not provided in most studies and age differences between women who were vaccinated and those who were not vaccinated might affect the risk of recurrence of disease. Women who are vaccinated might be younger than those who are not vaccinated, and increased recurrence of disease could partly be a result of older age. Also, confounders such as smoking, associated with a higher risk of recurrence, were not controlled for in many studies.
Variability in diagnostic methods, length of follow-up, and HPV vaccine types and timing among the studies could also influence the accuracy of the effect estimate. The median of median length of follow-up was 36 months and therefore we could not assess whether the effect estimate would be sustained in the long term. Although subgroup analyses according to median length of follow-up (<24 v ≥24 months) showed similar effect estimates, pooling risk ratios at different time points was not possible. The short follow-up is an intrinsic limitation of our meta-analysis and similar meta-analyses, and this factor needs to be looked at in randomised studies with a long follow-up interval. Most recurrences present in the first 24 months,60 however, and no clear biological reason exists to explain why a longer length of follow-up would reverse this trend.
Heterogeneity in several meta-analyses was high, as indicated by the τ2 estimates and the wide prediction intervals, highlighting the uncertainty around the observed effect estimates. Also, the difference in effect estimates between the random and fixed effect models indicates the presence of small study effects. The use of adjusted study specific effects, where available, might have also contributed to this heterogeneity. Assessment of publication bias was restricted by the limited number of studies for some comparisons, further highlighting the need for a randomised trial. Although the subgroup and sensitivity analyses according to type of vaccine, timing of vaccination, attrition bias, and overall risk of bias showed consistent results and reduced this heterogeneity to some extent, the number of included studies in these subgroups was low. Furthermore, distinguishing between incident and persistent infections was not possible because persistence was assessed in only one study. Studies that used the nonavalent vaccine Gardasil 9 that might offer wider protection were not available. An analysis with data from an intention to treat analysis of randomised controlled trials was also not possible because of lack of data. For many of the secondary outcomes, the number of studies and participants was also low, precluding clear conclusions to be drawn on the effectiveness of the vaccine. The quality of evidence ranged from very low to moderate, as assessed by GRADE, owing to the possible presence of publication bias, high risk of bias of the included studies, and inconsistency.
Conclusions
Women who have been treated for high grade CIN have a lifelong residual high risk of cervical cancer and other malignancies related to HPV infection. New strategies to reduce the risk in these women might shorten and simplify screening programmes and eliminate cervical cancer more rapidly.5 6 Large, appropriately powered randomised controlled trials are required to establish the effectiveness of adjuvant HPV vaccination at the time of local surgical treatment of CIN. Given that the incidence of recurrence of high grade disease is low in quality assured national screening programmes, such as in the UK, absolute risks and a cost effectiveness analysis would be important in determining the implementation strategy of HPV vaccination after treatment. The NOVEL (Nonavalent Prophylactic HPV Vaccine (Gardasil 9) After Local Conservative, NCT03979014) trial, an ongoing, large, appropriately powered randomised controlled trial, is expected to report results and give further insight on the effect of Gardasil 9 on persistent incident, recurrent, and prevalent HPV infections and cost effectiveness.61 If the study reports benefit, the results could be applicable to women with multifocal disease, other diseases related to HPV infection, and individuals with a diagnosis of malignancies related to HPV infection.
What is already known on this topic
Women with cervical intraepithelial neoplasia (CIN) undergoing local surgical treatment have a high risk of recurrent preinvasive and invasive cervical disease and other diseases related to human papillomavirus (HPV) infection
Women who develop high grade CIN might be particularly sensitive to HPV infection and rapidly re-acquire infections
The use of prophylactic HPV vaccination at the time of local surgical treatment for CIN might reduce the risk of future recurrence
What this study adds
Prophylactic HPV vaccination at the time of local treatment for CIN might reduce the risk of recurrence of high grade preinvasive cervical, but the evidence is inconclusive and the GRADE assessment for the quality of evidence ranged from very low to moderate
The effect of HPV vaccination on the risk of HPV infection and other non-cervical diseases related to HPV infection treated surgically is unclear because of the scarcity of data and the moderate to high overall risk of bias of the available studies
Large, appropriately powered randomised controlled trials are required to establish the effectiveness of HPV vaccination at the time of surgical treatment of cervical preinvasive disease based on failure rates and costs in different settings
Web extra.
Extra material supplied by authors
Web appendix: Supplementary material
Contributors: The study was conceived by MK and designed by MK, KSK, AAV, and IK. The data were acquired and collated by KSK, AA, SJB, MP, and MK, and analysed by KSK, IK, AA, SJB, MP, MK, and AAV. The manuscript was drafted and revised critically for important intellectual content by all authors (KSK, IK, SJB, AA, MP, EP, JD, PN, BS, PS, AAV, and MK). All authors gave final approval of the version to be published. The corresponding author (MK) attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. The guarantor (MK) accepts full responsibility for the work, had access to the data, and controlled the decision to publish.
Funding: This work was supported by the National Institute for Health and Care Research (NIHR) EME (17/11/45). The researchers were also supported by Imperial Healthcare NHS Trust NIHR Biomedical Research Centre (P83204) (MK and KSK); Imperial Charity Fellowship (RFP02122_8) (MP); and CRUK Early Detection (EDDPJT-May21/100001) (MK and MP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the NIHR EME for the submitted work; the authors declare no conflict of interest with regards to the presented work; a number of authors are investigators of the NIHR EME funded NOVEL trial (MK, KSK, PS, JD, BS, PN, and IK); this trial is also supported by MSD who supplied the vaccines for the trial.
The lead authors (KSK and MK) affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Dissemination to participants and related patient and public communities: The results will be disseminated to the lay audience through a press release, social media, through the Imperial College website, and through the authors' involvement with charities, public presentations, and interviews.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Schiller JT, Lowy DR. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat Rev Microbiol 2012;10:681-92. 10.1038/nrmicro2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paavonen J, Naud P, Salmerón J, et al. HPV PATRICIA Study Group . Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009;374:301-14. 10.1016/S0140-6736(09)61248-4 [DOI] [PubMed] [Google Scholar]
- 3. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. Wiley Online Library, 2019: 3202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soutter WP, Sasieni P, Panoskaltsis T. Long-term risk of invasive cervical cancer after treatment of squamous cervical intraepithelial neoplasia. Int J Cancer 2006;118:2048-55. 10.1002/ijc.21604 [DOI] [PubMed] [Google Scholar]
- 5. Kalliala I, Athanasiou A, Veroniki AA, et al. Incidence and mortality from cervical cancer and other malignancies after treatment of cervical intraepithelial neoplasia: a systematic review and meta-analysis of the literature. Ann Oncol 2020;31:213-27. 10.1016/j.annonc.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strander B, Hällgren J, Sparén P. Effect of ageing on cervical or vaginal cancer in Swedish women previously treated for cervical intraepithelial neoplasia grade 3: population based cohort study of long term incidence and mortality. BMJ 2014;348:f7361. 10.1136/bmj.f7361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet 2006;367:489-98. 10.1016/S0140-6736(06)68181-6 [DOI] [PubMed] [Google Scholar]
- 8. Kyrgiou M, Athanasiou A, Paraskevaidi M, et al. Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: systematic review and meta-analysis. BMJ 2016;354:i3633. 10.1136/bmj.i3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kyrgiou M, Mitra A, Arbyn M, et al. Fertility and early pregnancy outcomes after treatment for cervical intraepithelial neoplasia: systematic review and meta-analysis. BMJ 2014;349:g6192. 10.1136/bmj.g6192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kyrgiou M, Mitra A, Arbyn M, et al. Fertility and early pregnancy outcomes after conservative treatment for cervical intraepithelial neoplasia. Cochrane Database Syst Rev 2015;(9):CD008478. 10.1002/14651858.CD008478.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kyrgiou M, Athanasiou A, Kalliala IEJ, et al. Obstetric outcomes after conservative treatment for cervical intraepithelial lesions and early invasive disease. Cochrane Database Syst Rev 2017;11:CD012847. 10.1002/14651858.CD012847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Athanasiou A, Veroniki A, Efthimiou O, et al. Comparative effectiveness and risk of preterm birth of local treatments for cervical intraepithelial neoplasia and stage IA1 cervical cancer: a systematic review and network meta-analysis. Lancet Oncol 2022. 10.1016/S1470-2045(22)00334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harro CD, Pang Y-YS, Roden RB, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst 2001;93:284-92. 10.1093/jnci/93.4.284 [DOI] [PubMed] [Google Scholar]
- 14. Olsson S-E, Kjaer SK, Sigurdsson K, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin 2009;5:696-704. 10.4161/hv.5.10.9515 [DOI] [PubMed] [Google Scholar]
- 15. Luna J, Plata M, Gonzalez M, et al. Long-term follow-up observation of the safety, immunogenicity, and effectiveness of Gardasil™ in adult women. PLoS One 2013;8:e83431. 10.1371/journal.pone.0083431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hildesheim A, Gonzalez P, Kreimer AR, et al. Costa Rica HPV Vaccine Trial (CVT) Group . Impact of human papillomavirus (HPV) 16 and 18 vaccination on prevalent infections and rates of cervical lesions after excisional treatment. Am J Obstet Gynecol 2016;215:212.e1-15. 10.1016/j.ajog.2016.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joura EA, Garland SM, Paavonen J, et al. FUTURE I and II Study Group . Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data. BMJ 2012;344:e1401. 10.1136/bmj.e1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghelardi A, Parazzini F, Martella F, et al. SPERANZA project: HPV vaccination after treatment for CIN2. Gynecol Oncol 2018;151:229-34. 10.1016/j.ygyno.2018.08.033 [DOI] [PubMed] [Google Scholar]
- 19. Sand FL, Kjaer SK, Frederiksen K, Dehlendorff C. Risk of cervical intraepithelial neoplasia grade 2 or worse after conization in relation to HPV vaccination status. Int J Cancer 2020;147:641-7. [DOI] [PubMed] [Google Scholar]
- 20. Zhao S, Hu S, Xu X, et al. Impact of HPV-16/18 AS04-adjuvanted vaccine on preventing subsequent infection and disease after excision treatment: post-hoc analysis from a randomized controlled trial. BMC Infect Dis 2020;20:846. 10.1186/s12879-020-05560-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pieralli A, Bianchi C, Auzzi N, et al. Indication of prophylactic vaccines as a tool for secondary prevention in HPV-linked disease. Arch Gynecol Obstet 2018;298:1205-10. 10.1007/s00404-018-4926-y [DOI] [PubMed] [Google Scholar]
- 22. Karimi-Zarchi M, Allahqoli L, Nehmati A, Kashi AM, Taghipour-Zahir S, Alkatout I. Can the prophylactic quadrivalent HPV vaccine be used as a therapeutic agent in women with CIN? A randomized trial. BMC Public Health 2020;20:274. 10.1186/s12889-020-8371-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Page M, McKenzie J, Bossuyt P, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. MetaArXiv 2020. 10.31222/osf.io/v7gm2. [DOI] [PMC free article] [PubMed]
- 24. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 26. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383-94. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 27. Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons, 2019. 10.1002/9781119536604. [DOI] [Google Scholar]
- 28. Veroniki AA, Jackson D, Viechtbauer W, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods 2016;7:55-79. 10.1002/jrsm.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med 2001;20:3875-89. 10.1002/sim.1009 [DOI] [PubMed] [Google Scholar]
- 30. Sidik K, Jonkman JN. A simple confidence interval for meta-analysis. Stat Med 2002;21:3153-9. 10.1002/sim.1262. [DOI] [PubMed] [Google Scholar]
- 31. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 32. Mavridis D, Sutton A, Cipriani A, Salanti G. A fully Bayesian application of the Copas selection model for publication bias extended to network meta-analysis. Stat Med 2013;32:51-66. 10.1002/sim.5494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 2006;11:193-206. 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 35. Veroniki AA, Jackson D, Viechtbauer W, et al. Recommendations for quantifying the uncertainty in the summary intervention effect and estimating the between-study heterogeneity variance in random-effects meta-analysis. In: Chandler J, McKenzie J, Boutron I, et al., eds. Cochrane Methods. Cochrane Database of Systematic Reviews, 2015. [Google Scholar]
- 36. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 37. Viechtbauer W. Confidence intervals for the amount of heterogeneity in meta-analysis. Stat Med 2007;26:37-52. 10.1002/sim.2514. [DOI] [PubMed] [Google Scholar]
- 38. Jackson D, Turner R. Power analysis for random-effects meta-analysis. Res Synth Methods 2017;8:290-302. 10.1002/jrsm.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schwarzer G. meta: An R package for meta-analysis. R News 2007;7:40-5. [Google Scholar]
- 40. Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: Evolution, critique and future directions. Stat Med 2009;28:3049-67. 10.1002/sim.3680 [DOI] [PubMed] [Google Scholar]
- 41. Coskuner ER, Ozkan TA, Karakose A, Dillioglugil O, Cevik I. Impact of the quadrivalent HPV vaccine on disease recurrence in men exposed to HPV infection: a randomized study. J Sex Med 2014;11:2785-91. 10.1111/jsm.12670 [DOI] [PubMed] [Google Scholar]
- 42. Kang WD, Choi HS, Kim SM. Is vaccination with quadrivalent HPV vaccine after loop electrosurgical excision procedure effective in preventing recurrence in patients with high-grade cervical intraepithelial neoplasia (CIN2-3)? Gynecol Oncol 2013;130:264-8. 10.1016/j.ygyno.2013.04.050 [DOI] [PubMed] [Google Scholar]
- 43. Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis 2012;54:891-8. 10.1093/cid/cir1036 [DOI] [PubMed] [Google Scholar]
- 44. Grzes BHJ, Ciszek M. Minimally invasive surgery with the complementing immunotherapy in the treatment of intraepithelial neoplasia of cervix in women of child-bearing age. Onkologia Polska 2011;14:125-30. [Google Scholar]
- 45. Ortega-Quiñonero P, Remezal-Solano M, Carazo-Díaz M, et al. Impact of the human papillomavirus vaccination on patients who underwent conization for high-grade cervical intraepithelial neoplasia. Eur J Gynaecol Oncol 2019;40:402-7. [Google Scholar]
- 46. Vinnytska A. EP1090 Use of HPV-vaccine in prevention of recurrent HSIL after LEEP in women of reproductive age. Int J Gynecol Cancer 2019;29:A571. 10.1136/ijgc-2019-ESGO.1132 [DOI] [Google Scholar]
- 47. Petrillo M, Dessole M, Tinacci E, et al. Efficacy of HPV vaccination in women receiving LEEP for cervical dysplasia: a single institution’s experience. Vaccines (Basel) 2020;8:45. 10.3390/vaccines8010045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Del Pino M, Martí C, Torras I, et al. HPV vaccination as adjuvant to conization in women with cervical intraepithelial neoplasia: a study under real-life conditions. Vaccines (Basel) 2020;8:245. 10.3390/vaccines8020245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bogani G, Raspagliesi F, Sopracordevole F, et al. Assessing the long-term role of vaccination against HPV after loop electrosurgical excision procedure (LEEP): a propensity-score matched comparison. Vaccines (Basel) 2020;8:717. 10.3390/vaccines8040717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ghelardi A, Marrai R, Bogani G, et al. Surgical treatment of vulvar HSIL: adjuvant HPV vaccine reduces recurrent disease. Vaccines (Basel) 2021;9:83. 10.3390/vaccines9020083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Garland SM, Paavonen J, Jaisamrarn U, et al. HPV PATRICIA Study Group . Prior human papillomavirus-16/18 AS04-adjuvanted vaccination prevents recurrent high grade cervical intraepithelial neoplasia after definitive surgical therapy: Post-hoc analysis from a randomized controlled trial. Int J Cancer 2016;139:2812-26. 10.1002/ijc.30391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Giannella L, Mfuta K, Fodero C, Prandi S. Outcome of nonpersonalized human papillomavirus vaccinations during postconization follow-up: a report of two cases. J Reprod Med 2015;60:455-7. [PubMed] [Google Scholar]
- 53. Moscato GM, Di Matteo G, Ciotti M, Di Bonito P, Andreoni M, Moschese V. Dual response to human papilloma virus vaccine in an immunodeficiency disorder: resolution of plantar warts and persistence of condylomas. J Eur Acad Dermatol Venereol 2016;30:1212-3. 10.1111/jdv.13133 [DOI] [PubMed] [Google Scholar]
- 54.Şenol T, Yerçok Y, Özkaya E, et al. Efficacy of quadrivalent HPV vaccine for vulvar condyloma recurrence. 2016. https://acikerisim.medipol.edu.tr/xmlui/handle/20.500.12511/1038
- 55. Kreuter A, Wieland U. Lack of efficacy in treating condyloma acuminata and preventing recurrences with the recombinant quadrivalent human papillomavirus vaccine in a case series of immunocompetent patients. J Am Acad Dermatol 2013;68:179-80. 10.1016/j.jaad.2011.11.970 [DOI] [PubMed] [Google Scholar]
- 56. Lichter K, Krause D, Xu J, et al. Adjuvant human papillomavirus vaccine to reduce recurrent cervical dysplasia in unvaccinated women: a systematic review and meta-analysis. Obstet Gynecol 2020;135:1070-83. 10.1097/AOG.0000000000003833 [DOI] [PubMed] [Google Scholar]
- 57. Jentschke M, Kampers J, Becker J, Sibbertsen P, Hillemanns P. Prophylactic HPV vaccination after conization: A systematic review and meta-analysis. Vaccine 2020;38:6402-9. 10.1016/j.vaccine.2020.07.055 [DOI] [PubMed] [Google Scholar]
- 58. Di Donato V, Caruso G, Petrillo M, et al. Adjuvant HPV vaccination to prevent recurrent cervical dysplasia after surgical treatment: a meta-analysis. Vaccines (Basel) 2021;9:410. 10.3390/vaccines9050410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bartels HC, Postle J, Rogers AC, Brennan D. Prophylactic human papillomavirus vaccination to prevent recurrence of cervical intraepithelial neoplasia: a meta-analysis. Int J Gynecol Cancer 2020;30:777-82. 10.1136/ijgc-2020-001197 [DOI] [PubMed] [Google Scholar]
- 60. Arbyn M, Redman CWE, Verdoodt F, et al. Incomplete excision of cervical precancer as a predictor of treatment failure: a systematic review and meta-analysis. Lancet Oncol 2017;18:1665-79. 10.1016/S1470-2045(17)30700-3 [DOI] [PubMed] [Google Scholar]
- 61.Kyrgiou M. Nonavalent prophylactic HPV vaccine (GARDASIL9) after local conservative The NOVEL Trial (NOVEL). 2019. https://clinicaltrials.gov/ct2/show/NCT03979014?term=NOVEL&draw=2&rank=1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary material
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.