Abstract
Understanding SARS-CoV-2 transmission within and among communities is critical for tailoring public health policies to local context. However, analysis of community transmission is challenging due to a lack of high-resolution surveillance and testing data. Here, using contact tracing records for 644,029 cases and their contacts in New York City during the second pandemic wave, we provide a detailed characterization of the operational performance of contact tracing and reconstruct exposure and transmission networks at individual and ZIP code scales. We find considerable heterogeneity in reported close contacts and secondary infections and evidence of extensive transmission across ZIP code areas. Our analysis reveals the spatial pattern of SARS-CoV-2 spread and communities that are tightly interconnected by exposure and transmission. We find that higher vaccination coverage and reduced numbers of visitors to points-of-interest are associated with fewer within- and cross-ZIP code transmission events, highlighting potential measures for curtailing SARS-CoV-2 spread in urban settings.
Introduction
Within metropolitan areas, infection risk and disease burden due to SARS-CoV-2, the causative agent of COVID-19, are characterized by spatial heterogeneity at neighborhood scales1–3. Communities with substantial local infections can sustain the spread of SARS-CoV-2, seed infections in interconnected neighborhoods, and spark resurgences of cases following the relaxation of non-pharmaceutical interventions (NPIs), such as masking and social distancing4. In densely populated urban settings, public health tactics may need to be uniquely tailored to specific geographic areas and/or communities that most support the persistence and spatial dispersion of SARS-CoV-2 infections. Development of such tailored tactics requires improved understanding of both transmission patterns at fine geographical scales and the factors shaping the intensity of community outbreaks. Examples of previously utilized targeted intervention include limiting indoor dining and gathering, increasing testing availability, encouraging home quarantine for exposed contacts, requiring face masks indoors, and closing nonessential businesses in high-risk communities. While the transmission patterns of SARS-CoV-2 at global, national, and regional levels have been reported5–12, research on community-level transmission is often challenging due to limited availability of high-resolution surveillance and testing data, the lack of routine case interviews, and the difficulty identifying transmission events. In addition, the effect of public health interventions on community transmission of SARS-CoV-2 in metropolitan areas has not been well evaluated.
Data collected through contact tracing efforts have provided valuable insights into the transmission dynamics of SARS-CoV-213–17; however, most contact tracing during the early phase of the pandemic mainly focused on specific local outbreaks, which cannot support population-level analysis of community transmission. Here, we use detailed data from confirmed and probable cases18 and case investigations during the second pandemic wave in New York City (NYC) to quantify community spread of COVID-19 at small spatial scales from October 2020 to May. Unlike the initial outbreak during the spring of 2020, the second pandemic wave was fully captured by contact tracing. Additionally, contact tracing operation and individual protective measures such as mask-wearing and social distancing remained relatively stable during this period of the pandemic (in contrast with the post-Omicron era when protective measures were largely abandoned). As a result, data collected during the second pandemic wave may better inform understanding of SARS-CoV-2 community transmission in NYC and the operational performance of contact tracing during a public health emergency.
Results
Contact tracing in NYC
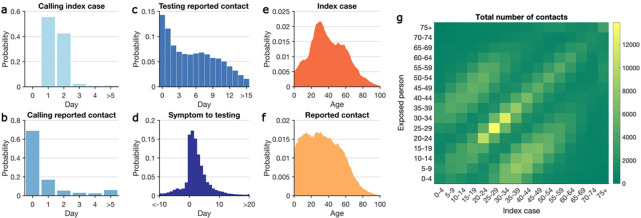
The NYC Test & Trace Corps initiative was launched in June 202019. Established as an operation to provide contact tracing, testing, and resources to support isolation and quarantine, the contact tracing program was integrated with a set of intervention efforts designed to limit morbidity and mortality from COVID-19 in NYC (Supplementary Information). We analyzed data obtained from case investigations and COVID-19 testing results (molecular and antigen) collected between October 1, 2020 and May 10, 2021 (Extended Data Fig. 1, Supplementary Information). During this period, 691,834 confirmed and probable cases were reported to the New York City Department of Health and Mental Hygiene (DOHMH)20. After excluding cases residing in residential congregate settings, cases were sent to the NYC Test & Trace Corps for contact tracing. Among these cases, 644,029 were reached by tracers and 450,415 completed an interview. In total, 779,011 contacts with confirmed and probable cases were self-reported via case investigations, of whom 20.9% (162,659/779,011) were subsequently tested. The median time from specimen collection to reporting results to DOHMH was 2 days. 97% of index patients were called by tracers within two days of reporting to DOHMH (Fig. 1a) and 68.4% of contacts were called the day of reporting to the Test & Trace team (Fig. 1b). Among tested contacts, 66.6% sought testing within one week of notification (Fig. 1d). For traced symptomatic infections, 86.7% were tested after symptom onset, and 13.3% were tested before symptom development (Fig. 1d).
Figure 1.
Key statistics of contact tracing in NYC. Panels (a-d) show the distributions of: (a) time between reporting date for index cases and being called by contact tracers; (b) time between calling index cases and notifying exposed persons; (c) time between notifying exposed persons and specimen sampling of notified individuals who were tested; (d) time from symptom onset to specimen sampling for symptomatic COVID infections. A negative value implies that testing preceded symptom onset. Age distributions of index cases (e) and self-reported contacts (f). The contact mixing matrix (g) shows the total number of exposures among age groups reported during the study period.
Adults aged 20 to 49 years old constituted the majority of index cases (Fig. 1e), a finding in agreement with the age distribution of confirmed infections in the United States21. Self-reported contacts were more uniformly distributed among the population under 50 years old (Fig. 1f). The age-stratified contact matrix highlights more frequent interactions among individuals of similar age and inter-generation mixing within the household (Fig. 1g), a pattern also observed in other countries22.
Exposure and transmission networks
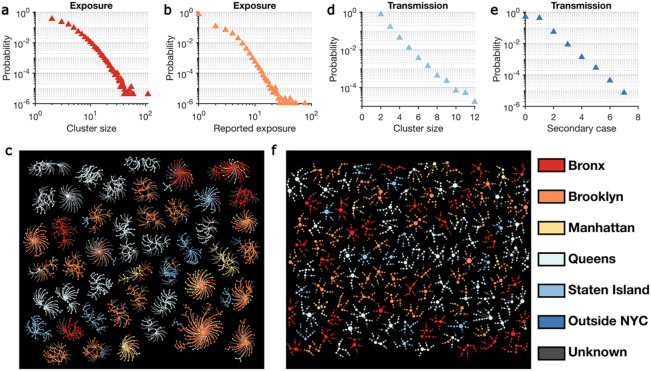
We reconstructed the self-reported exposure network at the individual level for the study period. The exposure network was highly fragmented, with 947,042 individuals in 242,486 disjoint clusters. Cluster size showed considerable heterogeneity (Fig. 2a), as did the number of contacts reported by each index case (Fig. 2b). We visualize several large exposure clusters in Fig. 2c, color-coded by the home borough of each person. Exposure clusters exhibit diverse structures ranging from hub-and-spoke networks with a single spreader to networks with multiple spreaders. Over half of the clusters shown in Fig. 2c were in Queens and Brooklyn. Within the large exposure clusters in Fig. 2c, 1,195 index patients (59.4%) reported contacts living in the same borough, but 817 (40.6%) cross-borough contacts were also recorded.
Figure 2.
Structure of exposure and transmission networks. (a) and (b) show the distributions of cluster size and number of close contacts reported by each index case in the exposure network. Exposure clusters with more than 35 individuals are visualized in (c). The exposure network is undirected. Index cases and reported close contacts are connected. Node size is proportional to the number of connected individuals. Colors indicate the home location of each person (five boroughs in NYC, outside NYC, and unknown). The distributions of cluster size and the number of secondary cases in the transmission network are shown in (d) and (e), respectively. Panel (f) visualizes transmission clusters with more than six infected individuals. Node size represents the number of secondary cases. Arrows indicate the direction of transmission.
We further reconstructed transmission chains by linking the contact tracing records and the laboratory-confirmed cases (molecular and antigen). Due to asymptomatic and pre-symptomatic shedding23–25, index cases were not necessarily the source of infections in these putative transmission events. To infer the direction of transmission, we estimated the infection date of lab-positive cases. For symptomatic cases, infection date was estimated using an empirical incubation period distribution obtained from a prior study17; for asymptomatic cases, we used specimen collection date to estimate infection date using a model of viral load dynamics coupled with a Bayesian inference (Extended Data Fig. 2)26. We sampled an ensemble of possible transmission networks compatible with the estimated chronological order of infections and selected the network with maximum likelihood based on transmission probabilities across age groups (Extended Data Table 1, Extended Data Fig. 3). More details on the transmission network reconstruction are provided in the Supplementary Information.
During the study period, we identified 58,474 potential transmission clusters formed by exposures that resulted in lab-confirmed infections. On average, these transmission clusters had a mean size of 2.3 individuals, representing 19.6% (135,478/691,834) recorded cases during the study period. However, transmission cluster size and the number of secondary cases linked to each index case had large variance (Fig. 2d–e) – only 0.20% of transmission clusters involved more than 6 infections. The largest transmission cluster identified consisted of 12 cases, and the maximum number of secondary cases for each single index case was 7. Transmission clusters with at least 6 infections are visualized in Fig. 2f.
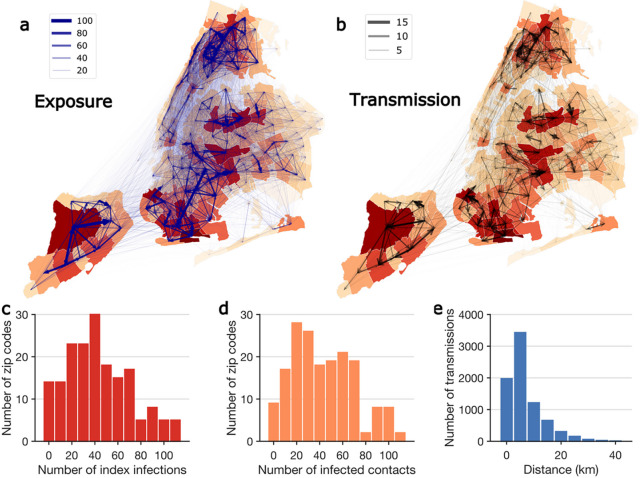
To quantify the spatial spread of SARS-CoV-2 in NYC at fine geographical scales, we mapped exposure and transmission networks across modified ZIP code tabulation areas (MODZCTAs, referred to as ZIP codes hereafter; Fig. 3a–b). Among 72,191 transmission events where place of residence was known, 7,826 (10.8%) included multiple ZIP codes. We observed several local clusters of ZIP codes that were tightly interconnected by exposure and transmission, centered around locations with high community prevalence. Infections in those high-prevalence ZIP code clusters were linked to self-reported contacts in nearby and far locations (Fig. 3a), which may have facilitated the spread of COVID-19 across the city (Fig. 3b). Among the cross-ZIP code transmission chains, we examined distributions of index cases who initiated transmission (Fig. 3c) and the infected contacts (Fig. 3d) across ZIP codes. A distinct skew in the distribution suggests that certain ZIP codes were more involved in the spatial spread of COVID-19. Geographically, most cross-ZIP code transmission events occurred within 10 km; however, long-distance transmission up to 40 km was also evident (Fig. 3e).
Figure 3.
Spatial transmission of SARS-CoV-2 in NYC. (a) and (b) show the exposures and transmission events across ZIP codes in NYC identified from contact tracing data. Arrows indicate direction of exposure (from index cases to reported close contacts) and transmission (from index infections to infected contacts). Arrow thickness indicates the number of exposures and transmission events. ZIP code area color represents the cumulative number of confirmed cases during the study period (yellow to red – low to high). For cross-ZIP code transmission events, the distributions of index infections and infected contacts across ZIP code areas are presented in (c) and (d). Panel (e) shows the distribution of distance between home ZIP codes of index infections and infected contacts in cross-ZIP code transmission events. The population weighted centroids for ZIP code areas were used to compute the distance.
Evaluation of intervention measures
During the period from October 2020 to March 2021, a dynamic zone-based control strategy was adopted in New York State to limit viral spread in communities with high case growth rates while avoiding undue harm to the economy27. Three tiers of zones (yellow, orange, and red) were identified based on a set of metrics, collectively defined by test positivity rate, hospital admissions per capita, and hospital capacity27,28. Local restrictions on business and services were imposed based on zone conditions. Compliance to these restrictions can be reflected by population mobility in each ZIP code. In December 2020, vaccines became available to the population at highest risk for severe outcomes associated with COVID-19 in NYC and were subsequently available to all eligible individuals over 15 years old during early April 2021. With the support of the detailed contact tracing data, we evaluated the impact of these public health interventions on community transmission of SARS-CoV-2 in NYC.
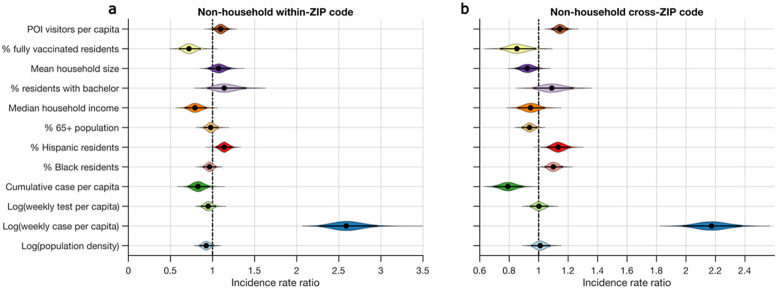
We assessed the associations of the numbers of non-household within- and cross-ZIP code transmission events across NYC with demographic, socioeconomic, disease surveillance, vaccination coverage, and human mobility features (Supplementary Information). As non-household transmission contributed to the expansion of SARS-CoV-2 outside the household, we focused on 4,642 non-household transmission events. We used aggregated foot traffic records derived from mobile phone data29 documenting weekly numbers of individuals visiting points-of-interest (POIs, e.g., restaurants, grocery stores, gyms, and bars) in each ZIP code as an indicator of human mobility and compliance with the zone-based local restrictions (Supplementary Information). We used conditional autoregressive (CAR) models30 to assess the effects of the above factors on within- and cross-ZIP code transmission (Fig. 4). Specifically, for both within- and cross-ZIP code transmission, we fitted Poisson generalized linear mixed models (GLMM) with random effects and CAR priors to account for the inherent spatial-temporal autocorrelation in disease transmission data30,31 (Supplementary Information, Extended Data Figs. 4–5).
Figure 4.
Effects of various features on the transmission of SARS-CoV-2 in NYC. Incidence rate ratios (exponentiated coefficients) for non-household within-ZIP code transmission and cross-ZIP code transmission are shown for 12 covariates in (a) and (b), respectively (Deviance information criterion, DIC=6,342 for a and DIC=12,644 for b). Coefficients were estimated using a Poisson generalized linear mixed model controlling for spatial-temporal autocorrelations. We used the log-transformed population as the offset in the regression model. Covariates were standardized and are shown on the y-axis. The incidence rate ratio quantifies the multiplicative change in the number of transmission events per each covariate increase of one standard deviation, controlling for other covariates. The violin plots show the distributions of incidence rate ratios. Black dots and horizontal black lines highlight the median estimates and 95% CIs.
We found that higher vaccination coverage and fewer POI visitors were associated with reduced non-household within- and cross-ZIP code transmission in the same week (Fig. 4). Estimates of coefficients are provided in Extended Data Table 2. The model identifies a strong effect of vaccination on SARS-CoV-2 transmission: a 12.48% newly vaccinated population was associated with reductions of 28.0% (95% CI: 14.0% – 40.0%) and 14.8% (1.7% – 26.4%) for within- and cross-ZIP code non-household transmission events, respectively. In contrast, a 0.12 per capita increase of POI visitors was associated with increases of 9.6% (0.3% – 19.3%) and 14.4% (8.7% – 20.2%) for within- and cross-ZIP code transmission outside households, respectively. We further found that both within- and cross-ZIP code transmission had strong positive associations with log weekly cases per capita (. Higher percentage of Hispanic residents and lower cumulative cases per capita were associated with higher non-household transmission ( ). For cross-ZIP code transmission, cumulative cases per capita had a stronger effect than vaccination and POI visitors (Fig. 4b, Extended Data Table 2), indicating that prior infections may result in reduced cross-ZIP code transmission in locations with a higher attack rate. These findings reveal how health inequities related to COVID-19 manifest across NYC communities. Results also indicate that promoting vaccination and capacity limits or temporary limits on local businesses, schools, and other POIs in high-prevalence communities were effective in reducing SARS-CoV-2 transmission in NYC. These findings were corroborated with an alternate random-effect model (Supplementary Information), and testing of effect lags of one week and two weeks (Extended Data Figs. 6–8).
Discussion
Here, leveraging detailed test and tracing data, we performed an analysis of ZIP code level SARS-CoV-2 transmission in NYC. The observed heterogeneity of SARS-CoV-2 spread at community scales implies that NPIs focusing on neighborhoods with extensive community transmission could potentially be more cost-effective. However, because communities with high test positivity were typically high poverty areas3, during isolation and quarantine resources (such as food delivery, medication delivery, and access to safe isolation places) should be provided to address the disproportionate impact of the pandemic on these communities. Our statistical analyses suggest that the combination of vaccination and reactive, zone-based intervention measures implemented in NYC likely reduced the spread of COVID-19 during the second wave.
This study has several limitations. Firstly, the contact tracing data were biased to household exposure, and voluntarily reported close contacts, especially outside the household, were incomplete. As a result, identified clusters of exposure and transmission are largely confined to small networks, limiting the detection of complete transmission networks, including super-spreading events. However, the spatial transmission pattern is less affected by the selection bias if such bias is similar across ZIP code areas. Secondly, some communities may have a lower response rate to the calls from tracers. Further studies are needed to quantify the factors associated with the lower response rate for improving future contact tracing effectiveness. Thirdly, due to missing and incorrect personal identifying information, the matching to close contacts and their test results may be incomplete.
With the global circulation of new variants of concern, such as Omicron and its sublinages32, our findings can inform control management in other urban settings beyond NYC. Specifically, public health authorities should consider the community-level spatial dispersion of SARS-CoV-2 when designing control tactics, which can be analyzed in real time using contact tracing data. Our analysis on the exposure network may inform a better definition of the proper geographical units for observation and interventions based on actual human interactions and disease transmission in NYC and elsewhere. Coordinated interventions targeting identified clusters of ZIP codes currently supporting the spatial transmission of SARS-CoV-2 could potentially produce more effective outbreak control. The findings may also support future pandemic preparedness and response. The operational performance of contact tracing can be used as a benchmark in urban settings and support modeling studies33–36 of the potential effects of contact tracing on emerging infectious disease containment.
Supplementary Material
Acknowledgements:
This study was supported by funding from the National Institutes of Health grant R01AI163023 (JS), Centers for Disease Control and Prevention U01CK000592 (JS, SP), Council of State and Territorial Epidemiologists NU38OT00297 (SP, WY) and a gift from the Morris-Singer Foundation (JS). We thank Sharon Greene, Celia Quinn, Hannah Helmy and Jeffrey Sachs for comments and discussions. We also thank SafeGraph for providing human mobility data.
Footnotes
Competing Interest: J.S. and Columbia University disclose partial ownership of SK Analytics. J.S. discloses consulting for BNI. All other authors declare no competing interests.
Code Availability: Custom code supporting this study will be made publicly available at GitHub (https://github.com/SenPei-CU/).
Additional Information: Supplementary Information is available for this paper. Correspondence and requests for materials should be addressed to S.P. (sp3449@cumc.columbia.edu). Reprints and permissions information is available at www.nature.com/reprints.
Contributor Information
Sen Pei, Columbia University.
Sasikiran Kandula, Mailman School of Public Health, Columbia University.
Jaime Cascante Vega, Columbia University.
Wan Yang, Columbia University.
Steffen Foerster, New York City Department of Health and Mental Hygiene.
Corinne Thompson, New York City Department of Health and Mental Hygiene.
Jennifer Baumgartner, New York City Department of Health and Mental Hygiene.
Shama Ahuja, New York City Department of Health and Mental Hygiene.
Kathleen Blaney, New York City Department of Health and Mental Hygiene.
Jay Varma, Weill Cornell Medical College.
Theodore Long, NYC Health + Hospitals.
Jeffrey Shaman, Mailman School of Public Health, Columbia University.
Data Availability:
COVID-19 surveillance data in NYC at the MOZCTA (modified ZIP code tabulation area) level are publicly available at the GitHub repository maintained by the NYC Department of Health and Mental Hygiene (DOHMH) (https://github.com/nychealth/coronavirus-data). Demographic and socioeconomic data for NYC zip code tabulation areas (ZCTA) are available from the 5-year American Community Survey (ACS) (https://www.census.gov/programs-surveys/acs/data.html). Contact tracing records and individual testing results are subject to restrictions for the protection of patient privacy. Requests for data access should be addressed to NYC DOHMH and NYC Health + Hospitals.
References
- 1.Chang S. et al. Mobility network models of COVID-19 explain inequities and inform reopening. Nature 589, 82–87 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Brauner J. M. et al. Inferring the effectiveness of government interventions against COVID-19. Science 371, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamb M. R., Kandula S. & Shaman J. Differential COVID-19 case positivity in New York City neighborhoods: Socioeconomic factors and mobility. Influenza Other Respir. Viruses 15, 209–217 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee E. C., Wada N. I., Grabowski M. K., Gurley E. S. & Lessler J. The engines of SARS-CoV-2 spread. Science 370, 406–407 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Davis J. T. et al. Cryptic transmission of SARS-CoV-2 and the first COVID-19 wave. Nature 600, 127–132 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pei S., Yamana T. K., Kandula S., Galanti M. & Shaman J. Burden and characteristics of COVID-19 in the United States during 2020. Nature 598, 338–341 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Plessis L. du et al. Establishment and lineage dynamics of the SARS-CoV-2 epidemic in the UK. Science 371, 708–712 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemey P. et al. Untangling introductions and persistence in COVID-19 resurgence in Europe. Nature 595, 713–717 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bedford T. et al. Cryptic transmission of SARS-CoV-2 in Washington state. Science 370, 571–575 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Reiche A. S. et al. Introductions and early spread of SARS-CoV-2 in the New York City area. Science 369, 297–301 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng X. et al. Genomic surveillance reveals multiple introductions of SARS-CoV-2 into Northern California. Science 369, 582–587 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraemer M. U. G. et al. Spatiotemporal invasion dynamics of SARS-CoV-2 lineage B.1.1.7 emergence. Science 373, 889–895 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park Y. J. et al. Contact Tracing during Coronavirus Disease Outbreak, South Korea, 2020. Emerg. Infect. Dis. 26, 2465–2468 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bi Q. et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect. Dis. 20, 911–919 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachdev D. D. et al. Outcomes of Contact Tracing in San Francisco, California-Test and Trace During Shelter-in-Place. JAMA Intern. Med. 181, 381–383 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun K. et al. Transmission heterogeneities, kinetics, and controllability of SARS-CoV-2. Science 371, eabe2424 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu S. et al. Infectivity, susceptibility, and risk factors associated with SARS-CoV-2 transmission under intensive contact tracing in Hunan, China. Nat. Commun. 12, 1533 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coronavirus Disease 2019 (COVID-19) 2021 Case Definition | CDC. https://ndc.services.cdc.gov/case-definitions/coronavirus-disease-2019-2021/. [Google Scholar]
- 19.Test & Trace Corps | NYC Health + Hospitals. https://www.nychealthandhospitals.org/test-and-trace/.
- 20.COVID-19: Data Trends and Totals - NYC Health. https://www1.nyc.gov/site/doh/covid/covid-19-data-totals.page.
- 21.Monod M. et al. Age groups that sustain resurging COVID-19 epidemics in the United States. Science 371, eabe8372 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prem K. et al. Projecting contact matrices in 177 geographical regions: An update and comparison with empirical data for the COVID-19 era. PLOS Comput. Biol. 17, e1009098 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He X. et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 26, 672–675 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Li R. et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science 368, 489–493 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cevik M. et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe 2, e13–e22 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larremore D. B. et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 surveillance. medRxiv 2020.06.22.20136309 (2020) doi: 10.1101/2020.06.22.20136309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Governor Cuomo Details COVID-19 Micro-Cluster Metrics. https://www.governor.ny.gov/news/governor-cuomo-details-covid-19-micro-cluster-metrics.
- 28.Governor Cuomo Announces Updated Zone Metrics, Hospital Directives and Business Guidelines. https://www.governor.ny.gov/news/governor-cuomo-announces-updated-zone-metrics-hospital-directives-and-business-guidelines. [Google Scholar]
- 29.Weekly Patterns | SafeGraph Docs. SafeGraph; https://docs.safegraph.com/docs/weekly-patterns. [Google Scholar]
- 30.Lee D., Rushworth A. & Napier G. Spatio-Temporal Areal Unit Modeling in R with Conditional Autoregressive Priors Using the CARBayesST Package. J. Stat. Softw. 84, 1–39 (2018).30450020 [Google Scholar]
- 31.Rushworth A., Lee D. & Mitchell R. A spatio-temporal model for estimating the long-term effects of air pollution on respiratory hospital admissions in Greater London. Spat. Spatio-Temporal Epidemiol. 10, 29–38 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Pulliam J. R. C. et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science 376, eabn4947 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kucharski A. J. et al. Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: a mathematical modelling study. Lancet Infect. Dis. 20, 1151–1160 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aleta A. et al. Modelling the impact of testing, contact tracing and household quarantine on second waves of COVID-19. Nat. Hum. Behav. 4, 964–971 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grantz K. H. et al. Maximizing and evaluating the impact of test-trace-isolate programs: A modeling study. PLOS Med. 18, e1003585 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gardner B. J. & Kilpatrick A. M. Contact tracing efficiency, transmission heterogeneity, and accelerating COVID-19 epidemics. PLOS Comput. Biol. 17, e1009122 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
COVID-19 surveillance data in NYC at the MOZCTA (modified ZIP code tabulation area) level are publicly available at the GitHub repository maintained by the NYC Department of Health and Mental Hygiene (DOHMH) (https://github.com/nychealth/coronavirus-data). Demographic and socioeconomic data for NYC zip code tabulation areas (ZCTA) are available from the 5-year American Community Survey (ACS) (https://www.census.gov/programs-surveys/acs/data.html). Contact tracing records and individual testing results are subject to restrictions for the protection of patient privacy. Requests for data access should be addressed to NYC DOHMH and NYC Health + Hospitals.