The rapid advancement in science with interdisciplinary collaboration is transforming our lives. Progress in science is changing innovation ecosystem. Discovery in basic sciences can be enabled to provide a need-based solution with high translational potential. New ideas are leading to incremental as well as disruptive innovations. The emerging technologies are creating novel products for informatics, communication, transport, energy, textile, agriculture, food, health care and other sectors. One of the classical examples of a disruptive innovation in transport sector is the driverless vehicle developed on the basis of collaboration among engineering, communication and artificial intelligence1. Similarly, in health sector, precision medicine with molecular targeting, minimally invasive procedures with imaging, regenerative medicine, nanomedicine and point-of-care devices with nano-bio sensors are replacing the conventional medical practices2. In cancer nanomedicine, the toxic chemotherapeutic drugs can be targeted to cancer cells, sparing the normal cells, resulting in better efficacy and significant reduction of toxicity3. Recently, tissue nano transfection technology has been introduced in the field of regenerative medicine which can reprogram the differentiation of one cell type to another by topical delivery of anti-sense oligonucleotides through skin with nano-electroporation4. These advanced technology-based products need new modalities for assessment of their quality, safety, efficacy and performance (QSEP) attributes. As these products are the results of advanced interdisciplinary research, their evaluation should be done according to the science pathway involved in the innovation process. Academicians and scientists from different fields need to work with the regulators to assess these products. The scientists working in this domain validating QSEP attributes of these novel products form emerging technologies can lead to development of the new discipline of ‘Regulatory Science’. There is an urgent need to develop this discipline to bridge the gap between scientific understanding and regulatory need for a product3,5. The present article explores the international progress in regulatory science and compares with the current Indian status. Capacity building in this area will help the regulator and policy maker to embrace the new technologies with higher economic benefit, reducing the time for translating fundamental science with proof of concept in the laboratory to application in clinical medicine, strengthening evidence-based regulatory practice.
Defining regulatory science
The US Food and Drug Administration (FDA) has defined regulatory science5 as ‘the science of developing new tools, standards and approaches to assess the safety, efficacy, quality, performance of all FDA-regulated products’. A more inclusive and simple definition of regulatory science may be, the science for understanding and study of regulation required to bring a product to market. The regulatory science should be a multidimensional multidisciplinary field which can protect the consumers’ interest as well as help to define the regulatory pathway. Success in developing this new field will depend on continuous collaboration among academia, industry and regulators along with legal, social as well as economic inputs empowering the innovation ecosystem in India. The concept of this interactive field is shown in Figure 1.
Fig. 1.
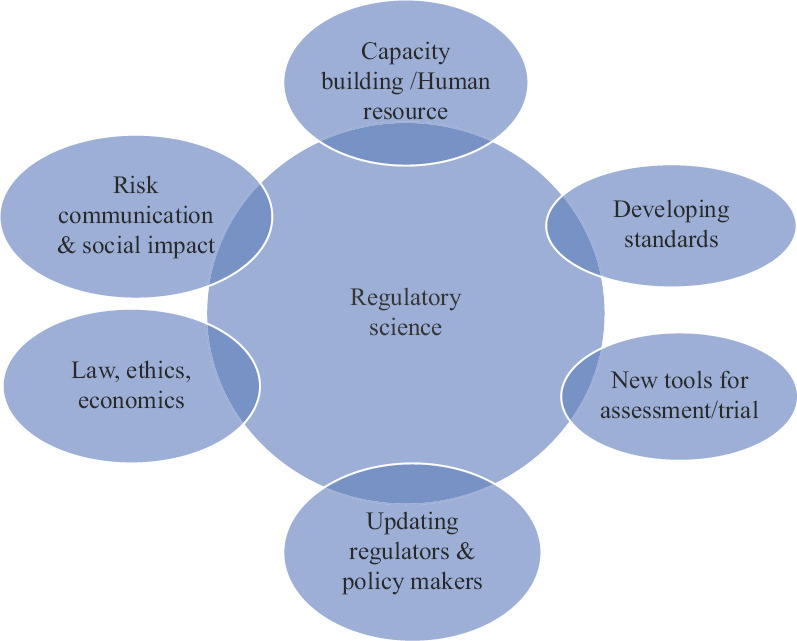
Schematic representation of the multidimensional approach for establishing regulatory science in India.
Applications of regulatory science
The rapid advances in molecular biology, genomics, transcriptomics, proteomics and metabolomics are leading to ‘Omics integrative approach’ for diagnosis and identification of molecular targets for therapy as part of precision medicine6. This practice can be further strengthened by bioinformatics, big data analysis and artificial intelligence7. The diagnostic tests and therapeutic products emerging through such a complex pathway can pose a greater regulatory challenge. So, understanding the gaps in the areas of standardization of such a complex test, pre-clinical assessment procedure, reviewing clinical utility and validity as well as post-marketing surveillance need regulatory science/research input8. Though the challenges are great, these gaps in knowledge and understanding can be bridged by development of goal-oriented collaboration among academia, industry and regulators9. The academic medical centres can be developed in India with commitment for Good Laboratory Practice and Good Clinical Practice with quality-enabled ‘Biobank Facilities’. The large patient population in India can be utilized as a huge clinical and biological resource following ethical guidelines, empowering translational research and product development. Although complete harmonization of all biobanks in India may not be possible, initiative may be taken to develop few major biobanks in different parts of India with well-documented uniform quality-controlled processes of sample collection, processing, analysis and preservation8. The Genomics for Understanding Rare Diseases: India Alliance Network (GUaRDIAN) is a good example of the effort of academic institutions in research with translational capability in molecular diagnosis10. However, more such collaborations are needed for developing novel, commercially viable, affordable molecular diagnostics with regulatory approval.
Currently, on an average, it takes about 12 years with an expenditure of approximately US$ 2.6 billion for R&D (Research & Development) and getting regulatory approval for a new medicine11. The regulatory science approach can reduce the time and expenditure for new drug development12. With similar aim, the USFDA in 2011 created the division of Applied Regulatory Science under the Center for Drug Evaluation and Research (CDER). This division is actively working with experts from multiple disciplines for scientific evaluation and validation of all modalities of translational research which include in vitro and in vivo experiments, in silico computational modelling and informatics, clinical research, experimental medicine and post-market analysis. This regulatory science approach has helped CDER for better regulatory enforcement with less time with development of new regulatory assessment tools in the areas of clinical pharmacology and chemical and biomedical informatics13. An important example of the strength of translational research is the rapid development of more than 90 vaccine candidates for the current worldwide pandemic of COVID-19 (SARS-CoV2) in five months after the onset of infection14. There was an urgent need for vaccine against this highly contagious infection causing pandemic like SARS-CoV2, as there is no specific therapeutic drug available. The different steps for making of a successful vaccine candidate include developing global regulatory science agenda for a vaccine with exchange of data, previous experience, experimental and bioinformatic tools and capability of validation of new technologies such as nanoadjuvants15.
Another potential application of regulatory science is in the medical device development which can transform the current healthcare practices in India as well as other developing countries. The Ayushman Bharat Pradhan Mantri Jan Arogya Yojana and the path to universal health coverage in India16 will be most successful if low-cost, high-efficiency diagnostic devices with novel technologies can be developed indigenously with QSEP attributes comparable to international standards with regulatory approval. The schematic pathway explaining the scope of regulatory science intervention for the development of medical devices with complex multidisciplinary technologies integrating hardware and software is shown in Fig. 2. The USFDA has created the Center for Devices and Radiological Health with a strong interdisciplinary regulatory science framework which looks after seven priority areas including, (i) advancing innovation of medical devices and evaluation of new and emerging technologies, (ii) improvement of device quality and manufacturing process, (iii) analysis of medical device performance, (iv) enhancement of medical device safety, (v) development of novel ways to use clinical data for evaluating medical devices, (vi) improving technology to protect against emerging infectious diseases and terrorism, and (vii) improvement of health of pediatric and other special populations17. India has a large number of start-up companies and innovators who are working on the development of novel medical devices with diverse functionalities and applications. Regulatory science input can help them to reduce their development time in different steps as mentioned in Fig. 2. There are many reputed basic, engineering and medical science institutions in India with good research infrastructure, which can be harnessed in collaborative networks along with a regulatory agency and industry with specific goal like the USFDA. This will strengthen the initiative of Department of Health Research, Government of India, which has set up a health technology assessment body, ‘Health Technology Assessment in India’ (https://dhr.gov.in/health-technology-assessment-india-htain). The networking groups can be formed in some of the specific areas such as device designing and assessment of performance using computer modelling, safety and reproducibility of nanotechnology interventions in devices, safety of endoscopic and minimally invasive devices for various interventions, accuracy of next generation sequencing-based tests for clinical practice, safety and reproducibility of imaging instruments and reliability of ‘smart’ implants/devices and other necessary domains.
Fig. 2.
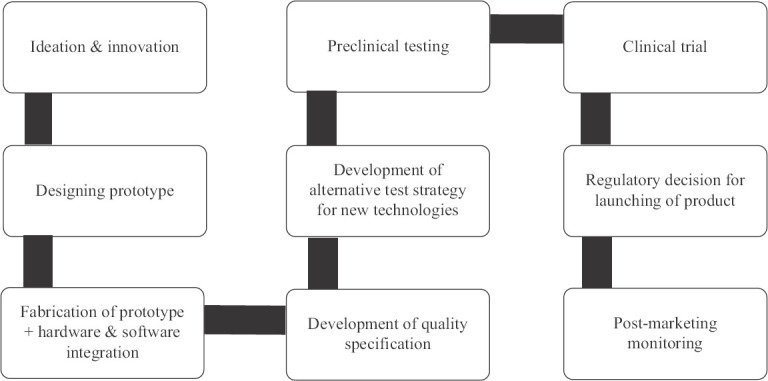
Flow diagram of proposed regulatory science path for assessment of new generation of novel medical devices/tools.
Regulating nanotechnology-based products is a huge challenge all over the world. A large segment of nanomedicine/nanopharmaceutical products in the market are for cancer management3. Although there is a significant advantage of nanotechnology intervention for targeted therapy, imaging as well as a combination of both (theragnosis), the complexity of nano-bio interaction; characterization of nanoparticles and safety, efficacy and reproducibility issues need in-depth studies as well as development of alternative test strategies3,17. Nanotechnology is also being used in agriculture, food, nutraceuticals and food packaging, Nano-agri products are being developed for fertiliser, pesticide, seed coating, diagnostic and sensing applications. Recently, the Indian safety guideline for nanopharmaceuticals has been developed18 and a similar safety guideline for Nano-agri products has also been developed. Considering the huge scope of innovation and commercialization of nanotechnology-based products, development of nano-specific regulatory science infrastructure in India will be helpful not only for ensuring safety of the consumers, but also for prevention of possible environmental contamination and ecotoxicity. One relevant example is the wide application of silver nanoparticle (AgNP) as a potent antimicrobial agent in diverse products for wound care, personal protection equipment, disinfectants, food packaging, textile and others. However, there is no uniform international regulatory guideline for AgNP-based products, though the danger of environmental contamination with ecotoxicity has been mentioned by most of the regulatory agencies19.
Pharmacovigilance (PV) and post-marketing surveillance is one of the most important tools for ensuring safety of the drugs as well as devices. The USA and Europe have developed a robust PV system with continuous upgrading with introduction of data analytics and communication technologies as a part of regulatory science intervention5,11,13,17,20. India being one of the largest producers of pharmaceuticals in the world and a major hub of clinical research needs to strengthen this area20. With the availability of good communication systems and a wide use of mobile phones by citizens, the possibility of utilizing mHealth (mobile health) and big-data integration may be considered for PV purpose, maintaining all ethical guidelines for data handling and sharing21.
Human resource development for regulatory science
The most important step for establishing regulatory science in India will be the plan for generating specialized ‘Human Resources’ who can modify, validate and improve each stage of drug and device development according to the regulatory need17,22,23. Resource persons from different basic science backgrounds and professional specialities such as medicine, pharmacy, engineering, agriculture, law, information technology, economics, statistics, etc. who can be trained to develop different domain-specific expertise and skills in a competency-based education system should be identified24. The candidates can be trained with interdisciplinary complementary major and minor course modules to develop knowledge and competency to explore regulatory issues for different kinds of products17. The training should include latest trend in regulation and quality policies in domestic and global scenarios25. Few examples of the important training areas are ‘quality systems and statistical process control’, ‘quality assurance for drugs and biologicals’, ‘quality assurance in medical devices and combination products’, ‘regulatory need for medical diagnostics, devices and imaging’, ‘regulation for food and dietary supplements’, ‘medical products and law’, ‘planning and management of preclinical and clinical trials’ and ‘regulatory need for agriculture products’. The courses should be designed with active collaboration among academia, industry and regulatory bodies and critically monitored by the agency looking after such regulatory policies in India. A stringent quality control of such courses is essential for preparing and training human resources who can enhance our capability for commercialization of emerging technology-based products. Considering the dynamism of the innovation and complexity of products, the course should also be remodelled and redesigned from time to time. The doctoral courses may be designed to address futuristic regulatory challenges such as animal sparing technology or assay development for safety assessment of non-biologic-complex-drug, etc. In India, few universities impart courses and teaching modules in regulatory affairs, regulatory toxicology and clinical research, which may not be adequate for developing comprehensive competency in regulatory science as mentioned earlier.
Career prospect in regulatory science
The trained experts in regulatory science from different disciplines with new knowledge and skills for assessment of QSEP may have great career prospects in industries, regulatory bodies and various ecosystems for innovation, product development, marketing and post-marketing surveillance. These experts are likely to be in great demand in India and globally depending on their training and area of expertise. They can be instrumental in developing new regulation and policy to introduce new technology-based products in the market.
Need for regulatory science in the Indian context
The economic progress of India depends on its strength of innovation and development of novel products or technologies in different areas. Indian researchers and innovators are successful in generating leads with proof of concept at laboratory level. However, the major bottleneck to translate such research to products for commercialization is the lack critical implementation of regulatory guideline or generation of validated data and information needed for the approval procedure (Table). To generate the data for QSEP attributes for novel technology-based products, new drugs and devices, trained competent workforce is needed. Regulatory science is not only important for the industry or regulatory bodies, but it is also equally beneficial for the consumers, protecting their safety and ensuring quality and efficacy of the products. With the development of regulatory science, India can achieve a significant capability to regulate the new generation of high-quality, cost-effective innovation-based products with a greater social and economic impact. Thus, regulatory science can be a game changer, empowering Indian innovation with global recognition.
Table.
Proposed areas of capacity building for development of regulatory science in India
| Areas | Expected benefit |
|---|---|
| Introduction of concept of Good Laboratory Practice and SOP Driven Research in Academic and Research Institutions | High-quality data generation with reproducibility, helping expeditious translation |
| Development of academic medical Centres, stimulating closer academia–industry interaction with regulatory interface | Enhancement of capability of safety and efficacy assessment of complex molecular diagnostic systems and molecular/nano medicines |
| Facility for development of gene-manipulated animals for exploration and validation | Capacity building in the development of genomic medicine |
| Development of quality-enabled harmonized Biobanks in different parts of the country with virtual connectivity | Expeditious testing capability with large number of samples for validation of diagnostics/prognostic biomarkers/tools |
| Development of computational model for regulatory interventions such as structure activity relationship, cell/organ/system biology and clinical trial simulation. | Augmentation of the capability of risk identification and prediction |
| Strengthening the application of AI, machine learning and big data analysis in drug discovery and health technology assessment | Reduction of time between exploration and product development with regulatory approval |
| Organized strategy for “competence-based education” in regulatory science in multidisciplinary environment | Increase in critical human resources for evaluation of novel emerging technology-based products in innovation pathway enhancing evidence-based regulatory approval |
SOP, standard operating procedure; AI, artificial intelligence
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.Martínez-Díaz M, Soriguera F. Autonomous vehicles: theoretical and practical challenges. Transportation Res Procedia. 2018;33:275–82. [Google Scholar]
- 2.Modi S, Prajapati R, Inwati GK, Deepa N, Tirth V, Yadav VK, et al. Recent trends in fascinating applications of nanotechnology in allied health sciences. Crystals. 2022;12:1–16. [Google Scholar]
- 3.Nature Nanotechnology. The two direction of cancer nanomedicine. [accessed on December 31, 2020]. Available from: nature.com/articles/s41565-019-0597-5.pdf .
- 4.Gallego-Perez D, Pal D, Ghatak S, Malkoc V, Higuita-Castro N, Gnyawali S, et al. Topical tissue nano-transfection mediates non-viral stroma reprogramming and rescue. Nat Nanotechnol. 2017;12:974–9. doi: 10.1038/nnano.2017.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration. Why you should care about regulatory science? [accessed on December 31, 2020;]. Available from: https://www.fda.gov/consumers/consumer-updates/why-you-should-care-about-regulatory-science .
- 6.Karczewski KJ, Snyder MP. Integrative omics for health and disease. Nat Rev Genet. 2018;19:299–310. doi: 10.1038/nrg.2018.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perakakis N, Yazdani A, Karniadakis GE, Mantzoros C. Omics, big data and machine learning as tools to propel understanding of biological mechanisms and to discover novel diagnostics and therapeutics. Metabolism. 2018;87:A1–9. doi: 10.1016/j.metabol.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adamo JE, Bienvenu RV, 2nd, Fields FO, Ghosh S, Jones CM, Liebman M, et al. The integration of emerging omics approaches to advance precision medicine: How can regulatory science help? J Clin Transl Sci. 2018;2:295–300. doi: 10.1017/cts.2018.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva PJ, Schaibley VM, Ramos KS. Academic medical centers as innovation ecosystems to address population -omics challenges in precision medicine. J Transl Med. 2018;16:28. doi: 10.1186/s12967-018-1401-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sivasubbu S, Scaria V GUaRDIAN Consortium. Genomics of rare genetic diseases-experiences from India. Hum Genomics. 2019;14:52. doi: 10.1186/s40246-019-0215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borup G, Bach KF, Schmiegelow M, Wallach-Kildemoes H, Bjerrum OJ, Westergaard N. A paradigm shift towards patient involvement in medicines development and regulatory science: Workshop proceedings and commentary. Ther Innov Regul Sci. 2016;50:304–11. doi: 10.1177/2168479015622668. [DOI] [PubMed] [Google Scholar]
- 12.Lee M, Ly H, Möller CC, Ringel MS. Innovation in Regulatory Science Is Meeting Evolution of Clinical Evidence Generation. Clin Pharma Therap. 2019:105, 886–98. doi: 10.1002/cpt.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rouse R, Kruhlak N, Burkhart K, Patel V, Strauss DG. Translating new science into the drug review process: The US FDA’s division of applied regulatory science. Therap Innov Regul Sci. 2018;52:244–55. doi: 10.1177/2168479017720249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callaway E. Scores of coronavirus vaccines are in competition – How will scientists choose the best? Nature. 2020 doi: 10.1038/d41586-020-01247-2. doi: 10.1038/d41586-020-01247-2. [DOI] [PubMed] [Google Scholar]
- 15.Elmgren L, Li X, Wilson C, Ball R, Wang J, Cichutek K, et al. A global regulatory science agenda for vaccines. Vaccine. 2013;31(Suppl 2):B163–75. doi: 10.1016/j.vaccine.2012.10.117. [DOI] [PubMed] [Google Scholar]
- 16.Angell BJ, Prinja S, Gupt A, Jha V, Jan S. The Ayushman Bharat Pradhan Mantri Jan Arogya Yojana and the path to universal health coverage in India: Overcoming the challenges of stewardship and governance. PLoS Med. 2019;16:e1002759. doi: 10.1371/journal.pmed.1002759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration. Regulatory science in FDA’s center for devices and radiological health: A vital framework for protecting and promoting public health. [accessed on December 31, 2020]. Available from: https://www.fda.gov/about-fda/cdrh-reports/regulatory-science-report#: ~: text=The%20report%2C%20%22Regulatory%20Science%20in,the%20same%20time%20providing%20the .
- 18.Central Drugs Standard Control Organization. Department of Biotechnology. Indian Council for Medical Research. Ministry of Health & Family Welfare Government of India. Guidelines for evaluation of nanopharmaceuticals in India. [accessed on December 31, 2020]. Available from: http://dbtindia.gov.in/sites/default/files/uploadfiles/Guidelines_For_Evaluation_of_Nanopharmaceu ticals_in_India_24.10.19.pdf .
- 19.Faunce T, Watal A. Nanosilver and global public health: International regulatory issues. Nanomedicine (Lond) 2010;5:617–32. doi: 10.2217/nnm.10.33. [DOI] [PubMed] [Google Scholar]
- 20.Jose J, Rafeek NR. Pharmacovigilance in India in comparison with the USA and European Union: Challenges and perspectives. Ther Innov Regul Sci. 2019;53:781–6. doi: 10.1177/2168479018812775. [DOI] [PubMed] [Google Scholar]
- 21.Madanian S, Parry DT, Airehrour D, Cherrington M. mHealth and big-data integration: Promises for healthcare system in India. BMJ Health Care Inform. 2019;26:e100071. doi: 10.1136/bmjhci-2019-100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondo T, Hayashi Y, Sato J, Sekine S, Hoshino T, Sato D. Evolving vision of regulatory science in the global medical community. Clin Pharmacol Ther. 2020;107:136–9. doi: 10.1002/cpt.1604. [DOI] [PubMed] [Google Scholar]
- 23.Volkers N. All in the details: Careers in regulatory science. Science. 2010 doi: 10.1126/science.caredit.a1000037. [Google Scholar]
- 24.Calvin-Naylor NA, Jones CT, Wartak MM, Blackwell K, Davis JM, Divecha R, et al. Education and training of clinical and translational study investigators and research coordinators: A competency-based approach. J Clin Transl Sci. 2017;1:16–25. doi: 10.1017/cts.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pire-Smerkanich N, Locke D. Options in regulatory affairs –Specialties and skill sets. Regul Rapporteur. 2015;12:20–3. [Google Scholar]


