Abstract
Background
The cause of infertility remains unclear in a significant proportion of reproductive-age couples who fail to conceive naturally. Chromosomal aberrations have been identified as one of the main genetic causes of male and female infertility. Structural chromosomal aberrations may disrupt the functioning of various genes, some of which may be important for fertility. The present study aims to identify candidate genes and putative functional interaction networks involved in male and female infertility using cytogenetic data from cultured peripheral blood lymphocytes of infertile patients.
Methods
Karyotypic analyses was done in 201 infertile patients (100 males and 101 females) and 201 age and gender matched healthy controls (100 males and 101 females) after 72 h peripheral lymphocyte culturing and GTG banding, followed by bioinformatic analysis using Cytoscape v3.8.2 and Metascape.
Results
Several chromosomal regions with a significantly higher frequency of structural aberrations were identified in the infertile males (5q2, 10q2, and 17q2) and females (6q2, 16q2, and Xq2). Segregation of the patients based on type of infertility (primary v/s secondary infertility) led to the identification of chromosomal regions with a significantly higher frequency of structural aberrations exclusively within the infertile males (5q2, 17q2) and females (16q2) with primary infertility. Cytoscape identified two networks specific to these regions: a male specific network with 99 genes and a female specific network with 109 genes. The top enriched GO terms within the male and female infertility networks were “skeletal system morphogenesis” and “mRNA transport” respectively. PSME3, PSMD3, and CDC27 were the top 3 hub genes identified within the male infertility network. Similarly, UPF3B, IRF8, and PSMB1 were the top 3 hub genes identified with the female infertility network. Among the hub genes identified in the male- and female-specific networks, PSMB1, PSMD3, and PSME3 are functional components of the proteasome complex. These hub genes have a limited number of reports related to their respective roles in maintenance of fertility in mice model and humans and require validation in further studies.
Conclusion
The candidate genes predicted in the present study can serve as targets for future research on infertility.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12920-022-01320-x.
Keywords: Male infertility, Female infertility, Karyotyping, In-silico, Network analysis, Protein–protein interaction
Background
Human beings, despite a substantial growth in their population, can be considered a relatively infertile species [1]. Infertility is defined as a condition marked by the inability of a couple to conceive after one year of unprotected intercourse [2].
An estimated 8% to 12% couples of reproductive age worldwide are affected by infertility. Approximately 50% of total cases involve a diagnosis of infertility in the male partner [2, 3]. Male infertility can be caused by various mechanical, lifestyle and genetic factors. The genetic causes of male infertility include structural and numerical chromosome abnormalities, Y chromosome deletions, single gene disorders, and multifactorial causes [2]. Among the genetic factors, chromosomal anomalies have been identified as one of the main causes of male infertility [4]. With at least 2000 genes believed to be involved in spermatogenesis, the number of genetic anomalies associated with male infertility is growing steadily [5].
On the other hand, female infertility can be caused by developmental, endocrine, immunological, metabolic, microbial, surgical or genetic factors [6, 7]. The genetic causes of female infertility include chromosomal aberrations due to meiotic non-disjunction errors, copy number variants (CNV’s), single gene disorders and polygenic disorders [7].
Cytogenetic aberrations are included among the main genetic causes of infertility [4, 8]. Therefore, identification of chromosomal loci frequently involved in aberrations can help in identifying the genes/pathways involved in infertility using in-silico tools. Keeping these facts in mind, the present study uses a combination of cytogenetics and bioinformatic tools for the prediction of candidate genes which are actively involved in the pathogenesis of infertility.
Methods
Cytogenetic analysis
In order to study the cytogenetic aberrations associated with infertility, 201 infertile patients (100 males and 101 females) and 201 age and gender matched controls (100 males and 101 females) from a North-Indian population of Punjab, India were karyotyped after 72 h peripheral blood lymphocyte culturing and GTG-banding. The inclusion and exclusion criteria for recruitment of the infertile patients are given in Additional file 1: Table S1. The phenotypic presentations of the infertile males and females are given in Additional file 1: Table S2. The patients included in the present study were clinically diagnosed as infertile after failure to conceive via natural methods, with medications and also experiencing in-vitro fertilization (IVF) failure. These include a subset of patients wherein the fertility assessment parameters (spermiogram in males, hormonal profiles and reproductive imaging in females) were within the standard clinical limits in both the male and female partners undergoing IVF (Additional file 1: Table S2).
The cytogenetic analysis of the cultured peripheral blood lymphocytes of the patients and controls involved scoring of chromosomal aberrations as total metaphases showing any chromosomal aberration (TAM), metaphases showing only numerical aberrations (TMNA), metaphases showing only structural aberrations (TMSA), and metaphases showing both structural and numerical aberrations (TM(NA + SA)) in 50 to 100 metaphases per subject. The comparison of frequency of chromosomal aberrations in cases and controls was done using Student’s t-test. The cut off p-value adopted for statistical significance was 0.05.
Bioinformatic analysis
The cytogenetic analysis helped in the identification of several chromosomal regions with a significantly higher frequency of structural aberrations among the infertile patients as compared to controls. The genes harbored within these loci were assessed by in-silico tools to predict candidate genes and pathways which might be impaired in infertile males and females. The cytogenetic loci observed within these regions were used as the input query for National Centre for Biotechnology Information (NCBI) Gene database (https://www.ncbi.nlm.nih.gov/gene) to identify the constituent genes. The data provided by NCBI Gene was filtered according to species (“Homo sapiens”), chromosome number (chromosomal regions not queried were removed) and number of exons (only genes containing one or more exons were included).
Cytoscape v3.8.2 [9] was used to generate various interactive biological networks from the genes annotated to the different chromosomal regions. In the present study, the ‘Gene Set/Mutation Analysis’ tool of the ‘Reactome Functional Interaction (FI)’ Cytoscape plugin [10] was used to generate the different interaction networks. For this purpose, the 2019 ‘Reactome FI Network’ dataset and ‘Show genes not linked to others’ options were used to create interaction networks without the addition of any linker gene. The cytoHubba plugin [11] within Cytoscape was used to identify the various hub genes within the male and female networks. The set of genes located within the different networks was used as the input for the web-based tool, Metascape [12], to identify the genes and pathways enriched within the infertile males and females.
Results
Cytogenetic analysis
The comparison of chromosomal aberrations between the infertile cases and age-matched controls revealed a significantly higher mean frequency of aberrations among the infertile cases (Table 1). A similar trend was observed upon segregating the cases and controls by gender and type of infertility (primary versus secondary infertility) (Tables 2, 3). Among the infertile patients, 5 males and 8 females were identified as carriers of constitutional anomalies. These patients were removed from further analysis resulting in 188 infertile patients (95 males and 93 females) and 188 age-matched controls (95 males and 93 females) remaining for further analysis. A significantly high mean frequency of structural aberrations (deletions, chromatid/chromosomal breaks and gaps) was identified in certain chromosomal regions within these subsets of patients (Table 4). The representative karyotypes for a subset of infertile patients and healthy controls are depicted in Additional file 1: Table S3.
Table 1.
Cytogenetic profile of infertile cases and healthy controls
| Variable | Male cases | Male controls | p-value | Female cases | Female controls | p-value |
|---|---|---|---|---|---|---|
| No. of subjects | 100 | 100 | – | 101 | 101 | – |
| Age (Mean ± SD) in years | 34.61 ± 7.21 | 34.91 ± 7.66 | 0.7758 | 32.58 ± 6.28 | 34.88 ± 7.45 | 0.0186 |
| Mean (%) aberrant metaphases | 28.37 ± 13.46 | 11.28 ± 7.28 | < 0.0001 | 29.16 ± 14.02 | 13.60 ± 7.03 | < 0.0001 |
| Mean (%) metaphases with structural aberrations | 16.81 ± 10.99 | 5.44 ± 5.37 | < 0.0001 | 17.94 ± 12.71 | 5.30 ± 4.37 | < 0.0001 |
| Mean (%) metaphases with numerical aberrations | 7.82 ± 6.18 | 4.98 ± 3.98 | 0.0002 | 7.51 ± 5.32 | 7.03 ± 4.72 | 0.4984 |
| Mean (%) metaphases with both structuraland numerical aberrations | 3.57 ± 3.15 | 0.59 ± 1.01 | < 0.0001 | 4.16 ± 3.80 | 1.43 ± 2.29 | < 0.0001 |
Significant p-values (< 0.05), calculated by t-test, are shown in bold
Table 2.
Comparison of cytogenetic profiles of primary infertility cases (male and female) with age and gender matched controls
| Variable | Male primary infertility cases | Age-matched male controls | p-value | Female primary infertility cases | Age-matched female controls | p-value |
|---|---|---|---|---|---|---|
| No. of subjects | 66 | 66 | – | 66 | 66 | – |
| Age (Mean ± SD) in years | 33.94 ± 6.93 | 34.24 ± 7.23 | 0.8081 | 31.80 ± 6.0 | 33.94 ± 7.28 | 0.0676 |
| Mean (%) aberrant metaphases | 26.96 ± 12.52 | 11.31 ± 7.02 | < 0.0001 | 27.22 ± 12.34 | 13.44 ± 7.12 | < 0.0001 |
| Mean (%) metaphases with structural aberrations | 16.17 ± 10.74 | 5.29 ± 5.06 | < 0.0001 | 16.74 ± 11.87 | 5.42 ± 4.83 | < 0.0001 |
| Mean (%) metaphases with numerical aberrations | 7.24 ± 5.50 | 5.34 ± 4.27 | 0.0284 | 7.4 ± 5.33 | 6.96 ± 4.80 | 0.6191 |
| Mean (%) metaphases with both structural and numerical aberrations | 3.25 ± 2.99 | 0.55 ± 1.04 | < 0.0001 | 3.49 ± 2.9 | 1.34 ± 2.15 | < 0.0001 |
Significant p-values (< 0.05), calculated by t-test, are shown in bold
Table 3.
Comparison of cytogenetic profiles of secondary infertility cases (male and female) with age and gender-matched controls
| Variable | Male secondary infertility cases | Age-matched male controls | p-value | Female secondary infertility cases | Age-matched female controls | p-value |
|---|---|---|---|---|---|---|
| No. of subjects | 34 | 34 | – | 35 | 35 | – |
| Age (Mean ± SD) in years | 35.91 ± 7.66 | 36.21 ± 8.39 | 0.8781 | 34.06 ± 6.62 | 36.66 ± 7.55 | 0.1302 |
| Mean (%) aberrant metaphases | 31.12 ± 14.9 | 11.23 ± 7.88 | < 0.0001 | 32.67 ± 16.24 | 13.91 ± 6.92 | < 0.0001 |
| Mean (%) metaphases with structural aberrations | 18.29 ± 11.3 | 5.73 ± 5.99 | < 0.0001 | 20.12 ± 14.01 | 5.06 ± 3.38 | < 0.0001 |
| Mean (%) metaphases with numerical aberrations | 8.6 ± 7.4 | 4.29 ± 3.29 | 0.0028 | 7.51 ± 5.39 | 7.16 ± 4.62 | 0.7714 |
| Mean (%) metaphases with both structural and numerical aberrations | 4.3 ± 3.4 | 0.68 ± 0.94 | < 0.0001 | 5.38 ± 4.85 | 1.60 ± 2.55 | 0.0001 |
Significant p-values (< 0.05), calculated by t-test, are shown in bold
Table 4.
List of chromosomal regions with a significantly higher frequency of structural aberrations in infertile males and females
| Gender | Chromosome/chromosomal arm/chromosomal region | Frequency of aberrations in infertility cases (Mean ± SD) |
Frequency of aberrations in healthy age-matched controls (Mean ± SD)* |
p-value |
|---|---|---|---|---|
| Male | 5q2 | 1.67 ± 0.58 | 1.00 ± 0.00 | < 0.0001 |
| 10q2 | 1.50 ± 0.71 | 1.00 ± 0.00 | < 0.0001 | |
| 17q2 | 1.33 ± 0.58 | 1.00 ± 0.00 | < 0.0001 | |
| Female | 6q2 | 1.17 ± 0.41 | 1.00 ± 0.00 | < 0.0001 |
| 16q2 | 1.50 ± 0.71 | 1.00 ± 0.00 | < 0.0001 | |
| Xq2 | 2.00 ± 1.18 | 1.00 ± 0.00 | < 0.0001 |
Significant p-values (<0.05), calculated by t-test, are highlighted in bold
*The zero values were omitted during the calculation of Mean and Standard Deviation due to presence of a high number of zeros in the data
Upon segregating the patients based on type of infertility (primary vs. secondary infertility), chromosomal regions with a significantly high mean frequency of structural aberrations were identified only within the primary infertility patients (Table 5). The cytogenetic loci affected within these regions (Tables 4 and 5) were subjected to bioinformatic analysis.
Table 5.
List of chromosomal regions with a significantly higher frequency of structural aberrations in males and females diagnosed with primary infertility
| Gender | Chromosome/chromosomal arm/chromosomal region | Frequency of aberrations in infertility cases (Mean ± SD) |
Frequency of aberrations in healthy age-matched controls (Mean ± SD)* |
p-value |
|---|---|---|---|---|
| Male | 5q2 | 1.50 ± 0.71 | 1.00 ± 0.00 | < 0.0001 |
| 17q2 | 1.50 ± 0.71 | 1.00 ± 0.00 | < 0.0001 | |
| Female | 16q2 | 1.50 ± 0.71 | 1.00 ± 0.00 | < 0.0001 |
Significant p-values (<0.05), calculated by t-test, are highlighted in bold
*The zero values were omitted during the calculation of Mean and Standard Deviation due to presence of a high number of zeros in the data.
Bioinformatic analysis
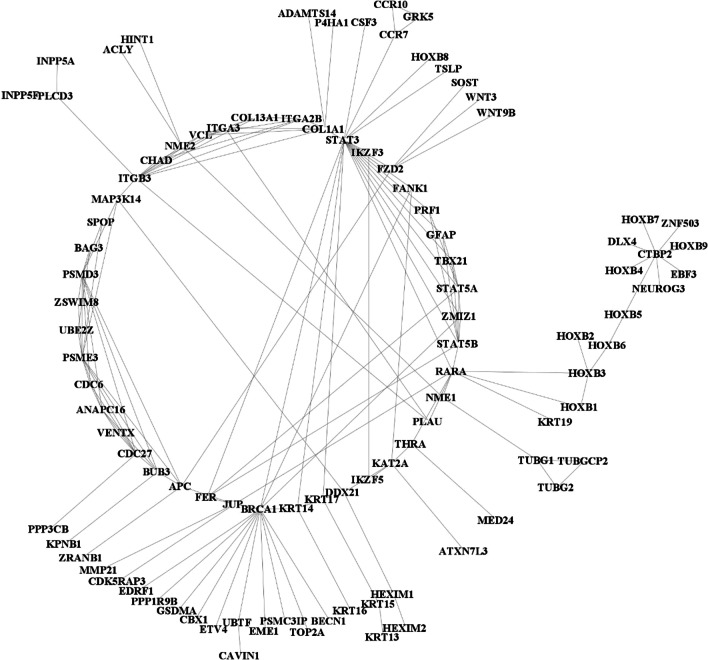
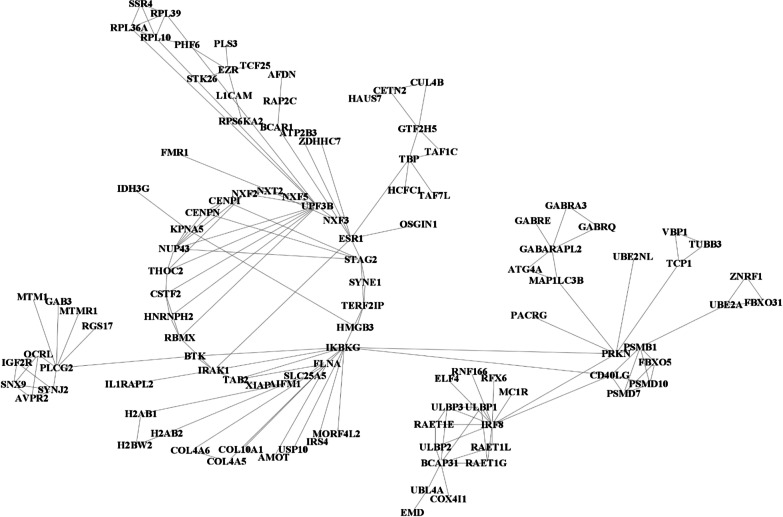
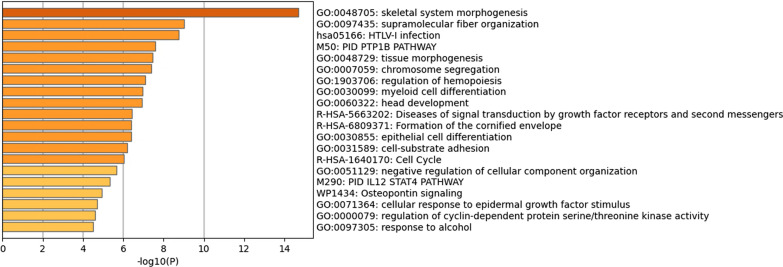
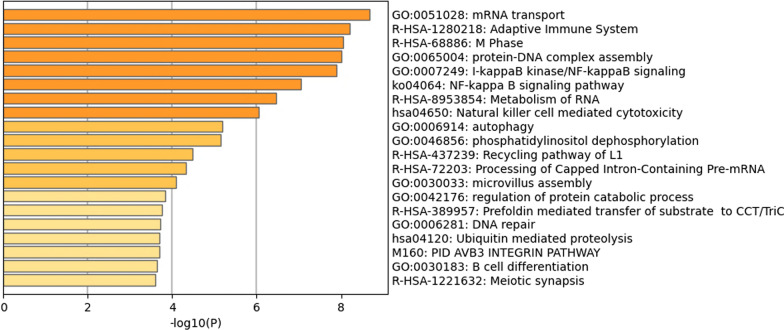
NCBI Gene returned a list of the genes present at the cytogenetic loci queried: 731 genes in the male dataset and 901 genes in the female dataset. Querying Reactome FI with the aforementioned gene sets led to the generation of a network of 99 genes in the male-specific network (Fig. 1) and 109 genes in the female-specific network (Fig. 2). Further analysis by cytoHubba led to the identification of hub genes within the male (PSMD3, PSME3, and CDC27) and female (UPF3B, IRF8, and PSMB1) networks. Metascape identified “skeletal system morphogenesis” as the top enriched term within the male infertility network (Fig. 3) and “mRNA transport” as the top enriched term within the female infertility network (Fig. 4).
Fig. 1.
Biological interaction network generated using Cytoscape v3.8.2 for the male infertility dataset
Fig. 2.
Biological interaction network generated using Cytoscape v3.8.2 for the female infertility dataset
Fig. 3.
A list of the top 20 enriched gene ontology categories identified by Metascape for the male infertility dataset
Fig. 4.
A list of the top 20 enriched gene ontology categories identified by Metascape for the female infertility dataset
Discussion
The human interactome is a highly complex network of functionally interacting cellular components, including a multitude of genes, proteins, metabolites, and RNA molecules [13]. It is now believed that many diseases manifest as a result of disruption of biological cascades due to altered interaction of various network components [14].
Among the structural aberrations identified in the present study, deletions, chromatid/chromosomal gaps and breaks were the most frequentin the infertile patients. In the present study, terminal deletions were observed in the 6q, 16q, and Xq region in infertile females. Deletions, either terminal or interstitial, result in loss of chromosomal segments and a subsequent haploinsufficiency of the gene(s) located in the deleted segments [15].Besides deletions, chromatid/chromosomal gaps and breaks were observed in both males (5q2, 10q2, and 17q2) and females (6q2 and Xq2). These aberrations occur as a consequence of DNA damage through exposure to physical and/or chemical agents, or as a result of recombination events [16]. If left unrepaired, chromosomal breaks can result in deletions (small- or large-scale) and translocations [17].
In the bio-informatic analysis in the present study, the top enriched gene ontology (GO) category within the male infertility network was GO:0048705—“skeletal system morphogenesis” (Fig. 3). The genes enriched within this category included APC, BRCA1, CHAD, COL1A1, COL13A1, FZD2, HOXB1, HOXB2, HOXB3, HOXB4, HOXB5, HOXB6, HOXB7, HOXB8, HOXB9, ITGA3, ITGB3, KAT2A, KRT19, MMP21, NEUROG3, PLCD3, RARA, TBX21, THRA, WNT3, WNT9B, ZMIZ1. Twenty-two genes enriched within this category have published reports on roles in maintenance of male fertility (APC, BRCA1, COL1A1, COL13A1, FZD2, HOXB1, HOXB2, HOXB4, HOXB5, HOXB6, HOXB7, HOXB8, HOXB9, ITGA3, KAT2A, KRT19, NEUROG3, RARA, THRA, WNT3, WNT9B, ZMIZ1) (Additional file 1: Table S4). Among the male infertility network, sixty-two genes had literature published on roles in male fertility (Additional file 1: Table S4).
The top enriched category within the female infertility network was GO:0051028—“mRNA transport” (Fig. 4). The genes enriched within this category included CETN2, CSTF2, EMD, EZR, FLNA, FMR1, HCFC1, IKBKG, KPNA5, NUP43, NXF2, NXF3, NXF5, NXT2, SLC25A5, TAB2, TBP, TCP1, THOC2, UPF3B. A dozen genes enriched within this category have published reports on roles in maintenance of female fertility (CETN2, CSTF2, EZR, FLNA, FMR1, HCFC1, IKBKG, NUP43, NXF5, SLC25A5, TAB2, UPF3B) (Additional file 1: Table S5). Among the female infertility network, sixty-eight genes had literature published on roles in female infertility (Additional file 1: Table S5).
In the male infertility network, the top 3 hub genes identified were PSME3, PSMD3, and CDC27. Research on mice models have shown that double knockout of Psme3 and Psme4 results in complete infertility in males [18]. In an additional report, male mice with PSME3 (also known as REGγ) deficiency exhibited subfertility due to a decrease in the activity and concentration of spermatozoa [19]. The comparison of gene expression profiles of high motility sperm samples between healthy normozoospermic and asthenozoospermic individuals showed that PSMD3, CDC27 and many other proteins involved in protein polyubiquitination were significantly downregulated in asthenozoospermic individuals [20]. The ubiquitin–proteasome system (UPS) has been reported to play an important role in sperm capacitation and fertilization [21]. Therefore, the UPS components involved in the sperm proteasome can be considered as potential candidates for further research on male infertility.
In the female infertility network, the top 3 hub genes identified were UPF3B, IRF8 and PSMB1. Copy number variation in the 6q27 region (which includes PSMB1) have been speculated to be the cause of premature ovarian failure (POF) in a patient from a POF cohort [22]. In recent publications, IRF8 positive cells were reported to be increased during the proliferative phase of the menstrual cycle in the endometrium of women with endometriosis [23]. Additionally, IRF8 and MEF2C have been reported to be regulated at both mRNA and protein level in the endometrial epithelium during the window of implantation [24]. Upf3b was predicted to be a target gene for the rno-miR-141-5p microRNA. This miRNA was reported to possibly play a role in modulating endometrial receptivity in rats with endometriosis [25]. Currently, limited reports are available on the roles of these genes in maintenance of female fertility, warranting further research on these candidates.
Analysis of the predicted loss-of-function (pLOF) variants in the Genome Aggregation Database (gnomAD) browser [26] suggests that the hub genes, CDC27, PSMD3, PSME3 (male-specific network), PSMB1, UPF3B (female-specific network) are intolerant to loss-of-function variants. In the clinical setting, microarray-based comparative genomic hybridization (aCGH) coupled with multiplex ligation-dependent probe amplification (MLPA) would be a better alternative to identify genomic imbalances within infertile patients having structural aberrations (especially deletions) within the chromosomal regions harboring these genes [27].
There are few limitations associated with the present study. A cytogenetic approach has been used in the present study to identify possible candidate genes located in chromosomal regions with a high mean frequency of structural aberrations in infertile patients, compared to healthy control individuals. GTG banding has been used for cytogenetic analysis. Compared to other microscopy-based alternatives, G-banding has a lower resolution [28].Finally, there is no expression-based data for the present dataset which can reveal the differentially expressed genes associated with the infertility subsets.
Conclusion
The present study has identified several candidate genes associated with male and female infertility based on information of aberrations available from chromosomal analysis in G-banded cultured peripheral blood lymphocytes. Among the hub genes, the PSMB1 (female-specific network), PSMD3, and PSME3 (male-specifc network) are components of the proteasome complex. Currently, limited research has been conducted in human infertility on the roles of most of the genes predicted in the present study with a majority of the available reports limited to murine models. Therefore, future research may focus on determining the role of these genes in the maintenance of human fertility.
Supplementary Information
Additional file 1: Clinical characteristics and karyotypes of study participants with supporting literature.
Acknowledgements
The authors would like to thank Dr. Sonia Kamboj (Genesis Fertility and Surgical Centre, Jalandhar, Punjab, India) for allowing access to blood samples and clinical details of the patients. Financial support from Council of Scientific & Industrial Research (CSIR), India (Junior Research Fellowship) to JSS and University Grants Commission (UGC), India via UGC Minor Project (F. No. 8-1 (338)/2010 (MRP/NRCB)) to BS is highly acknowledged.
Abbreviations
- aCGH
Array-based comparative genomic hybridization
- CHAD
Chondroadherin
- CNV’s
Copy number variations
- EMD
Emerin
- EZR
Ezrin
- FI
Functional interaction
- gnomAD
Genome aggregation database
- GO
Gene ontology
- GTG
G-bands after trypsin and Giemsa
- HOXB3
Homeobox B3
- IVF
In-vitro fertilization
- KPNA5
Karyopherin subunit alpha 5
- MEF2C
Myocyte enhancer factor 2C
- MLPA
Multiplex ligation-dependent probe amplification
- MMP21
Matrix metallopeptidase 21
- NCBI
National Centre for Biotechnology Information
- NXF2
Nuclear RNA export factor 2
- NXF3
Nuclear RNA export factor 3
- NXT2
Nuclear transport factor 2 like export factor 2
- PALB2
Partner and localizer of BRCA2
- PLCD3
Phospholipase C delta 3
- pLOF
Predicted loss-of function
- POF
Premature ovarian failure
- STAT3
Signal transducer and activator of transcription 3
- T1LCs
T1 prospermatogonia-like cells
- TBP
TATA-box binding protein
- TAM
Total metaphases showing any chromosomal aberration
- TCP1
T-complex 1
- THOC2
THO complex 2
- TM(NA + SA)
Total metaphases showing both structural and numerical aberrations.
- TMNA
Total metaphases showing only numerical aberrations
- TMSA
Total metaphases showing only structural aberrations
- UPS
Ubiquitin–proteasome system
Author contributions
VS and KG contributed in the study design. BS collected samples and performed cytogenetic analysis. JSS analyzed the data using bioinformatic methods. JSS and VS prepared the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by funding from Council of Scientific & Industrial Research (CSIR) (Junior Research Fellowship) provided to JSS and by University Grants Commission (UGC) via UGC Minor Project (F. No. 8–1(338)/2010 (MRP/NRCB)) provided to BS.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
Declarations
Ethics approval and consent to participate
This study was conducted after approval by the Institutional Ethics Committee, Guru Nanak Dev University, Amritsar, Punjab, India in accordance with the tenets of the Declaration of Helsinki (Letter No: 301/HG dated 07–04-2022). Informed consent was obtained from all participants before conducting the study. The methodology was in accordance with the guidelines and regulations of Indian Council of Medical Research (ICMR) National Ethical Guidelines for Biomedical and Health Research for human subjects.
Consent for publication
Not applicable.
Competing interests
Corresponding author, Vasudha Sambyal and co-author, Kamlesh Guleria are Associate Editors of the journal BMC Medical Genomics. Rest of the authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aitken RJ, Curry BJ. Redox regulation of human sperm function: from the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid Redox Signal. 2011;14:367–381. doi: 10.1089/ars.2010.3186. [DOI] [PubMed] [Google Scholar]
- 2.Griffin DK, Finch KA. The genetic and cytogenetic basis of male infertility. Hum Fertil. 2005;8:19–26. doi: 10.1080/14647270400016407. [DOI] [PubMed] [Google Scholar]
- 3.Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10. doi: 10.1016/j.clinbiochem.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Martin RH. Cytogenetic determinants of male fertility. Hum Reprod Update. 2008;14:379–390. doi: 10.1093/humupd/dmn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krausz C, Riera-Escamilla A. Genetics of male infertility. Nat Rev Urol. 2018;15:369–384. doi: 10.1038/s41585-018-0003-3. [DOI] [PubMed] [Google Scholar]
- 6.Shah K, Sivapalan G, Gibbons N, Tempest H, Griffin DK. The genetic basis of infertility. Reproduction. 2003;126:13–25. doi: 10.1530/rep.0.1260013. [DOI] [PubMed] [Google Scholar]
- 7.Zorrilla M, Yatsenko AN. The genetics of infertility: current status of the field. Curr Genet Med Rep. 2013;1:247–260. doi: 10.1007/s40142-013-0027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gekas J, Meens R, Gondry J, Mathieu M, Thepot F. Value of karyotyping women patients of couples referred for sterility. Gynecol Obstet Fertil. 2003;31:66–69. doi: 10.1016/S1297-9589(02)00008-5. [DOI] [PubMed] [Google Scholar]
- 9.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu G, Dawson E, Duong A, Haw R, Stein L. ReactomeFIViz: a cytoscape app for pathway and network-based data analysis. F1000Res. 2014;3:146. doi: 10.12688/f1000research.4431.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8:1–7. doi: 10.1186/1752-0509-8-S4-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10:1–10. doi: 10.1038/s41467-018-07882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barabási AL, Gulbahce N, Loscalzo J. Network medicine: a network-based approach to human disease. Nat Rev Genet. 2011;12:56–68. doi: 10.1038/nrg2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirk IK, Weinhold N, Belling K, Skakkebæk NE, Jensen TS, Leffers H, et al. Chromosome-wise protein interaction patterns and their impact on functional implications of large-scale genomic aberrations. Cell Syst. 2017;4:357–364. doi: 10.1016/j.cels.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Theisen A, Shaffer LG. Disorders caused by chromosome abnormalities. Appl Clin Genet. 2010;3:159. doi: 10.2147/TACG.S8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eid MM, Temtamy SA. Cytogenetic studies of chromosomal breakage diseases. Middle East J Med Genet. 2013;2:11–22. doi: 10.1097/01.MXE.0000422776.59740.e2. [DOI] [Google Scholar]
- 17.Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008;18:134–147. doi: 10.1038/cr.2007.111. [DOI] [PubMed] [Google Scholar]
- 18.Huang L, Haratake K, Miyahara H, Chiba T. Proteasome activators, PA28γ and PA200, play indispensable roles in male fertility. Sci Rep. 2016;6:23171. doi: 10.1038/srep23171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao X, Chen H, Liu J, Shen S, Wang Q, Clement TM, et al. The REGγ-proteasome regulates spermatogenesis partially by P53-PLZF signaling. Stem Cell Reports. 2019;13:559–571. doi: 10.1016/j.stemcr.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caballero-Campo P, Lira-Albarrán S, Barrera D, Borja-Cacho E, Godoy-Morales HS, Rangel-Escareño C, et al. Gene transcription profiling of astheno-and normo-zoospermic sperm subpopulations. Asian J Androl. 2020;22:608–615. doi: 10.4103/aja.aja_143_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmerman S, Sutovsky P. The sperm proteasome during sperm capacitation and fertilization. J Reprod Immunol. 2009;83:19–25. doi: 10.1016/j.jri.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Tšuiko O, Noukas M, Žilina O, Hensen K, Tapanainen JS, Mägi R, et al. Copy number variation analysis detects novel candidate genes involved in follicular growth and oocyte maturation in a cohort of premature ovarian failure cases. Hum Reprod. 2016;31:1913–1925. doi: 10.1093/humrep/dew142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hey-Cunningham AJ, Wong C, Hsu J, Fromm PD, Clark GJ, Kupresanin F, et al. Comprehensive analysis utilizing flow cytometry and immunohistochemistry reveals inflammatory changes in local endometrial and systemic dendritic cell populations in endometriosis. Hum Reprod. 2021;36:415–428. doi: 10.1093/humrep/deaa318. [DOI] [PubMed] [Google Scholar]
- 24.Chi RPA, Wang T, Adams N, Wu SP, Young SL, Spencer TE, et al. Human endometrial transcriptome and progesterone receptor cistrome reveal important pathways and epithelial regulators. J Clin Endocrinol Metab. 2020;105:e1419–e1439. doi: 10.1210/clinem/dgz117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai H, Zhu XX, Li ZF, Zhu YP, Lang JH. MicroRNA dysregulation and steroid hormone receptor expression in uterine tissues of rats with endometriosis during the implantation window. Chin Med J. 2018;131:2193–2204. doi: 10.4103/0366-6999.240808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, Collins RL, Laricchia KM, Ganna A, Birnbaum DP, Gauthier LD. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faundes V, Santa María L, Morales P, Curotto B, Parraguez MM. Distal 7q11. 23 duplication, an emerging microduplication syndrome: a case report and further characterisation. Mol Syndromol. 2016;7:287–291. doi: 10.1159/000448698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bridge JA. Advantages and limitations of cytogenetic, molecular cytogenetic, and molecular diagnostic testing in mesenchymal neoplasms. J Orthop Sci. 2008;13:273–282. doi: 10.1007/s00776-007-1215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanwar PS, Zhang L, Teixeira JM. Adenomatous polyposis coli (APC) is essential for maintaining the integrity of the seminiferous epithelium. Mol Endocrinol. 2011;25:1725–1739. doi: 10.1210/me.2011-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kierszenbaum AL, Rivkin E, Tres LL. Expression of Fer testis (FerT) tyrosine kinase transcript variants and distribution sites of FerT during the development of the acrosome-acroplaxome-manchette complex in rat spermatids. Dev Dyn. 2008;237:3882–3891. doi: 10.1002/dvdy.21789. [DOI] [PubMed] [Google Scholar]
- 31.Bansal SK, Gupta N, Sankhwar SN, Rajender S. Differential genes expression between fertile and infertile spermatozoa revealed by transcriptome analysis. PLoS ONE. 2015;10:e0127007. doi: 10.1371/journal.pone.0127007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alkhaled Y, Laqqan M, Tierling S, Lo Porto C, Hammadeh ME. DNA methylation level of spermatozoa from subfertile and proven fertile and its relation to standard sperm parameters. Andrologia. 2018;50:e13011. doi: 10.1111/and.13011. [DOI] [PubMed] [Google Scholar]
- 33.Hu X, Shen B, Liao S, Ning Y, Ma L, Chen J, et al. Gene knockout of Zmym3 in mice arrests spermatogenesis at meiotic metaphase with defects in spindle assembly checkpoint. Cell Death Dis. 2017;8:e2910–e2910. doi: 10.1038/cddis.2017.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Aurora M, Ferlin A, Garolla A, Franchi S, D’Onofrio L, Trubiani O, et al. Testis transcriptome modulation in Klinefelter patients with hypospermatogenesis. Sci Rep. 2017;7:45729. doi: 10.1038/srep45729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovac J, Addai J, Lipshultz L, Lamb D. 2047 microarray analysis reveals multiple genes associated with male infertility. J Urol. 2013;189:e840–e840. doi: 10.1016/j.juro.2013.02.2466. [DOI] [Google Scholar]
- 36.Ramathal C, Angulo B, Sukhwani M, Cui J, Durruthy-Durruthy J, Fang F, et al. DDX3Y gene rescue of a Y chromosome AZFa deletion restores germ cell formation and transcriptional programs. Sci Rep. 2015;5:1–13. doi: 10.1038/srep15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sujit KM, Sarkar S, Singh V, Pandey R, Agrawal NK, Trivedi S, et al. Genome-wide differential methylation analyses identifies methylation signatures of male infertility. Hum Reprod. 2018;33:2256–2267. doi: 10.1093/humrep/dey319. [DOI] [PubMed] [Google Scholar]
- 38.Dong WW, Huang HL, Yang W, Liu J, Yu Y, Zhou SL, et al. Testis-specific Fank1 gene in knockdown mice produces oligospermia via apoptosis. Asian J Androl. 2014;16:124. doi: 10.4103/1008-682X.122592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Zhang X, Zhang Y, Zeng W, Zhao S, Liu M. Normal spermatogenesis in Fank1 (fibronectin type 3 and ankyrin repeat domains 1) mutant mice. PeerJ. 2019;7:e6827. doi: 10.7717/peerj.6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Razavi SM, Sabbaghian M, Jalili M, Divsalar A, Wolkenhauer O, Salehzadeh-Yazdi A. Comprehensive functional enrichment analysis of male infertility. Sci Rep. 2017;7:1–14. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcello MR, Evans JP. Multivariate analysis of male reproductive function in Inpp5b−/− mice reveals heterogeneity in defects in fertility, sperm–egg membrane interaction and proteolytic cleavage of sperm ADAMs. Mol Hum Reprod. 2010;16:492–505. doi: 10.1093/molehr/gaq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaucher AV, Oatley MJ, Oatley JM. NEUROG3 is a critical downstream effector for STAT3-regulated differentiation of mammalian stem and progenitor spermatogonia. Biol Reprod. 2012;86:164. doi: 10.1095/biolreprod.111.097386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao K, Liu Y, Xiong Z, Hu L, Xiong CL. Tissue-specific inhibition of urokinase-type plasminogen activator expression in the testes of mice by inducible lentiviral RNA interference causes male infertility. Reprod Fertil Dev. 2017;29:2149–2156. doi: 10.1071/RD16477. [DOI] [PubMed] [Google Scholar]
- 44.Chan CC, Shui HA, Wu CH, Wang CY, Sun GH, Chen HM, et al. Motility and protein phosphorylation in healthy and asthenozoospermic sperm. J Proteome Res. 2009;8:5382–5386. doi: 10.1021/pr9003932. [DOI] [PubMed] [Google Scholar]
- 45.Roa-Espitia AL, Hernández-Rendón ER, Baltiérrez-Hoyos R, Muñoz-Gotera RJ, Cote-Vélez A, Jiménez I, et al. Focal adhesion kinase is required for actin polymerization and remodeling of the cytoskeleton during sperm capacitation. Biol Open. 2016;5:1189–1199. doi: 10.1242/bio.017558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hilz S, Fogarty EA, Modzelewski AJ, Cohen PE, Grimson A. Transcriptome profiling of the developing male germ line identifies the miR-29 family as a global regulator during meiosis. RNA Biol. 2017;14:219–235. doi: 10.1080/15476286.2016.1270002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beverdam A, Koopman P. Expression profiling of purified mouse gonadal somatic cells during the critical time window of sex determination reveals novel candidate genes for human sexual dysgenesis syndromes. Hum Mol Genet. 2006;15:417–431. doi: 10.1093/hmg/ddi463. [DOI] [PubMed] [Google Scholar]
- 48.Fang N, Cao C, Wen Y, Wang X, Yuan S, Huang X. MicroRNA profile comparison of testicular tissues derived from successful and unsuccessful microdissection testicular sperm extraction retrieval in non-obstructive azoospermia patients. Reprod Fertil Dev. 2019;31:671–682. doi: 10.1071/RD17423. [DOI] [PubMed] [Google Scholar]
- 49.Martin-Hidalgo D, Serrano R, Zaragoza C, Garcia-Marin LJ, Bragado MJ. Human sperm phosphoproteome reveals differential phosphoprotein signatures that regulate human sperm motility. J Proteomics. 2020;215:103654. doi: 10.1016/j.jprot.2020.103654. [DOI] [PubMed] [Google Scholar]
- 50.Elzeiny D, Monir R, El. Sabakhawy K, Selim MK, Zalata A. Relationship between DYNLT1 and Beclin1 expression and the fertilising potential of human spermatozoa. Andrologia. 2019;51:e13380. doi: 10.1111/and.13380. [DOI] [PubMed] [Google Scholar]
- 51.Simhadri S, Peterson S, Patel DS, Huo Y, Cai H, Bowman-Colin C, et al. Male fertility defect associated with disrupted BRCA1-PALB2 interaction in mice. J Biol Chem. 2014;289:24617–24629. doi: 10.1074/jbc.M114.566141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Charaka V, Tiwari A, Pandita RK, Hunt CR, Pandita TK. Role of HP1β during spermatogenesis and DNA replication. Chromosoma. 2020;129:215–226. doi: 10.1007/s00412-020-00739-4. [DOI] [PubMed] [Google Scholar]
- 53.Rival C, Guazzone VA, Von Wulffen W, Hackstein H, Schneider E, Lustig L, et al. Expression of co-stimulatory molecules, chemokine receptors and proinflammatory cytokines in dendritic cells from normal and chronically inflamed rat testis. Mol Hum Reprod. 2007;13:853–861. doi: 10.1093/molehr/gam067. [DOI] [PubMed] [Google Scholar]
- 54.Zhou R, Lv X, Chen T, Chen Q, Tian H, Yang C, et al. Construction and external validation of a 5-gene random forest model to diagnose non-obstructive azoospermia based on the single-cell RNA sequencing of testicular tissue. Aging (Albany NY) 2021;13:24219. doi: 10.18632/aging.203675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spiller C, Wilhelm D, Koopman P. Cell cycle analysis of fetal germ cells during sex differentiation in mice. Biol Cell. 2009;101:587–598. doi: 10.1042/BC20090021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He Z, Feng L, Zhang X, Geng Y, Parodi DA, Suarez-Quian C, et al. Expression of Col1a1, Col1a2 and procollagen I in germ cells of immature and adult mouse testis. Reproduction. 2005;130:333–341. doi: 10.1530/rep.1.00694. [DOI] [PubMed] [Google Scholar]
- 57.Jiang L, Zheng T, Huang J, Mo J, Zhou H, Liu M, et al. Association of semen cytokines with reactive oxygen species and histone transition abnormalities. J Assist Reprod Genet. 2016;33:1239–1246. doi: 10.1007/s10815-016-0756-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun TC, Zhang Y, Yu K, Li Y, Yu H, Zhou SJ, et al. LncRNAs induce oxidative stress and spermatogenesis by regulating endoplasmic reticulum genes and pathways. Aging (Albany NY) 2021;13:13764. doi: 10.18632/aging.202971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eo J, Song H, Lim H. Etv5, a transcription factor with versatile functions in male reproduction. Clin Exp Reprod Med. 2012;39:41. doi: 10.5653/cerm.2012.39.2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dube E, Hermo L, Chan PT, Cyr DG. Alterations in the human blood-epididymis barrier in obstructive azoospermia and the development of novel epididymal cell lines from infertile men. Biol Reprod. 2010;83:584–596. doi: 10.1095/biolreprod.110.084459. [DOI] [PubMed] [Google Scholar]
- 61.Butruille L, Batailler M, Cateau ML, Sharif A, Leysen V, Prévot V, et al. Selective depletion of Adult GFAP-expressing tanycytes leads to hypogonadotropic hypogonadism in males. Front Endocrinol. 2022 doi: 10.3389/fendo.2022.869019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hwang YS, Suzuki S, Seita Y, Ito J, Sakata Y, Aso H, et al. Reconstitution of prospermatogonial specification in vitro from human induced pluripotent stem cells. Nat Commun. 2020;11:5656. doi: 10.1038/s41467-020-19350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oluwayiose OA, Wu H, Saddiki H, Whitcomb BW, Balzer LB, Brandon N, et al. Sperm DNA methylation mediates the association of male age on reproductive outcomes among couples undergoing infertility treatment. Sci Rep. 2021;11:3216. doi: 10.1038/s41598-020-80857-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmad M, Shah AA. In silco prediction of target genes for up-regulated microRNAs in male infertile patients. J Fertil In Vitro IVF Worldw Reprod Med Genet Stem Cell Biol. 2019;10:2375–4508. [Google Scholar]
- 65.Korhonen HM, Yadav RP, Da Ros M, Chalmel F, Zimmermann C, Toppari J, et al. DICER regulates the formation and maintenance of cell-cell junctions in the mouse seminiferous epithelium. Biol Reprod. 2015;93:139–141. doi: 10.1095/biolreprod.115.131938. [DOI] [PubMed] [Google Scholar]
- 66.Kosir R, Juvan P, Perse M, Budefeld T, Majdic G, Fink M, et al. Novel insights into the downstream pathways and targets controlled by transcription factors CREM in the testis. PLoS ONE. 2012;7:e31798. doi: 10.1371/journal.pone.0031798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luense LJ, Donahue G, Lin-Shiao E, Rangel R, Weller AH, Bartolomei MS, et al. Gcn5-mediated histone acetylation governs nucleosome dynamics in spermiogenesis. Dev Cell. 2019;51:745–758. doi: 10.1016/j.devcel.2019.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cafe SL, Nixon B, Dun MD, Roman SD, Bernstein IR, Bromfield EG. Oxidative stress dysregulates protein homeostasis within the male germ line. Antioxid Redox Signal. 2020;32:487–503. doi: 10.1089/ars.2019.7832. [DOI] [PubMed] [Google Scholar]
- 69.Zhu Z, Xu W, Dai J, Chen X, Zhao X, Fang P, et al. The alteration of protein profile induced by cigarette smoking via oxidative stress in mice epididymis. Int J Biochem Cell Biol. 2013;45:571–582. doi: 10.1016/j.biocel.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 70.Dorus S, Wasbrough ER, Busby J, Wilkin EC, Karr TL. Sperm proteomics reveals intensified selection on mouse sperm membrane and acrosome genes. Mol Biol Evol. 2010;27:1235–1246. doi: 10.1093/molbev/msq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.D’Aurora M, Ferlin A, Di Nicola M, Garolla A, De Toni L, Franchi S, et al. Deregulation of sertoli and leydig cells function in patients with Klinefelter syndrome as evidenced by testis transcriptome analysis. BMC Genom. 2015;16:1–9. doi: 10.1186/s12864-015-1356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pini T, Haywood M, McCallie B, Lane SL, Schoolcraft WB, Katz-Jaffe M. Liquid chromatography-tandem mass spectrometry reveals an active response to DNA damage in human spermatozoa. F S Sci. 2021;2:153–163. doi: 10.1016/j.xfss.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 73.Al-Agha AE, Ahmed IA, Nuebel E, Moriwaki M, Moore B, Peacock KA, et al. Primary ovarian insufficiency and azoospermia in carriers of a homozygous PSMC3IP stop gain mutation. J Clin Endocrinol Metab. 2018;103:555–563. doi: 10.1210/jc.2017-01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Noman MAA, Kyzer JL, Chung SS, Wolgemuth DJ, Georg GI. Retinoic acid receptor antagonists for male contraception: current status. Biol Reprod. 2020;103:390–399. doi: 10.1093/biolre/ioaa122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci USA. 2004;101:4661–4666. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hernandez A. Thyroid hormone deiodination and action in the gonads. Curr Opin Endocr Metab Res. 2018;2:18–23. doi: 10.1016/j.coemr.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alikhani M, Mirzaei M, Sabbaghian M, Parsamatin P, Karamzadeh R, Adib S, et al. Quantitative proteomic analysis of human testis reveals system-wide molecular and cellular pathways associated with non-obstructive azoospermia. J Proteomics. 2017;162:141–154. doi: 10.1016/j.jprot.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 78.Cai Z, Zhang J, Xiong J, Ma C, Yang B, Li H. New insights into the potential mechanisms of spermatogenic failure in patients with idiopathic azoospermia. Mol Hum Reprod. 2020;26:469–484. doi: 10.1093/molehr/gaaa033. [DOI] [PubMed] [Google Scholar]
- 79.Basu S, Arya SP, Usmani A, Pradhan BS, Sarkar RK, Ganguli N, et al. Defective Wnt3 expression by testicular Sertoli cells compromise male fertility. Cell Tissue Res. 2018;371:351–363. doi: 10.1007/s00441-017-2698-5. [DOI] [PubMed] [Google Scholar]
- 80.Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 81.Llonch S, Barragán M, Nieto P, Mallol A, Elosua-Bayes M, Lorden P, et al. Single human oocyte transcriptome analysis reveals distinct maturation stage-dependent pathways impacted by age. Aging Cell. 2021;20:e13360. doi: 10.1111/acel.13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lamp M, Peters M, Reinmaa E, Haller-Kikkatalo K, Kaart T, Kadastik U, et al. Polymorphisms in ESR1, ESR2 and HSD17B1 genes are associated with fertility status in endometriosis. Gynecol Endocrinol. 2011;27:425–433. doi: 10.3109/09513590.2010.495434. [DOI] [PubMed] [Google Scholar]
- 83.Matsumoto H, Daikoku T, Wang H, Sato E, Dey SK. Differential expression of ezrin/radixin/moesin (ERM) and ERM-associated adhesion molecules in the blastocyst and uterus suggests their functions during implantation. Biol Reprod. 2004;70:729–736. doi: 10.1095/biolreprod.103.022764. [DOI] [PubMed] [Google Scholar]
- 84.Steuerwald NM, Bermúdez MG, Wells D, Munné S, Cohen J. Maternal age-related differential global expression profiles observed in human oocytes. Reprod Biomed Online. 2007;14:700–708. doi: 10.1016/S1472-6483(10)60671-2. [DOI] [PubMed] [Google Scholar]
- 85.Martin JH, Aitken RJ, Bromfield EG, Nixon B. DNA damage and repair in the female germline: contributions to ART. Hum Reprod Update. 2019;25:180–201. doi: 10.1093/humupd/dmy040. [DOI] [PubMed] [Google Scholar]
- 86.McKinnon KE, Getsios S, Woodruff TK. Distinct follicular and luteal transcriptional profiles in engineered human ectocervical tissue dependent on menstrual cycle phase. Biol Reprod. 2020;103:487–496. doi: 10.1093/biolre/ioaa056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lane SL, Parks JC, Khan SA, Yuan Y, Schoolcraft WB, Katz-Jaffe MG. Restoring ovarian antioxidant balance to combat female reproductive aging. Fertil Steril. 2021;116:e40. doi: 10.1016/j.fertnstert.2021.07.117. [DOI] [Google Scholar]
- 88.Lira-Albarrán S, Larrea-Schiavon MF, González L, Durand M, Rangel C, Larrea F. The effects of levonorgestrel on FSH-stimulated primary rat granulosa cell cultures through gene expression profiling are associated to hormone and folliculogenesis processes. Mol Cell Endocrinol. 2017;439:337–345. doi: 10.1016/j.mce.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 89.Abedel-Majed MA, Romereim SM, Davis JS, Cupp AS. Perturbations in lineage specification of granulosa and theca cells may alter corpus luteum formation and function. Front Endocrinol. 2019;10:832. doi: 10.3389/fendo.2019.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Adriaenssens T, Wathlet S, Segers I, Verheyen G, De Vos A, Van der Elst J, et al. Cumulus cell gene expression is associated with oocyte developmental quality and influenced by patient and treatment characteristics. Hum Reprod. 2010;25:1259–1270. doi: 10.1093/humrep/deq049. [DOI] [PubMed] [Google Scholar]
- 91.Giritharan G, Talbi S, Donjacour A, Di Sebastiano F, Dobson AT, Rinaudo PF. Effect of in vitro fertilization on gene expression and development of mouse preimplantation embryos. Reproduction. 2007;134:63–72. doi: 10.1530/REP-06-0247. [DOI] [PubMed] [Google Scholar]
- 92.Sapkota Y, Steinthorsdottir V, Morris AP, Fassbender A, Rahmioglu N, De Vivo I, et al. Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat Commun. 2017;8:1–12. doi: 10.1038/ncomms15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaur S, Archer KJ, Devi MG, Kriplani A, Strauss JF, III, Singh R. Differential gene expression in granulosa cells from polycystic ovary syndrome patients with and without insulin resistance: identification of susceptibility gene sets through network analysis. J Clin Endocrinol Metab. 2012;97:E2016–E2021. doi: 10.1210/jc.2011-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koks S, Velthut A, Sarapik A, Altmäe S, Reinmaa E, Schalkwyk LC, et al. The differential transcriptome and ontology profiles of floating and cumulus granulosa cells in stimulated human antral follicles. Mol Hum Reprod. 2010;16:229–240. doi: 10.1093/molehr/gap103. [DOI] [PubMed] [Google Scholar]
- 95.González-Foruria I, Santulli P, Chouzenoux S, Carmona F, Batteux F, Chapron C. Soluble ligands for the NKG2D receptor are released during endometriosis and correlate with disease severity. PLoS ONE. 2015;10:e0119961. doi: 10.1371/journal.pone.0119961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu H. Expressions of natural cytotoxicity receptor, NKG2D and NKG2D ligands in endometriosis. J Reprod Immunol. 2019;136:102615. doi: 10.1016/j.jri.2019.102615. [DOI] [PubMed] [Google Scholar]
- 97.Chi RPA, Wang T, Adams N, Wu SP, Young SL, Spencer TE, et al. Transcriptional and Progesterone Receptor Binding Profiles of the Human Endometrium Reveal Important Pathways and Regulators in the Epithelium During the Window of Implantation. bioRxiv, 2019; 10.1101/680181.
- 98.Taylor DM, Pike JW, Kasabwala K, Northrop LE, Treff NR, Scott RT. A genome-wide association scan identifies several maternal susceptbility loci for embryo aneuploidy. Fertil Steril. 2010;94:S183. doi: 10.1016/j.fertnstert.2010.07.713. [DOI] [Google Scholar]
- 99.Xu B, Zhang YW, Tong XH, Liu YS. Characterization of microRNA profile in human cumulus granulosa cells: Identification of microRNAs that regulate Notch signaling and are associated with PCOS. Mol Cell Endocrinol. 2015;404:26–36. doi: 10.1016/j.mce.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 100.Yuan X, Tian G, Pei X, Hu X, Wu J. Spermidine induces cytoprotective autophagy of female germline stem cells in vitro and ameliorates aging caused by oxidative stress through upregulated sequestosome-1/p62 expression. Cell Biosci. 2021;11:1–14. doi: 10.1186/s13578-020-00515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Salilew-Wondim D, Wang Q, Tesfaye D, Schellander K, Hoelker M, Hossain MM, et al. Polycystic ovarian syndrome is accompanied by repression of gene signatures associated with biosynthesis and metabolism of steroids, cholesterol and lipids. J Ovarian Res. 2015;8:1–14. doi: 10.1186/s13048-015-0151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ametzazurra A, Matorras R, Garcia-Velasco JA, Prieto B, Simón L, Martinez A, et al. Endometrial fluid is a specific and non-invasive biological sample for protein biomarker identification in endometriosis. Hum Reprod. 2009;24:954–965. doi: 10.1093/humrep/den450. [DOI] [PubMed] [Google Scholar]
- 103.Chen Q, Hang Y, Zhang T, Tan L, Li S, Jin Y. USP10 promotes proliferation and migration and inhibits apoptosis of endometrial stromal cells in endometriosis through activating the Raf-1/MEK/ERK pathway. Am J Physiol Cell Physiol. 2018;315:C863–C872. doi: 10.1152/ajpcell.00272.2018. [DOI] [PubMed] [Google Scholar]
- 104.Timofeeva A, Drapkina Y, Fedorov I, Chagovets V, Makarova N, Shamina M, et al. Small noncoding RNA signatures for determining the developmental potential of an embryo at the morula stage. Int J Mol Sci. 2020;21:9399. doi: 10.3390/ijms21249399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sheng X, Liu C, Yan G, Li G, Liu J, Yang Y, et al. The mitochondrial protease LONP1 maintains oocyte development and survival by suppressing nuclear translocation of AIFM1 in mammals. EBioMedicine. 2022;75:103790. doi: 10.1016/j.ebiom.2021.103790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Matsumoto H, Fukui E, Yoshizawa M, Sato E, Daikoku T. Differential expression of the motin family in the peri-implantation mouse uterus and their hormonal regulation. J Reprod Dev. 2012;58:649–653. doi: 10.1262/jrd.2012-075. [DOI] [PubMed] [Google Scholar]
- 107.Park MR, Choi YJ, Kwon DN, Park C, Bui HT, Gurunathan S, et al. Intraovarian transplantation of primordial follicles fails to rescue chemotherapy injured ovaries. Sci Rep. 2013;3:1–11. doi: 10.1038/srep01384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Athavale DM, Barré A, Kranyak AC, Lal A, Blalock JL, Zimmerman S, et al. Pro-apoptotic gene expression in blastocoel fluid from euploid day-5 embryos is associated with negative pregnancy outcomes. Fertil Steril. 2019;112:e261. doi: 10.1016/j.fertnstert.2019.07.788. [DOI] [Google Scholar]
- 109.Wang W, Hafner KS, Flaws JA. In utero bisphenol A exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse. Toxicol Appl Pharmacol. 2014;276:157–164. doi: 10.1016/j.taap.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fortuño C, Labarta E. Genetics of primary ovarian insufficiency: a review. J Assist Reprod Genet. 2014;31:1573–1585. doi: 10.1007/s10815-014-0342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Katari S, Aarabi M, Kintigh A, Mann S, Yatsenko SA, Sanfilippo JS, et al. Chromosomal instability in women with primary ovarian insufficiency. Hum Reprod. 2018;33:531–538. doi: 10.1093/humrep/dey012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mehine M, Mäkinen N, Heinonen HR, Aaltonen LA, Vahteristo P. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril. 2014;102:621–629. doi: 10.1016/j.fertnstert.2014.06.050. [DOI] [PubMed] [Google Scholar]
- 113.Stigliani S, Moretti S, Anserini P, Casciano I, Venturini PL, Scaruffi P. Storage time does not modify the gene expression profile of cryopreserved human metaphase II oocytes. Hum Reprod. 2015;30:2519–2526. doi: 10.1093/humrep/dev232. [DOI] [PubMed] [Google Scholar]
- 114.Jiang B, Zhao W, Yuan J, Qian Y, Sun W, Zou Y, et al. Lack of Cul4b, an E3 ubiquitin ligase component, leads to embryonic lethality and abnormal placental development. PLoS ONE. 2012;7:e37070. doi: 10.1371/journal.pone.0037070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang JJ, Liu X, Chen L, Zhang S, Zhang X, Hao C, et al. Advanced maternal age alters expression of maternal effect genes that are essential for human oocyte quality. Aging (Albany NY) 2020;12:3950. doi: 10.18632/aging.102864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Madawala RJ, Poon CE, Dowland SN, Murphy CR. Actin crosslinking protein filamin A during early pregnancy in the rat uterus. Reprod Fertil Dev. 2016;28:960–968. doi: 10.1071/RD14240. [DOI] [PubMed] [Google Scholar]
- 117.Pastore LM, Johnson J. The FMR1 gene, infertility, and reproductive decision-making: a review. Front Genet. 2014;5:195. doi: 10.3389/fgene.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sliz A, Locker KC, Lampe K, Godarova A, Plas DR, Janssen EM, et al. Gab3 is required for IL-2–and IL-15–induced NK cell expansion and limits trophoblast invasion during pregnancy. Sci Immunol. 2019;4:eaav3866. doi: 10.1126/sciimmunol.aav3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Desai A, Madar IH, Asangani AH, Al Ssadh H, Tayubi IA. Influence of PCOS in obese vs. non-obese women from mesenchymal progenitors stem cells and other endometrial cells: an in silico biomarker discovery. Bioinformation. 2017;13:111. doi: 10.6026/97320630013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Azevedo MA, Jr, Silva IDCG. Identification of differentially expressed genes in pathways of cerebral neurotransmission of anovulatory mice. Genet Mol Res. 2017 doi: 10.4238/gmr16039622. [DOI] [PubMed] [Google Scholar]
- 121.Strieby A, McCallie BR, Parks JC, Schoolcraft WB, Katz-Jaffe MG. Blastocysts from women of advanced maternal age have compromised transcription of key implantation and development genes. Fertil Steril. 2014;102:e20. doi: 10.1016/j.fertnstert.2014.07.074. [DOI] [Google Scholar]
- 122.Li J, Ren L, Li M, Yang C, Chen J, Chen Q. Screening of potential key genes related to tubal factor infertility based on competitive endogenous RNA network. Genet Test Mol Biomark. 2021;25:325–333. doi: 10.1089/gtmb.2020.0083. [DOI] [PubMed] [Google Scholar]
- 123.Sun PR, Jia SZ, Lin H, Leng JH, Lang JH. Genome-wide profiling of long noncoding ribonucleic acid expression patterns in ovarian endometriosis by microarray. Fertil Steril. 2014;101:1038–1046. doi: 10.1016/j.fertnstert.2013.12.035. [DOI] [PubMed] [Google Scholar]
- 124.Kim MK, Seok HH, Kim YS, Chin MU, Sung SR, Lee WS, et al. Molecular genetic and cytogenetic characterization of a partial Xp duplication and Xq deletion in a patient with premature ovarian failure. Gene. 2014;534:54–59. doi: 10.1016/j.gene.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 125.Diez-Fraile A, Lammens T, Tilleman K, Witkowski W, Verhasselt B, De Sutter P, et al. Age-associated differential microRNA levels in human follicular fluid reveal pathways potentially determining fertility and success of in vitro fertilization. Hum Fertil. 2014;17:90–98. doi: 10.3109/14647273.2014.897006. [DOI] [PubMed] [Google Scholar]
- 126.Zhang T, Xi Q, Wang D, Li J, Wang M, Li D, et al. Mitochondrial dysfunction and endoplasmic reticulum stress involved in oocyte aging: an analysis using single-cell RNA-sequencing of mouse oocytes. J Ovarian Res. 2019;12:1–9. doi: 10.1186/s13048-018-0475-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Finas D, Huszar M, Agic A, Dogan S, Kiefel H, Riedle S, et al. L1 cell adhesion molecule (L1CAM) as a pathogenetic factor in endometriosis. Hum Reprod. 2008;23:1053–1062. doi: 10.1093/humrep/den044. [DOI] [PubMed] [Google Scholar]
- 128.Xu X, Guan R, Gong K, Xie H, Shi L. Circ_FURIN knockdown assuages testosterone-induced human ovarian granulosa-like tumor cell disorders by sponging miR-423-5p to reduce MTM1 expression in polycystic ovary syndrome. Reprod Biol Endocrinol. 2022;20:1–12. doi: 10.1186/s12958-021-00875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang G, Feenstra B, Bacelis J, Liu X, Muglia LM, Juodakis J, et al. Genetic associations with gestational duration and spontaneous preterm birth. N Engl J Med. 2017;377:1156–1167. doi: 10.1056/NEJMoa1612665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Luo J, Zhu L, Zhou N, Zhang Y, Zhang L, Zhang R. Construction of circular RNA–MicroRNA–messenger RNA regulatory network of recurrent implantation failure to explore its potential pathogenesis. Front Genet. 2021 doi: 10.3389/fgene.2020.627459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Massad-Costa AM, da Silva IDCG, Affonso R, Soares JM, Jr, Nunes MG, De Lima GR, et al. Gene analysis in patients with premature ovarian failure or gonadal dysgenesis: a preliminary study. Maturitas. 2007;57:399–404. doi: 10.1016/j.maturitas.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 132.Tolmacheva EN, Kashevarova AA, Nazarenko LP, Minaycheva LI, Skryabin NA, Lopatkina ME, et al. Delineation of clinical manifestations of the inherited Xq24 microdeletion segregating with sXCI in mothers: two novel cases with distinct phenotypes ranging from UBE2A deficiency syndrome to recurrent pregnancy loss. Cytogenet Genome Res. 2020;160:245–254. doi: 10.1159/000508050. [DOI] [PubMed] [Google Scholar]
- 133.Kim KH, Lee KA. Maternal effect genes: findings and effects on mouse embryo development. Clin Exp Reprod Med. 2014;41:47. doi: 10.5653/cerm.2014.41.2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yatsenko SA, Mittal P, Wood-Trageser MA, Jones MW, Surti U, Edwards RP, et al. Highly heterogeneous genomic landscape of uterine leiomyomas by whole exome sequencing and genome-wide arrays. Fertil Steril. 2017;107:457–466. doi: 10.1016/j.fertnstert.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 135.Antsiferova YS, Sotnikova NY, Bogatova IK, Boitsova AV. Changes of apoptosis regulation in the endometrium of infertile women with tubal factor and endometriosis undergoing in vitro fertilization treatment. JBRA Assist Reprod. 2014;18:2. doi: 10.5935/1518-0557.20140084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Clinical characteristics and karyotypes of study participants with supporting literature.
Data Availability Statement
All data generated or analyzed during this study are included in this article.