Abstract
Alcohol induces neuroinflammation but its role in cognitive impairment and impulsivity in alcohol use disorder (AUD) has been poorly investigated. We used proton magnetic resonance spectroscopy to measure brain glutamate levels and diffusion-weighted imaging to measure functional anisotropy (FA) in the thalamus and ventral anterior cingulate cortex (vACC) in 15 recently detoxified patients with AUD and 14 matched controls. Compared to controls, AUD patients showed higher glutamate levels (p=0.04) and lower FA in the thalamus (p=0.04) but not in the vACC. In AUD, thalamic glutamate levels (r=0.62, p=0.019) and FA (r=−0.55, p=0.034) were associated with severity of drinking (drinks/week). Compared to controls, AUD patients showed higher scores on Conners’ Adult ADHD Rating Scale for impulsivity (p=0.03), which correlated with glutamate levels in the thalamus (r=0.58, p=0.03) and vACC (r=0.55, p=0.036). In a second cohort of AUD patients (n=32), Glu in dorsal ACC (dACC) also correlated with Barrett Impulsiveness Scale total score (r=.43, p=0.014). We interpret the elevated thalamic glutamate levels and the parallel reduction in FA in AUD—which correlated with drinking severity—as possible evidence of neurotoxicity from neuroinflammation. The association of Glu with impulsivity suggests that neurotoxic effects of chronic alcohol exposure in the thalamus and dACC may contribute to impulsivity.
Keywords: Inflammation, neurotoxicity, withdrawal, abstinence, neuroimaging
1. Introduction
Alcohol use disorder (AUD) is associated with impairments in multiple cognitive domains, including response inhibition and impulsivity (Freeman et al., 2018; Goldstein et al., 2001; Kopera et al., 2012; Loeber et al., 2010; Zehra et al., 2019). These cognitive deficits in AUD have been associated with alterations in brain structure (Gropper et al., 2016; Pfefferbaum et al., 2009; Tomasi et al., 2019; Wiers et al., 2015) and function (Kim et al., 2018; Shokri-Kojori et al., 2017; Volkow et al., 2017).
AUD affects several neurotransmitter systems, including dopamine (Volkow et al., 2006; Volkow et al., 1996), GABA, and glutamate (Glu) (Hillmer et al., 2015; Prisciandaro et al., 2019). In animal models of alcohol dependence, microdialysis studies reported elevated brain Glu in various brain regions including the cortex and thalamus (Fliegel et al., 2013), with greater increases during acute than prolonged alcohol withdrawal. Clinical studies of Glu levels using proton magnetic resonance spectroscopy (1H-MRS) (Hillmer et al., 2015) have generally shown higher Glu (Hermann et al., 2012) or glutamate + glutamine (combined: Glx) levels (Yeo et al., 2013) in the anterior cingulate cortex (ACC) of AUD patients studied during withdrawal compared to controls. However, some studies found either no differences (Bauer et al., 2013; Cheng et al., 2018) or lower ACC Glu in AUD patients compared to controls (Mon et al., 2012; Thoma et al., 2011). Interestingly, ACC Glu levels in AUD patients correlated inversely with the number of heavy drinking days in the 14 days preceding the MRS scan (Cheng et al., 2018). Glu changes in other brain regions have been reported in AUD including elevated Glu in the nucleus accumbens (Bauer et al., 2013) and reduced Glx in the occipital cortex (Bagga et al., 2014). Binge drinking in rats induced neuroinflammation (i.e., active microglia) in the hippocampus, which was found to be mediated by elevated extracellular Glu levels (Ward et al., 2009). Nevertheless, administration of N-acetylcysteine (NAC), which restores glutamate homestasis in the synapse, was shown to protect against the neuroinflammatory changes from chronic alcohol exposure in rats (Schneider et al., 2017). In humans, elevated Glu levels have been associated with neuroinflammatory markers—such as cytokines and C-reactive protein—in mood disorders (Haroon et al., 2017).
Diffusion MRI has also been used to assess alcohol’s effects on the brain (Chen et al., 2017). In healthy individuals, a study using diffusion tensor imaging (DTI) reported that acute alcohol administration reduced mean diffusivity in the frontal lobe, thalamus, and middle cerebellar peduncle (Kong et al., 2012). In non-smoking individuals with AUD, 1-month of abstinence was associated with increased fractional anisotropy (FA) in temporal white matter and with decreased diffusivity throughout the cortex suggestive of microstructural recovery (Gazdzinski et al., 2010). In treatment-seeking AUD patients, those who resumed heavy drinking showed lower FA and higher diffusivity in the frontal lobes compared to those who maintained abstinence, suggesting that white matter injury increased the risk for relapse (Sorg et al., 2012).
Functional consequences of DTI changes in AUD were shown on an alcohol cue reactivity task (Monnig et al., 2013). Specifically, FA values of nine white matter tracts (including the corpus callosum and cingulate gyrus) in heavy drinkers were inversely correlated with BOLD activation in several brain regions including the thalamus and ACC. These results suggest that impaired white matter integrity in frontoparietal and corticolimbic networks is associated with an individual’s frontal lobe function, which would impact their ability to control their alcohol consumption. DTI-based measures have been associated with impulsive behavior in other substance use disorders (SUD). For instance, chronic marijuana smokers exhibited lower FA in frontal white matter compared to non-smokers and this decreased fiber integrity was associated with higher impulsivity (Gruber et al., 2011). Methamphetamine (Meth)-dependent patients similarly displayed high levels of impulsivity that correlated with FA and axial and radial diffusivity in frontal white matter tracts (Uhlmann et al., 2016). In addition, current Meth users had greater impulsivity than past users and their brain microstructural abnormalities differed, which could reflect different stages of neuroinflammation or iron-induced neurodegeneration (Andres et al., 2016).
In the current study, we aimed to assess (1) the effect of chronic alcohol exposure on brain Glu levels in the ACC and thalamus using 1H-MRS and their association with impulsivity, (2) the effect of chronic alcohol exposure on FA using diffusion-weighted imaging (DWI) in the same brain regions, and (3) the association between Glu concentrations and FA in the ACC and thalamus. We hypothesized that, in both brain regions, patients with AUD compared to healthy controls (HC) would show (1) elevated concentrations of Glu which would positively correlate with impulsivity, (2) lower FA, and (3) negative associations between Glu and FA indicating underlying inflammation related to chronic alcohol use.
2. Methods
2.1. Study 1
2.1.1. Participants
Fifteen patients with AUD and 14 HC completed the first study. The two groups did not differ in age, gender proportion, or BMI (Table 1). Patients were screened to exclude major medical, neurological and psychiatric disorders, head trauma with loss of consciousness greater than 30 minutes, chronic use of psychoactive medications, current or past diagnosis of SUD (other than alcohol abuse and/or dependence in the AUD group, or current tobacco smoking in either group) as assessed by the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (American Psychiatric Association, 2000) or DSM-5 (American Psychiatric Association, 2013), and metallic implants which are contraindicated for MRI. Women were neither pregnant nor breastfeeding and were studied in the mid follicular phase. AUD patients were abstinent from alcohol an average of 3.5 days at the time of the scans (range 1–7 days). All patients had a negative urine drug screen on the days of testing and were free of psychoactive medications within 24 hours of study procedures (except for benzodiazepines used to relieve withdrawal symptoms in 4 AUD patients). All patients provided written informed consent to participate in the study, which was approved by the Institutional Review Board at the National Institutes of Health (Combined Neurosciences White Panel) and were scanned between June 2015 and April 2017.
Table 1.
Demographics and clinical characteristics of AUD and HC groups.
| AUD (n=15) | Controls (n=14) | P-value | |
|---|---|---|---|
|
| |||
| Age (years) | 47.1 (9.7) | 48.0 (11.4) | 0.8 |
| Years of education | 12.3 (2.3) | 15.1 (2.3) | 0.002 |
| WASI IQ | 90.2 (15.8) | 99.4 (16.1) | 0.13 |
| BMI | 26.4 (3.7) | 29.0 (4.7) | 0.11 |
| Gender | 4 females | 4 females | 0.9 |
| Smoking status | 8 smokers 2 ex-smokers 5 non-smokers |
0 smokers 1 ex-smoker 13 non-smokers |
<0.0001 |
| LDH (kg) | 1526 (1420) | 33 (52) | 0.001 |
| TLFB Drinks/week | 66.3 (34.9) | 1.0 (1.6) | <0.0001 |
| TLFB # Drinking days | 79.3 (15.2) | 6.6 (9.2) | <0.0001 |
| ADS | 14.1 (8.5) | 0.2 (0.4) | <0.0001 |
| CAARS A inattention | 46.7 (9.9) | 38.2 (5.7) | 0.01 |
| CAARS B hyperactivity | 47.1 (8.2) | 44.3 (8.7) | 0.01 |
| CAARS C impulsivity | 42.9 (7.2) | 37.2 (5.9) | 0.03 |
Abbreviations: ADS Alcohol Dependence Scale, BMI body mass index, CAARS Conners’ Adult ADHD Rating Scale, IQ intelligence quotient, LDH lifetime drinking history, TLFB Timeline Follow-back, WASI Wechsler Abbreviated Scale of Intelligence.
On the day of screening, patients completed the Timeline Follow-Back (TLFB) to assess daily alcohol consumption in the 90 days prior to the study (Sobell and Sobell, 1996), the Lifetime Drinking History (LDH) to assess total lifetime alcohol consumption (Skinner and Sheu, 1982), the Alcohol Dependence Scale (ADS) to assess the severity of dependence (Skinner and Allen, 1982), and the Wechsler Abbreviated Scale of Intelligence (WASI-II) subtests Matrix Reasoning and Vocabulary as a proxy for general intelligence (Wechsler, 1999).
Patients completed the Conners Adult ADHD Rating Scale (CAARS) long version as a measure of inattention, hyperactivity and impulsivity (Conners, 1998).
2.1.2. Image Acquisition and Processing
2.1.2.1. MRI
Patients underwent MRI on a 3.0T Magnetom Prisma scanner (Siemens Healthineers USA, Inc., Malvern, PA) equipped with a 20-channel head coil. T1-weighted 3D magnetization-prepared gradient-echo (MP-RAGE, TR/TE = 2200/4.25 ms; FA = 9°, 1 mm isotropic resolution) and T2-weighted multi-slice spin-echo (TR/TE = 8000/72ms; 1.1 mm in-plane resolution; 94 slices, 1.7-mm slice thickness; matrix = 192) pulse sequences were used to acquire high-resolution anatomical brain images.
We used the minimal preprocessing pipelines (Glasser et al., 2013) of the Human Connectome Project (HCP) for the spatial normalization of the structural scans to the stereotactic space of the Montreal Neurological Institute (MNI). Freesurfer version 5.3.0 (http://surfer.nmr.mgh.harvard.edu) was used to automatically segment the anatomical MRI scans into cortical and subcortical gray matter regions of interest (ROIs). Standard preprocessing steps were adopted in the Freesurfer pipeline (Fischl et al., 2002; Fischl et al., 1999; Ségonne et al., 2004).
2.1.2.2. DWI
Patients also underwent diffusion-weighted imaging (TR/TE = 7.4s/73ms, 2.2 mm isotropic voxels; 70 slices; Field-of-view = 240mm; GRAPPA phase encoding acceleration factor = 2; bandwidth = 2391 Hz/pixel) consisting of 2 runs (PA and AP) each of which contained a total of 45 volumes (3 volumes of b=0, 6 volumes of b = 200, 6 volumes of b = 500, and 30 volumes of b=1100 s/mm2). Diffusion data were preprocessed in FSL (Jenkinson et al., 2012) and according to Andersson et al., 2003, including: intensity normalization across runs, EPI distortion correction, eddy current and motion correction, and gradient nonlinearity correction. The resulting diffusion images were registered to the MNI152 standard space with 2×2×2mm isotropic resolution. For each ROI, the mean FA value was calculated.
2.1.2.3. 1H-MRS
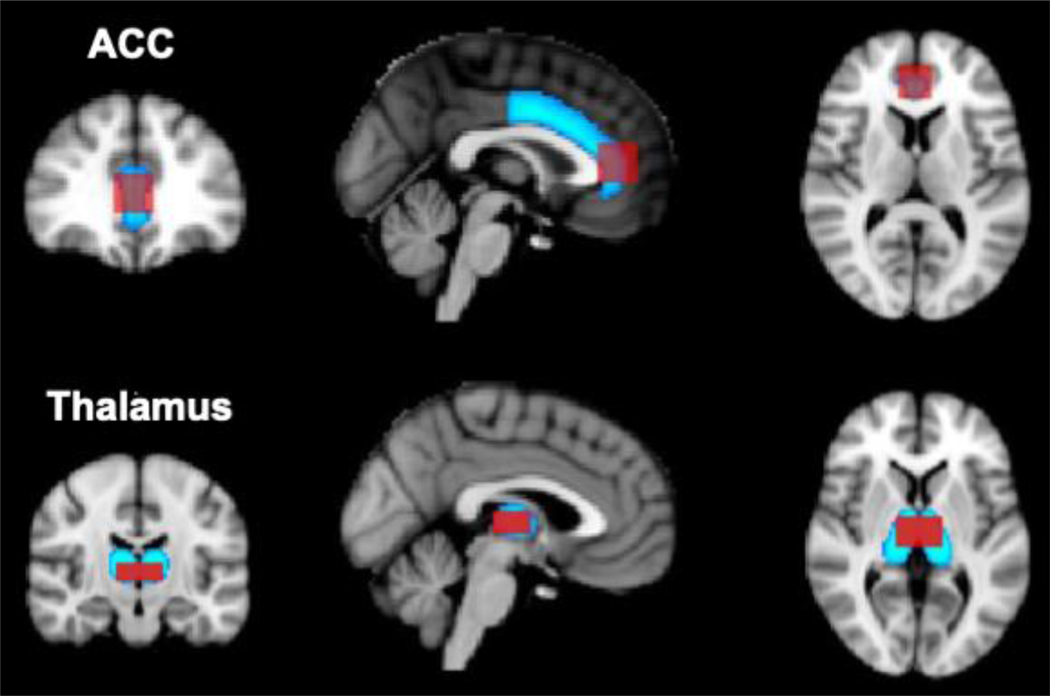
Localized proton magnetic resonance spectroscopy (1H-MRS) was performed in two ROIs: the ventral ACC (vACC) and thalamus (Fig. 1). Dimensions were 2×2×2cm for the vACC and 3×1.3×2cm for the thalamus. A Point Resolved Spectroscopy (PRESS) pulse sequence (TE/TR=30/3000sec, 64 averages) was used to acquire water-suppressed spectra. The PRESS sequence (TR=10s, 1 average) was also used to collect 7 water-unsuppressed data at different TEs (0.03, 0.05, 0.08, 0.12, 0.20, 0.50, 1.00 s). The water-unsuppressed spectra were used to measure the T2 decay of the water signal and correct the metabolite concentrations for the partial volume of cerebrospinal fluid (Ernst et al., 1993; Kreis et al., 1993). Spectral fitting of the MRS datasets was carried with the LCModel program (Provencher, 1993) to determine absolute and relative metabolite concentrations for Glu and total creatine (tCr). There were no group differences for tCr and absolute Glu measures were used for further analysis. The results from LCModel spectral analysis were inspected for nonrandom residuals as well as baseline fitting and a Cramér–Rao lower bound (CRLBs) of 20% for each individual peak was used as a quality criterion (Provencher, 1993). One Glu spectrum in the vACC (HC participant) and one in the thalamus (AUD patient) did not meet this criterion and were excluded from analyses. The CRLBs for Glu in all participants were between 5–13% (vACC) and 7–19% (thalamus). Additional spectral quality measures included mean±SD signal-to-noise ratio (vACC: 23.9±6.9; thalamus: 12.4±3.9) and full width at half maximum (FWHM) (vACC: 0.07±0.04 ppm; thalamus: 0.09±0.02 ppm).
Fig. 1. 1H-MRS voxel locations projected on to FA ROIs in the ACC and thalamus.
MPRAGE images and anatomical landmarks were used to prescribe isotropic 8-mL MRS voxels in the ACC and thalamus (in red). These custom ROIs were used for subsequent MRS analyses. Bilateral FA ACC and thalamus ROIs (in blue) were selected for the DWI data analysis from FSL’s Harvard–Oxford Cortical and Subcortical Structural probabilistic atlases (Desikan et al., 2006). Both DWI ROI masks were thresholded in FSL at 50% signal intensity.
2.1.3. Statistical Analyses
There were no >3SD of the mean outliers for Glu, FA or impulsivity measures. All measures were normally distributed as per K-S test (all p>0.07). Two-sample t-tests were used to compare Glu concentrations and FA values between healthy controls and AUD patients to test our hypothesis that Glu levels would be elevated and FA reduced in the ACC and thalamus of AUD patients. Pearson’s correlations were used to test our hypothesis that Glu levels and FA in the vACC and thalamus would be associated with the CAARS score of impulsivity (with a significance threshold of p<0.05). Moreover, within the AUD group, we explored associations between Glu and FA measures with CAARS inattention and restlessness, and with alcohol history measures (i.e., days since last alcohol use, lifetime drinking in kg, and drinks/day).
2.2. Study 2
2.2.1. Participants
We aimed to study the association between brain Glu and impulsivity in a second replication cohort of n=32 AUD patients, who were scanned between October 2017 and March 2020. Clinical characteristics of the study population can be found in Supplementary Table 1. Exclusion crtieria were the same as reported for the AUD participants in study 1. All patients were treatment-seeking AUD patients undergoing detoxification in the NIAAA treatment center. AUD patients were abstinent from alcohol an average of 4.8 days at the time of the scans (range 1–11 days). All patients had a negative urine drug screen on the days of testing and were free of psychoactive medications within 24 hours of study procedures (except for benzodiazepines used to relieve withdrawal symptoms in n=7 AUD patients). All patients provided written informed consent to participate in the study, which was approved by the Institutional Review Board at the National Institutes of Health (Combined Neurosciences White Panel).
2.2.2. Impulsivity scale
Participants underwent the same study questionnaires as cohort 1, except for the Barrett Impulsiveness Scale (BIS-11; Patton et al., 1995). The BIS-11 consists of 30 items and assesses general impulsivity (total score) as well as the subfactors Attentional, Motor and Nonplanning impulsivity.
2.2.3. 1H-MRS
The same 1H-MRS scanning procedures were used as in study 1. The 2×2×2cm cm voxel was placed in the dorsal ACC (dACC), rather than in the vACC used in study 1 (see Supplementary Figure 1 for voxel position of the dACC).
2.2.4. Statistical Analyses
There were no >3SD of the mean outliers for Glu or BIS-11 impulsivity. We correlated Glu measures with the BIS-11 total score using Pearson’s correlations, and post-hoc with the BIS-11 second order factors to explore the contribution of subfactors of impulsivity (i.e., Attentional, Motor and Nonplanning) to variance in Glu. Alpha was set at 0.05
3. Results
3.1. Glu concentrations and impulsivity
3.1.1. Cohort 1
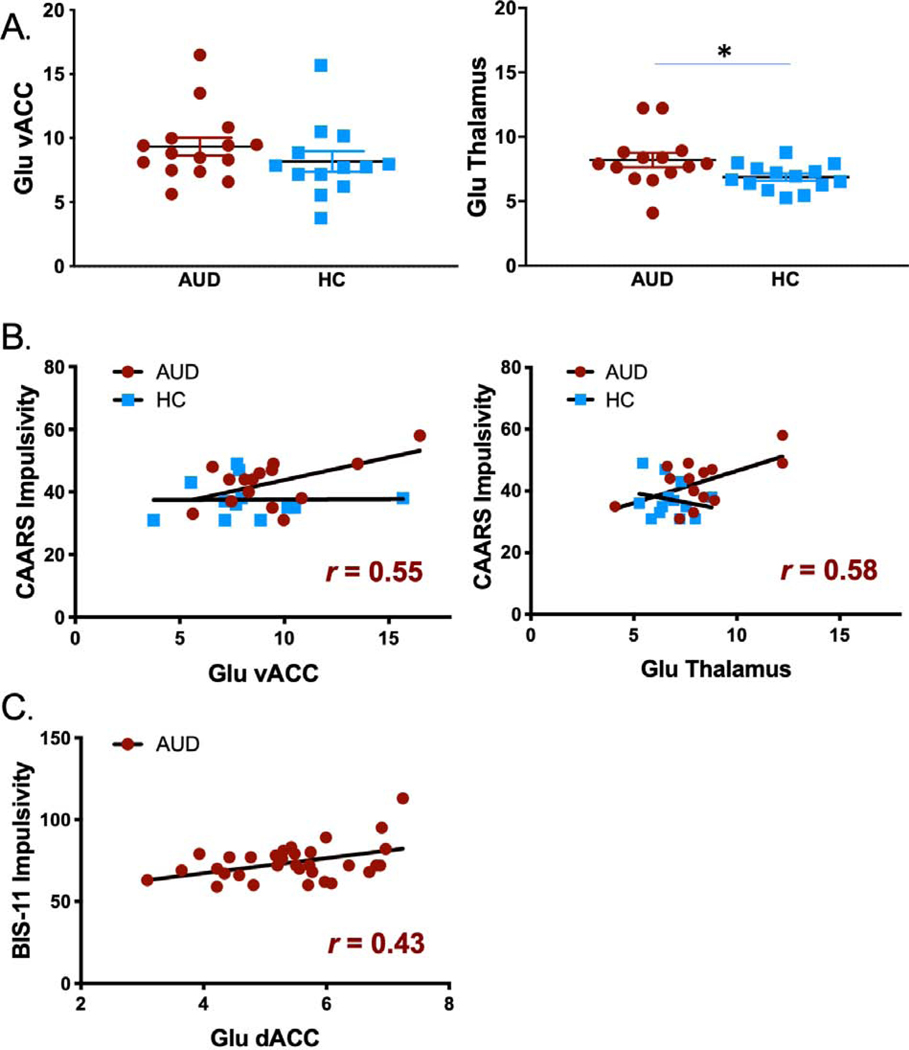
AUD: 8.2±2.1 SD, mean HC: 6.9±1.0 SD; t(26)=2.2, p=0.04) but not in the vACC (p=0.3) (see Fig. 2A). AUD patients had higher scores than controls on the CAARS impulsivity scale (t(26)=2.2, p=0.03). Within the AUD group, Glu levels in thalamus and vACC correlated positively with CAARS impulsivity (r=0.58 p=0.03 and r=0.55 p=0.036) (Fig. 2B). CAARS inattention and hyperactivity/restlessness scores were also higher in AUD patients than HC (Table 1), but did not correlate with Glu measures in the thalamus or vACC.
Fig. 2. Comparison of Glu concentrations and subject impulsivity.
(A) Group comparisons of Glu concentrations in the ACC and thalamus. (B) Correlations between Glu concentrations and impulsivity across healthy and AUD patients in the ACC and thalamus (* p < 0.05).
3.1.2. Cohort 2
In the n=32 AUD patients in cohort 2 Glu concentrations in dACC correlated with BIS-11 total score (r=.43, p=.014; Fig. 2C), which was driven by a significant effect of the subscales Attentional (r=.51, p=0.003) and Motor impulsivity (r=.42, p=.020), but not Nonplanning impulsivity (r=.25, p=.17) (data not shown).
3.2. FA in vACC and thalamus
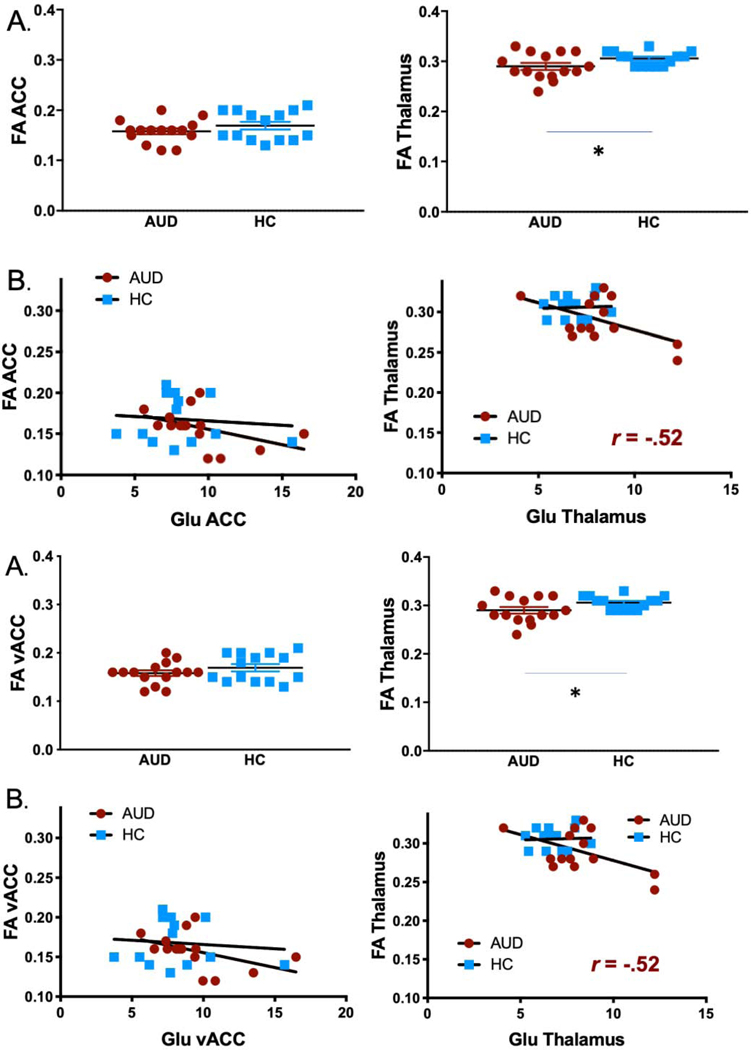
FA was lower in AUD patients than HC in the thalamus (AUD: 0.29±0.026, HC: 0.31±0.015; t(df)=2.1, p=0.04) but not in the vACC (p=0.3) (Fig. 3A). The assocations between FA and impulsivity in the thalamus or ACC were not significant in either group (all p > 0.5).
Fig. 3. Comparison of Glu concentrations and FA measures.
(A) Group comparisons of FA in the ACC and thalamus. Thalamic FA was lower in AUD patients compared to HC (p=0.04). (B) Correlation between Glu concentrations and FA values across healthy and AUD patients in the ACC and thalamus. Glu and FA in the thalamus correlated at trend level in the AUD group (r=−0.52, p=0.058).
3.3. Relationship between Glu and FA
Within the AUD group only, Glu levels and FA correlated at trend level in the thalamus (r=−0.52, p=0.058) but not in the vACC (r=−0.43, p=0.11) (Fig. 3B).
3.4. Correlations with drinking behavior
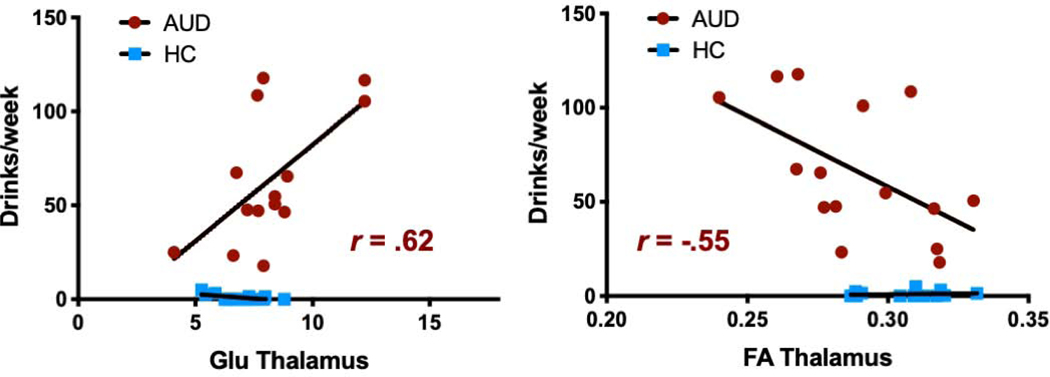
Within the AUD group, the number of drinks per week in the last 90 days correlated with both Glu concentrations (r=0.62, p=0.019) and FA (r=−0.55, p=0.034) in the thalamus (Fig. 4). Moreover, LDH in kilograms correlated with Glu in the thalamus (r=0.54, p=0.049) (data not shown). There were no associations between Glu measures and days of abstinence. Moreover, within the AUD group, neither smoking status nor cigarettes per day was associated with Glu or FA.
Fig. 4. Correlation of drinking severity with Glu concentrations and FA.
Within the AUD group, number of drinks per week in the last 90 days correlated positively with Glu concentrations (r=0.62, p=0.019) and negatively with FA (r=−0.55, p=0.034) in the thalamus.
4. Discussion
The main findings of our study are: 1) AUD patients showed higher Glu levels and lower FA in the thalamus compared to HC, 2) Glu in thalamus and in dACC (but not vACC) were positively associated with measures of impulsivity, and 3) both elevated Glu and lower FA in the thalamus in AUD patients were associated with the severity of alcohol drinking behavior in the weeks preceding the study. Elevated Glu levels have been associated with alcohol-induced neurotoxicity and neuroinflammation (Frischknecht et al., 2017) whereas reductions in thalamic FA have been associated with degenerative changes (Cavallari et al., 2014; Santos et al., 2019). Therefore, we suggest our findings reflect neurotoxic changes in the thalamus that are possibly triggered by chronic alcohol exposure due to neuroinflammation. In an exploratory analysis, we found that the higher thalamic Glu levels are associated with higher impulsivity scores in AUD patients, which suggests that alcohol-induced neurotoxic changes in the thalamus may contribute to impulsivity in AUD. Although we had hypothesized that elevated Glu levels and lower FA would occur in the ACC as well, we did not find any group differences in this region. Nevertheless, we found that in a larger, independent cohort of n=32 patients with AUD Glu in dACC was associated with increased impulsivity as well.
To our knowledge, this is the first study in humans to report elevated levels of Glu and lower FA in the thalamus of recently detoxified AUD patients compared to controls. Our thalamic results are consistent with preclinical findings of elevated Glu in the thalamus of alcohol-dependent animals studied during acute withdrawal (Fliegel et al., 2013). A recent clinical study showed that in moderate drinkers, acute alcohol administration elevated Glx in the thalamus relative to baseline (Monnig et al., 2019). However, the latter study did not evaluate if Glx remained elevated post-intoxication and withdrawal. Our study did not find an association with days since last intoxication (ranged 1–7 days), which indicates that elevated Glu levels persisted for at least one week of detoxification. On the other hand, both elevated Glu and reductions in FA in the thalamus were associated with the severity of recent drinking behavior, which suggest that these abnormalities were related to alcohol exposures. Lower FA has been reported in AUD in various white and gray matter structures (Monnig et al., 2013; Pandey et al., 2018; Wang et al., 2016) including the thalamus (Monnig et al., 2013), although one study reported higher thalamic FA in AUD patients compared to controls (Pandey et al., 2018). Acute alcohol exposure was also found to reduce the apparent diffusion coefficient—a measure of the magnitude of mean diffusion (Kong et al., 2012).
Since both increased Glu levels and reduced FA have been associated with neuroinflammation, including in an animal model of alcohol dependence (Fliegel et al., 2013), our findings may reflect neuroinflammatory changes associated with chronic alcohol exposure to the thalamus. Indeed, using autoradiography, we previously showed that chronic alcohol exposure in rodents was associated with increases in thalamic binding of the TSPO ligands [3 H]PBR28 and [3 H]PK11195, which are markers of inflammation (Tyler et al., 2019). Other imaging methods, including resting state functional connectivity, have also documented that acute and chronic alcohol administration significantly affects the thalamus (Shokri-Kojori et al., 2017; Wang et al., 2016). Therefore, the thalamus may be particularly vulnerable to the effects of alcohol.
Glu levels in both the thalamus and dACC (but not vACC) of the AUD patients correlated positively with impulsivity. This finding is consistent with studies that found positive associations between Glu in the ACC and impulsivity in neuropsychiatric disorders that are characterized by high impulsivity, including cocaine use (Schmaal et al., 2012) and borderline personality (Hoerst et al., 2010) and attention-deficit-hyperactivity disorders (Ende et al., 2016). Impulsive behavior in AUD patients may follow a similar mechanism in which acute and chronic alcohol use alters Glu neurotransmission. This in turn may serve as a biomarker (or predictor) of AUD-related behaviors. Indeed, elevations in impulsivity (Gropper et al., 2016) as well as Glu (Hermann et al., 2012) were observed during acute alcohol withdrawal in AUD patients compared to controls. While Glu levels normalized after a few weeks of abstinence in AUD patients (Hermann et al., 2012), future research is needed to investigate whether this is accompanied by decreased impulsivity. Pharmacological intervention with NAC normalizes Glu levels and decreased neuroinflammation in alcohol-dependent rodents (Schneider et al., 2017), as well as alcohol self-administration, extinction responding, and relapse (Lebourgeois et al., 2019). In human cocaine abusers, NAC also normalized elevated Glu levels, and impulsivity at baseline was a predictor for medication-induced reductions in Glu. However, impulsivity was not assessed after treatment (Schmaal et al., 2012). Future studies are needed to investigate whether NAC may also decrease Glu levels in AUD and whether it has an impact on impulsive behavior.
Despite previous findings of elevated Glu and Glx levels in the ACC of AUD patients (Hermann et al., 2012; Yeo et al., 2013), in the current study we did not find any group differences in Glu levels in thr ACC. The reason for these discrepancies is unclear but might involve low power due to the small sample size of our study or differences in the clinical and demographic characteristics of the patients. However, other than a younger age for the AUD group in Yeo et al., 2013 (mean age is 31, rather than 47 in our study and 46 in Hermann et al., 2012), we could not identify any other group differences between our study and previous studies.
Study Limitations
Given our limited sample size, we see our first study as exploratory and in need of further replications. All results from study 1 were presented without corrections for multiple comparisons and although all data were normally distributed and we removed > 3SD outliers, the correlationsal measures of thalamic Glu with impulsivity and thala mic FA would lack significane if 2SD outliers were removed. The small sample size may have also have limited the power to detect group differences in the vACC, or associations with impulsivity. Nevertheless, we were able to replicate the association between elevated brain Glu and impulsivity in AUD in an independent and larger cohort of 32 AUD patients – although in a different brain area (dACC) and with a different scale used (i.e., BIS-11 rather than CAARS). In addition, our measurement of Glu using the PRESS sequence may have suffered from glutamine contamination; future studies using edited MRS sequences that can measure glutamate separate from glutamine (e.g., TE-averaged PRESS or HERMES) are needed to replicate our findings. Moreover, a higher proportion of AUD patients than HC were tobacco smokers which confounds the interpretation of our findings. Although we did not find an association between FA or Glu and smoking status or quantity, we cannot completely rule out the contribution of smoking to our findings. Finally, we did not obtain peripheral markers of neuroinflammation, which would have allowed us to assess associations with Glu and FA.
In conclusion, we found elevated Glu and decreased FA in the thalamus, but not in vACC, associated with impulsivity (Glu in thalamus and dACC) and severity of drinking behavior in AUD patients, which we interpret as evidence of thalamic neurotoxicity. These findings suggest that the thalamus and dACC are vulnerable to the neurotoxic effects of chronic alcohol use and implicate these effects in impulsivity.
Supplementary Material
Highlights.
Patients with Alcohol Use Disorder show higher glutamate (Glu) and lower FA in the thalamus than controls
Chronic alcohol exposure in the thalamus may contribute to impulsivity
Past drinking behavior correlated with higher Glu and lower FA in the thalamus
Acknowledgements
We thank Karen Torres, Minoo McFarland, Lori Talagala, Joelle Sarlls, Ted George, Kimberly Herman, and Nancy Diazgranados for their contributions.
Funding and Disclosure
This work was accomplished with support from the National Institute on Alcohol Abuse and Alcoholism (Y1AA-3009). Authors have no competing financial interests in relation to the work described.
Footnotes
Conflict of Interest
All authors declare they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association, 2000. Diagnostic and statistical manual of mental disorders: DSM-IV-TR, 4 ed, Washington, DC. [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders, 5th ed, Washington DC. [Google Scholar]
- Andres T., Ernst T., Oishi K., Greenstein D., Nakama H., Chang L., 2016. Brain Microstructure and Impulsivity Differ between Current and Past Methamphetamine Users. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 11, 531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagga D, Khushu S, Modi S, Kaur P, Bhattacharya D, Garg ML, Singh N, 2014. Impaired visual information processing in alcohol-dependent subjects: a proton magnetic resonance spectroscopy study of the primary visual cortex. Journal of studies on alcohol and drugs 75, 817–826. [DOI] [PubMed] [Google Scholar]
- Bauer J, Pedersen A, Scherbaum N, Bening J, Patschke J, Kugel H, Heindel W, Arolt V, Ohrmann P, 2013. Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 38, 1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari M, Moscufo N, Meier D, Skudlarski P, Pearlson GD, White WB, Wolfson L, Guttmann CR, 2014. Thalamic fractional anisotropy predicts accrual of cerebral white matter damage in older subjects with small-vessel disease. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 34, 1321–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XR, Zeng JY, Shen ZW, Kong LM, Zheng WB, 2017. Diffusion Kurtosis Imaging Detects Microstructural Changes in the Brain after Acute Alcohol Intoxication in Rats. Biomed Res Int 2017, 4757025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Kellar D, Lake A, Finn P, Rebec GV, Dharmadhikari S, Dydak U, Newman S, 2018. Effects of Alcohol Cues on MRS Glutamate Levels in the Anterior Cingulate. Alcohol and alcoholism 53, 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK, 1998. Rating scales in attention-deficit/hyperactivity disorder: use in assessment and treatment monitoring. The Journal of clinical psychiatry 59 Suppl 7, 24–30. [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31, 968–980. [DOI] [PubMed] [Google Scholar]
- Ende G, Cackowski S, Van Eijk J, Sack M, Demirakca T, Kleindienst N, Bohus M, Sobanski E, Krause-Utz A, Schmahl C, 2016. Impulsivity and Aggression in Female BPD and ADHD Patients: Association with ACC Glutamate and GABA Concentrations. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 41, 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Kreis R, Ross BD, 1993. Absolute quantitation of water and metabolites in the human brain. I: compartments and water. Journal of Magnetic Resonance B 102, 1–8. [Google Scholar]
- Fischl B, Salat D, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale A, 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno M, Dale A, 1999. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 9, 195–207. [DOI] [PubMed] [Google Scholar]
- Fliegel S, Brand I, Spanagel R, Noori HR, 2013. Ethanol-induced alterations of amino acids measured by in vivo microdialysis in rats: a meta-analysis. In silico pharmacology 1, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman CR, Wiers CE, Sloan ME, Zehra A, Ramirez V, Wang GJ, Volkow ND, 2018. Emotion Recognition Biases in Alcohol Use Disorder. Alcoholism, clinical and experimental research. [DOI] [PubMed] [Google Scholar]
- Frischknecht U., Hermann D., Tunc-Skarka N., Wang GY., Sack M., van Eijk J., Demirakca T., Falfan-Melgoza C., Krumm B., Dieter S., Spanagel R., Kiefe F., Mann KF., Sommer WH., Ende G., Weber-Fahr W., 2017. Negative Association Between MR-Spectroscopic Glutamate Markers and Gray Matter Volume After Alcohol Withdrawal in the Hippocampus: A Translational Study in Humans and Rats. Alcoholism, clinical and experimental research 41, 323–333. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Mon A, Yeh PH, Meyerhoff DJ, 2010. Cerebral white matter recovery in abstinent alcoholics--a multimodality magnetic resonance study. Brain : a journal of neurology 133, 1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M, Sotiropoulos S, Wilson J, Coalson T, Fischl B, Andersson J, Xu J, Jbabdi S, Webster M, Polimeni J, Van Essen D, Jenkinson M, Consortium W-MH, 2013. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, Wang GJ, Fowler JS, Rajaram S, 2001. Addiction changes orbitofrontal gyrus function: involvement in response inhibition. Neuroreport 12, 2595–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropper S, Spengler S, Stuke H, Gawron CK, Parnack J, Gutwinski S, Wiers CE, Bermpohl F, 2016. Behavioral impulsivity mediates the relationship between decreased frontal gray matter volume and harmful alcohol drinking: A voxel-based morphometry study. Journal of psychiatric research 83, 16–23. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Silveri MM, Dahlgren MK, Yurgelun-Todd D, 2011. Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Experimental and clinical psychopharmacology 19, 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Miller AH, Sanacora G, 2017. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 42, 193–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, Tunc-Skarka N, Perreau-Lenz S, Hansson AC, Krumm B, Kiefer F, Spanagel R, Mann K, Ende G, Sommer WH, 2012. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biological psychiatry 71, 1015–1021. [DOI] [PubMed] [Google Scholar]
- Hillmer AT, Mason GF, Fucito LM, O’Malley SS, Cosgrove KP, 2015. How Imaging Glutamate, gamma-Aminobutyric Acid, and Dopamine Can Inform the Clinical Treatment of Alcohol Dependence and Withdrawal. Alcoholism, clinical and experimental research 39, 2268–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerst M, Weber-Fahr W, Tunc-Skarka N, Ruf M, Bohus M, Schmahl C, Ende G, 2010. Correlation of glutamate levels in the anterior cingulate cortex with self-reported impulsivity in patients with borderline personality disorder and healthy controls. Archives of general psychiatry 67, 946–954. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM, 2012. FSL. NeuroImage 62, 782–790. [DOI] [PubMed] [Google Scholar]
- Kim SW, Wiers CE, Tyler R, Shokri-Kojori E, Jang YJ, Zehra A, Freeman C, Ramirez V, Lindgren E, Miller G, Cabrera EA, Stodden T, Guo M, Demiral SB, Diazgranados N, Park L, Liow JS, Pike V, Morse C, Vendruscolo LF, Innis RB, Koob GF, Tomasi D, Wang GJ, Volkow ND, 2018. Influence of alcoholism and cholesterol on TSPO binding in brain: PET [(11)C]PBR28 studies in humans and rodents. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 43, 1832–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LM., Zheng WB., Lian GP., Zhang HD., 2012. Acute effects of alcohol on the human brain: diffusion tensor imaging study. AJNR. American journal of neuroradiology 33, 928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopera M, Wojnar M, Brower K, Glass J, Nowosad I, Gmaj B, Szelenberger W, 2012. Cognitive functions in abstinent alcohol-dependent patients. Alcohol 46, 665–671. [DOI] [PubMed] [Google Scholar]
- Kreis R, Ernst T, Ross BD, 1993. Absolute quantitation of water and metabolites in the human brain. II: metabolite concentrations. Journal of Magnetic Resonance B 102, 9–19. [Google Scholar]
- Lebourgeois S, Gonzalez-Marin MC, Antol J, Naassila M, Vilpoux C, 2019. Evaluation of N-acetylcysteine on ethanol self-administration in ethanol-dependent rats. Neuropharmacology 150, 112–120. [DOI] [PubMed] [Google Scholar]
- Loeber S, Duka T, Welzel Marquez H, Nakovics H, Heinz A, Mann K, Flor H, 2010. Effects of repeated withdrawal from alcohol on recovery of cognitive impairment under abstinence and rate of relapse. Alcohol and alcoholism 45, 541–547. [DOI] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Meyerhoff DJ, 2012. Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug and alcohol dependence 125, 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnig MA, Caprihan A, Yeo RA, Gasparovic C, Ruhl DA, Lysne P, Bogenschutz MP, Hutchison KE, Thoma RJ, 2013. Diffusion tensor imaging of white matter networks in individuals with current and remitted alcohol use disorders and comorbid conditions. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors 27, 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnig MA, Woods AJ, Walsh E, Martone CM, Blumenthal J, Monti PM, Cohen RA, 2019. Cerebral Metabolites on the Descending Limb of Acute Alcohol: A Preliminary 1H MRS Study. Alcohol and alcoholism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AK, Ardekani BA, Kamarajan C, Zhang J, Chorlian DB, Byrne KN, Pandey G, Meyers JL, Kinreich S, Stimus A, Porjesz B, 2018. Lower Prefrontal and Hippocampal Volume and Diffusion Tensor Imaging Differences Reflect Structural and Functional Abnormalities in Abstinent Individuals with Alcohol Use Disorder. Alcoholism, clinical and experimental research 42, 1883–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES, 1995. Factor structure of the Barratt Impulsiveness Scale. J. Clin. Psychol 51, 768–774. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV, 2009. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biological psychiatry 65, 680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisciandaro JJ, Schacht JP, Prescot AP, Renshaw PF, Brown TR, Anton RF, 2019. Brain Glutamate, GABA, and Glutamine Levels and Associations with Recent Drinking in Treatment-Naive Individuals with Alcohol Use Disorder Versus Light Drinkers. Alcoholism, clinical and experimental research 43, 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher S, 1993. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic Resonance in Medicine 30, 672. [DOI] [PubMed] [Google Scholar]
- Santos TEG, Baggio JAO, Rondinoni C, Machado L, Weber KT, Stefano LH, Santos AC, Pontes-Neto OM, Leite JP, Edwards DJ, 2019. Fractional Anisotropy of Thalamic Nuclei Is Associated With Verticality Misperception After Extra-Thalamic Stroke. Front Neurol 10, 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Veltman DJ, Nederveen A, van den Brink W, Goudriaan AE, 2012. N-acetylcysteine normalizes glutamate levels in cocaine-dependent patients: a randomized crossover magnetic resonance spectroscopy study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 37, 2143–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R Jr., Bandiera S., Souza DG., Bellaver B., Calett G., Quincozes-Santos A., Elisabetsky E., Gomez R., 2017. N-acetylcysteine Prevents Alcohol Related Neuroinflammation in Rats. Neurochem Res 42, 2135–2141. [DOI] [PubMed] [Google Scholar]
- Ségonne F, Dale A, Busa E, Glessner M, Salat D, Hahn H, Fischl B, 2004. A hybrid approach to the skull stripping problem in MRI. Neuroimage 22, 1060–1075. [DOI] [PubMed] [Google Scholar]
- Shokri-Kojori E, Tomasi D, Wiers CE, Wang GJ, Volkow ND, 2017. Alcohol affects brain functional connectivity and its coupling with behavior: greater effects in male heavy drinkers. Molecular psychiatry 22, 1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Allen BA, 1982. Alcohol dependence syndrome: measurement and validation. Journal of abnormal psychology 91, 199–209. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ, 1982. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. Journal of studies on alcohol 43, 1157–1170. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 1996. Timeline Followback: User’s Guide - A calendar method for assessing alcohol and drug use. Addiction Research Foundation, Toronto. [Google Scholar]
- Sorg SF, Taylor MJ, Alhassoon OM, Gongvatana A, Theilmann RJ, Frank LR, Grant I, 2012. Frontal white matter integrity predictors of adult alcohol treatment outcome. Biological psychiatry 71, 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma R, Mullins P, Ruhl D, Monnig M, Yeo RA, Caprihan A, Bogenschutz M, Lysne P, Tonigan S, Kalyanam R, Gasparovic C, 2011. Perturbation of the glutamate-glutamine system in alcohol dependence and remission. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 36, 1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi DG, Wiers CE, Shokri-Kojori E, Zehra A, Ramirez V, Freeman C, Burns J, Kure Liu C, Manza P, Kim SW, Wang GJ, Volkow ND, 2019. Association Between Reduced Brain Glucose Metabolism and Cortical Thickness in Alcoholics: Evidence of Neurotoxicity. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler RE, Kim SW, Guo M, Jang YJ, Damadzic R, Stodden T, Vendruscolo LF, Koob GF, Wang GJ, Wiers CE, Volkow ND, 2019. Detecting neuroinflammation in the brain following chronic alcohol exposure in rats: A comparison between in vivo and in vitro TSPO radioligand binding. The European journal of neuroscience 50, 1831–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann A, Fouche JP, Lederer K, Meintjes EM, Wilson D, Stein DJ, 2016. White matter microstructure and impulsivity in methamphetamine dependence with and without a history of psychosis. Human brain mapping 37, 2055–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, Wong C, Ma Y, Logan J, Goldstein R, Alexoff D, Thanos PK, 2006. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Archives of general psychiatry 63, 999–1008. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K, 1996. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcoholism, clinical and experimental research 20, 1594–1598. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wiers CE, Shokri-Kojori E, Tomasi D, Wang GJ, Baler R, 2017. Neurochemical and metabolic effects of acute and chronic alcohol in the human brain: Studies with positron emission tomography. Neuropharmacology 122, 175–188. [DOI] [PubMed] [Google Scholar]
- Wang J., Fan Y., Dong Y., Ma M., Ma Y., Dong Y., Niu Y., Jiang Y., Wang H., Wang Z., Wu L., Sun H., Cui C., 2016. Alterations in Brain Structure and Functional Connectivity in Alcohol Dependent Patients and Possible Association with Impulsivity. PloS one 11, e0161956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RJ, Colivicchi MA, Allen R, Schol F, Lallemand F, de Witte P, Ballini C, Corte LD, Dexter D, 2009. Neuro-inflammation induced in the hippocampus of ‘binge drinking’ rats may be mediated by elevated extracellular glutamate content. Journal of neurochemistry 111, 1119–1128. [DOI] [PubMed] [Google Scholar]
- Wechsler D, 1999. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Wiers CE, Gawron CK, Gropper S, Spengler S, Stuke H, Lindenmeyer J, Walter H, Bermpohl F, 2015. Decreased gray matter volume in inferior frontal gyrus is related to stop-signal task performance in alcohol-dependent patients. Psychiatry research 233, 125–130. [DOI] [PubMed] [Google Scholar]
- Yeo RA, Thoma RJ, Gasparovic C, Monnig M, Harlaar N, Calhoun VD, Kalyanam R, Mayer AR, Durazzo TC, Hutchison KE, 2013. Neurometabolite concentration and clinical features of chronic alcohol use: a proton magnetic resonance spectroscopy study. Psychiatry research 211, 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehra A, Lindgren E, Wiers CE, Freeman C, Miller G, Ramirez V, Shokri-Kojori E, Wang GJ, Talagala L, Tomasi D, Volkow ND, 2019. Neural correlates of visual attention in alcohol use disorder. Drug and alcohol dependence 194, 430–437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.