Abstract
Pancreatic islets consist of several endocrine cell types that maintain glucose homeostasis. Type 1 diabetes (T1D) results from autoimmune-mediated destruction of insulin producing beta cells in pancreatic islets. Islet transplantation is a treatment for certain individuals with T1D. Islet transplantation in rodents, as an experimental model of the clinical scenario, requires consistency of islet quantity and quality to obtain reproducible results. In this study, we investigated the yield and function of the isolated islets from rats of different ages. Pancreata were harvested from young (10–20 week-old), intermediate (21–40 week-old) and old (> 41 week-old) male rats and islets were isolated using a standard protocol. Islet number, morphometry, viability, function, and metabolism were characterized. Islet yield, normalized to body weight, decreased as a function of increasing donor age. Islets from pancreata from young animals were larger and less fragmented compared to islets from organs from intermediate and older animals. Islet viability following overnight culture was the same for islets derived from young and intermediate aged donors but less for islets from old donors. Glucose-stimulated insulin secretion was decreased in islets from older donors. Islet metabolism following glucose challenge, as measured by oxygen consumption, revealed that islets from old donors were metabolically slower and lagged in response to glucose-stimuli. These data demonstrate that increasing donor age has a negative impact on isolated islet yield and quality.
Keywords: Islet isolation, Islet yield, Aging, Insulin-secreting function, Islet metabolism
1. Introduction
Pancreatic islets consist of endocrine cells that function to maintain glucose homeostasis (Brissova et al., 2005). Deterioration of beta cells, a dominant cell type in islets, leads to diabetes. Type 1 diabetes (T1D) is characterized by autoimmune-induced depletion of insulin-secreting pancreatic beta cells (Chiang et al., 2014). Islet transplantation is a recognized treatment for some individuals with T1D and can achieve normoglycemia and restore hypoglycemia awareness (Leitao et al., 2008). Islet transplantation is performed by islet infusion into the recipient’s portal vein (Shapiro et al., 2000, 2006; Ryan et al., 2002). The transplanted islets are obtained from donated cadaveric pancreata that undergo tissue digestion within GMP guidelines (Ricordi et al., 1989). Various factors impact islet quality and isolation yield including the donor’s body mass index and cause of death (Kim et al., 2005), and these, in turn, influence clinical outcomes. Fully defining such factors is an important step in islet approval prior to transplantation.
While clinical islet transplantation is an option for individuals with T1D, there remains room for improvement (Rother and Harlan, 2004; Hirshberg et al., 2003; Casey et al., 2002). In this regard, animal models of islet transplantation provide pre-clinical insight into the factors that predict and determine successful transplant engraftment and function. As with human islets, yield and quality of islets obtained from rodents are subject to modification by donor characteristics as well as isolation methods (de Haan et al., 2004; Wolters et al., 1990). In pancreatic islet research, consistent yield and quality of isolated islets should be warranted for reliability and reproducibility in experiments.
Glucose tolerance decreases with age, and diabetes is an age-associated disease (Chen et al., 1985; Karve and Hayward, 2010). Aging is accompanied by increased cell senescence which may adversely impact islet yield and quality. Herein, we investigated the relationship between islet yield and quality and donor age in a rat model.
2. Materials and methods
2.1. Islet isolation procedures
Islets were isolated from male Lewis (LEW) rat pancreata using our standard procedure (Ito et al., 2010). Under general anesthesia, 9 mL of ice cold Hanks’ balanced salt solution (HBSS; Sigma-Aldrich, St. Louis, MO, USA) supplemented with collagenase (2.5 mg/mL, Sigma-Aldrich) and HEPES (100 mM, Irvine Scientific, Santa Ana, CA, USA) was injected into the pancreatic duct through the common bile duct. The distended pancreas was dissected free from surrounding connective tissue and placed in ice-cold HBSS-collagenase solution. This was followed by enzymatic digestion at 37 °C for 10 min. To stop the digestion, 30 mL ice-cold HBSS supplemented with 10 mM HEPES, 1% fetal bovine serum (FBS; Atlanta Biologicals, Flower Branch, GA, USA) and Penicillin-Streptomycin-Glutamine (Thermo Fisher Scientific, Waltham, MA, USA) was added. The digested pancreas was centrifuged at 300 ×g for 3 min. Pellets were washed and the resultant subjected to density gradient centrifugation in HBSS solution and Histopaque-1077 (density: 1.077 g/mL, Sigma-Aldrich) for 25 min at 300 ×g and 24 °C. Islets were hand-picked for purity. All isolations were performed by a single investigator. The use of animals and animal procedures in this project was approved by City of Hope/Beckman Research Institute Institutional Animal Care and Use Committee.
2.2. Retrospective analysis of age, islet isolation yield, and body weight of the donor rats
LEW rats from 13 to 71 weeks old were used as islet donors. Body weight was measured on the day of islet isolation. The number of islets was determined by a volume-based method as described previously (Ito et al., 2010; Komatsu et al., 2016a). Briefly, the length of packed islets in a PE50 tube after centrifugation (160 ×g for 2 min) was converted to the islet number based on an established standard curve. Relations between the factors of age, islet isolation yield, and body weight of the donor rats were retrospectively analyzed in 41 consecutive isolations obtained from 128 donors. In isolations from multiple donors, animals of similar age (within 2 weeks difference) were utilized, and averages of body weight and islet yield were analyzed. Isolations were categorized into 3 groups according to the donor age: young 10–20 week-old (n = 22, average age = 17.3 week-old); intermediate aged 21–40 week-old (n = 9, average age = 25.1 week-old); and old > 41 week-old (n = 10, average age = 52.9 week-old).
2.3. Retrospective analysis of multiple donor effect on isolated islet yield
As described above, rat pancreases were placed on ice during enzymatic digestion. Generally, organ dissection takes 10 min, therefore, when more than one donor is used, some pancreases undergo longer intervals of cold incubation compared to single organ isolations. Thus, the effect of multiple organ isolation on the islet yield was analyzed. Isolations were classified into two groups for the analyses: 1–3 donors (n = 14) and > 4 donors (n = 27). To examine the effect of multiple organ isolation in the different age groups sub-analysis was performed according to the three age groups described above.
2.4. Islet preparation for viability, function and metabolism assessment
Prospective studies were employed to determine the viability, function and metabolism of isolated islets. In preparation for these assays, islets from each age group were cultured overnight at 37 °C in CMRL 1066 culture media (Corning Life Sciences, Corning, NY) supplemented with 0.5% human serum albumin, 0.1 μg/mL insulin-like growth factor-1 (Cell Sciences, Newburyport MA), 10 U/mL heparin sodium (Sagent Pharmaceuticals, Schaumburg, IL) and 0.5 mg/mL of Alpha1–Proteinase Inhibitor (ARALAST; Baxter, Deerfield, IL) in a 5% CO2-incubator.
2.5. Assessment of islet viability
Islet cell viability was assessed by fluorescein diacetate (FDA, for live cells) and propidium iodide (PI, for dead cells) staining as described (Komatsu et al., 2016b). Briefly, FDA/PI-stained islet suspensions containing 100 islets were plated in wells of a 96-well plate to capture multiple images covering the entire area of the well (IX50, Olympus, Tokyo, Japan). Images were assembled to assess the viability of all islets in the well by cellSens software (Olympus). Islet viability was calculated using the following formula: Viability (%) = 100 - (Area_PI/ [Area_FDA + Area_PI])*100], with (Area_FDA) defined as the FDA-positive area and (Area_PI) defined as the PI-positive area.
2.6. Isolated islet size analysis
Islet size was measured using the same pictures captured for viability assays (cellSens, Olympus). All islets used in the viability assay (100 islets per donor) were measured to calculate average islet size. Average size in each rat donor was calculated and analyzed in the three age groups.
2.7. Isolated islet shape analysis
Islet shape was assessed post-isolation using microscopic images taken for viability assays (cellSens, Olympus). Shape factor, which numerically describes the shape of a particle under two dimensional images in a microscope (Podczeck and Newton, 1994), was calculated for all islets. Shape factor values can range from 0 to 1, where the value of 1.0 indicates a perfect spheroid. Average islet shape factor for islets from each rat donor was assessed and analyzed according to the three age groups.
2.8. Functional assessment of isolated islets: insulin secretion by glucose stimulation
Glucose stimulated insulin secretion (GSIS) was assessed using islets cultured overnight. Approximately 100 islets per well in a 24-well plate cell culture insert (Millipore Sigma, Burlington, MA, USA) were incubated in 1 mL of Krebs-Ringer buffer (KRB) solution containing 2.8 mM glucose for 1 h, followed by a 1 h incubation in 1 mL of KRB solution containing 28 mM glucose. The buffer was collected after each incubation to measure insulin concentration using a rat insulin ELISA kit (Mercodia, Uppsala, Sweden). After GSIS, islets on the cell inserts were collected and DNA extraction was performed (ZR Genomic DNA-Tissue MiniPrep kit, Zymo Research, Irvine, CA, USA) to quantify the total DNA content. Insulin secretion was normalized to the DNA content of each sample. The ratio between high insulin secretion and low insulin secretion was used to calculate the stimulation index (SI_GSIS).
2.9. Metabolic assessment of isolated islets: oxygen consumption rate
Islets cultured overnight were used to assess the oxygen consumption rate (OCR). Islet OCR was measured using a Seahorse XFe analyzer (Seahorse Bioscience, North Billerica, MA, USA) as described (Komatsu et al., 2019). One hundred islets per donor were plated onto wells in a Seahorse XFe islet capture plate. Islet OCR was measured at basal level (3 mM glucose for 40 min), upon glucose stimulation (20 mM glucose for 180 min), and during mitochondrial respiration inhibition (Oligomycin 5 nM for 90 min). Measurements were repeated every 7.5 min until the end of the experiment. Basal OCR was calculated as the average OCR obtained on incubation for 30 min in a 3 mM glucose solution. All OCR values measured were normalized to the basal OCR and expressed as % to basal OCR. The maximum OCR was obtained at least 1 h after the initiation of glucose stimulation. The OCR fold increase was defined as the maximum OCR/basal OCR (SI_OCR). To assess the responsiveness of islet cell metabolic change induced by the high glucose environment, the rate of change in OCR was taken as the change in OCR within 7.5 and 15 min of high glucose challenge (ΔOCR_7.5 min and ΔOCR_15min), as well as the maximum OCR increase (ΔOCR_max) after glucose stimulation (Supplemental Fig. 1). The % OCR change in response within these time intervals was calculated as %ΔOCR_7.5 min/ΔOCR_max and %ΔOCR_15min/ΔOCR_max, respectively.
2.10. In vivo islet transplantation with different donor age groups
Syngeneic rat islet transplantation was performed using a subcutaneously transplantable O2 transporting device as previously reported (Komatsu et al., 2018). Female LEW rats weighing 180–200 g were used as recipients, and islets for transplantations were prepared from male luciferase (LUC) transgenic LEW donors of different ages (10–20 weeks-old and > 41 weeks-old). Briefly, 600 islets were loaded on the device which was then implanted in a subcutaneous location. In vivo islet graft viability was determined on post-transplant day 7 (Komatsu et al., 2016c). After injection of 15 mg luciferin/kg of body weight, bioluminescence of the LUC (+) islet grafts was captured using the Lago X platform (Spectral Instruments Imaging, Tucson, AZ, USA). Bioluminescent intensity between 1 and 2 min was taken as a marker of islet viability. Assessments were performed in 6 animals transplanted with islets from donors 10–20 week-old and in 3 animals transplanted with islets from donors > 41 week-old.
2.11. Statistical analysis
Data are reported as the mean ± standard error (SEM). Statistical analysis was performed using the JMP 13 program (SAS Institute, Cary, NC). For statistical comparison, Student’s t-tests were carried out. Correlations were analyzed using fitted regression lines and coefficient of determination (R2) values, and statistical significance was calculated using F-tests. p < 0.05 denotes statistical significance.
3. Results
3.1. Body weight increases with age in male LEW rats
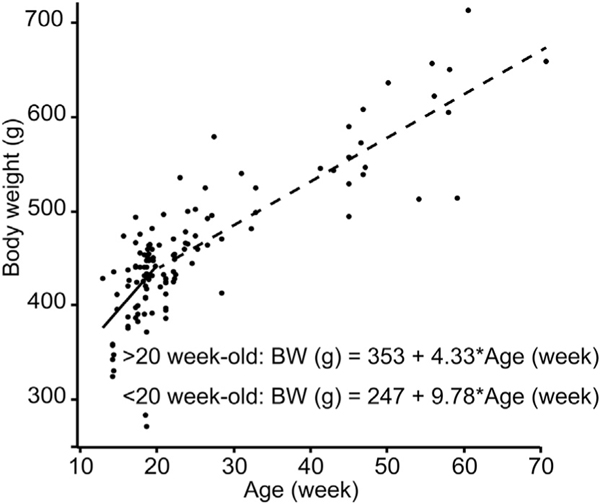
To clarify the relationship between body weight and aging in pancreata donors, we plotted the data of the 128 rats employed in 41 isolations (Fig. 1). Until 20 weeks of age, donors showed rapid growth (+9.8 g/week; fitted line: Body weight [g] = 246.9 + 9.78 × Age [week], R2 = 0.166). Beyond 20 weeks of age, organ donors demonstrated slower weight gain (+4.3 g/week; fitted line: Body weight [g] = 353.1 + 4.33 × Age [week], R2 = 0.663).
Fig. 1.
Relationship between body weight and age in male LEW rats. Isolation data of 128 rats employed in 41 isolations were plotted. < 20 week-old donors (n = 72) showed rapid body weight increase (+9.78 g/week), and > 20 week-old donors (n = 56) showed slower body weight increase (+4.33 g/week).
3.2. Islet yield is decreased in pancreata from older compared to younger donors
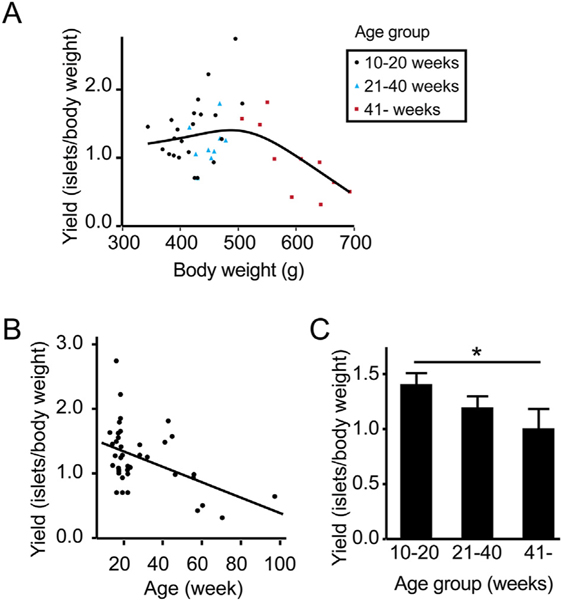
To assess if islet yield is affected by aging, we analyzed the data of 41 islet isolations. Fig. 2A shows the relations between the donor body weight and the number of islets isolated normalized to body weight (islet number/body weight [g]). Islet yield from donors between 350 and 500 g body weight ranged from 1.0 to 1.5 islet/g and the yield decreased in donors > 500 g body weight. When normalized yield was plotted according to the age (Fig. 2B), a linear decrease in yield was noted as donor age increased (Islet yield = 1.577–0.01193*Age, R2 = 0.218, p = 0.0024). Indeed, islet yields from organs from the oldest donor cohort were significantly lower compared to yields obtained from organs from the youngest donor group (1.40 ± 0.10 islets/ body weight [g] in the 10–20 week-old cohort, 1.19 ± 0.10 islets/body weight [g] in the 21–40 week-old cohort, 0.96 ± 0.16 islets/body weight [g] in the > 41 week-old cohort; p = 0.0342, Fig. 2C).
Fig. 2.
Islet yield normalized by the body weight is decreased by aging. (A) Relationship between donor body weight and isolated islet number normalized to donor body weight demonstrated greater yields in donors < 500 g body weight. (B) Relationship between the donor age and isolated islet number demonstrated a linear decrease in islet yield by age (R2 = 0.218, p = 0.0024). (C) Normalized islet yield assessment in different age groups (10–20 week-old, n = 22; 21–40 week-old, n = 9; and > 41 week-old, n = 10). Decreased normalized islet yield was demonstrated in older donors; > 41 week-old donors demonstrated significantly lower normalized islet yield compared to 10–20 week-old donors (p = 0.0342).
3.3. Effect of multiple donor isolations on islet yield
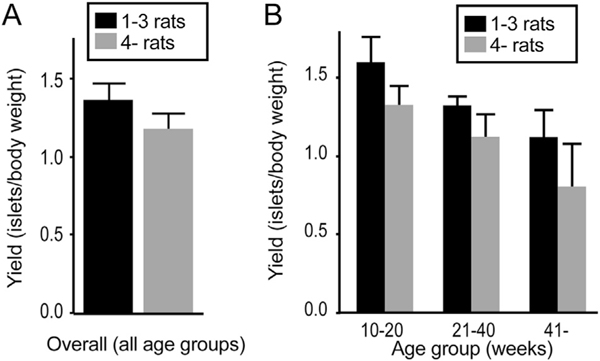
Unlike islet isolation in clinical transplantations, experimental islet isolation is often performed upon several organs simultaneously exposing islets to longer periods of cold ischemia. To examine the effect of multiple donor isolations on islet yield, we retrospectively analyzed the data of 41 isolations in two groups (≤3 donors and > 4 donors; Fig. 3A). The two groups did not show a statistical difference in normalized islet yield. Furthermore, we considered the role of donor age in relation to multiple organ isolation yield. Organ donor age did not significantly alter islet yield between the less and more organ groups, but > 4 donors demonstrated a trend towards lower normalized islet yield compared to ≤3 donors in all age groups (Fig. 3B).
Fig. 3.
Effect of multiple donor isolations on islet yield. (A) Retrospective analysis of multiple donor isolations on normalized islet yield. No statistical significance was noted between ≤3 organs (n = 14) and > 4 organs (n = 27). (B) Analysis of three age groups of multiple donor isolations on normalized islet yield; > 4 donors demonstrated a trend towards lower normalized islet yield compared to ≤3 donors although there was no statistical significance between ≤3 donors and > 4 donors.
3.4. The morphology and viability of isolated islet is limited by organ donor age
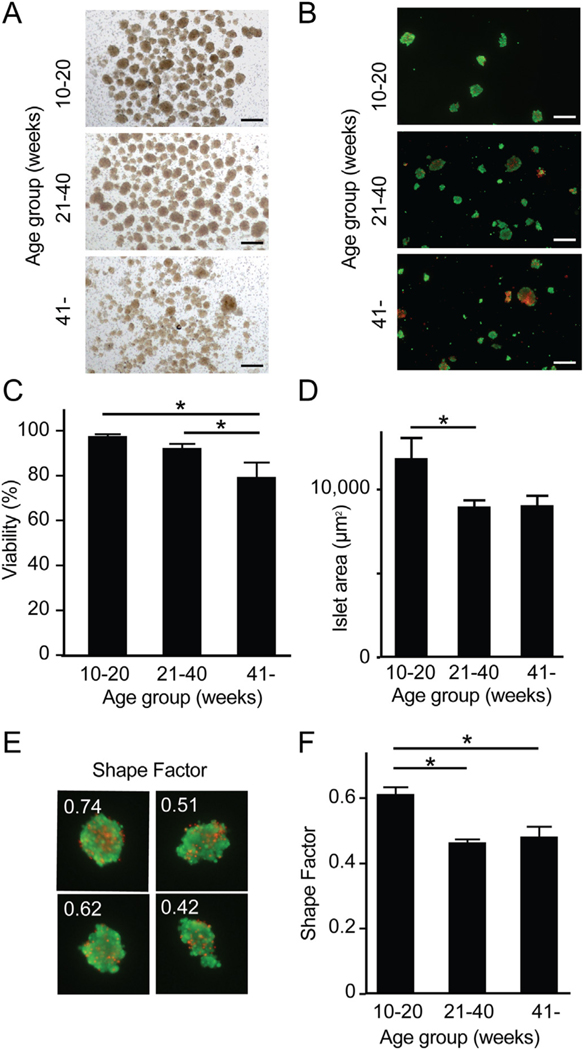
Analysis of the morphology of isolated islets from different age groups demonstrated less demarcated islet structure and higher light permeability due to the smaller islet structure in islets from > 41 week-old donors (Fig. 4A). We compared the viability of isolated islets among the three groups (Live/Dead staining, Fig. 4B). Area-based viability assessment revealed that islets isolated from young donors (10–20 week-old) demonstrated significantly higher viability (97.8%) compared to islets from organs from intermediate 21–40 week-old (92.5%, p = 0.0275) and aged > 41 week-old donors (79.5%, p = 0.0053) (Fig. 4C). Average islet sizes were 11,837 μm2, 8952 μm2 and 9035 μm2 from organs from young, intermediate and old donors respectively (Fig. 4D). On the assumption that the isolated islets were spheres, islet size corresponded to islet diameters of 122.8 μm, 106.8 μm and 107.2 μm, respectively. Isolated islet size was significantly larger in those isolated from organs from young versus old donors (p = 0.0399). Isolated islet shape was evaluated using shape factors as a marker of islet integrity with a higher shape factor signifying less fragmentation. Representative islets with different shape factors are shown in Fig. 4E. Shape factor values were increased in islets from young 10–20 week-old donors (0.61), compared to islets from intermediate 21–40 week-old (0.46, p = 0.0007) and aged > 41 week-old donors (0.48, p = 0.0024) (Fig. 4F). The smaller size and lower shape factor noted in islets obtained from intermediate and old organ donors are consistent with islet fragmentation.
Fig. 4.
Viability and integrity of islets isolated from young and older organ donors. (A) Morphology of islets in bright field. Scale bar: 500 μm. (B) Viability staining of isolated islets between the three groups. Green and red indicate live and dead cells. Scale bar: 500 μm. (C) Analysis of viability revealed that > 41 week-old donors had significantly lower viability (79.5%, n = 3) compared to 21–40 week-old donors (92.5%, p = 0.0275, n = 4) and 10–20 week-old donors (97.8%, p = 0.0053, n = 4). (D) Islet size (area) was measured in the images acquired to assess viability. Islet area in 10–20 week-old donors (11,837 μm2) were significantly larger in comparison to > 41 week-old donors (9035 μm2, p = 0.0399). (E) Representative shape factor of isolated islets calculated by imaging software (cellSens). Higher numbers imply more uniform spherical shape. (F) Analysis of shape factor demonstrated a significant difference in 10–20 week-old donors (0.61) when compared to 21–40 week-old (0.46, p = 0.0007) and > 41 week-old donors (0.48, p = 0.0024). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Islets from older organ donors show less insulin-secreting function
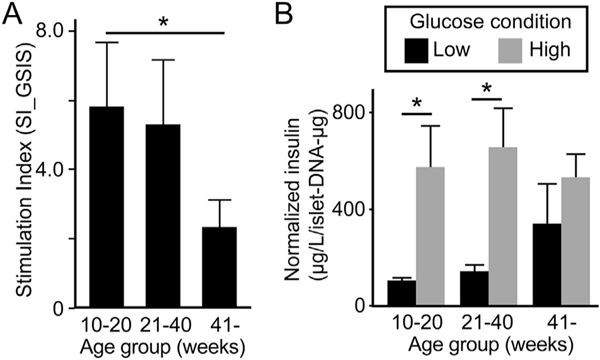
Isolated islet function was evaluated under varying glucose concentrations. The stimulation index (SI_GSIS) (Fig. 5A) is the ratio of islet insulin secretion in response to low (basal) and high glucose conditions. The SI_GSIS decreased with increasing age of donors being 5.81, 5.30 and 2.34 in islets from young, intermediate and old donors. The SI_GSIS was significantly lower in islets from the oldest donors compared to the youngest (p = 0.0472). Analysis of absolute insulin secretion under low and high glucose found islets from young and intermediate organs donors increased insulin secretion after a glucose challenge whereas islets from old organ donors did not (Fig. 5B).
Fig. 5.
Insulin-secreting function of isolated islets. (A) Stimulation index by GSIS (SI_GSIS) demonstrated age-associated decrease with > 41 week-old donors (2.34) having significantly lower SI compared to 10–20 week-old donors (5.81, p = 0.0472). n = 4 in 10–20 week-old and 21–40 week-old, and n = 3 in > 41 week-old donors. (B) Absolute insulin secretion analysis in low (basal) and high glucose conditions demonstrated significantly higher insulin secretion in the glucose-stimulated environment than under basal conditions in 10–20 week-old (p = 0.0335) and 21–40 week-old (p = 0.0207) donors but not in > 41 week-old donors (p = 0.3740).
3.6. Metabolic increment in response to glucose is less in islets from older donors
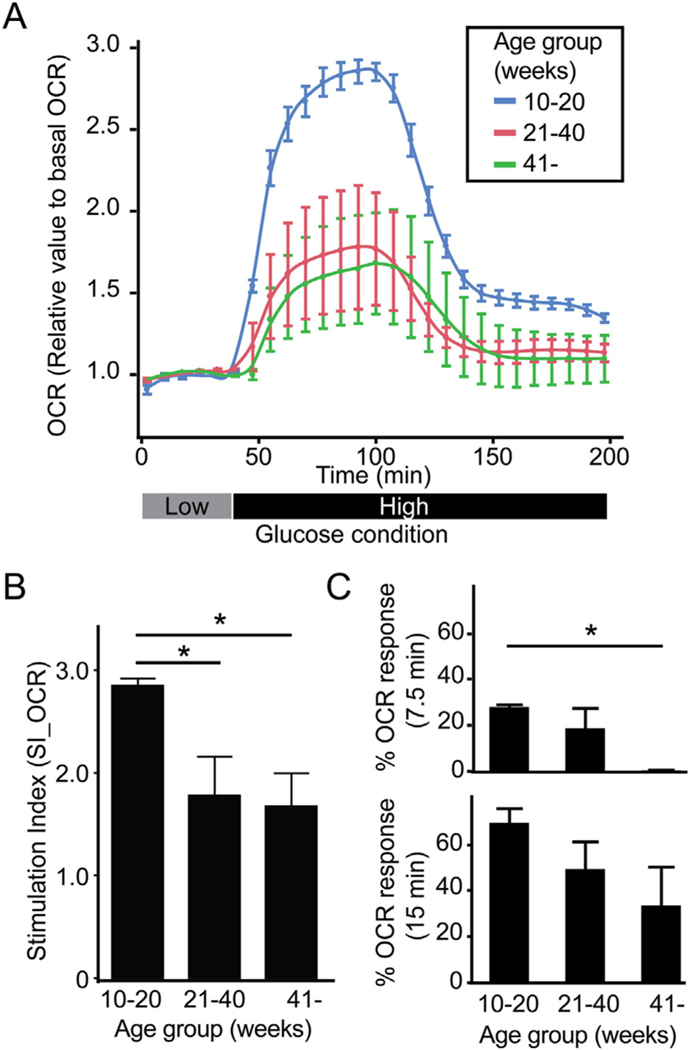
Islet oxygen consumption (OCR) following glucose challenge is a predictor of islet function post-transplantation (Papas et al., 2015; Sweet et al., 2008). We measured the OCR of isolated islets cultured in low and high glucose to determine the stimulation index (SI_OCR, Fig. 6A). The SI_OCR of islets from young organ donors was significantly higher (2.87) compared to islets from intermediate (1.80, p = 0.0212) and aged donors (1.69, p = 0.0228) (Fig. 6B). Response of islet OCR in the first 7.5 min (%ΔOCR_7.5 min/ΔOCR_max) after a glucose challenge provides insight into cellular metabolism. As expected, islets from young organ donors displayed a rapid increase in OCR (27.9% of maximum) within 7.5 min of exposure to high glucose (Fig. 6C, upper). Conversely, islets from intermediate aged and old donors showed a less rapid increase in OCR after glucose challenge (18.6%, p = 0.0610 and 0.26%, p = 0.0111 respectively versus young donor islets). These differences persisted over time. The 15 min OCR (%ΔOCR_15min/ΔOCR_max) post-glucose challenge was 69.4% of maximum for islets from young donors versus 49.2% (p = 0.2363 compared to young islets) for islets from intermediate aged donors and 33.4% for islets from old donors (p = 0.0610 compared to young islets) (Fig. 6C, bottom).
Fig. 6.
Glucose-mediated metabolic flux is less in islets from older organ donors. (A) OCR of isolated islets measured in low followed by high glucose. (B) SI_OCR in 10–20 week-old donors (2.87, n = 4) was significantly higher in comparison to 21–40 week-old (1.80, p = 0.0212, n = 4) and > 41 week-old donors (1.69, p = 0.0228, n = 3). (C) Responsiveness of islet cell metabolism to high glucose calculated as %ΔOCR_7.5 min/ΔOCR_max and %ΔOCR_15min/ ΔOCR_max demonstrated decreased metabolic flux with advancing donor age.
3.7. Islet grafts from old donors showed lower viability than those from young donors
To prospectively assess the effect of donor age on the islet viability following transplantation, we performed syngeneic transplantion of 600 LUC (+) islets into a subcutaneous site in LUC (−) recipients employing an O2 transporting device as reported (Komatsu et al., 2018). Measurement of viability on post-transplant day 7 showed that islets from young donors (10–20 week-old) tended to have greater viability compared to islets from old donors (> 41 week-old) (p = 0.4055, Supplemental Fig. 2A and B).
4. Discussion
Herein, we investigated the role that organ donor age played on isolated islet yield, function and integrity. We found that isolated islet yield significantly decreased according to the age of the donor. Importantly, age functioned in a dose-dependent manner to decrease islet viability, glucose sensitivity, insulin release and OCR. These data indicate that age may be an important factor contributing to the variability in results from pre-clinical studies of islet transplantation. They also point to the need in pre-clinical experiments to rigorously age-match donor organs to improve uniformity in islet function post-transplantation.
In the pancreas of LEW rats, beta cell mass rapidly increases by 7 months of age and continues to gradually increase until 20 months (Montanya et al., 2000). The period of rapid increase in pancreatic beta cell mass (~7 month of age and 500 g body weight) corresponds to our data of increased islet yield from pancreata from animals with a body weight up to 500 g. Similarly, islet yield from pancreata of Wistar and LEW rat donors was maximum at body weights of 451–500 g (Tze and Tai, 1982). Whole pancreas weight increases in relation to age and body weight and islets become larger and hyperplastic (Montanya et al., 2000; Reaven et al., 1987). Together these studies indicated that the islet yield from donors > 500 g weight decreased regardless of increased pancreas weight, which is consistent to our data (Fig. 2A). However, it remains unknown why islet yield decreases when organs are from donors > 500 g weight. We hypothesized that this was due to increased fibrotic infiltration of islets. Hyperplastic islets from older donors contain increased fibrous matrix with islets divided into multiple nodules (Dillberger, 1994; Hajdu et al., 1968; Hajdu and Rona, 1967; Imaoka et al., 2007). Digestion of pancreas tissue by collagenase is the key step in the islet isolation (Lacy and Kostianovsky, 1967) and ideally lyses collagen only on the islet surface to acquire well-demarcated, sphere-shaped islets. However, the infiltrated fibrotic tissue in the hyperplastic islets may be susceptible to digestion, leading to smaller, fragmented islets. Consistent with this hypothesis, isolated islet shape quantification confirmed that fragmentation is increased in islets from older, heavier donors.
In addition to exerting a negative effect on isolated islet yield, viability and integrity, donor age impacted islet function. Standard GSIS and OCR analysis found islets from young donors out performed islets from older donors in all categories. Not only was the maximum islet glucose response less with donor age, so was the rate of response. That is, islets from older donors secreted less insulin and took longer to do so after glucose challenge. Similarly, islets from aged Sprague-Dawley rats displayed a decrease of insulin secretory function, as measured by GSIS, compared to islets from younger donors (Reaven et al., 1979). Related to this, aged Wister rats showed glucose intolerance and increased islet apoptosis compared to younger animals (Gu et al., 2012). Insulin secretion requires aerobic glycolysis and alters KATP channel activity and depolarization (Schuit et al., 1997; Gregg et al., 2016). It is not surprising then that islets with slower insulin secretion also showed decreased OCR. Others noted a relationship between donor age and islet functional response in Wister rats; a ten minute exposure to high glucose stimulated more islets to secrete insulin from young versus old animals (Perfetti et al., 1995). In people glucose intolerance is age-related being more common in the elderly (Chen et al., 1985; Karve and Hayward, 2010). The slower response of islets from older donors to a glucose challenge that we observed provides an in vitro example of clinical glucose intolerance. Changes in mitochondrial respiration, cell membrane electrical potential and calcium handling have been linked to age-related decreases in islet function (Gregg et al., 2016; Westacott et al., 2017; Li et al., 2014). However, the age-related loss in capacity noted in adult islets is reversed in islets from neonates that show less insulin response to the glucose (Smith et al., 2018), and after birth increase insulin release on glucose challenge.
By way of caveats, some issues arise as a result of the culture conditions we employed. Specifically, isolated islets were cultured overnight to permit cell recovery and mimic the clinical scenario. We utilized CMRL-based culture media with 0.5% HSA, which is the same media employed in our clinical protocol. This media has a lower albumin concentration compared to conventional cell culture media to minimize contamination with non-recipient-derived albumin. However, while acceptable for brief culture intervals, this restricted media may not be appropriate for long-term islet culture.
Although our study focused on LEW rats the results support those in other settings. Isolated islets from human cadaveric donors demonstrated a negative correlation between donor age and islet function as measured by GSIS (Ihm et al., 2006). Not only donor age, but also BMI, pancreas condition and enzyme digestion are important factors for isolated islet yield (Niclauss et al., 2011; Ponte et al., 2007; Nano et al., 2005; Hanley et al., 2008). In translational studies, the diabetes reversal rate in mice transplanted with human islets was significantly higher in animals that received islets from young donors compared to animals transplanted with islets from old donors (Ihm et al., 2006). Related to this, a North American Islet Donor Score age > 75 years is considered unfavorable and predicts less clinical success (Wang et al., 2016). Additionally, organ donor age negatively correlates with glucose challenge-mediated changes in C-peptide levels following islet allotransplantation (Ihm et al., 2006). In dogs fractional islet volume is negatively correlated with age (Saladino and Getty, 1972; van der Burg et al., 1994). Among the neonatal, juvenile and adult porcine islets, adult porcine islets demonstrated the highest functionality (Smith et al., 2018; Nagaraju et al., 2015). Thus, islet yield and quality are influenced by aging among different species and in people.
5. Conclusions
Islet yield in rat pancreatic islet isolations was negatively correlated with organ donor age. Islet morphology, function, and metabolism, were also all negatively impacted by advanced donor organ age. These biological and physiological data are important, not only for the experimental rat islet transplantation studies, but also for clarification of the aging process of pancreatic islets.
Supplementary Material
Acknowledgements
NG and HK designed the study, collected and analyzed data, and wrote the manuscript. MS and LM collected data. YM and HK reviewed and edited the manuscript. This study was supported by a grant from the Nora Eccles Treadwell Foundation to YM.
Abbreviations:
- FBS
fetal bovine serum
- GSIS
Glucose-stimulated insulin secretion
- HBSS
Hanks’ balanced salt solution
- LEW rats
Lewis rats
- SI
stimulation index
- T1D
Type 1 diabetes
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.exger.2019.110739.
Declaration of competing interest
The authors have no conflicts of interests to report.
References
- Brissova M, Fowler MJ, Nicholson WE, et al. , 2005. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J. Histochem. Cytochem. 53 (9), 1087–1097. [DOI] [PubMed] [Google Scholar]
- Casey JJ, Lakey JR, Ryan EA, et al. , 2002. Portal venous pressure changes after sequential clinical islet transplantation. Transplantation 74 (7), 913–915. [DOI] [PubMed] [Google Scholar]
- Chen M, Bergman RN, Pacini G, et al. , 1985. Pathogenesis of age-related glucose intolerance in man: insulin resistance and decreased beta-cell function. J. Clin. Endocrinol. Metab. 60 (1), 13–20. [DOI] [PubMed] [Google Scholar]
- Chiang JL, Kirkman MS, Laffel LM, et al. , 2014. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 37 (7), 2034–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan BJ, Faas MM, Spijker H, et al. , 2004. Factors influencing isolation of functional pancreatic rat islets. Pancreas 29 (1), e15–e22. [DOI] [PubMed] [Google Scholar]
- Dillberger JE, 1994. Age-related pancreatic islet changes in Sprague-Dawley rats. Toxicol. Pathol. 22 (1), 48–55. [DOI] [PubMed] [Google Scholar]
- Gregg T, Poudel C, Schmidt BA, et al. , 2016. Pancreatic beta-cells from mice offset age-associated mitochondrial deficiency with reduced KATP channel activity. Diabetes 65 (9), 2700–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Du Y, Liu Y, et al. , 2012. Effect of aging on islet beta-cell function and its mechanisms in Wistar rats. Age (Dordr.) 34 (6), 1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdu A, Rona G, 1967. Morphological observations on spontaneous pancreatic islet changes in rats. Diabetes 16 (2), 108–110. [DOI] [PubMed] [Google Scholar]
- Hajdu A, Herr F, Rona G, 1968. The functional significance of a spontaneous pancreatic islet change in aged rats. Diabetologia 4 (1), 44–47. [DOI] [PubMed] [Google Scholar]
- Hanley SC, Paraskevas S, Rosenberg L, 2008. Donor and isolation variables predicting human islet isolation success. Transplantation 85 (7), 950–955. [DOI] [PubMed] [Google Scholar]
- Hirshberg B, Rother KI, Digon BJ 3rd, et al. , 2003. Benefits and risks of solitary islet transplantation for type 1 diabetes using steroid-sparing immunosuppression: the National Institutes of Health experience. Diabetes Care 26 (12), 3288–3295. [DOI] [PubMed] [Google Scholar]
- Ihm SH, Matsumoto I, Sawada T, et al. , 2006. Effect of donor age on function of isolated human islets. Diabetes 55 (5), 1361–1368. [DOI] [PubMed] [Google Scholar]
- Imaoka M, Satoh H, Furuhama K, 2007. Age- and sex-related differences in spontaneous hemorrhage and fibrosis of the pancreatic islets in Sprague-Dawley rats. Toxicol. Pathol. 35 (3), 388–394. [DOI] [PubMed] [Google Scholar]
- Ito T, Itakura S, Todorov I, et al. , 2010. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation 89 (12), 1438–1445. [DOI] [PubMed] [Google Scholar]
- Karve A, Hayward RA, 2010. Prevalence, diagnosis, and treatment of impaired fasting glucose and impaired glucose tolerance in nondiabetic U.S. adults. Diabetes Care 33 (11), 2355–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SC, Han DJ, Kang CH, et al. , 2005. Analysis on donor and isolation-related factors of successful isolation of human islet of Langerhans from human cadaveric donors. Transplant. Proc. 37 (8), 3402–3403. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Kang D, Medrano L, et al. , 2016a. Isolated human islets require hyperoxia to maintain islet mass, metabolism, and function. Biochem. Biophys. Res. Commun. 470 (3), 534–538. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Omori K, Parimi M, et al. , 2016b. Determination of islet viability using a zinc-specific fluorescent dye and a semi-automated assessment method. Cell Transplant. 25 (10), 1777–1786. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Kang DY, Lin H, et al. , 2016c. MEMS oxygen transport device for islet transplantation in the subcutaneous site. Micro & Nano Letters 11 (10), 632–635. [Google Scholar]
- Komatsu H, Cook CA, Gonzalez N, et al. , 2018. Oxygen transporter for the hypoxic transplantation site. Biofabrication 11 (1), 015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu H, Rawson J, Medrano L, et al. , 2019. Optimizing temperature and oxygen supports long-term culture of human islets. Transplantation 103 (2), 299–306. [DOI] [PubMed] [Google Scholar]
- Lacy PE, Kostianovsky M, 1967. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 16 (1), 35–39. [DOI] [PubMed] [Google Scholar]
- Leitao CB, Tharavanij T, Cure P, et al. , 2008. Restoration of hypoglycemia awareness after islet transplantation. Diabetes Care 31 (11), 2113–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Trifunovic A, Kohler M, et al. , 2014. Defects in beta-cell Ca2+ dynamics in ageinduced diabetes. Diabetes 63 (12), 4100–4114. [DOI] [PubMed] [Google Scholar]
- Montanya E, Nacher V, Biarnes M, et al. , 2000. Linear correlation between beta-cell mass and body weight throughout the lifespan in Lewis rats: role of beta-cell hyperplasia and hypertrophy. Diabetes 49 (8), 1341–1346. [DOI] [PubMed] [Google Scholar]
- Nagaraju S, Bottino R, Wijkstrom M, et al. , 2015. Islet xenotransplantation: what is the optimal age of the islet-source pig? Xenotransplantation 22 (1), 7–19. [DOI] [PubMed] [Google Scholar]
- Nano R, Clissi B, Melzi R, et al. , 2005. Islet isolation for allotransplantation: variables associated with successful islet yield and graft function. Diabetologia 48 (5), 906–912. [DOI] [PubMed] [Google Scholar]
- Niclauss N, Bosco D, Morel P, et al. , 2011. Influence of donor age on islet isolation and transplantation outcome. Transplantation 91 (3), 360–366. [DOI] [PubMed] [Google Scholar]
- Papas KK, Bellin MD, Sutherland DE, et al. , 2015. Islet oxygen consumption rate (OCR) dose predicts insulin independence in clinical islet autotransplantation. PLoS One 10 (8), e0134428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti R, Rafizadeh CM, Liotta AS, et al. , 1995. Age-dependent reduction in insulin secretion and insulin mRNA in isolated islets from rats. Am. J. Phys. 269 (6 Pt 1), E983–E990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podczeck F, Newton JM, 1994. A shape factor to characterize the quality of spheroids. J. Pharm. Pharmacol. 46 (2), 82–85. [DOI] [PubMed] [Google Scholar]
- Ponte GM, Pileggi A, Messinger S, et al. , 2007. Toward maximizing the success rates of human islet isolation: influence of donor and isolation factors. Cell Transplant. 16 (6), 595–607. [DOI] [PubMed] [Google Scholar]
- Reaven EP, Gold G, Reaven GM, 1979. Effect of age on glucose-stimulated insulin release by the beta-cell of the rat. J. Clin. Invest. 64 (2), 591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven EP, Curry DL, Reaven GM, 1987. Effect of age and sex on rat endocrine pancreas. Diabetes 36 (12), 1397–1400. [DOI] [PubMed] [Google Scholar]
- Ricordi C, Lacy PE, Scharp DW, 1989. Automated islet isolation from human pancreas. Diabetes 38 (Suppl. 1), 140–142. [DOI] [PubMed] [Google Scholar]
- Rother KI, Harlan DM, 2004. Challenges facing islet transplantation for the treatment of type 1 diabetes mellitus. J. Clin. Invest. 114 (7), 877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EA, Lakey JR, Paty BW, et al. , 2002. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes 51 (7), 2148–2157. [DOI] [PubMed] [Google Scholar]
- Saladino CF, Getty R, 1972. Quantitative study on the islets of langerhans of the beagle as a function of age. Exp. Gerontol. 7 (2), 91–97. [DOI] [PubMed] [Google Scholar]
- Schuit F, De Vos A, Farfari S, et al. , 1997. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J. Biol. Chem. 272 (30), 18572–18579. [DOI] [PubMed] [Google Scholar]
- Shapiro AM, Lakey JR, Ryan EA, et al. , 2000. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 343 (4), 230–238. [DOI] [PubMed] [Google Scholar]
- Shapiro AM, Ricordi C, Hering BJ, et al. , 2006. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 355 (13), 1318–1330. [DOI] [PubMed] [Google Scholar]
- Smith KE, Purvis WG, Davis MA, et al. , 2018. In vitro characterization of neonatal, juvenile, and adult porcine islet oxygen demand, beta-cell function, and transcriptomes. Xenotransplantation 25 (6), e12432. [DOI] [PubMed] [Google Scholar]
- Sweet IR, Gilbert M, Scott S, et al. , 2008. Glucose-stimulated increment in oxygen consumption rate as a standardized test of human islet quality. Am. J. Transplant. 8 (1), 183–192. [DOI] [PubMed] [Google Scholar]
- Tze WJ, Tai J, 1982. Effect of body weight on islet isolation in rats. Transplantation 34 (1), 68. [DOI] [PubMed] [Google Scholar]
- van der Burg MP, Guicherit OR, Frolich M, et al. , 1994. Impact of donor-related variables on islet isolation outcome in dogs. Diabetologia 37 (1), 111–114. [DOI] [PubMed] [Google Scholar]
- Wang LJ, Kin T, O‘Gorman D, et al. , 2016. A multicenter study: North American islet donor score in donor pancreas selection for human islet isolation for transplantation. Cell Transplant. 25 (8), 1515–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westacott MJ, Farnsworth NL, St Clair JR, et al. , 2017. Age-dependent decline in the coordinated [Ca(2+)] and insulin secretory dynamics in human pancreatic islets. Diabetes 66 (9), 2436–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters GH, van Suylichem PT, van Deijnen JH, et al. , 1990. Factors influencing the isolation process of islets of Langerhans. Horm. Metab. Res. Suppl. 25, 20–26. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.