Abstract
Unvaccinated COVID-19 patients display a large spectrum of symptoms, ranging from asymptomatic to severe symptoms, the latter even causing death. Distinct Natural killer (NK) and CD4+ and CD8+ T cells immune responses are generated in COVID-19 patients. However, the phenotype and functional characteristics of NK cells and T-cells associated with COVID-19 pathogenesis versus protection remain to be elucidated. In this study, we compared the phenotype and function of NK cells SARS-CoV-2-specific CD4+ and CD8+ T cells in unvaccinated symptomatic (SYMP) and unvaccinated asymptomatic (ASYMP) COVID-19 patients. The expression of senescent CD57 marker, CD45RA/CCR7differentiation status, exhaustion PD-1 marker, activation of HLA-DR, and CD38 markers were assessed on NK and T cells from SARS-CoV-2 positive SYMP patients, ASYMP patients, and Healthy Donors (HD) using multicolor flow cytometry. We detected significant increases in the expression levels of both exhaustion and senescence markers on NK and T cells from SYMP patients compared to ASYMP patients and HD controls. In SYMP COVID-19 patients, the T cell compartment displays several alterations involving naive, central memory, effector memory, and terminally differentiated T cells. The senescence CD57 marker was highly expressed on CD8+ TEM cells and CD8+ TEMRA cells. Moreover, we detected significant increases in the levels of pro-inflammatory TNF-α, IFN-γ, IL-6, IL-8, and IL-17 cytokines from SYMP COVID-19 patients, compared to ASYMP COVID-19 patients and HD controls. The findings suggest exhaustion and senescence in both NK and T cell compartment is associated with severe disease in critically ill COVID-19 patients.
Keywords: SARS-CoV-2, COVID-19, senescence, NK cells, CD8+ T cells
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a β-Coronavirus that was first detected in 2019 in Wuhan, China. In the ensuing months, it has been transmitted worldwide. As of July 2022, more than 568 million people have contracted coronavirus disease (COVID-19), the pandemic that has killed approximately 6.38 million people globally (1). COVID-19, caused by SARS CoV-2, has a wide range of clinical manifestations, ranging from asymptomatic to severe symptomatic disease (2). Therefore, understanding the clinical and immunological characteristics of unvaccinated ASYMP and SYMP COVID-19 patients holds significance in elucidating the immunopathogenesis of COVID-19 and informing the development of effective immune treatments. Within 2–14 days after SARS-CoV-2 exposure, newly infected individuals may develop fever, fatigue, myalgia, and respiratory symptoms, including cough and shortness of breath (3, 4). While the majority (80–85%) of newly infected individuals are asymptomatic (i.e., patients who remain symptomless despite being SARS-CoV-2-positive), a minority of individuals are symptomatic, especially the elderly and those with compromised health, that develop severe pulmonary inflammatory disease and may need a rapid medical intervention to prevent acute respiratory distress syndrome and death (5–10).
Innate and adaptive immune responses are of great significance for the control of viral infections. NK cells exert the primary control during acute viral infection, but CD4+ and cytotoxic CD8+ T lymphocytes (CTLs) are critical for the long-term surveillance (11). Recently, De Biasi et al. reported an increase in the CD57 expression on CD8+ T cells (12, 13). CD57 is a key marker of in vitro replicative senescence and is associated with prolonged chronic infection (14). Immunosenescence includes a shift towards less functional T cells in the immune system (15). However, CD57 expression is reported to be a marker of mature NK cells. The phenotypes and differentiation status associated with replicative senescent T lymphocytes are not well-defined. Like T-cells, NK cell expression of CD57 could be considered as a marker of terminal differentiation (16). Furthermore, the expression of CD57 aids in identifying the final stages of peripheral NK cell maturation, and the expression of CD57 increases with age and chronic infections (16).
Reports show that repeated T cell activation is associated with terminally differentiated cells and the corresponding upregulation of CD57 (13, 17). It is observed that shortened telomeres are features of senescent cells, and replicative senescence results in a low proliferative capacity of the cells, eventually leading to an inability to eradicate infection (18).
Understanding the spectrum of innate and adaptive immune responses against SARS-CoV2, disease severity, and cellular immunosenescence in SARS-CoV-2 infected symptomatic versus asymptomatic individuals can ultimately inform the identification of new therapeutic targets. To attain this goal, we phenotypically and functionally characterized the senescence markers (CD57), differentiation status (CD45RA/CCR7), exhaustion marker (PD-1), and activation marker (HLA-DR and CD38) from patients with SARS-CoV-2 positive symptomatic and asymptomatic patients, and Healthy controls using multicolor flow cytometry.
In this report, we show 1) a decreased CD56bright NK cell population and higher frequency of mature/terminally differentiated NK cells (CD57+) in SYMP patients; 2) the activation status, senescence, and exhaustion profile were significantly increased in COVID-19 SYMP individuals; 3) SARS-CoV-2 specific senescent T cells with an effector memory phenotype (CD57+CD8+ TEM and CD57+CD8+ TEMRA cells) was detected in COVID-19 SYMP individuals; 4) COVID-19 patients displayed increased cytokine storm detectable in the plasma samples.
Our findings demonstrate that increased T cell exhaustion and senescence markers in unvaccinated ASYMP COVID-19 patients compared to unvaccinated ASYMP COVID-19 patients and Healthy Controls. Furthermore, T cell senescence markers were highly expressed on CD8+ TEM and CD8+ TEMRA cells than on TNaive and TCM cells. These results suggest that the upregulation of exhaustion and senescence pathways during symptomatic COVID-19 may affect both NK and T cell compartments, leading to inefficient clearance of SARS-CoV-2 infection and severe disease.
MATERIALS & METHODS
Human study population:
All clinical investigations in this study were conducted according to the Declaration of Helsinki principles. All subjects were enrolled at the University of California, Irvine, under approved Institutional Review Board-approved protocols (IRB#−2020–5779). Written informed consent was received from all participants before inclusion. Twenty COVID-19 patients (Asymptomatic and Symptomatic) and ten unexposed Healthy individuals, who had never been exposed to SARS-CoV-2 or COVID-19 patients, were enrolled in this study (Table 1). Thirty percent were Caucasian, and 70% were non-Caucasian. Forty-four percent were females, and 60% were males with an age range of 21–67 years old (median 39). None of the symptomatic patients were on anti-viral or anti-inflammatory drug treatments during blood sample collections.
Table 1.
Cohorts of HLA-A2/HLA-DR positive, SARS-CoV-2 seropositive Symptomatic and Asymptomatic individuals enrolled in this study
| Subject-level Characteristic |
All Subjects (n = 20) |
|---|---|
| Gender [no. (%)]: | |
| Female | 8 (40%) |
| Male | 12 (60%) |
| Race [no. (%)]: | |
| Caucasian | 6 (30%) |
| Non-Caucasian | 14 (70%) |
| Age [median (range) yr.]: | 39 (21–67 yr.) |
| SARS-CoV-2 status [no. (%)]: | |
| SARS-CoV-2-seropositive | 20 (100%) |
| HLA [no. (%)] | |
| HLA-A2-positive | 20 (100%) |
| HLA-DR-positive | 20 (100%) |
| COVID-19 Disease Status [no. (%)] | |
| Asymptomatic (ASYMP) | 10 (100%) |
| Symptomatic (SYMP) | 10 (100%) |
Detailed clinical and demographic characteristics of the symptomatic versus asymptomatic COVID-19 patients and the unexposed Healthy individuals concerning age, gender, HLA-A*02:01, and HLA-DR distribution, COVID-19 disease severity, comorbidity, and biochemical parameters are detailed in Table 1.
HLA-A2 typing:
The HLA-A2 status was confirmed by PBMCs staining with 2 μL of anti-HLA-A2 mAb (clone BB7.2) (BD Pharmingen, Franklin Lakes, NJ), at 4°C for 30 minutes. The cells were washed and analyzed by flow cytometry using an LSRII (Becton Dickinson, Franklin Lakes, NJ). The acquired data were analyzed with FlowJo software (BD Biosciences, San Jose, CA).
Tetramer/ peptide staining:
Fresh PBMCs were analyzed for the frequency of CD8+ T cells recognizing the SARS-CoV-2 peptide/tetramer complexes, as we previously described in (19). The cells were incubated with SARS-CoV-2 peptide/tetramer complex for 30–45 min at 37°C. The cell preparations were then washed with FACS buffer and stained with FITC-conjugated anti-human CD8 mAb (BD Pharmingen). Finally, the cells were washed and fixed with 1% paraformaldehyde in PBS and subsequently acquired on a BD LSRII. Data were analyzed using FlowJo version 9.5.6 (Tree Star).
Human peripheral blood mononuclear cells (PBMC) isolation:
SARS-COV-2 positive individuals were recruited at the UC Irvine Medical Center. Between 40 –50 mL of blood was drawn into yellow-top Vacutainer® Tubes (Becton Dickinson). The plasma samples were isolated and stored at −80°C for the detection of various cytokines using Luminex. PBMCs were isolated by gradient centrifugation using a leukocyte separation medium (Life Sciences, Tewksbury, MA). The cells were then washed in PBS, and re-suspended in a complete culture medium consisting of RPMI1640, 10% FBS (Bio-Products, Woodland, CA) supplemented with 1x penicillin/streptomycin/L-glutamine, 1x sodium pyruvate, 1x non-essential amino acids, and 50 μM of 2-mercaptoethanol (Life Technologies, Rockville, MD). For future testing, freshly isolated PBMCs were also cryopreserved in 90% FCS and 10% DMSO in liquid nitrogen.
Human T cells flow cytometry assays:
The following anti-human antibodies were used for the flow cytometry assays: CD3 Percp, CD8 APC-Cy7, CD57 PE-Cy7, PD-1 A647, CD45RA FITC, CCR7 BV786, HLA-DR BUV385, CD38 A700, CD56 APC (BioLegend, San Diego, CA). For surface staining, mAbs against cell markers were added to a total of 1 × 106 cells in 1X PBS containing 1% FBS and 0.1% sodium azide (FACS buffer) for 45 minutes at 4°C. After washing with FACS buffer, cells were permeabilized for 20 minutes on ice using the Cytofix/Cytoperm Kit (BD Biosciences) and then washed twice with Perm/Wash Buffer (BD Biosciences). Intracellular cytokine mAbs were then added to the cells and incubated for 45 minutes on ice in the dark. Finally, cells were washed with Perm/Wash and FACS Buffer and fixed in PBS containing 2% paraformaldehyde (Sigma-Aldrich, St. Louis, MO). For each sample, 100,000 total events were acquired on the BD LSRII. Ab capture beads (BD Biosciences) were used as individual compensation tubes for each fluorophore in the experiment. We used fluorescence minus controls for each fluorophore to define positive and negative populations when initially developing staining protocols. In addition, we further optimized gating by examining known negative cell populations for background expression levels. The gating strategy was similar to that used in our previous work (20). Briefly, we gated on single cells, dump cells, viable cells (Aqua Blue), lymphocytes, CD3+ cells, and human epitope-specific CD8+ T cells using HSV-specific tetramers. Data analysis was performed using FlowJo version 9.9.4 (TreeStar, Ashland, OR). Statistical analyses were done using GraphPad Prism version 5 (La Jolla, CA).
Statistical analyses:
Data for each assay were compared by analysis of variance (ANOVA) and Student’s t-test using GraphPad Prism version 5.03. ANOVA and multiple comparison procedures identified differences between the groups, as we previously described in (21). Data are expressed as the mean ± SD. Results were considered statistically significant at p < 0.05.
RESULTS
1. Composition of NK cell subsets in COVID-19 SYMP patients show a decreased CD56bright NK cell population and higher frequency of mature/terminally differentiated NK cells (CD57+) compared to Healthy individuals:
NK cells are a subset of innate immune lymphocytes composing 5% to 20% of PBMCs in humans and play an important role in the defense against viral infections. These cells are reduced in numbers but less consistently than T cells, particularly in severely sick patients. Therefore, we first investigated the phenotypic status of NK cells in SARS-CoV-2 positive asymptomatic (ASYMP) and symptomatic (SYMP) patients and Healthy Controls. The characteristics of the SYMP, ASYMP and Healthy control study populations used in this study, concerning age, sex, HLA-A*02:01 frequency distribution, SARS-CoV-2 positivity, and status of COVID-19 disease are presented in Table 1. These SARS-CoV-2 positive individuals were divided into two groups: 1) HLA-A*02:01–positive SARS-CoV-2–infected ASYMP individuals, with no detectable levels of any clinical COVID-19 disease; and 2) HLA-A*02:01–positive SARS-CoV-2–infected SYMP individuals with a well-documented COVID-19 clinical disease.
We analyzed the NK cell population following a gating strategy as shown in Fig. 1A. The NK cell population was further categorized into CD56dim and CD56bright cells, and their relative frequency was evaluated in ASYMP, SYMP and Healthy individuals. Analysis of NK cell phenotype showed no difference in mature CD56dim subset in SARS-CoV-2 positive ASYMP and SYMP patients and Healthy controls (Fig. 1B, top panel). However, SYMP patients significantly reduced immature CD56bright NK cells (Fig. 1B, bottom panel; P = 0.02) compared to Healthy controls.
Figure 1: Composition of NK cell subsets in COVID-19 SYMP individuals shows a decreased CD56bright NK cell population and a higher frequency of mature/terminally differentiated NK cells (CD57+) than in Healthy individuals.
A gating strategy for defining of NK cell population is shown using FACS. (A) Using forward scatter (FSC) and side scatters (SSC), the lymphocyte populations were gated. Singlets were gated after gating the lymphocytes population, and NK cells were defined as CD3− CD56+ cells. The NK cell population was further categorized into CD56dim and CD56bright cells, and their relative frequency of senescence (CD57+) was evaluated. (B) Representative FACS data of the frequencies of CD56dim NK cells and CD56bright NK cells detected in PBMCs from COVID-19 ASYMP individuals SYMP individuals and Healthy controls (left panel). Average frequencies of PBMC-derived CD56dim NK cells (top) and CD56bright NK cells (bottom) were detected from ASYMP, SYMP and Healthy individuals (right panel). (C) Representative FACS data of the frequencies of CD57 gated on CD56dim NK cells and the frequencies of CD57 gated on CD56bright NK cells detected in PBMCs from COVID-19 ASYMP individual, SYMP individual, and Healthy control (left panel). Average frequencies of PBMCs-derived CD56dim NK cells (top) and CD56bright NK cells (bottom) were detected from ASYMP, SYMP and Healthy individuals (right panel). The results are representative of two independent experiments on each individual. The indicated P values, calculated using an unpaired t-test, show statistical significance between SYMP and Healthy individuals.
CD57 expression on NK cells defines a mature phenotype, and their expression of CD57 could also be considered a marker of terminal differentiation, although not associated with senescence in this population. It is highly expressed on CD56dim cells, representing mature NK cells, whereas less than 1% of CD56bright NK cells, considered immature, also express CD57 (Fig. 1C). There was a significant increase in the mature (CD56dim/CD57+) and immature subset (CD56bright/CD57+) (Fig. 1C, top and bottom panel; P = 0.03 and P = 0.01 respectively) in SYMP patients with COVID-19 compared with Healthy controls.
Collectively, these data indicate different states of maturation within the CD56dim and CD56bright NK-cell subset and its correlation with COVID-19.
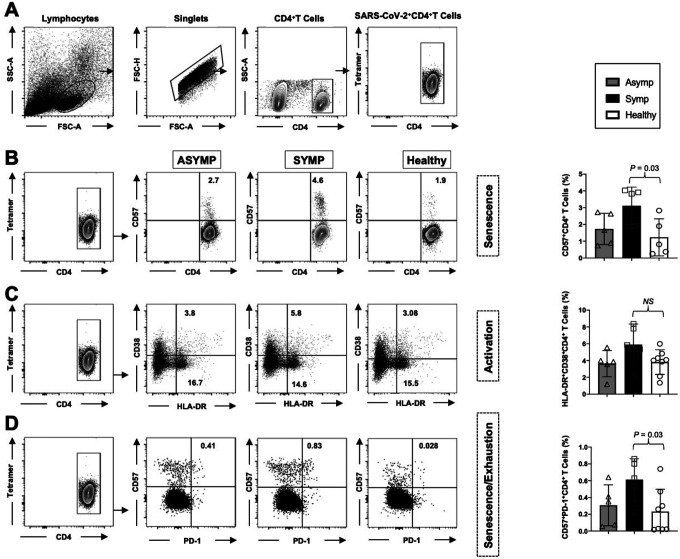
2. The activation status, senescence, and exhaustion profile were significantly increased in COVID-19 SYMP individuals compared to Healthy individuals within CD4+T cells:
CD4+ T cells in COVID-19 are activated as characterized by the expression of cellular markers like HLA-DR and CD38. Therefore, we next evaluated the degree of CD4+ T cell activation in COVID-19 positive ASYMP and SYMP patients and Healthy Controls. Within the CD4+ population, we analyzed markers commonly related to T cell activation (HLA-DR and CD38). The gating strategy used to analyze markers related to activation status, senescence, and exhaustion together within CD4+ T cells is demonstrated in Fig. 2A. The expression of CD57 correlates with senescence in human CD4+ and CD8+ T cells. Therefore, we compared the frequency of CD57+ on total CD4+T cells in COVID-19 ASYMP, SYMP and Healthy individuals. PBMC-derived CD57+CD4+ T cells detected from COVID-19 SYMP individuals showed an increased frequency compared to Healthy individuals (Fig. 2B; P = 0.03).
Figure 2: The activation status, senescence, and exhaustion profile were significantly increased in COVID-19 SYMP individuals compared to Healthy individuals within CD4+ T cells.
Expression of CD38 and HLA-DR was detected to analyze the activation status of CD4+ T cells. Expression of CD57 and PD-1 was detected to analyze the senescence/exhaustion status of CD4+ T cells. (A) The gating strategy was used to analyze markers related to activation status, senescence, and exhaustion together within CD4+ T cells. Activated cells are CD38+HLA-DR+; exhausted/senescent are PD1+CD57+. (B) Representative FACS data of the frequencies of CD57+ CD4+ T cells detected in PBMCs from COVID-19 ASYMP, SYMP and Healthy individuals (left panel). Average frequencies of PBMC-derived CD57+ CD4+ T cells were detected from ASYMP, SYMP and Healthy individuals (right panel). (C) Representative FACS data of the frequencies of HLA-DR+CD38+ CD4+ T cells detected in PBMCs from ASYMP individual, SYMP individual and Healthy control (left panel). Average frequencies of PBMC-derived HLA-DR+CD38+ CD4+ T cells were detected from ASYMP SYMP and Healthy individuals (right panel). (D) Representative FACS data of the frequencies of CD57+ PD-1+CD4+ T cells detected in PBMCs from COVID-19 ASYMP, SYMP and Healthy individuals (left panel). Average frequencies of PBMCs-derived CD57+ PD-1+CD4+ T cells were detected from ASYMP, SYMP and Healthy individuals (right panel). The results are representative of two independent experiments on each individual. The indicated P values, calculated using an unpaired t-test, show statistical significance between SYMP and Healthy individuals.
The levels of CD4+T-cell activation were also evaluated in SARS-CoV-2 infected ASYMP, SYMP patients, and healthy controls. We found an increasing trend reflected by higher proportions of HLA-DR+CD38+CD4+ T cells in SYMP patients compared to healthy controls, however with no statistical significance (Fig. 2C).
Furthermore, we evaluated the expression of the senescence/exhaustion molecules on CD4+ T cells by analyzing markers CD57 and PD-1 (Fig. 2D). The proportion of CD57+PD-1+CD4+ T cells was significantly higher in COVID-19 positive SYMP patients than in healthy controls and ASYMP patients. However, no statistical difference was detected in the proportion of CD57+PD-1+CD4+ T cells in ASYMP and SYMP patients (Fig. 2D; P = 0.03).
Taken together, findings from COVID-19 positive SYMP individuals indicate the involvement of activated CD4+ T cells and T cell exhaustion/senescence in the immunopathogenesis of SARS-CoV2 infection.
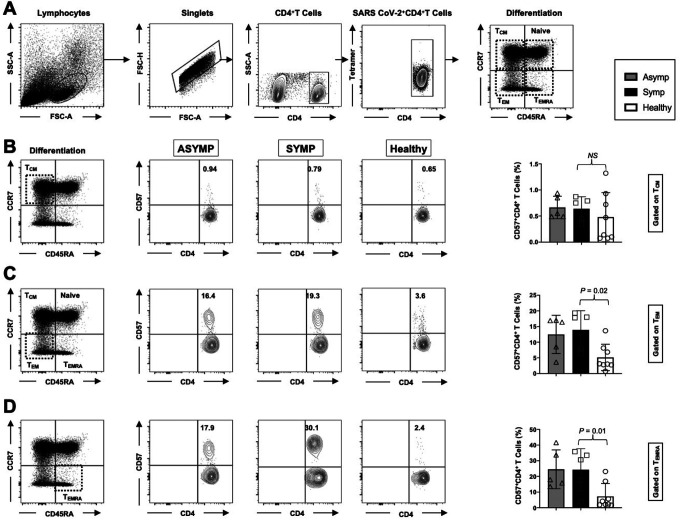
3. Frequent SARS-CoV-2 specific senescent CD4+ T cells with an effector memory phenotype (CD57+CD4+ TEM and CD57+CD4+ TEMRA cells) detected in COVID-19 SYMP individuals compared to Healthy individuals:
SARS-CoV-2 specific memory CD4+ T cells were also categorized into three major phenotypically distinct effector memory (TEM), central memory (TCM) and a subset of effector memory T cells re-expresses CD45RA subpopulations termed as TEMRA. Moreover, we studied the expression levels of CD57 on the memory CD4+ T cell subpopulations at various stages of differentiation: central memory T cells, (CD45RAlowCCR7highCD4+ TCM cells); effector memory T cells, (CD45RAlowCCR7lowCD4+ TEM cells) and TEMRA T cells (CD45RAhighCCR7lowCD4+ TEMRA cells). In the peripheral blood of HLA-A*02:01 positive, SARS-CoV-2 positive ASYMP, SYMP and Healthy individuals, we compared the CD57 expression in CD4+ T cells and divided them into TNAIVE, TCM, TEM, and TEMRA phenotypes (Fig. 3A). Similar percentages of CD57+CD45RAlowCCR7highCD4+ TCM cells were detected in ASYMP, SYMP and Healthy individuals (Fig. 3B; left and right panel). There was an increase in the CD57+CD45RAlowCCR7lowCD4+ TEM cells in SYMP individuals compared to Healthy controls (Fig. 3C; left and right panel (P=0.02)). Significantly higher percentages of CD57+CD45RAhighCCR7lowCD4+ TEMRA cells (Fig. 3D; left and right panel) were detected in SYMP individuals compared to Healthy individuals (P = 0.01). Altogether, the phenotypic properties of SARS-CoV-2 specific memory CD4+ T cells revealed a clear dichotomy in memory CD4+ T cell sub-populations in SYMP versus Healthy individuals. SYMP individuals appeared to develop frequent effector memory CD57+CD4+ TEMRA and CD57+CD4+ TEM cells compared to Healthy and ASYMP individuals. By maintaining high frequencies of the SARS-CoV-2-specific CD57+CD4+ TEMRA cells and CD57+CD4+ TEM cells, the SYMP individuals may not be protected against infection and/or COVID-19 disease.
Figure 3: Frequent SARS-CoV-2 specific senescent CD4+ T cells with an effector memory phenotype (CD57+CD4+ TEM and CD57+CD4+ TEMRA cells) detected in COVID-19 SYMP individuals compared to Healthy individuals.
The phenotype of CD4+ T cells and the gating strategy shown in Fig. 2A were analyzed in TNAIVE, TCM, TEMRA, and TEM phenotypes in PBMCs from COVID-19 ASYMP SYMP and Healthy individuals. Representative FACS data (left panel) and the frequencies of CD57 (right panel) (B) gated on CD45RAlowCCR7highCD4+ TCM cells, (C) gated on CD45RAlowCCR7lowCD4+ TEM cells, and (D) and CD45RAhighCCR7lowCD4+ TEMRA cells detected in COVID-19 ASYMP individual, SYMP individual and Healthy individual. The results are representative of two independent experiments on each individual. The indicated P values, calculated using an unpaired t-test, show statistical significance between SYMP and Healthy individuals.
4. Frequent SARS-COV-2 S1220–1228 and S958–966 epitope-specific CD57+CD8+T cells detected in COVID-19 SYMP individuals compared to ASYMP and Healthy individuals:
As described earlier SARS-CoV-2 positive individuals were segregated into two groups: 1) HLA-A*02:01–positive SARS-CoV-2–infected ASYMP individuals, and 2) HLA-A*02:01–positive SARS-CoV-2–infected SYMP individuals with a well-documented COVID-19 clinical disease. We next compared the frequency of CD57+ on total CD8+T cells in HLA-A*02:01 positive COVID-19 ASYMP, SYMP and Healthy individuals. We have used a gating strategy to analyze markers related to senescence (CD57+) gated within CD8+ T cells from COVID-19 ASYMP, SYMP and Healthy individuals. Average frequencies of PBMC-derived CD57+CD8+ T cells detected from COVID-19 SYMP individuals showed an increased frequency compared to Healthy individuals (Fig. 4A, P = 0.03). We then compared the frequency of SARS-CoV-2 peptide/tetramer complex specific CD8+T cells. The representative dot plots in Fig. 4B indicate an increased frequency of CD57+CD8+ T cells, specific to S1220–1228 epitope in COVID-19 SYMP individuals compared to ASYMP Healthy individuals (P = 0.01). Similarly, Fig. 4C depicts the high frequencies of CD57+CD8+ T cells detected in COVID-19 SYMP individuals against another peptide/tetramer complex S958–966 epitope (P = 0.02). Altogether, these results indicate that SYMP individuals develop frequent SARS-CoV-2-specific CD57+CD8+ T cells compared to ASYMP and Healthy individuals.
Figure 4: Frequent SARS-COV-2 S1220–1228 and S958–966 epitope-specific CD57+CD8+ T cells detected in COVID-19 SYMP individuals compared to ASYMP and Healthy individuals.
The frequency of CD57+ on total CD8+ T cells and SARS-CoV-2 peptide/tetramer complex specific CD8+T cells were analyzed in HLA-A*02:01 positive COVID-19 ASYMP, SYMP and Healthy individuals. (A) Gating strategy used to analyze markers related to senescence gated within CD8+ T cells from COVID-19 ASYMP, SYMP and Healthy individuals (left panel), and average frequencies of PBMC-derived CD8+ T cells detected from COVID-19 ASYMP, SYMP and Healthy individuals (right panel). (B) Representative FACS data of the frequencies of CD57+CD8+ T cells, specific to S1220–1228 epitope, detected in PBMCs from HLA-A*02:01 positive COVID-19 ASYMP, SYMP and Healthy individuals (left panel). Average frequencies of PBMC-derived CD8+ T cells, specific to S1220–1228 epitope, were detected from COVID-19 ASYMP, SYMP and Healthy individuals (right panel). (C) Representative FACS data of the frequencies of CD57+CD8+ T cells, specific to S958–966 epitope, detected in PBMCs from HLA-A*02:01 positive COVID-19 ASYMP, SYMP and Healthy individuals (left panel). Average frequencies of PBMC-derived CD57+CD8+ T cells, specific to S958–966 epitope, were detected from ASYMP, SYMP and Healthy individuals (right panel). The results are representative of two independent experiments on each individual. The indicated P values, calculated using an unpaired t-test, show statistical significance between SYMP and Healthy individuals.
5. Frequent SARS-CoV-2 S1220–1228 epitope-specific senescent CD8+ T cells with an effector memory phenotype (CD57+CD8+ TEM and CD57+CD8+ TEMRA cells) detected in COVID-19 SYMP individuals compared to Healthy individuals:
Similar to the CD4+T cell memory response, SARS-CoV-2 specific memory CD8+ T cells are also categorized into three major phenotypically distinct effector memory (TEM), central memory (TCM) and a subset of effector memory T cells re-expresses CD45RA subpopulations termed as TEMRA. Furthermore, we examined the expression levels of CD57 on the memory CD8+ T cell subpopulations at various stages of differentiation: central memory T cells, (CD45RAlowCCR7highCD8+ TCM cells); effector memory T cells, (CD45RAlowCCR7lowCD8+ TEM cells) and TEMRA T cells (CD45RAhighCCR7lowCD8+ TEMRA cells). In the peripheral blood of HLA-A*02:01 positive, SARS-CoV-2 positive ASYMP, SYMP and Healthy individuals, we compared the CD57 expression in CD8+ T cells specific to S1220–1228 epitope and divided them into TNAIVE, TCM, TEM, and TEMRA phenotypes (Fig. 5A). Similar percentages of CD57+CD45RAlowCCR7highCD8+ TCM cells were detected in ASYMP, SYMP and Healthy individuals (Fig. 5B; left and right panel). There was a significant increase in the CD57+CD45RAlowCCR7lowCD8+ TEM cells in SYMP individuals compared to Healthy controls (Fig. 5C; left and right panel). Significantly higher percentages of CD57+CD45RAhighCCR7lowCD8+ TEMRA cells (Fig. 5D; left and right panel) were detected in SYMP individuals compared to Healthy individuals (P = 0.004). Altogether, the phenotypic properties of SARS-CoV-2 specific S1220–1228 epitope epitope-specific memory CD8+ T cells revealed a clear dichotomy in memory CD8+ T cell sub-populations in SYMP versus Healthy individuals. SYMP individuals appeared to develop frequent SARS-CoV-2 specific effector memory CD57+CD8+ TEMRA and CD57+CD8+ TEM cells compared to Healthy and ASYMP individuals. By maintaining high frequencies of the SARS-CoV-2-specific CD57+CD8+ TEMRA cells and CD57+CD8+ TEM cells, the SYMP individuals may not be protected against infection and/or COVID-19 disease.
Figure 5: Frequent SARS-CoV-2 S1220–1228 epitope-specific senescent CD8+ T cells with an effector memory phenotype (CD57+CD8+ TEM and CD57+CD8+ TEMRA cells) detected in COVID-19 SYMP individuals compared to Healthy individuals.
The phenotype of CD8+ T cells specific to S1220–228 peptide/tetramer shown in Fig. 5A was analyzed in terms of TNAIVE, TCM, TEMRA, and TEM phenotypes in PBMCs from HLA-A*02:01 positive COVID-19 ASYMP, SYMP and Healthy individuals. Representative FACS data (left panel) and the frequencies of CD57 (right panel) gated on CD45RAlowCCR7highCD8+ TCM cells (B), gated on CD45RAlowCCR7lowCD8+ TEM cells (C), and CD45RAhighCCR7lowCD8+ TEMRA cells (D) detected in COVID-19 ASYMP, SYMP and Healthy individuals. The results are representative of two independent experiments on each individual. The indicated P values, calculated using an unpaired t-test, show statistical significance between SYMP and Healthy individuals.
6. The activation status, senescence, and exhaustion profile were significantly increased in COVID-19 SYMP individuals compared to Healthy individuals within CD8+ T cells:
Most viral infections induce activation of CD8+T cells that can be detected by increases in the co-expression of CD38 and Human leukocyte antigen-DR isotype (HLA-DR). HLA-DR is constitutively expressed by antigen-presenting cells (APCs) and is involved in the presentation of antigens to T-cells. Most T-cells do not express it, but notably, a subset of activated T-cells becomes HLA-DR+ during an immune response. In contrast, CD38 is constitutively expressed by naive T-cells, down-regulated in resting memory cells, and then elevated again in activated cells. Thus, we evaluated the degree of CD8+ T-cell activation in COVID-19 positive ASYMP and SYMP patients and Healthy Controls. Within the CD8+ population, we analyzed markers commonly related to T cell activation (HLA-DR and CD38). Our gating strategy was used to analyze markers related to activation status, senescence, and exhaustion together within SARS-CoV-2 specific CD8+ T cells (Fig. 6A). The levels of T-cell activation were significantly higher (hyperactivated) in SARS-CoV-2 infected SYMP patients than in healthy controls, as reflected by higher proportions of HLA-DR+CD38+CD8+ T cells (Fig. 6B, P = 0.02).
Figure 6: The activation status, senescence, and exhaustion profile were significantly increased in COVID-19 SYMP individuals compared to Healthy individuals within CD8+T cells.
Expression of CD38 and HLA-DR was detected to analyze the activation status of CD8+ T cells. Expression of CD57 and PD-1 was detected to analyze the senescence/exhaustion status of CD8+ T cells. (A) Gating strategy used to analyze markers related to activation status, senescence, and exhaustion together within SARS-CoV-2 specific CD8+ T cells. Activated cells are CD38+HLA-DR+; exhausted/senescent are PD1+CD57+. FACS was used to determine the expression level of various markers on tetramer gated CD8+ T cells specific to the S1220–1228 epitope. (B) Representative FACS data of the frequencies of HLA-DR+CD38+ CD8+ T cells, specific to S1220–1228 epitope detected in PBMCs from HLA-A*02:01 positive COVID-19 ASYMP individuals, SYMP individuals, and Healthy controls (left panel). Average frequencies of PBMCs-derived HLA-DR+CD38+ CD8+ T cells, specific to S1220–1228 epitope, were detected from COVID-19 ASYMP, SYMP and Healthy individuals (right panel). (C) Representative FACS data of the frequencies of CD57+ PD-1+CD8+ T cells, specific to S1220–1228 epitope, detected in PBMCs from HLA-A*02:01 positive COVID-19 ASYMP individuals, SYMP individuals, and Healthy individuals (left panel). Average frequencies of PBMCs-derived CD57+ PD-1+CD8+ T cells, specific to the S1220–1228 epitope, were detected from ASYMP, SYMP and Healthy individuals (right panel). The results are representative of two independent experiments on each individual. The indicated P values, calculated using an unpaired t-test, show statistical significance between SYMP and Healthy individuals.
We evaluated the senescence/exhaustion molecules expression on circulating T cells by analyzing markers CD57 and PD-1 (Fig. 6C). We found that the proportion of CD57+PD-1+CD8+ T cells was significantly higher in COVID-19 positive SYMP patients than in Healthy controls and ASYMP patients. Still, there was no statistical difference in the proportion of CD57+PD-1+CD8+ T cells in ASYMP and SYMP patients (Fig. 6C, P = 0.009).
Taken together, findings from COVID-19 positive SYMP individuals indicate the involvement of hyperactivated CD8+T cells and T cell exhaustion/senescence in the immunopathogenesis of SARS-CoV2 infection.
7. Elevated Plasma levels of selective cytokines in COVID-19 ASYMP and SYMP individuals compared to Healthy controls:
Many studies have previously reported that hyper-inflammatory response induced by SARS-CoV-2 is a major cause of disease severity and death. Therefore, we implemented a multiplex cytokine assay (Luminex) to measure inflammatory cytokines known to contribute to pathogenic inflammation (IL)-6, IL-8, tumor necrosis factor (TNF)-α, interferon (IFN)-γ and IL-17. in the plasma samples of COVID-19 in ASYMP, SYMP and Healthy individuals. The cytokines assessed in this study had different detection ranges, with IL-6 and IL-8 having the most dynamic profile followed by TNF-α, IL-17, and IFN-γ. We found that TNF-α and IFN-γ (P = 0.001) were significantly elevated in COVID-19 symptomatic patients compared to Healthy controls (Fig. 7A and 7B). Similarly, we found that IL-6 and IL-8 (P = 0.01) were significantly elevated in COVID-19 symptomatic patients compared to Healthy controls (Fig. 7C and 7D). Interleukin (IL)-17 is one of the many cytokines released during SARS-CoV-2 infection. IL-17 plays a crucial role in neutrophil recruitment and activation. Neutrophils subsequently can migrate to the lung and are heavily involved in the pathogenesis of COVID-19. We found that SARS-CoV-2 positive ASYMP and SYMP individuals had significantly higher levels of IL-17 than Healthy controls (P = 0.04) (Fig. 7E).
Figure 7: Elevated Plasma levels of selective cytokines in COVID-19 ASYMP and SYMP individuals compared to Healthy controls.
Cytokine expression levels of two replicates per sample were measured in plasma samples of COVID-19 ASYMP, SYMP and Healthy individuals using Luminex. (A) Bar graphs with individual values showing the average amount of TNF-α (pg/ml) produced from ASYMP, SYMP and Healthy individuals. (B) Bar graphs with individual values showing the average amount of IFN-γ (pg/ml) produced from ASYMP, SYMP and Healthy individuals. (C) Bar graphs with individual values show the average IL-6 (pg/ml) produced from ASYMP, SYMP and Healthy individuals. (D) Bar graphs with individual values show the average IL-8 (pg/ml) produced from ASYMP, SYMP and Healthy individuals. (E) Bar graphs with individual values show the average IL-17 (pg/ml) produced from ASYMP, SYMP and Healthy individuals.
The vast majority of SYMP patients demonstrated elevated cytokines or cytokine storm compared to Healthy controls. In contrast, the cytokine levels were not significantly different in COVID-19 ASYMP and SYMP individuals.
Overall, our findings report that NK, CD4+ T cells, and CD8+ T cells’ phenotypic and functional characteristics in severe COVID-19 infection were compatible with activation of dysfunction/exhaustion pathways.
DISCUSSION
COVID-19 is characterized by enhanced lymphopenia in the peripheral blood and altered T cells phenotypes shown by a spectrum of activation and exhaustion. However, antigen-specific T cell responses are emerging as a critical mechanism for both virus clearance and the most plausible pathway to long-term immunological memory that would protect against re-infection. As a result, T cell responses are of great importance in the development of vaccines (22). Moreover, post-infection changes in the composition and function of T cell subsets have significant ramifications on the patients’ long-term immunological functions (23). The impairment of effector T cell responses has been associated with the overexpression of inhibitory and senescent markers on T cells. Therefore, the main objective of this research study was to detect T-cell immune signatures in peripheral blood, including those of innate cells, and to determine how important indicators of activation and exhaustion are related to the development of symptomatic COVID-19. Factors influencing the formation and nature of protective immunity and severity of COVID-19 are still unknown. Nevertheless, data defining disease phenotypes have the prospect of informed development of new therapeutic approaches for treating individuals infected with SARS-CoV-2 and developing novel vaccines. CD57 is a marker on some cell subsets, including T cells (15, 24, 25). A costimulatory molecule like CD28 (that provides signaling for T cell activation) is expressed by naïve T cells after antigen recognition that may bind to B7 proteins to provide co-stimulatory signals (26–28). However, repeated T-cell stimulation and activation leads to gradual loss of CD28, a distinct characteristic of memory or terminally differentiated cells, and subsequent upregulation of CD57 (29–31). These senescent cells are characterized by loss of CD27 and display low proliferative capacity of the cells (32), eventually leading to an inability to eradicate infection.
The CD57 antigen is commonly used to identify populations of late-differentiated ‘senescent’ cells with defined cell phenotypes and effector functions (33, 34). In this report, we examined the patterns of expression of CD57 on NK cells and SARS-CoV-2-specific T-cells and determined increased expression of these exhaustive and senescent markers in symptomatic individuals compared to those with asymptomatic infections and Healthy controls. While CD57 is now well-recognized as a marker for terminally differentiated T-cells, it was originally thought to identify cells with natural killer activity. The expression of CD57 varies among NK cell subsets. NK cells are innate effector lymphocytes that respond to acute viral infections but might also contribute to immunopathology. NK cells are typically divided into CD56bright NK cells and CD56dim NK cells, which rapidly respond during diverse acute viral infections in humans, including against dengue virus, hantavirus, tick-borne encephalitis virus, and yellow fever virus, among others (35–37). Although a similar analysis of NK cells has not been performed in acute SARS-CoV-2 infection-causing COVID-19, early reports from the pandemic (in line with our findings) have indicated low circulating NK cell numbers in patients with moderate and severe disease (38–40). The SARS-CoV-2 infection has also been linked to reduced NK cell counts during the acute phase of infection. We determined a terminally differentiated phenotype with up-regulated levels of CD57 molecules in NK cells from SYMP COVID-19 patients.
CD57 is expressed by CD16posCD56dim cytotoxic NK cells and CD16posCD56neg inflammatory NK cells, whereas CD16negCD56bright regulatory NK cells do not express this marker even during chronic infections (41–43). The acquisition of CD57 thus follows the natural differentiation of NK cells (from regulatory to cytotoxic to inflammatory NK cells). Thus, like T cells, NK cell expression of CD57 could be considered a marker of terminal differentiation, albeit not associated with senescence in this population.
The majority of prior studies into the biology of CD57 focused on the antigen’s significance in distinct T cell subsets. In the late phases of differentiation, CD57 has been found both on CD4 and CD8 T cells (15). CD57 identifies terminally differentiated cells with decreased proliferative responses in CD8 T lymphocytes. T cell senescent markers were more associated with CD8+ T cells than CD4+T cells, consistent with our results that accumulate at lower frequencies for CD4+T cells in the human periphery (44). Our findings herein indicate an increased expression of CD57+T cell subsets in symptomatic patients. It was previously shown that PD-1+CD57+CD8+T cells had increased sensitivity to apoptosis mediated by PD-1 (45).
The increased expression of CD57 and PD-1 double-positive markers on CD8+ T cells in COVID-19 suggests that these cells are at a higher risk of apoptosis. The fraction of T cells that express CD57 increased in the symptomatic individuals, suggesting that the observed phenotypic changes may lower the T cell repertoire’s responsiveness to SARS-CoV-2 antigens, resulting in an impaired ability to eliminate the infection. CD57+ memory T cells accumulate in peripheral blood throughout life, especially after infection with CMV (46). These associations with age and persistent antigenic drive were mechanistically linked in an in vitro study, which reported that replicative senescent memory CD8+ T cells expressed CD57 (15). However, an earlier study had reached a different conclusion (47), and later experiments showed that CD57+ memory CD8+ T cells could proliferate in vitro in the presence of certain growth factors, potentially mimicking the in vivo microenvironment (48). Recent studies suggest that TEMRA cells are fairly resistant to apoptosis and remain in the CD8+ lineage for an estimated half-life of about 25 years, assuming simple exponential decline without phenotypic change (49, 50). CD8+ TEMRA cells that expressed CD57 were recently reported to be more sensitive to cell death than CD8+ TEMRA cells that lacked CD57 in response to severe stimulation with supraphysiological doses of phytohemagglutinin and interleukin-2 (51). Compared to asymptomatic individuals and Healthy controls, symptomatic patients had increased CD57 expression on CD8+ TEMRA+ memory cells. Compared to asymptomatic infections, the phenotypic abnormality of T cells during COVID-19 infections is more apparent in symptomatic individuals and is associated with higher expression levels of exhaustive and senescent markers.
The findings of this research contribute to the current knowledge of the innate and adaptive immune landscape in asymptomatic and symptomatic COVID-19 patients. However, we recognize limitations that could be addressed with bigger sample numbers and matched control groups. Furthermore, the phenotype and activity of immune cells from the lungs performing a direct role in establishing symptomatic infections, are unknown. As a result, the immunophenotypic traits in the lungs may not completely mirror the hierarchy of immunodominant circulating immune cells in the blood.
In conclusion, this study presents an in-depth analysis of NK and T cell phenotypic and functional characteristics that are associated with COVID-19 severe disease. The finding will inform future immunotherapies to alleviate the symptoms of severe COVID-19 severe disease.
IMPORTANCE.
Unvaccinated COVID-19 patients display a large spectrum of symptoms, ranging from asymptomatic to severe symptoms, the latter even causing death. Distinct Natural killer (NK) and CD4+ and CD8+ T cells immune responses are generated in COVID-19 patients. In this study, we detected significant increases in the expression levels of both exhaustion and senescence markers on NK and T cells from unvaccinated symptomatic (SYMP) compared to unvaccinated asymptomatic (ASYMP) COVID-19 patients. Moreover, we detected significant increases in the levels of pro-inflammatory TNF-α, IFN-γ, IL-6, IL-8, and IL-17 cytokines from SYMP COVID-19 patients, compared to ASYMP COVID-19 patients. The findings suggest exhaustion and senescence in both NK and T cell compartment is associated with severe disease in critically ill COVID-19 patients.
TWEET.
Significant exhaustion and senescence in both NK and T cells were detected in unvaccinated symptomatic COVID-19 patients, suggesting a weakness in both innate and adaptive immune systems leads to severe disease in critically ill COVID-19 patients.
ACKNOWLEDGEMENTS
This work is supported by the Fast-Grant PR12501 from Emergent Ventures, a grant from Herbert Family Trust, by Public Health Service Research grants AI158060, AI150091, and AI143348, AI147499, AI143326, AI138764, AI124911 and AI110902 from the National Institutes of Allergy and Infectious Diseases (NIAID) to LBM.
The authors would like to thank Dr. Dale Long from the NIH Tetramer Facility (Emory University, Atlanta, GA) for providing the Tetramers used in this study. We thank UC Irvine Center for Clinical Research (CCR) and Institute for Clinical & Translational Science (ICTS) for providing human blood samples used in this study. A special thanks to Dr. Delia F. Tifrea for her continuous efforts and dedication in providing COVID-19 samples that are crucial for this clinical research. We also thank those who contributed directly or indirectly to this COVID-19 vaccine project: Dr. Steven A. Goldstein, Dr. Michael J. Stamos, Dr. Suzanne B. Sandmeyer, Jim Mazzo, Dr. Daniela Bota, Dr. Beverly L. Alger, Dr. Dan Forthal, Dr. Tahseen Muzaffar, Dr. Ilhem Messaoudi, Anju Subba, Janice Briggs, Marge Brannon, Beverley Alberola, Jessica Sheldon, Rosie Magallon, and Andria Pontello.
This work is supported by Public Health Service Research R01 Grants EY026103, EY019896, and EY024618 from the National Eye Institute (NEI) and R21 Grant AI110902 from the National Institutes of Allergy and Infectious Diseases (NIAID) (to L.BM.), and in part by The Discovery Center for Eye Research (DCER) and the Research to Prevent Blindness (RPB) grant
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists
REFERENCE
- 1.Dong E, Du H, Gardner L. 2020. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 20:533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM. 2020. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 3.Benhadou F, Del Marmol V. 2020. Improvement of SARS-CoV-2 symptoms following Guselkumab injection in a psoriatic patient. J Eur Acad Dermatol Venereol 34:e363–e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liguori C, Pierantozzi M, Spanetta M, Sarmati L, Cesta N, Iannetta M, Ora J, Mina GG, Puxeddu E, Balbi O, Pezzuto G, Magrini A, Rogliani P, Andreoni M, Mercuri NB. 2020. Subjective neurological symptoms frequently occur in patients with SARS-CoV2 infection. Brain Behav Immun 88:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Tawfiq JA, Al-Homoud AH, Memish ZA. 2020. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med Infect Dis 34:101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breslin N, Baptiste C, Miller R, Fuchs K, Goffman D, Gyamfi-Bannerman C, D’Alton M. 2020. Coronavirus disease 2019 in pregnancy: early lessons. Am J Obstet Gynecol MFM 2:100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moriyama M, Hugentobler WJ, Iwasaki A. 2020. Seasonality of Respiratory Viral Infections. Annu Rev Virol 7:83–101. [DOI] [PubMed] [Google Scholar]
- 8.Neher RA, Dyrdak R, Druelle V, Hodcroft EB, Albert J. 2020. Potential impact of seasonal forcing on a SARS-CoV-2 pandemic. Swiss Med Wkly 150:w20224. [DOI] [PubMed] [Google Scholar]
- 9.Nishiura H, Linton NM, Akhmetzhanov AR. 2020. Serial interval of novel coronavirus (COVID-19) infections. Int J Infect Dis 93:284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang R, Li Y, Zhang AL, Wang Y, Molina MJ. 2020. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A 117:14857–14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song H, Josleyn N, Janosko K, Skinner J, Reeves RK, Cohen M, Jett C, Johnson R, Blaney JE, Bollinger L, Jennings G, Jahrling PB. 2013. Monkeypox virus infection of rhesus macaques induces massive expansion of natural killer cells but suppresses natural killer cell functions. PLoS One 8:e77804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zingaropoli MA, Nijhawan P, Carraro A, Pasculli P, Zuccala P, Perri V, Marocco R, Kertusha B, Siccardi G, Del Borgo C, Curtolo A, Ajassa C, Iannetta M, Ciardi MR, Mastroianni CM, Lichtner M. 2021. Increased sCD163 and sCD14 Plasmatic Levels and Depletion of Peripheral Blood Pro-Inflammatory Monocytes, Myeloid and Plasmacytoid Dendritic Cells in Patients With Severe COVID-19 Pneumonia. Front Immunol 12:627548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Biasi S, Meschiari M, Gibellini L, Bellinazzi C, Borella R, Fidanza L, Gozzi L, Iannone A, Lo Tartaro D, Mattioli M, Paolini A, Menozzi M, Milic J, Franceschi G, Fantini R, Tonelli R, Sita M, Sarti M, Trenti T, Brugioni L, Cicchetti L, Facchinetti F, Pietrangelo A, Clini E, Girardis M, Guaraldi G, Mussini C, Cossarizza A. 2020. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun 11:3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinti M, Appay V, Campisi J, Frasca D, Fulop T, Sauce D, Larbi A, Weinberger B, Cossarizza A. 2016. Aging of the immune system: Focus on inflammation and vaccination. Eur J Immunol 46:2286–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101:2711–20. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen CM, White MJ, Goodier MR, Riley EM. 2013. Functional Significance of CD57 Expression on Human NK Cells and Relevance to Disease. Front Immunol 4:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pangrazzi L, Reidla J, Carmona Arana JA, Naismith E, Miggitsch C, Meryk A, Keller M, Krause AAN, Melzer FL, Trieb K, Schirmer M, Grubeck-Loebenstein B, Weinberger B. 2020. CD28 and CD57 define four populations with distinct phenotypic properties within human CD8(+) T cells. Eur J Immunol 50:363–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Deursen JM. 2014. The role of senescent cells in ageing. Nature 509:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prakash S, Srivastava R, Coulon PG, Dhanushkodi NR, Chentoufi AA, Tifrea DF, Edwards RA, Figueroa CJ, Schubl SD, Hsieh L, Buchmeier MJ, Bouziane M, Nesburn AB, Kuppermann BD, BenMohamed L. 2021. Genome-Wide B Cell, CD4(+), and CD8(+) T Cell Epitopes That Are Highly Conserved between Human and Animal Coronaviruses, Identified from SARS-CoV-2 as Targets for Preemptive Pan-Coronavirus Vaccines. J Immunol 206:2566–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chentoufi AA, Zhang X, Lamberth K, Dasgupta G, Bettahi I, Nguyen A, Wu M, Zhu X, Mohebbi A, Buus S, Wechsler SL, Nesburn AB, BenMohamed L. 2008. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J Immunol 180:426–37. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Chentoufi AA, Dasgupta G, Nesburn AB, Wu M, Zhu X, Carpenter D, Wechsler SL, You S, BenMohamed L. 2009. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol 2:129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah VK, Firmal P, Alam A, Ganguly D, Chattopadhyay S. 2020. Overview of Immune Response During SARS-CoV-2 Infection: Lessons From the Past. Front Immunol 11:1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikolich-Zugich J. 2008. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol 8:512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strauss-Albee DM, Horowitz A, Parham P, Blish CA. 2014. Coordinated regulation of NK receptor expression in the maturing human immune system. J Immunol 193:4871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, Birch KE, Cook JE, Jackson SE, Salmon M, Rustin MH, Akbar AN. 2005. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol 175:8218–25. [DOI] [PubMed] [Google Scholar]
- 26.Effros RB. 1997. Loss of CD28 expression on T lymphocytes: a marker of replicative senescence. Dev Comp Immunol 21:471–8. [DOI] [PubMed] [Google Scholar]
- 27.Effros RB, Pawelec G. 1997. Replicative senescence of T cells: does the Hayflick Limit lead to immune exhaustion? Immunol Today 18:450–4. [DOI] [PubMed] [Google Scholar]
- 28.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. 1992. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature 356:607–9. [DOI] [PubMed] [Google Scholar]
- 29.Li G, Larregina AT, Domsic RT, Stolz DB, Medsger TA Jr., Lafyatis R, Fuschiotti P. 2017. Skin-Resident Effector Memory CD8(+)CD28(−) T Cells Exhibit a Profibrotic Phenotype in Patients with Systemic Sclerosis. J Invest Dermatol 137:1042–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu HT, Park S, Shin EC, Lee WW. 2016. T cell senescence and cardiovascular diseases. Clin Exp Med 16:257–63. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, Xue X, Hunt P, Karim R, Kern DM, Hodis HN, Deeks SG. 2011. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis 203:452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olsson J, Wikby A, Johansson B, Lofgren S, Nilsson BO, Ferguson FG. 2000. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev 121:187–201. [DOI] [PubMed] [Google Scholar]
- 33.Neidleman J, Luo X, Frouard J, Xie G, Gill G, Stein ES, McGregor M, Ma T, George AF, Kosters A, Greene WC, Vasquez J, Ghosn E, Lee S, Roan NR. 2020. SARS-CoV-2-Specific T Cells Exhibit Phenotypic Features of Helper Function, Lack of Terminal Differentiation, and High Proliferation Potential. Cell Rep Med 1:100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, He T, Xue L, Guo H. 2021. Senescent T cells: a potential biomarker and target for cancer therapy. EBioMedicine 68:103409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bjorkstrom NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M, Michaelsson J, Malmberg KJ, Klingstrom J, Ahlm C, Ljunggren HG. 2011. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med 208:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmer CL, Cornillet M, Sola-Riera C, Cheung KW, Ivarsson MA, Lim MQ, Marquardt N, Leo YS, Lye DC, Klingstrom J, MacAry PA, Ljunggren HG, Rivino L, Bjorkstrom NK. 2019. NK cells are activated and primed for skin-homing during acute dengue virus infection in humans. Nat Commun 10:3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blom K, Braun M, Pakalniene J, Lunemann S, Enqvist M, Dailidyte L, Schaffer M, Lindquist L, Mickiene A, Michaelsson J, Ljunggren HG, Gredmark-Russ S. 2016. NK Cell Responses to Human Tick-Borne Encephalitis Virus Infection. J Immunol 197:2762–71. [DOI] [PubMed] [Google Scholar]
- 38.Jiang Y, Wei X, Guan J, Qin S, Wang Z, Lu H, Qian J, Wu L, Chen Y, Chen Y, Lin X. 2020. COVID-19 pneumonia: CD8(+) T and NK cells are decreased in number but compensatory increased in cytotoxic potential. Clin Immunol 218:108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazzoni A, Salvati L, Maggi L, Capone M, Vanni A, Spinicci M, Mencarini J, Caporale R, Peruzzi B, Antonelli A, Trotta M, Zammarchi L, Ciani L, Gori L, Lazzeri C, Matucci A, Vultaggio A, Rossi O, Almerigogna F, Parronchi P, Fontanari P, Lavorini F, Peris A, Rossolini GM, Bartoloni A, Romagnani S, Liotta F, Annunziato F, Cosmi L. 2020. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J Clin Invest 130:4694–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang F, Nie J, Wang H, Zhao Q, Xiong Y, Deng L, Song S, Ma Z, Mo P, Zhang Y. 2020. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J Infect Dis 221:1762–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ, Nixon DF, Lanier LL. 2010. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood 116:3865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bjorkstrom NK, Ljunggren HG, Sandberg JK. 2010. CD56 negative NK cells: origin, function, and role in chronic viral disease. Trends Immunol 31:401–6. [DOI] [PubMed] [Google Scholar]
- 43.Bjorkstrom NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, Bjorklund AT, Flodstrom-Tullberg M, Michaelsson J, Rottenberg ME, Guzman CA, Ljunggren HG, Malmberg KJ. 2010. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood 116:3853–64. [DOI] [PubMed] [Google Scholar]
- 44.Valenzuela HF, Effros RB. 2002. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol 105:117–25. [DOI] [PubMed] [Google Scholar]
- 45.Petrovas C, Chaon B, Ambrozak DR, Price DA, Melenhorst JJ, Hill BJ, Geldmacher C, Casazza JP, Chattopadhyay PK, Roederer M, Douek DC, Mueller YM, Jacobson JM, Kulkarni V, Felber BK, Pavlakis GN, Katsikis PD, Koup RA. 2009. Differential association of programmed death-1 and CD57 with ex vivo survival of CD8+ T cells in HIV infection. J Immunol 183:1120–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gratama JW, Fridell E, Lenkei R, Oosterveer MA, Ljungstrom I, Tanke HJ, Linde A. 1989. Correlation between cytomegalovirus and toxoplasma gondii serology and lymphocyte phenotypes in peripheral blood and cord blood. Scand J Infect Dis 21:611–6. [DOI] [PubMed] [Google Scholar]
- 47.Izquierdo M, Balboa MA, Fernandez-Ranada JM, Figuera A, Torres A, Iriondo A, Lopez-Botet M. 1990. Relation between the increase of circulating CD3+ CD57+ lymphocytes and T cell dysfunction in recipients of bone marrow transplantation. Clin Exp Immunol 82:145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chong LK, Aicheler RJ, Llewellyn-Lacey S, Tomasec P, Brennan P, Wang EC. 2008. Proliferation and interleukin 5 production by CD8hi CD57+ T cells. Eur J Immunol 38:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta S, Su H, Bi R, Agrawal S, Gollapudi S. 2005. Life and death of lymphocytes: a role in immunesenescence. Immun Ageing 2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ladell K, Hellerstein MK, Cesar D, Busch R, Boban D, McCune JM. 2008. Central memory CD8+ T cells appear to have a shorter lifespan and reduced abundance as a function of HIV disease progression. J Immunol 180:7907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verma K, Ogonek J, Varanasi PR, Luther S, Bunting I, Thomay K, Behrens YL, Mischak-Weissinger E, Hambach L. 2017. Human CD8+ CD57− TEMRA cells: Too young to be called “old”. PLoS One 12:e0177405. [DOI] [PMC free article] [PubMed] [Google Scholar]