Abstract
Background
Carotid patch angioplasty may reduce the risk of acute occlusion or long‐term restenosis of the carotid artery and subsequent ischaemic stroke in people undergoing carotid endarterectomy (CEA). This is an update of a Cochrane Review originally published in 1995 and updated in 2008.
Objectives
To assess the safety and efficacy of routine or selective carotid patch angioplasty with either a venous patch or a synthetic patch compared with primary closure in people undergoing CEA. We wished to test the primary hypothesis that carotid patch angioplasty results in a lower rate of severe arterial restenosis and therefore fewer recurrent strokes and stroke‐related deaths, without a considerable increase in perioperative complications.
Search methods
We searched the Cochrane Stroke Group trials register, CENTRAL, MEDLINE, Embase, two other databases, and two trial registries in September 2021.
Selection criteria
Randomised controlled trials and quasi‐randomised trials comparing carotid patch angioplasty with primary closure in people undergoing CEA.
Data collection and analysis
Two review authors independently assessed eligibility and risk of bias; extracted data; and determined the certainty of evidence using the GRADE approach. Outcomes of interest included stroke, death, significant complications related to surgery, and artery restenosis or occlusion during the perioperative period (within 30 days of the operation) or during long‐term follow‐up.
Main results
We included 11 trials involving 2100 participants undergoing 2304 CEA operations. The quality of trials was generally poor. Follow‐up varied from hospital discharge to five years. Compared with primary closure, carotid patch angioplasty may make little or no difference to reduction in risk of any stroke during the perioperative period (odds ratio (OR) 0.57, 95% confidence interval (CI) 0.31 to 1.03; P = 0.063; 8 studies, 1769 participants; very low‐certainty evidence), but may lower the risk of any stroke during long‐term follow‐up (OR 0.49, 95% CI 0.27 to 0.90; P = 0.022; 7 studies, 1332 participants; very low‐certainty evidence). In the included studies, carotid patch angioplasty resulted in a lower risk of ipsilateral stroke during the perioperative period (OR 0.31, 95% CI 0.15 to 0.63; P = 0.001; 7 studies, 1201 participants; very low‐certainty evidence), and during long‐term follow‐up (OR 0.32, 95% CI 0.16 to 0.63; P = 0.001; 6 studies, 1141 participants; very low‐certainty evidence). The intervention was associated with a reduction in the risk of any stroke or death during long‐term follow‐up (OR 0.59, 95% CI 0.42 to 0.84; P = 0.003; 6 studies, 1019 participants; very low‐certainty evidence). In addition, the included studies suggest that carotid patch angioplasty may reduce the risk of perioperative arterial occlusion (OR 0.18, 95% CI 0.08 to 0.41; P < 0.0001; 7 studies, 1435 participants; low‐certainty evidence), and may reduce the risk of restenosis during long‐term follow‐up (OR 0.24, 95% CI 0.17 to 0.34; P < 0.00001; 8 studies, 1719 participants; low‐certainty evidence). The studies recorded very few arterial complications, including haemorrhage, infection, cranial nerve palsies and pseudo‐aneurysm formation, with either patch or primary closure. We found no correlation between the use of patch angioplasty and the risk of either perioperative or long‐term stroke‐related death or all‐cause death rates.
Authors' conclusions
Compared with primary closure, carotid patch angioplasty may reduce the risk of perioperative arterial occlusion and long‐term restenosis of the operated artery. It would appear to reduce the risk of ipsilateral stroke during the perioperative and long‐term period and reduce the risk of any stroke in the long‐term when compared with primary closure. However, the evidence is uncertain due to the limited quality of included trials.
Plain language summary
Patch angioplasty versus primary closure for carotid endarterectomy
Key messages Eleven trials involving 2100 people now suggest a possible benefit from using a special procedure (patch angioplasty) following carotid endarterectomy.
What is carotid endarterectomy? About 20% of strokes result from narrowing of the carotid artery (the main artery supplying blood to the brain). A narrowed carotid artery can be treated with a surgical procedure called carotid endarterectomy, which involves cutting the artery open and removing fatty substances called plaques. This widens the artery and so reduces the risk of stroke. However, there is a small possibility that the operation itself can cause a stroke.
What is primary closure, and what is patch angioplasty? After removing the plaques from the artery, the surgeon can simply bring the two edges of the hole together and sew it closed (primary closure), or can close the hole with a patch, sewing the edges of the hole to the edges of the patch to widen the artery further (patch angioplasty). This patch can be made of synthetic material or can be a piece of the patient's own vein.
What did we want to find out? We wanted to find out if people who have people patch angioplasty after carotid endarterectomy – compared with those who have primary closure – have less chance of having a stroke or dying in the short or long term after the operation, or have less chance of their artery narrowing again, without suffering many more complications around the time of the operation.
What did we do? We searched for studies that compared patch angioplasty and primary closure in people who had carotid endarterectomy. We compared and summarised the results, and rated our confidence in the evidence, based on factors such as study methods and study size.
What did we find? We found 11 studies involving 2100 participants undergoing 2304 carotid endarterectomy operations. The studies were conducted all around the world.
Main results Patch angioplasty lowered the risk of stroke in the short and long term after surgery compared with primary closure. Patch angioplasty may reduce the risk of the artery becoming blocked and the risk of the patient having a stroke or dying in the long term.
Main limitations of the evidence Some studies monitored participants for up to five years, while others stopped monitoring participants after they left hospital. This makes us uncertain about the evidence.
How up to date is this evidence? The evidence is current to September 2021.
Summary of findings
Summary of findings 1. Main comparison of patch angioplasty versus primary closure.
| Patch angioplasty versus primary closure following carotid endarterectomy | ||||||
| Patient or population: people undergoing carotid endarterectomy, whether initial indication for endarterectomy was symptomatic or asymptomatic carotid disease Settings: hospitals with carotid centres Intervention: any type of patch angioplasty Comparison: primary closure | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) |
Relative effect (95% CI) |
No. of participants (studies) |
Certainty of the evidence (GRADE) |
Comments | |
| Assumed risk | Corresponding risk | |||||
| Primary closure | Patch angioplasty | |||||
| Any stroke (fatal or non‐fatal; contralateral, ipsilateral or brainstem; haemorrhage or infarct) within 30 days of operation | Total | OR 0.57 (0.31, 1.03) | 1769 (8 studies) | ⊕⊝⊝⊝
Very lowa,b |
No studies could be blinded for surgeons due to the nature of the intervention. | |
| 32 per 1000 | 19 per 1000 | |||||
| Ipsilateral stroke (fatal or non‐fatal; haemorrhage or infarct) within 30 days of operation | Total | OR 0.31 (0.15, 0.63) | 1201 (7 studies) | ⊕⊝⊝⊝
Very lowa,b |
No studies could be blinded for surgeons due to the nature of the intervention. | |
| 79 per 1000 | 14 per 1000 | |||||
| Occlusion of the operated artery within 30 days of operation | Total | OR 0.18 (0.08, 0.41) | 1435 (7 studies) | ⊕⊕⊝⊝
Lowa,b,d |
No studies could be blinded for surgeons due to the nature of the intervention. | |
| 31 per 1000 | 5 per 1000 | |||||
| Any stroke (fatal or non‐fatal; contralateral, ipsilateral or brainstem; haemorrhage or infarct) during long‐term follow‐up, including events during first 30 days | Total | OR 0.49 (0.27, 0.90) | 1332 (7 studies) | ⊕⊝⊝⊝
Very lowa,b |
No studies could be blinded for surgeons due to the nature of the intervention. | |
| 46 per 1000 | 23 per 1000 | |||||
| Ipsilateral stroke (fatal or non‐fatal; haemorrhage or infarct) during long‐term follow‐up, including events during first 30 days | Total | OR 0.32 (0.16, 0.63) | 1141 (6 studies) | ⊕⊝⊝⊝
Very lowa,b |
No studies could be blinded for surgeons due to the nature of the intervention. | |
| 48 per 1000 | 16 per 1000 | |||||
| Any stroke or death during long‐term follow‐up, including events during first 30 days | Total | OR 0.59 (0.42, 0.84) | 1019 (6 studies) | ⊕⊝⊝⊝
Very lowa,b |
No studies could be blinded for surgeons due to the nature of the intervention. | |
| 296 per 1000 | 130 per 1000 | |||||
| Restenosis (> 50%) or occlusion of operated artery during long‐term follow‐up, including events during first 30 days | Total | OR 0.24 (0.17, 0.34) | 1719 (8 studies) | ⊕⊕⊝⊝
Lowa,c,d |
No studies could be blinded for surgeons due to the nature of the intervention. | |
| 138 per 1000 | 43 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for serious risk of bias concerns as studies did not blind surgeons and participants and did not report blinding of outcome assessment (AbuRahma 1996; Al‐Rawi 2006; De Vleeschauwer 1987; Eikelboom 1988; İyigün 2019; Katz 1994; Lord 1989; Mannheim 2005; Myers 1994; Pratesi 1986; Ranaboldo 1993); and due to selective reporting (AbuRahma 1996 Al‐Rawi 2006; De Vleeschauwer 1987; Eikelboom 1988; İyigün 2019; Katz 1994; Lord 1989; Mannheim 2005; Myers 1994; Pratesi 1986; Ranaboldo 1993), incomplete outcome data (Eikelboom 1988; Katz 1994; Myers 1994; Ranaboldo 1993), and inadequate allocation concealment and random sequence generation (De Vleeschauwer 1987; Katz 1994). bDowngraded one level for imprecision due to wide confidence intervals (low event rates) and lack of events (De Vleeschauwer 1987; Eikelboom 1988 Katz 1994; Lord 1989). cDowngraded one level for inconsistency due to true heterogeneity according to the I² statistic and P value, confidence interval overlap, difference in point estimate, and between‐study variance (Analysis 2.6). dUpgraded one level for large effects (Analysis 1.6).
Background
Description of the condition
Large, well‐conducted randomised controlled trials (RCTs) have shown that carotid endarterectomy (CEA) reduces the risk of stroke in people with symptomatic, severe (greater than 70%) internal carotid artery stenosis (Rothwell 2003). There is also some evidence that it is beneficial for people with asymptomatic stenosis of 60% to 90% who are at high risk of late ipsilateral stroke (according to imaging characteristics); and that the rate of perioperative stroke or death associated with this procedure is less than 3%, with postoperative life expectancy exceeding five years (Aboyans 2018; ACAS 1995; Halliday 2004; Naylor 2018). Eleven RCTs found that CEA reduces long‐term mortality and ipsilateral stroke in people with asymptomatic carotid stenosis when compared with the best medical treatment (Barkat 2018).
Description of the intervention
Possible closure techniques after CEA include primary closure (direct suture) and patch angioplasty, where the surgeon closes the operated artery by suturing a venous or synthetic patch to the edges of the incision. The aim of carotid patch angioplasty is to maintain the diameter of the operated artery after CEA. However, the effect of this technique in terms of clinical outcomes remains unclear.
How the intervention might work
There are relatively few high‐quality prospective studies of restenosis following CEA, and these studies are difficult to compare because of differences in the definitions of stenosis and lengths of follow‐up. However, it appears that carotid restenosis of greater than 50% diameter reduction (as detected by Doppler ultrasound) occurs in 6% to 36% of patients during long‐term follow‐up (Bernstein 1990; Knudsen 1990; Ouriel 1987; Volteas 1994; Zierler 1982). Most stenoses occur in the first two years (Frericks 1998). Carotid patch angioplasty may reduce the risk of restenosis, and so reduce the long‐term risk of recurrent ipsilateral ischaemic stroke (Awad 1989; Ouriel 1987).
However, there is also some evidence that this technique does not improve perioperative and long‐term outcomes in people who have asymptomatic restenosis after CEA (Chung 2020; Edenfield 2019; Liu 2020). The risk of symptomatic restenosis appears to be much lower at around 2% to 4% (Das 1985; Frericks 1998). Patch angioplasty may also be associated with certain perioperative risks: routine patching involves a longer carotid occlusion time, two suture lines instead of one, and the use of a patch material, all of which may increase the risk of early re‐occlusion, arterial rupture, infection, or pseudoaneurysm formation (Awad 1989; Bernstein 1992). In addition, vein harvesting (required for venous patch angioplasty) may be associated with complications such as neuralgia, haemorrhage, and infection.
Why it is important to do this review
A survey of Great Britain and Ireland in a trial showed considerable variations among surgeons in the use of carotid patching, which may reflect uncertainty regarding its benefits: 65% of surgeons always used patching and 26% used selective patching dependent on internal carotid artery diameter (Harrison 2012). Analysis of the ECST trial data showed significant heterogeneity in frequency of use of patch angioplasty at an individual surgeon, national, and international level (ECST 1991). In total, 790 people who had undergone CEA had restenosis data at one and five years in the International Carotid Stenting Study (ICSS). Altogether, 64.7% had patch angioplasty and 29.4% primary closure. The cumulative incidence of more than 50% restenosis at one year and five years was 18.9% and 25.9 percent, respectively, in those who had patch angioplasty, versus 26.1%, and 37.2% in those who had primary closure (Cheng 2021). Given the uncertainty implied by such variation in practice, it is clearly important to establish whether routine or selective patching is more effective than, and as safe as, primary closure. Randomised controlled trials provide the most reliable evidence on which to base these assessments. We therefore performed a systematic review of all such trials that compared routine or selective patching with primary closure.
Note: the first version of this review included trials comparing one type of patch with another. These trials have now been included in a separate Cochrane Review (Bond 2003).
This is an update of a Cochrane Review originally published in 1995 and updated in 2009.
Objectives
To assess the safety and efficacy of routine or selective carotid patch angioplasty with either a venous patch or a synthetic patch compared with primary closure in people undergoing CEA. We wished to test the primary hypothesis that carotid patch angioplasty results in a lower rate of severe arterial restenosis and therefore fewer recurrent strokes and stroke‐related deaths, without a considerable increase in perioperative complications.
Methods
Criteria for considering studies for this review
Types of studies
We sought to identify all randomised trials of carotid patching. We included quasi‐randomised trials, in which allocation to different treatment regimens was not adequately concealed (e.g. allocation by alternation, date of birth, hospital number, day of the week, or by using an open random number list), and foreknowledge of treatment allocation might lead to biased treatment allocation and exaggerated treatment effects (Schulz 1995).
Types of participants
We included trials that enrolled anybody undergoing CEA, whether the initial indication for endarterectomy was symptomatic or asymptomatic carotid disease.
Types of interventions
We sought to identify all trials comparing routine carotid patch angioplasty (i.e. patching attempted in all participants) with primary closure. Any type of patch material was eligible (e.g. vein graft, Dacron, or polytetrafluoroethylene (PTFE)). We also intended to include trials comparing selective patch angioplasty (i.e. patching attempted only in people thought likely to benefit) with primary closure. Trials that compared one type of patch with another are included in a separate Cochrane Review (Bond 2003).
Types of outcome measures
We aimed to extract from each trial the number of participants originally allocated to each treatment group to allow an intention‐to‐treat analysis. Within each treatment group we then extracted the number of participants.
Primary outcomes
Any stroke (fatal or non‐fatal; contralateral, ipsilateral or brainstem; haemorrhage or infarct) within 30 days of the operation and during long‐term follow‐up
Stroke ipsilateral to the endarterectomy site (fatal or non‐fatal; haemorrhage or infarct) within 30 days of the operation and during long‐term follow‐up
Secondary outcomes
Death within 30 days of the operation and during long‐term follow‐up. We tried to classify each death as stroke‐related or not.
Occlusion of the operated artery within 30 days of the operation
Significant complications related to surgery, such as haemorrhage or rupture of the artery, infection of the endarterectomy site, cranial nerve palsy or pseudoaneurysm formation
Restenosis greater than 50% or occlusion of the operated artery during long‐term follow‐up
Search methods for identification of studies
We did not apply any language restriction in the searches, and we arranged translations of all possibly relevant publications where necessary.
Electronic searches
The Cochrane Stroke Group's Information Specialist searched the Cochrane Stroke Group's Trials Register (last search 13 September 2021). We also updated electronic searches and handsearched additional issues of relevant journals as follows.
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 8), in the Cochrane Library (searched 13 September 2021; Appendix 1)
MEDLINE Ovid (1946 to 13 September 2021; Appendix 2)
Embase Ovid (1980 to 13 September 2021; Appendix 3)
Web of Science Core Collection (last searched 13 September 2021; Appendix 4).
Searching other resources
We searched for ongoing trials in the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 13 September 2021), and the World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 13 September 2021), in accordance with Cochrane's MECIR standards (Higgins 2016).
We handsearched the following journals including conference supplements.
Annals of Surgery (1981 to 13 September 2021)
Annals of Vascular Surgery (1994 to 13 September 2021)
Cardiovascular Surgery (now Vascular; 1994 to 13 September 2021)
European Journal of Vascular Surgery (now European Journal of Vascular and Endovascular Surgery; 1987 to 13 September 2021)
Journal of Vascular Surgery (1994 to 13 September 2021
Stroke (1994 to 13 September 2021)
We reviewed the reference lists of all relevant studies, and we contacted experts in the field to identify further published and unpublished studies.
For the previous version of the review, we handsearched the following journals including conference supplements.
American Journal of Surgery (1994 to 13 September 2021)
British Journal of Surgery (1985 to 13 September 2021)
World Journal of Surgery (1978 to 13 September 2021)
We handsearched abstracts of the following meetings for the years 1995 to 13 September 2021.
AGM of the Vascular Surgical Society (UK)
AGM of the Association of Surgeons of Great Britain and Ireland
American Heart Association Stroke Conference
Annual Meeting of the Society for Vascular Surgery (USA)
The European Stroke Conference
Data collection and analysis
Selection of studies
Three review authors (SO, TB, and KR) independently read the titles and abstracts of records obtained from the searches, excluded obviously irrelevant studies, and selected those trials that met the inclusion criteria. We obtained the full‐text articles of potentially relevant studies on primary closure and patch angioplasty after CEA. The same three review authors screened all documents and independently extracted data, including details of methods, participants, setting, context, interventions, outcomes, results, publications, and investigators. We resolved all disagreements through discussion and performed meta‐analysis using Review Manager 5 (Review Manager 2020). The same three review authors also assessed the methodological quality of each trial.
Data extraction and management
Two review authors (SO, TB) independently reviewed and assessed all trials (so each trial received two assessments) and double‐checked all data extracted. We recorded data on:
randomisation method;
blinding of clinical and Doppler assessments;
whether outcomes were reported for all participants originally randomised to each group irrespective of whether they received the operation they were allocated to; and
number of participants lost to follow‐up.
We sought data on the number of outcome events for all participants originally randomised to allow an intention‐to‐treat analysis. For the 11 included trials, we extracted and cross‐checked all data. In addition, we extracted details about:
the trial participants;
inclusion and exclusion criteria;
comparability of the treatment and control groups for important prognostic factors;
type of patch;
type of anaesthetic;
use of shunts; and
use of antiplatelet therapy during follow‐up.
If any of the above data were not available from the publication, we sought further information by correspondence with the trialists. We resolved all disagreements through discussion with other review authors (BS, KR).
Assessment of risk of bias in included studies
Two review authors (SO, BS) independently assessed risk of bias (high risk, low risk, unclear risk) using the Cochrane risk of bias tool (RoB 1), and presented the results in risk of bias tables (Higgins 2011). We resolved all disagreements through discussion. Risks of bias included:
random sequence generation and allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias); and
incomplete outcome data (attrition bias).
Measures of treatment effect
Using Review Manager 5 (Review Manager 2020), we carried out statistical analyses to determine the effect estimates and describe the magnitude of the intervention effect in terms of differences in outcomes between the two groups. To quantify the effect of the intervention on our outcomes, all of which were dichotomous, we used odds ratios (ORs) together with the corresponding 95% confidence intervals (CIs).
We calculated proportional risk reductions based on weighted estimate of the OR using the Peto method (Deeks 2021). For rare events, the Peto one‐step OR method was found to be the least biased and most powerful method, with the best CI coverage, provided there was no substantial imbalance between treatment and comparator group sizes within studies, and treatment effects were not exceptionally large (Deeks 2021).
Unit of analysis issues
All selected trials included both unilateral and bilateral carotid endarterectomies. It was therefore possible for some participants to have primary closure on one side and carotid patching on the other side (İyigün 2019). Indeed, in one trial, if a participant required bilateral endarterectomies, each artery had to have a different procedure (Myers 1994). In most trials, the artery rather that the participant was randomised to a particular procedure (AbuRahma 1996; Al‐Rawi 2006; De Vleeschauwer 1987; Lord 1989; Mannheim 2005; Myers 1994; Pratesi 1986; Ranaboldo 1993); and only three trials were participant‐randomised (Eikelboom 1988; İyigün 2019; Katz 1994). Arterial randomisation is helpful for measuring outcomes such as haemorrhage, occlusion, ipsilateral events or complications within 30 days of surgery (as most participants waited at least this period between the first and second operation), but not for long‐term clinical outcome events such as death or any stroke. In participants with bilateral endarterectomies who had both patching and primary closure, it would not be possible to relate death or stroke to one particular procedure. Therefore, in trials where it was possible for a participant to have both procedures, we analysed death and any stroke only in those who had unilateral procedures or the same procedure in both arteries. These data were available from the authors in all except two trials (Lord 1989; Myers 1994). In trial, the authors no longer had the original data on participants with unilateral operations, so we excluded it from the analyses of these outcomes (Lord 1989). In the other trial, the number of participants undergoing unilateral endarterectomies was reported, and we were able to estimate the number of clinical events per participant in each group using the number of events per artery and the total number of deaths that were reported (Myers 1994).
We performed a separate analysis of only strokes ipsilateral to the operated artery for all arteries. However, the more clinically relevant outcome is the total number of strokes and not just ipsilateral strokes. We analysed arterial complications – such as occlusion, haemorrhage of the endarterectomy site, restenosis, infection at the operation site, or pseudoaneurysm formation – for all arteries rather than participants. Analyses based on arteries assumed that, in participants who had bilateral endarterectomies, outcome events in each carotid artery were independent. This is unlikely to be true, but as relatively few participants had bilateral procedures (10% overall), we felt it reasonable to perform such analyses. However, their results should be interpreted with caution.
Outcomes analysed per artery were:
ipsilateral stroke within 30 days of the operation and during long‐term follow‐up;
occlusion of the operated artery within 30 days of the operation;
rupture or haemorrhage of the endarterectomy site;
infection of the endarterectomy site;
cranial nerve palsy;
complications requiring reintervention within 30 days of the operation; and
restenosis or occlusion of the operated artery during long‐term follow‐up.
The outcomes analysed per participant included:
any stroke;
stroke‐related death;
all‐cause death; and
any stroke or death, all within 30 days of the operation and during long‐term follow‐up.
Dealing with missing data
When data were missing, we contacted the corresponding author or co‐author at the address given in the publication. If we received no response, we searched for the study group via the internet and contacted group members for missing information. In total, 58 participants were lost to follow‐up (Table 2). For the intention‐to‐treat analyses and the main analyses, we assumed that participants who were lost to follow‐up did not have an outcome event.
1. Number of cases lost to follow‐up.
| Study | Total participants | Total operations | Lost to FU at 30 days | Lost to FU at end | Number of exclusions | Cross‐over: patch to primary | Cross‐over: primary to patch | ||
| Patch angioplasty | Primary closure |
Patch angioplasty |
Primary closure | ||||||
| AbuRahma 1996 | 357 | 399 | 0 | 0 | 0 | 0 | 4 | 0 | 0 |
| Al‐Rawi 2006l | 315 | 328 | 0 | 0 | 7 | 8 | 10 | Data not available | Data not available |
| Eikelboom 1988 | 126 | 129 | 0 | 0 | 10 participants lost to Doppler FU but not clinical FU | 7 to Doppler FU but not clinical FU | 0 | 3 | 3 |
| İyigün 2019 | 137 | 137 | Data not available | Data not available | Data not available | Data not available | Data not available | Data not available | Data not available |
| Katz 1994 | 87 | 100 | 0 | 0 | 5 | 7 | 0 | 0 | 0 |
| Lord 1989 | 123 | 140 | 0 | 0 | 0 | 0 | 4 | Between 0 and 4 | Between 0 and 4 |
| Mannheim 2005 | 404 | 422 | 0 | 0 | Data not available | Data not available | Data not available | 0 | 0 |
| Myers 1994 | 136 (109 after exclusion of 27 people undergoing obligatory vein patching) | 152 (122 analysed, as 30 had obligatory vein patches) | 0 | 0 | 6 | 8 | 30 operations had obligatory vein patch closure and 16 participants had both sides done (total 46) | 0 | 0 |
| Pratesi 1986 | 90 | 100 | Data not available | Data not available | Data not available | Data not available | Data not available | Data not available | Data not available |
| Ranaboldo 1993 | 199 | 213 | 0 | 0 | 5 | 12 | 0 | 0 | 0 at 30‐day FU but 4 at 1‐year FU |
| De Vleeschauwer 1987 | 126 | 174 | 0 | 0 | Data not available | Data not available | 0 | 0 | 0 |
FU: follow‐up
Assessment of heterogeneity
We assessed between‐study heterogeneity using the I² statistic, which examines the percentage of total variation across studies due to heterogeneity rather than to chance (Higgins 2020). Thresholds for interpretation of the I² statistic can be misleading, in that the importance of inconsistency depends on several factors. A rough guide to interpretation in the context of meta‐analyses of randomised trials is as follows.
Values of I² over 75% indicate a high level of heterogeneity.
Values of I² equal or below 75% indicate a low‐to‐moderate level of heterogeneity.
An I² value of 0% indicates no heterogeneity.
Assessment of reporting biases
We performed an extensive literature search, and we are confident that we have identified all major relevant trials. We also contacted experts in this field. We searched for trials published in all languages, and we arranged translation of all possibly relevant publications when required. In addition, we searched all relevant ongoing clinical trials from ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) portal, and we handsearched relevant journals and reference lists. We had planned to compare study protocols with final study reports to evaluate selective reporting of outcomes. We used funnel plots to assess publication bias because more than 10 studies were included. However, none of the trials reported limits outside the 95% CI.
Data synthesis
We calculated proportional risk reductions based on a weighted estimate of the OR using the Peto method (Deeks 2021). Since all the outcome events assessed were rare, the ORs quoted will be similar to the relative risks. The Peto one‐step OR method was found to be the least biased and most powerful method for the rare event rate study (Deeks 2021). We calculated absolute risk reductions from the crude risks of each outcome in all trials combined (Deeks 2021).
Subgroup analysis and investigation of heterogeneity
We planned to explore heterogeneity by conducting the following subgroup analyses.
Age (younger than 65 years versus 65 to 74 years versus 75 years or older)
Sex (men versus women)
Diabetes versus no diabetes
Hypertension versus no hypertension
Previous myocardial infarction or angina versus no coronary artery disease
Peripheral arterial disease (PAD) versus no PAD
Current smoker versus non‐smoker
Asymptomatic versus symptomatic carotid stenosis
Contralateral carotid stenosis versus unilateral carotid stenosis
Contralateral carotid occlusion versus no occlusion
Preoperative antiplatelet therapy versus no antiplatelet therapy
Intraoperative shunt versus no shunt
Irregular or ulcerated symptomatic carotid plaque versus smooth plaque on the pre‐randomisation angiogram
We planned to use an established method for subgroup analyses (Deeks 2001). We will fulfil planned subgroup analyses when more studies are included in a single analysis, with sufficient information for each subgroup.
Analyses were stratified by patch type within the patch angioplasty group. Tests for overall effect and subgroup differences by patch type included:
venous patch versus primary closure;
synthetic patch versus primary closure; and
synthetic or venous patch versus primary closure.
Each subgroup was analysed for 10 perioperative and six long‐term outcomes; therefore, we examined a total of 16 groups of data. We did not investigate potential effect modifiers via subgroup analysis.
Sensitivity analysis
Before we had clearly defined the methods for this systematic review, we planned to undertake different sensitivity analyses to explore effects of certain methodological features, excluding, in turn, studies at high risk of selection bias, detection bias, attrition bias, reporting bias, and other bias (not peer reviewed).
Given that foreknowledge of treatment allocation might lead to biased treatment allocation and exaggerated treatment effects (Schulz 1995), in the original version of this review we performed separate sensitivity analyses of trials with adequate allocation concealment and those with inadequate allocation concealment. For the intention‐to‐treat analyses and the main analyses, we assumed that participants who were lost to follow‐up did not have an outcome event.
However, the current review authors considered these analysis to be unreasonably critical, so did not carry out any sensitivity analyses for this update.
Summary of findings and assessment of the certainty of the evidence
We created a summary of findings table for the seven main comparisons with GRADE Profiler 3.6 (GRADEpro GDT 2015), which imports data from RevMan 5 (Review Manager 2020). This table presents the results and the certainty of the evidence (high, moderate, low or very low) on the main outcomes according to the GRADE criteria (Schünemann 2011). We included the following important outcomes.
Any stroke (fatal or non‐fatal; contralateral, ipsilateral or brainstem; haemorrhage or infarct) within 30 days of the operation
Ipsilateral stroke (fatal or non‐fatal; haemorrhage or infarct) within 30 days of the operation
Occlusion of the operated artery within 30 days of the operation
Any stroke (fatal or non‐fatal; contralateral, ipsilateral or brainstem; haemorrhage or infarct) during long‐term follow‐up, including events during the first 30 days
Ipsilateral stroke (fatal or non‐fatal; haemorrhage or infarct) during long‐term follow‐up, including events during the first 30 days
Any stroke or death during long‐term follow‐up, including events during the first 30 days
Restenosis (greater than 50%) or occlusion of operated artery during long‐term follow‐up, including events during the first 30 days
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
We identified 27,755 records through database searching and 85 additional records from other sources in 2021. After automated and manual deduplication, we screened the titles and abstracts of 10,762 records, and retrieved the full‐text articles of 24 that we considered potentially eligible. Of these 24, we excluded 23 after reading the full‐text articles, because they did not meet our inclusion criteria. We included one new study in this update (İyigün 2019). The review now includes 11 studies of routine carotid patch angioplasty with either a venous patch or a synthetic patch compared to primary closure in people undergoing CEA. There are no studies awaiting classification or ongoing studies. See Figure 1.
1.
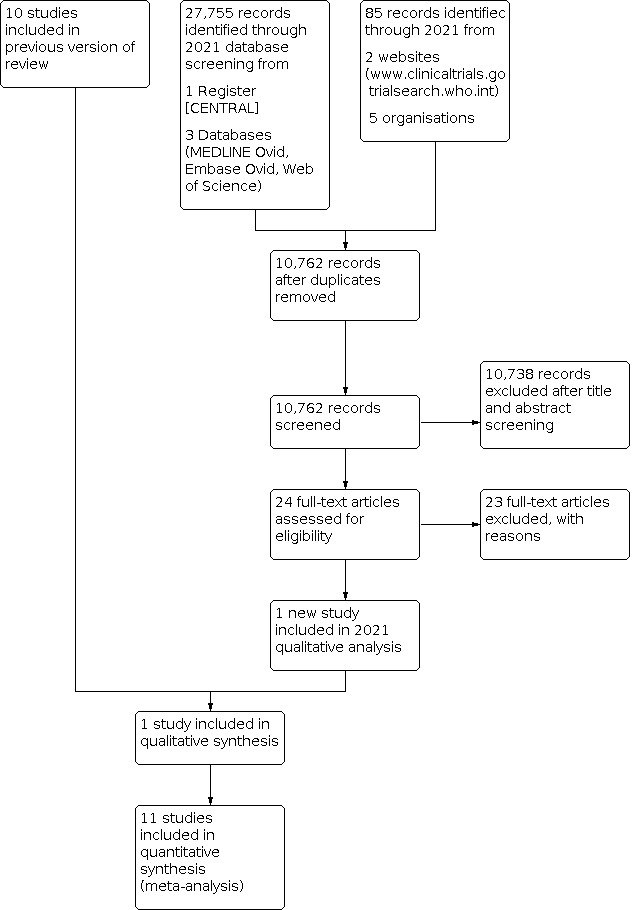
Study flow diagram.
Included studies
In the 2008 version of this review, we identified 10 trials that fulfilled the eligibility criteria (AbuRahma 1996; Al‐Rawi 2006; De Vleeschauwer 1987; Eikelboom 1988; Katz 1994; Lord 1989; Mannheim 2005; Myers 1994; Pratesi 1986; Ranaboldo 1993). For this update, we identified one additional RCT of sufficient standard that met our eligibility criteria (İyigün 2019). We did not identify any additional ongoing trials, and there are no trials presently awaiting assessment. In 2008, we added data from five new publications (AbuRahma 1998; AbuRahma 1999; AbuRahma 2000; Clagett 1989; De Letter 1994), to three included studies (AbuRahma 1996; Eikelboom 1988; Myers 1994): we added data from AbuRahma 1998, AbuRahma 1999, and AbuRahma 2000 to AbuRahma 1996; data from Clagett 1989 to Myers 1994; and data from De Letter 1994 to Eikelboom 1988.
All included trials compared routine patching with primary closure. Three of the trials used only saphenous vein patches (De Vleeschauwer 1987; Eikelboom 1988; Myers 1994), and three used only synthetic patches (Al‐Rawi 2006; Katz 1994; Mannheim 2005). Four trials used both vein and synthetic (PTFE or Dacron) patches (AbuRahma 1996; Lord 1989; Pratesi 1986; Ranaboldo 1993), but in three of these (İyigün 2019; Pratesi 1986; Ranaboldo 1993), results were not separated by the type of patch that the participant received. In the original review (Counsell 1996), the results of Lord 1989 were divided into vein patching versus control and synthetic patching versus control. However, we realised in hindsight that presenting the data in this way meant the number of operations with primary closure were counted twice in the overall analysis. Therefore, for this update, we have analysed these four trials as any patch versus no patch.
One trial included a group that was allocated to obligatory patching without randomisation (Myers 1994). We did not include this group of participants in our analysis. All operations in all trials were performed under general anaesthetic, and most also involved shunting. Most participants in all the trials were prescribed long‐term antiplatelet or anticoagulant therapy after the operation. All the trials with follow‐up beyond hospital discharge included Doppler ultrasound of the arteries during follow‐up (AbuRahma 1996; Al‐Rawi 2006; De Vleeschauwer 1987; Eikelboom 1988; İyigün 2019; Katz 1994; Lord 1989; Mannheim 2005; Myers 1994; Pratesi 1986; Ranaboldo 1993), and one also included intravenous digital subtraction angiography (Eikelboom 1988).
The average age of participants involved in these trials was about 67 years and there were approximately twice as many men as women. All trials included participants with asymptomatic carotid disease, with the proportion varying from 8% (Ranaboldo 1993), to 51% of all participants (Mannheim 2005). All trials compared routine patching in all participants in the treatment group with primary closure. In four trials, narrow carotid arteries were excluded before randomisation on the basis that it was not safe to close these with primary closure: Myers 1994 excluded 38/163 arteries because the internal diameter (assessed during the operation) was less than 5 mm; Katz 1994 excluded 1/110 patients because a preoperative angiogram showed the arterial diameter to be less than 3.5 mm; AbuRahma 1996 excluded 12/399 carotid endarterectomies because the internal carotid artery diameter was less than 4 mm; and Mannheim 2005 excluded 24/422 operations because the carotid artery internal diameter was too small or because interposition grafting was required. Only one participant randomised to primary closure required a patch because the artery was considered to be too narrow (Eikelboom 1988). Seven other participants randomised to primary closure required patching either because the degree of stenosis was very high (two participants; Ranaboldo 1993) or because the artery became occluded postoperatively (five participants; AbuRahma 1996). Seven participants from the patch groups did not receive a patch either because no vein was available (two participants; Eikelboom 1988), because rapid closure was required due to possible ischaemic changes shown by intraoperative electroencephalogram (one participant; Eikelboom 1988), or for reasons not described in the publications (four participants; Lord 1989). Follow‐up varied from until hospital discharge (Lord 1989), to five years (Eikelboom 1988; Myers 1994). The treatment groups were comparable for important prognostic factors in all trials that provided these data.
Excluded studies
We excluded two trials in the original review (Counsell 1996), and one trial in this update, for the reasons set out below.
For one unpublished trial (Gale 1988), an intention‐to‐treat analysis was not possible because one‐third of the 300 randomised participants did not receive their allocated operation and the results for these participants were not available.
In the discussion section of one of the included trials (Eikelboom 1988), the authors mention an unpublished trial (Hertzer 1987). However, the principal investigator of this unpublished trial informed us that it was not randomised or quasi‐randomised.
In this review update, we excluded one recent trial of patch angioplasty because it compared patch angioplasty and autoarterial remodelling of bifurcation of the common carotid artery (Ignatenko 2019).
Risk of bias in included studies
There were several significant biases in most of the included trials (Figure 2; Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Only four trials had adequate random sequence generation (AbuRahma 1996; Al‐Rawi 2006; Myers 1994; Ranaboldo 1993). Allocation concealment was adequate in only six trials (AbuRahma 1996; Al‐Rawi 2006; Lord 1989; Mannheim 2005; Myers 1994; Ranaboldo 1993), which used numbered, sealed, opaque envelopes as the method of randomisation. De Vleeschauwer 1987 used envelope randomisation, but the envelopes were not numbered or opaque, leading to a high risk of selection bias. Two trials used quasi‐random allocation based on the participant's hospital number (Eikelboom 1988), or social security number (Katz 1994).
Adequate blinding is important to reduce bias in the detection of certain outcome events. For instance, ultrasound assessment of restenosis should ideally be assessed blind, although experienced practitioners may be able to detect the slight dilatation associated with a carotid patch even when blinded. Correspondence with the authors confirmed that clinical assessment was definitely blinded in only three trials (AbuRahma 1996; De Vleeschauwer 1987; Ranaboldo 1993), but that restenosis was assessed blind in all except two trials (Katz 1994; Lord 1989).
As mentioned previously, one of the main flaws in eight of the trials was that a participant undergoing bilateral CEA could be randomised twice and have their two carotid arteries randomised to different treatment groups (AbuRahma 1996; Al‐Rawi 2006; De Vleeschauwer 1987; Lord 1989; Mannheim 2005; Myers 1994; Pratesi 1986; Ranaboldo 1993). In these trials, it was unclear from the published reports exactly how many participants (as opposed to arteries) were randomised to each group and how many participants with bilateral endarterectomies had different procedures on each artery (Table 2). We were able to obtain these data from all except one trial (Lord 1989). Demographic features (e.g. age, sex) as well as results were usually reported for each randomised artery rather than per participant. Participants undergoing bilateral CEA were randomised twice and had their two carotid arteries randomised to same or different treatment groups. True intention‐to‐treat analysis was only possible for three trials after we obtained additional data from the trial authors (AbuRahma 1996; Al‐Rawi 2006; Ranaboldo 1993). In the other trials, data on participants lost to follow‐up were not available, and in one trial (Lord 1989), four participants who did not have the procedure that they were randomised to receive were excluded from the analysis.
The newly included trial provided insufficient information to adequately assess risk of bias (İyigün 2019).
Allocation
Four trials had adequate generation of a randomised sequence and allocation concealment, and we judged them at low risk of bias (AbuRahma 1996; Al‐Rawi 2006; Myers 1994; Ranaboldo 1993). Seven trials did not report the random sequence generation method, and we considered them at unclear risk of bias (De Vleeschauwer 1987; Eikelboom 1988; İyigün 2019; Katz 1994; Lord 1989; Mannheim 2005; Pratesi 1986). Four trials did not report the allocation concealment method, and we judged them at unclear risk of bias (Eikelboom 1988; İyigün 2019; Katz 1994; Pratesi 1986). One included study was at high risk of selection bias because the envelopes used in randomisation were not numbered or opaque (De Vleeschauwer 1987)
Blinding
No studies reported blinding of participants. In Al‐Rawi 2006, a single surgeon performed primary closure and patch angioplasty, which eliminates the bias inherent in potential differences in experience or technique between surgeons. Because of the nature of the intervention, surgeons could not be blinded in any trial, and we therefore considered all of them at high risk of performance bias.
Incomplete outcome data
We judged four trials at high risk of attrition bias due to the large number of participants lost to follow‐up (Eikelboom 1988; Katz 1994; Myers 1994; Ranaboldo 1993). Few participants were lost to follow‐up in three trials (AbuRahma 1996; Al‐Rawi 2006; Lord 1989).
Four trials did not report the number of participants who were lost to follow‐up or who crossed over from one treatment arm to another (De Vleeschauwer 1987; İyigün 2019; Mannheim 2005; Pratesi 1986). We considered these trials at unclear risk of bias.
Selective reporting
Most trials did not report the findings for all study outcomes. This was inappropriate and is likely to have introduced reporting bias into the results. We therefore considered them at unclear risk of reporting bias (Al‐Rawi 2006; De Vleeschauwer 1987; Eikelboom 1988; İyigün 2019; Katz 1994; Lord 1989; Mannheim 2005; Myers 1994; Pratesi 1986; Ranaboldo 1993). Only in AbuRahma 1996 did the study authors publish findings on all study outcomes.
Other potential sources of bias
We judged all the included trials to be at low risk of 'other' bias as we identified no other sources of bias.
Effects of interventions
See: Table 1
We included data from 11 trials (2100 participants, 2304 CEAs) in this review. We performed meta‐analyses for the following 10 perioperative outcomes (occurring within 30 days of the operation).
Any stroke (fatal or non‐fatal; contralateral, ipsilateral or brainstem; haemorrhage or infarct; eight trials)
Stroke‐related death (seven trials)
Ipsilateral stroke (fatal or non‐fatal; haemorrhage or infarct; seven trials)
All‐cause death (nine trials)
Any stroke or death (eight trials)
Occlusion of operated artery (seven trials)
Rupture or haemorrhage of endarterectomy site (nine trials)
Infection of endarterectomy site (seven trials)
Cranial nerve palsy (four trials)
Complications requiring reintervention (seven trials)
We also performed meta‐analyses for the following six long‐term outcomes.
Any stroke (fatal or non‐fatal; contralateral, ipsilateral or brainstem; haemorrhage or infarct; seven trials)
Stroke‐related death (six trials)
Ipsilateral stroke (fatal or non‐fatal; haemorrhage or infarct; six trials)
All‐cause death (seven trials)
Any stroke or death (six trials)
Restenosis (greater that 50%) or occlusion of operated artery (eight trials)
The results presented may differ from those in the published reports where we attained additional information from the authors. There was no significant heterogeneity in any of the analyses as the values of I²were less than 75% in all cases.
Outcomes within 30 days of operation
Stroke
Any stroke (fatal or non‐fatal; contralateral, ipsilateral or brainstem; haemorrhage or infarct)
The overall perioperative risk of any stroke was 2.5% (45/1769). Patching may reduce the odds of any stroke, but the evidence is very uncertain (OR 0.57, 95% CI 0.31 to 1.03; P = 0.063; 8 studies, 1769 participants; very low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Patch versus primary closure: outcomes within 30 days of operation, Outcome 1: Any stroke (fatal or non‐fatal; contralateral, ipsilateral or brainstem; haemorrhage or infarct)
There was no heterogeneity among trials of the same type of patch material (I²= 0), but there was low‐to‐moderate heterogeneity among subgroups of the different patch materials (I²= 60.3). No trials recorded the severity of stroke in terms of residual disability. Only three strokes were fatal (one in the patch group, two in the primary closure group).
Stroke‐related death
Stroke‐related deaths were very rare (0.2%). In the seven trials that included this outcome (AbuRahma 1996; De Vleeschauwer 1987; Eikelboom 1988; Katz 1994; Lord 1989; Myers 1994; Mannheim 2005), there was only one stroke‐related death in the patch angioplasty group and two in the primary closure group. Patching did not reduce the odds of stroke‐related death, but the evidence is very uncertain (OR 0.47, 95% CI 0.05 to 4.56; P = 0.52; 7 studies, 1441 participants; very low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Patch versus primary closure: outcomes within 30 days of operation, Outcome 2: Stroke‐related death
Ipsilateral stroke (fatal or non‐fatal, haemorrhage or infarct)
If effective, patching would be expected to reduce mainly stroke ipsilateral to the operated artery. Seven trials recorded the number of ipsilateral strokes per artery randomised (AbuRahma 1996; De Vleeschauwer 1987; Eikelboom 1988; Katz 1994; Lord 1989; Myers 1994; Ranaboldo 1993), although in several instances we had to contact the study authors for additional data. The newly added trial did not provide this information (İyigün 2019). In total, 2.8% (33/1201) of operations were associated with an ipsilateral stroke. Carotid patching may reduce the relative odds of perioperative ipsilateral stroke, but the evidence is very uncertain (OR 0.31, 95% CI 0.15 to 0.63; P = 0.001; 7 studies, 1201 participants; very low‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Patch versus primary closure: outcomes within 30 days of operation, Outcome 3: Ipsilateral stroke (fatal or non‐fatal; haemorrhage or infarct)
All‐cause death
The evidence is uncertain about the effect of patch angioplasty versus primary closure on all‐cause death rate. There were only 11 deaths among 1869 participants (0.6%) in the nine trials with available data (AbuRahma 1996; Al‐Rawi 2006; De Vleeschauwer 1987; Eikelboom 1988; Katz 1994; Mannheim 2005; Myers 1994; Pratesi 1986; Ranaboldo 1993), so it remains unclear whether patching is associated with a higher or lower perioperative mortality than primary closure (OR 0.62, 95% CI 0.18 to 2.09; P = 0.44; 9 studies, 1869 participants; very low‐certainty evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1: Patch versus primary closure: outcomes within 30 days of operation, Outcome 4: Death from all causes
Stroke or death
Patching did not appear to reduce the combined stroke and death rate, but the evidence is very uncertain (OR 0.58, 95% CI 0.33 to 1.01; P = 0.056; 8 studies, 1769 participants; very low‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Patch versus primary closure: outcomes within 30 days of operation, Outcome 5: Any stroke or death
Arterial complications
As noted in the Methods section, these results should be interpreted with caution because, in participants who underwent bilateral endarterectomies, outcomes in each artery were probably not independent. We were unable to determine how many participants with bilateral endarterectomies had outcome events in both arteries.
Occlusion of operated artery
Four trials did not provide data on perioperative arterial occlusion (De Vleeschauwer 1987; İyigün 2019; Mannheim 2005; Pratesi 1986). Of the other trials, four used ultrasound (Duplex) scanning (AbuRahma 1996; Al‐Rawi 2006; Katz 1994; Ranaboldo 1993), two used intravenous digital subtraction angiography (Eikelboom 1988; Lord 1989), and one used ocular pneumoplethysmography (Myers 1994). At least 26 of the randomised arteries were not assessed within 30 days of the operation (14 patch, 12 primary closure) and these arteries were assumed to be not occluded for the purpose of this analysis. Patching may result in a large reduction (82%) in the odds of arterial occlusion (OR 0.18, 95% CI 0.08 to 0.41; P < 0.0001; 7 studies, 1435 participants; low‐certainty evidence; Analysis 1.6). The CI for this result is wide, because the estimate was based on small numbers of events (4/794 (0.5%) patching versus 20/641 (3.1%) primary closure), but the OR indicates a large effect. The consequences for the participants (in terms of stroke‐related death and non‐fatal stroke) resulting from this reduction in arterial occlusion are unclear.
1.6. Analysis.

Comparison 1: Patch versus primary closure: outcomes within 30 days of operation, Outcome 6: Occlusion of operated artery
Rupture or haemorrhage of endarterectomy site
The overall risk of rupture and haemorrhage in all participants combined was low (1.5%). Patching does not appear to reduce the risk of this outcome, but the evidence is very uncertain (OR 1.24, 95% CI 0.61 to 2.54; P = 0.55; 9 studies, 2031 participants; very low‐certainty evidence; Analysis 1.7). None of the arterial haemorrhages were associated with a fatal or major stroke.
1.7. Analysis.

Comparison 1: Patch versus primary closure: outcomes within 30 days of operation, Outcome 7: Rupture or haemorrhage of endarterectomy site
Infection of endarterectomy site
Only two trials included the outcome of infection at the endarterectomy site (Katz 1994; Mannheim 2005). These occurred in two participants in the synthetic patching group and six participants in the primary closure group. Patching does not appear to reduce local infection compared with primary closure, but the evidence is very uncertain (OR 0.38, 95% CI 0.09 to 1.54; P = 0.17; 7 studies, 1563 participants; very low‐certainty evidence; Analysis 1.8). There was low‐to‐moderate heterogeneity among subgroups of the different patch materials (I²= 61).
1.8. Analysis.

Comparison 1: Patch versus primary closure: outcomes within 30 days of operation, Outcome 8: Infection of endarterectomy site
Cranial nerve palsy
Five trials supplied data on this outcome (AbuRahma 1996; İyigün 2019; Katz 1994; Mannheim 2005; Myers 1994), and in one of these, no outcomes occurred (Katz 1994). The risk of nerve palsy was low (2.87%). Compared with primary closure, patching does not appear to affect the risk of cranial nerve palsy, but the evidence is very uncertain (OR 0.59, 95% CI 0.30 to 1.19; P = 0.14; 5 studies, 1184 participants; very low‐certainty evidence; Analysis 1.9).
1.9. Analysis.

Comparison 1: Patch versus primary closure: outcomes within 30 days of operation, Outcome 9: Cranial nerve palsy
Complications requiring reintervention
Seven studies recorded the number of complications (occlusion, haemorrhage, infection) that required reintervention within 30 days of the first operation (AbuRahma 1996; De Vleeschauwer 1987; Eikelboom 1988; Katz 1994; Lord 1989; Myers 1994; Ranaboldo 1993). Carotid patching may reduce complications requiring reintervention, but the evidence is very uncertain (OR 0.35, 95% CI 0.16 to 0.79; P = 0.01; 7 studies, 1281 participants; very low‐certainty evidence; Analysis 1.10).
1.10. Analysis.

Comparison 1: Patch versus primary closure: outcomes within 30 days of operation, Outcome 10: Complications requiring reintervention
Outcomes during long‐term follow‐up, including events during first 30 days
One trial followed up participants for 30 days only (Lord 1989). Because the participants of Lord 1989 were not followed up long‐term, we excluded it from these analyses. In the remaining trials, at least 56 participants (28 patch, 28 primary closure) were lost to follow‐up. We considered these participants to be event‐free for the main analyses. The shortest follow‐up in trials included in these analyses was one year (Al‐Rawi 2006; De Vleeschauwer 1987; Ranaboldo 1993).
Stroke
Any stroke (fatal or non‐fatal; ipsilateral, contralateral or brainstem; infarct or haemorrhage)
Patching may reduce the risk of any stroke during follow‐up, but the evidence is very uncertain (OR 0.49, 95% CI 0.27 to 0.90; P = 0.022; 7 studies, 1332 participants; very low‐certainty evidence; Analysis 2.1). A similar reduction was seen in fatal strokes (OR 0.27 95% CI 0.05 to 1.6; P = 0.15; 6 studies, 1019 participants; very low‐certainty evidence; Analysis 2.2), but this was based on only five events. There was no heterogeneity among trials of venous patch groups or synthetic and venous patch groups (I²= 0), but there was a low‐to‐moderate level of heterogeneity among trials of only synthetic patch closure (I²= 44), and a low‐to‐moderate level of heterogeneity among subgroups of the different patch materials (I²= 72.5).
2.1. Analysis.

Comparison 2: Patch versus primary closure: outcomes during long‐term follow‐up, including events during first 30 days, Outcome 1: Any stroke (fatal or non‐fatal; contralateral, ipsilateral or brainstem; haemorrhage or infarct)
2.2. Analysis.

Comparison 2: Patch versus primary closure: outcomes during long‐term follow‐up, including events during first 30 days, Outcome 2: Stroke‐related death
Ipsilateral stroke (fatal or non‐fatal; haemorrhage or infarct)
Thirty‐three strokes were definitely ipsilateral, and one other stroke was assumed ipsilateral (Eikelboom 1988). Patching may reduce the risk of ipsilateral stroke during long‐term follow‐up, but the evidence is very uncertain (OR 0.32, 95% CI 0.16 to 0.63; P = 0.001; 6 studies, 1141 participants; very low‐certainty evidence; Analysis 2.3).
2.3. Analysis.

Comparison 2: Patch versus primary closure: outcomes during long‐term follow‐up, including events during first 30 days, Outcome 3: Ipsilateral stroke (fatal or non‐fatal; haemorrhage or infarct)
All‐cause death
A total of 143 participants died during follow‐up (10.7%). Even if all participants lost to follow‐up were assumed to be alive, patching did not appear to reduce the risk of death, but the evidence is very uncertain (OR 0.78, 95% CI 0.54 to 1.12; P = 0.18; 7 studies, 1332 participants; very low‐certainty evidence; Analysis 2.4). Again, as outlined above, few of these deaths were directly attributable to stroke.
2.4. Analysis.

Comparison 2: Patch versus primary closure: outcomes during long‐term follow‐up, including events during first 30 days, Outcome 4: All‐cause death
Any stroke or death
The combined rate of any stroke or death was 13% in the patch group and 20.6% in the primary closure group. Patching may be associated with a reduction in the risk of any stroke or death, but the evidence is very uncertain (OR 0.59, 95% CI 0.42 to 0.84; P = 0.003; 6 studies, 1019 participants; very low‐certainty evidence; Analysis 2.5).
2.5. Analysis.

Comparison 2: Patch versus primary closure: outcomes during long‐term follow‐up, including events during first 30 days, Outcome 5: Any stroke or death
Arterial complications
As noted in the Methods section, these results should be interpreted with caution because, in participants who underwent bilateral endarterectomies, outcomes in each artery were probably not independent. We were unable to identify how many participants with bilateral endarterectomies had outcome events in both arteries.
Restenosis (greater than 50%) or occlusion of operated artery
Patching may result in a large reduction in the risk of arterial occlusion or restenosis, but the evidence is uncertain (OR 0.24, 95% CI 0.17 to 0.34; P < 0.00001; 8 studies, 1719 participants; low‐certainty evidence; Analysis 2.6). There was low‐to‐moderate heterogeneity among trials of venous patch angioplasty only and among trials of synthetic patch angioplasty only (I²= 15 and 66, respectively), but there was a high level of heterogeneity among trials that included both synthetic and venous patching (I²= 77), and a low‐to‐moderate level of heterogeneity among subgroups of the different patch materials (I²= 72.4).
2.6. Analysis.

Comparison 2: Patch versus primary closure: outcomes during long‐term follow‐up, including events during first 30 days, Outcome 6: Restenosis (> 50%) or occlusion of operated artery
Lack of data meant that it was not possible to correct for participants who had died during follow‐up. However, the results appear to be particularly robust and are likely to remain significant even if corrected for the small numbers who died. Another problem is that the clinical significance of a reduction in occlusion or restenosis is unknown: the important outcome from the patient's point of view is a reduction in the risk of stroke. The trial by Eikelboom and colleagues suggested that the reduction in restenosis or occlusion was confined to women, but this finding may be due to a chance subgroup effect or due to the fact that women had an increased absolute risk of restenosis and so the numbers who developed restenosis were greater (Eikelboom 1988).
Pseudoaneurysm formation
No pseudoaneurysms were documented during follow‐up of at least one year in 1141 arteries.
Discussion
Summary of main results
We included 11 trials investigating the effect of patch angioplasty compared with primary closure in people undergoing carotid endarterectomy. Meta‐analysis, involving 2100 participants (2304 CEAs), found that patch angioplasty may reduce the risk of ipsilateral stroke during the perioperative period and long‐term follow‐up, and the risk of any stroke and the combined risk of any stroke or death during long‐term follow‐up, but the evidence is uncertain. In addition, patch angioplasty may reduce perioperative occlusion and long‐term restenosis or occlusion of the operated artery, and perioperative complications requiring reintervention, but again, the evidence is uncertain. A new finding was not obtained from our review between the two techniques of closure at the endarterectomy site. However, 11 trials with the larger participants may increase the level of evidence in subset of outcome including increased the total number of the operations and reduced the imprecision. The limited evidence from this review of 11 trials suggests that carotid patch angioplasty may reduce the risk of perioperative occlusion and long‐term restenosis or occlusion of the operated artery, though the supporting evidence is of low certainty.
Overall completeness and applicability of evidence
The results of the original version of this systematic review, with six trials involving 794 participants and 882 operations, were considered to be inconclusive, although there appeared to be promising and potentially clinically important trends in favour of routine patching in terms of both short‐ and long‐term reductions in risk of ipsilateral stroke (Counsell 1996). The results were felt to be unreliable because they were based on a small number of outcome events (33 ipsilateral strokes in total), because there were a number of losses to follow‐up, and because the methodological quality of the trials was on the whole poor (Table 2). However, the updated review in 2008 included a total of 10 trials involving 1967 participants and 2157 operations (AbuRahma 1996; Al‐Rawi 2006; De Vleeschauwer 1987; Eikelboom 1988; Katz 1994; Lord 1989; Mannheim 2005; Myers 1994; Pratesi 1986; Ranaboldo 1993), and added perioperative and long‐term events (Rerkasem 2009a). The 2008 update of this review contributed additional weight to the conclusions drawn in the previous analyses (Rerkasem 2009a). In this current update, we added one new small trial (İyigün 2019), which has increased the number of events and participants in the cranial nerve injury outcome. Compared with primary closure, patch angioplasty may have little to no effect on cranial nerve palsy, though the evidence is of low certainty. One included trial, which investigated both synthetic and venous patch angioplasty compared with primary closure, demonstrated a significant reduction in ipsilateral stroke and occlusion of the operated artery during the perioperative period and long‐term follow‐up (AbuRahma 1996). However, the results are compromised by the poor quality of this trial. The results of our updated meta‐analysis suggest that using patch angioplasty during CEA may reduce ipsilateral stroke and restenosis or occlusion of the operated artery.
Quality of the evidence
The certainty of evidence for all outcomes was low to very low due to serious risk of bias concerns and imprecision.
There were significant methodological flaws in these trials that should be addressed in future trials. Many had inadequate methods of randomisation and blinding, which can seriously bias the results of trials (Schulz 1995). None of these trials could be blinded for surgeons due to the nature of the intervention. The blinding of participants and outcome assessment was not documented in all studies. It is well known that studies that have neurologists as assessors are associated with higher stroke and death rates (Rothwell 1995). Almost all trials were assessed as being at unclear risk of reporting bias. The trials were generally too small to achieve adequate statistical power and only three trials were analysed on a true intention‐to‐treat basis (AbuRahma 1996; Al‐Rawi 2006; Ranaboldo 1993), partly because there were significant losses to follow‐up. Problems arose with the randomisation of arteries rather than participants, and there was poor reporting of the numbers of ipsilateral strokes and disabling strokes in each treatment group. In addition, differences between trials with selected patient criteria, details of operative techniques, selective versus routine shunt during operation, and timing of follow‐up of participants led to inadequate certainty of evidence for each outcome.
The imprecision occurred in three secondary outcomes: perioperative occlusion of the operated artery, rupture or haemorrhage, and infection of the endarterectomy site. Though the confidence intervals were not very wide mathematically, the width was clinically important.
Potential biases in the review process
Attempts to obtain all relevant data were successful. Selection bias, performance bias, detection bias, attrition bias, and other bias were identified in all 11 trials. We searched systematically for all studies in all languages. We did not perform subgroup analyses of pooled data from included studies. We have reported all of the analyses that we performed.
Agreements and disagreements with other studies or reviews
The significant reductions in the risk of acute occlusion or long‐term restenosis with patching may be less useful than data on clinically important outcomes such as stroke. Acute occlusion, though feared, is not always associated with stroke. Similarly, restenosis detected by routine Duplex scanning may not be clinically important. In some cases, remodelling of the arterial wall after endarterectomy can be mistaken for stenosis, and in other cases, spontaneous regression of Duplex‐defined stenosis has occurred (Bernstein 1990; Ranaboldo 1993). One study showed no significant association between restenosis and recurrent neurological symptoms (Knudsen 1990), whilst in another, participants with restenosis greater than 50% had a better long‐term prognosis in terms of death or stroke than participants with no significant restenosis (Bernstein 1990).
Most surgeons agree that carotid patching can be useful in CEA, as they are faced with situations where this type of closure is either unavoidable or positively desirable, for example an artery with a very narrow internal diameter or a very long plaque (Eikelboom 1988). However, it is unclear how frequently such situations arise and how narrow an artery should be before it has to be patched. Only two trials in this review excluded narrow arteries on the grounds that they must be patched: Myers 1994 excluded 23% of arteries because they were less than 5 mm in diameter, whilst Katz 1994 excluded only 1% of arteries because they were less than 3.5 mm in diameter. In the other trials, very few participants had to cross over from primary closure to patching because the artery was deemed too narrow for primary closure. A British survey demonstrated that there is divided opinion on how often patching is required: some surgeons use it always; others rarely or never (Girn 2008). The trials of patch versus no patch included in this review tested the policy of routinely patching all arteries against a policy of never patching in participants with no definite indication for a patch. To date, no RCTs have tested the policy of selective patching of only those arteries thought to require a patch at the time of operation compared with no patching.
The potential benefit of patching may be restricted to narrow arteries (Golledge 1996). This would be analogous to CEA for symptomatic carotid stenosis where the benefit is restricted to those with severe artery stenosis (ECST 1991). We were unable to test this hypothesis because the results of the trials were not reported according to the degree of narrowing of the artery. The results of Myers 1994 (which excluded a significant number of arteries because they were less than 5 mm in diameter) were no worse than those of the other trials, which might suggest that there is little difference in the effect of patching between arteries greater than or less than 5 mm diameter. However, such indirect comparisons between trials are unreliable.
Currently, there is considerable evidence to support the use of routine or selective patching over primary closure during CEA (Cheng 2021; Counsell 1998; Naylor 2018; Rerkasem 2011). A previous meta‐analysis of 10 RCTs (2157 participants), comparing routine patching with primary closure (Rerkasem 2011), observed that:
routine patching was associated with significant reductions in perioperative ipsilateral stroke;
routine patching was associated with significant reductions in 30‐day operated artery thrombosis;
participants randomised to primary closure were three times more likely to return to theatre within 30 days; and
there was no significant difference between routine patching and routine primary closure regarding perioperative death, fatal stroke, combined death or stroke, and cranial nerve injury.
These findings are consistent with those of our review, which included one additional trial (İyigün 2019). The 2017 clinical practice guidelines of the European Society for Vascular Surgery (ESVS) recommend routine patch angioplasty over routine primary closure in people undergoing CEA (Naylor 2018). A recent study by the ICSS investigators found that long‐term restenosis at one and five years occurred more frequently after primary closure compared with patch closure in CEA operations (Cheng 2021). This conclusion supports the use of patch closure as the treatment of choice to avoid restenosis after CEA, although the study authors did not find a higher incidence of ipsilateral stroke in people who had primary closure.
Authors' conclusions
Implications for practice.
The results of this review provide some evidence to support routine patching during carotid endarterectomy (CEA), although the certainty of the evidence is low or very low and the numbers are still relatively small. No randomised controlled trials have studied the use of selective patching (such as for very narrow arteries), so no clear indication for selective patching can be given. Until more reliable evidence is available, individual surgeons (and patients) will have to interpret the current evidence and its limitations when deciding on the most appropriate approach.
Implications for research.
The potential benefit of routine patching could be clinically important, but in order to have reliable evidence on the risks and benefits of patching compared with primary closure, a large multicentre randomised controlled trial is required. This trial should concentrate on clinical outcomes (death and all strokes, particularly fatal or disabling strokes and ipsilateral strokes) as opposed to restenosis, and have long‐term follow‐up (perhaps five years). Assuming a 30‐day risk of stroke or death of 5%, the trial would need to recruit about 3000 people to have a 90% chance of detecting a reduction in the absolute risk of death or stroke to 2.5% (this number would also provide a greater than 90% chance of detecting a reduction in the risk of stroke or death at five years from 25% to 20%). Such a trial should use a secure method of randomisation and be performed on a true intention‐to‐treat basis with complete follow‐up of all participants. Participants rather than arteries should be randomised so that the number of deaths and strokes are reported on a participant basis rather than an artery basis. Clinical follow‐up should be blinded with independent assessment of strokes, preferably by neurologists (Rerkasem 2009b; Rothwell 1995). The results should be analysed according to the degree of narrowing of the artery and whether the participant has had a previous stroke or transient ischaemic attack. It would be possible to use a factorial design for such a trial so that some other procedure could be tested simultaneously, such as routine shunting. Until the benefit of carotid patching in terms of clinical outcomes for the patient is established, any future trials should include a control group of primary closure.
What's new
| Date | Event | Description |
|---|---|---|
| 13 September 2021 | New citation required but conclusions have not changed | The authorship of the review has changed. With the new trial comparing patch angioplasty closure versus primary closure in people undergoing carotid endarterectomy, the sample size and the number of outcome events of cranial nerve palsy have increased. There was no significant difference in all outcomes. The conclusion of the review has not changed. |
| 13 September 2021 | New search has been performed | We have updated the searches for this review. The searches have been complete to September 2021. We have included 1 new randomised controlled trial which published in 2021, comparing patch angioplasty closure versus primary closure in people undergoing carotid endarterectomy. The review now includes 11 randomised controlled trials with data for 2100 participants and 2304 operations. |
History
Protocol first published: Issue 3, 1996 Review first published: Issue 3, 1996
| Date | Event | Description |
|---|---|---|
| 9 September 2008 | Amended | Converted to new review format. |
| 28 February 2003 | New search has been performed | Differences between this review and the previous version: one new trial (357 patients and 399 operations), comparing primary closure with venous patching (saphenous or jugular vein) and with synthetic patching, has published its early and late results (AbuRahma 1996 and 1998) and has been included in the review. |
Acknowledgements
We would like to thank Dr Rick Bond, Professor Ali AbuRahma, Professor Ross Naylor and Professor Peter M Rothwell for their contribution to previous versions of this review. We also thank Hazel Fraser for searching the Cochrane Stroke Group trials register and for providing regular updates of newly identified trials; and Joshua Cheyne for searching the Cochrane Central Register of Controlled Trials (CENTRAL). This study was supported by the Research group in surgery, Faculty of Medicine, Thammasat University. This study was partially supported by Chiang Mai University.
Ongoing trials
If any reader is aware of any randomised trials that we have omitted, please contact Dr Saritphat Orrapin.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1MeSH descriptor: [Endarterectomy, Carotid] #2MeSH descriptor: [Carotid Arteries] #3MeSH descriptor: [Carotid Artery Diseases] #4MeSH descriptor: [Carotid Arteries] #5MeSH descriptor: [Carotid Artery Diseases] #6carotid:ti,ab,kw (Word variations have been searched) #7#4 or #5 or #66360 #8MeSH descriptor: [Endarterectomy] #9(endarterectom* or surg*):ti,ab,kw (Word variations have been searched)178437 #10#8 or #9178437 #11#7 and #102050
Appendix 2. MEDLINE Ovid search strategy
1. Endarterectomy, Carotid/ 2. exp Carotid Arteries/su [Surgery] 3. exp Carotid Artery Diseases/su [Surgery] 4. exp carotid arteries/ 5. exp carotid artery diseases/ 6. carotid.tw. 7. 4 or 5 or 6 8. endarterectomy/ 9. (endarterectom$ or surg$).tw. 10. 8 or 9 11. 7 and 10 12. 1 or 2 or 3 or 11 13. randomized controlled trial.pt. 14. controlled clinical trial.pt. 15. randomized.ab. 16. placebo.ab. 17. clinical trials as topic.sh. 18. random$.ab. 19. trial.ti. 20. or/13‐19 21. exp animals/ not humans.sh. 22. 12 and 20 23. 22 not 21 24. 202$.dp. or 20200523:20301231.(ep). [Date range for MEDLINE Ovid] 25. 23 and 24 26. limit 23 to ed=20200523‐20301231 27. 25 or 26
Appendix 3. Embase Ovid search strategy
1. carotid endarterectomy/ 2. carotid artery surgery/ 3. exp carotid artery disease/su 4. exp carotid artery/ 5. exp carotid artery disease/ 6. 4 or 5 7. artery surgery/ or endarterectomy/ or vascular surgery/ or surgery/ 8. 6 and 7 9. (carotid adj5 (endarterect$ or surgery)).tw. 10. 1 or 2 or 3 or 8 or 9 11. Clinical trial/ 12. randomized controlled trial/ 13. controlled study/ 14. randomization/ 15. random$.tw. 16. Prospective study/ 17. "Evaluation and follow up"/ or Follow up/ 18. versus.tw. 19. prospective.tw. 20. types of study/ 21. methodology/ 22. comparative study/ 23. ((intervention or experiment$) adj5 group$).tw. 24. Parallel design/ 25. intermethod comparison/ 26. (controls or control group$).tw. 27. (control$ adj trial$).tw. 28. patch$.tw. 29. or/11‐28 30. 10 and 29
Appendix 4. Search strategies for other databases
Database: Conference Proceedings Citation Index – Science (CPCI‐S) – Web of Science search strategy
TI=(carotid AND (endarterectom* OR surg*))
Database: ClinicalTrials.gov
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 19 March 2018) search strategy Interventional Studies | Endarterectomy | First posted from 23 May 2020 to 13 September 2021
Database: WHO International Clinical Trials Registry Platform (ICTRP; 13 September 2021)
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 19 March 2018) search strategy carotid AND endarterectomy OR carotid AND surgery
Data and analyses
Comparison 1. Patch versus primary closure: outcomes within 30 days of operation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Any stroke (fatal or non‐fatal; contralateral, ipsilateral or brainstem; haemorrhage or infarct) | 8 | 1769 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.57 [0.31, 1.03] |
| 1.1.1 Venous patch | 3 | 346 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.08, 1.72] |
| 1.1.2 Synthetic patch | 3 | 837 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.50, 3.07] |
| 1.1.3 Synthetic or venous patch | 2 | 586 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.11, 0.74] |
| 1.2 Stroke‐related death | 7 | 1441 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.05, 4.56] |
| 1.2.1 Venous patch | 3 | 346 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.06, 14.73] |
| 1.2.2 Synthetic patch | 2 | 509 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.2.3 Synthetic or venous patch | 2 | 586 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.46] |
| 1.3 Ipsilateral stroke (fatal or non‐fatal; haemorrhage or infarct) | 7 | 1201 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.31 [0.15, 0.63] |
| 1.3.1 Venous patch | 3 | 349 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.39 [0.09, 1.75] |
| 1.3.2 Synthetic patch | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.53 [0.05, 5.19] |
| 1.3.3 Synthetic or venous patch | 3 | 752 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.27 [0.11, 0.62] |
| 1.4 Death from all causes | 9 | 1869 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.18, 2.09] |
| 1.4.1 Venous patch | 3 | 346 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.79 [0.18, 17.57] |
| 1.4.2 Synthetic patch | 3 | 837 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 7.15] |
| 1.4.3 Synthetic or venous patch | 3 | 686 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.10, 2.24] |
| 1.5 Any stroke or death | 8 | 1769 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.58 [0.33, 1.01] |
| 1.5.1 Venous patch | 3 | 346 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.13, 2.25] |
| 1.5.2 Synthetic patch | 3 | 837 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.10 [0.45, 2.69] |
| 1.5.3 Synthetic or venous patch | 2 | 586 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.15, 0.78] |
| 1.6 Occlusion of operated artery | 7 | 1435 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.18 [0.08, 0.41] |
| 1.6.1 Venous patch | 2 | 255 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.01, 1.99] |
| 1.6.2 Synthetic patch | 2 | 428 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.06, 1.95] |
| 1.6.3 Synthetic or venous patch | 3 | 752 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.16 [0.06, 0.42] |
| 1.7 Rupture or haemorrhage of endarterectomy site | 9 | 2031 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [0.61, 2.54] |
| 1.7.1 Venous patch | 3 | 429 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.86 [0.14, 346.63] |
| 1.7.2 Synthetic patch | 3 | 850 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.39, 2.14] |
| 1.7.3 Synthetic or venous patch | 3 | 752 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.32 [0.56, 9.57] |
| 1.8 Infection of endarterectomy site | 7 | 1563 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.09, 1.54] |
| 1.8.1 Venous patch | 3 | 429 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.8.2 Synthetic patch | 2 | 522 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.09, 1.54] |
| 1.8.3 Synthetic or venous patch | 2 | 612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.9 Cranial nerve palsy | 5 | 1184 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.30, 1.19] |
| 1.9.1 Venous patch | 1 | 126 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.17, 3.50] |
| 1.9.2 Synthetic patch | 2 | 522 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.24, 2.35] |
| 1.9.3 Synthetic or venous patch | 2 | 536 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.43 [0.15, 1.25] |
| 1.10 Complications requiring reintervention | 7 | 1281 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.16, 0.79] |
| 1.10.1 Venous patch | 3 | 429 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.06, 15.64] |
| 1.10.2 Synthetic patch | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.53 [0.05, 5.19] |
| 1.10.3 Synthetic or venous patch | 3 | 752 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.12, 0.73] |
Comparison 2. Patch versus primary closure: outcomes during long‐term follow‐up, including events during first 30 days.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Any stroke (fatal or non‐fatal; contralateral, ipsilateral or brainstem; haemorrhage or infarct) | 7 | 1332 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.49 [0.27, 0.90] |
| 2.1.1 Venous patch | 3 | 346 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.42 [0.14, 1.30] |
| 2.1.2 Synthetic patch | 2 | 400 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.95 [0.59, 6.45] |
| 2.1.3 Synthetic or venous patch | 2 | 586 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.10, 0.62] |
| 2.2 Stroke‐related death | 6 | 1019 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.27 [0.05, 1.60] |
| 2.2.1 Venous patch | 3 | 346 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.05, 4.52] |
| 2.2.2 Synthetic patch | 1 | 87 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.2.3 Synthetic or venous patch | 2 | 586 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.04] |
| 2.3 Ipsilateral stroke (fatal or non‐fatal; haemorrhage or infarct) | 6 | 1141 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.32 [0.16, 0.63] |
| 2.3.1 Venous patch | 3 | 429 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.42 [0.12, 1.47] |
| 2.3.2 Synthetic patch | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.53 [0.05, 5.19] |
| 2.3.3 Synthetic or venous patch | 2 | 612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.10, 0.62] |
| 2.4 All‐cause death | 7 | 1332 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.54, 1.12] |
| 2.4.1 Venous patch | 3 | 346 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.45, 1.42] |
| 2.4.2 Synthetic patch | 2 | 400 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.39, 2.05] |
| 2.4.3 Synthetic or venous patch | 2 | 586 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.38, 1.26] |
| 2.5 Any stroke or death | 6 | 1019 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.42, 0.84] |
| 2.5.1 Venous patch | 3 | 346 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.40, 1.20] |
| 2.5.2 Synthetic patch | 1 | 87 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.23, 1.66] |
| 2.5.3 Synthetic or venous patch | 2 | 586 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.30, 0.86] |
| 2.6 Restenosis (> 50%) or occlusion of operated artery | 8 | 1719 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.24 [0.17, 0.34] |
| 2.6.1 Venous patch | 3 | 429 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.17, 0.66] |
| 2.6.2 Synthetic patch | 3 | 678 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.22, 0.98] |
| 2.6.3 Synthetic or venous patch | 2 | 612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.15 [0.09, 0.25] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AbuRahma 1996.
| Study characteristics | ||
| Methods | Allocation concealment: computer‐generated sealed envelopes (arteries randomised) Blinding of outcome assessment: duplex and clinical FU blinding unclear Cross‐overs: 4 participants in vein group underwent primary closure; 3 jugular vein participants had saphenous vein patching. Exclusion during trial: the 4 participants who crossed over from vein patch to primary closure Participants lost to FU: 3% | |
| Participants | Country: USA Number of participants: 357 Number of operations: 399, in 3 arms (130 vein, 134 PTFE, 135 primary closure) Sex: 50% male Mean age: 68 years % asymptomatic carotid disease: 33% % stenosis: unknown Comparability: age, sex, vascular risk factors and % asymptomatic disease similar in each group | |
| Interventions | Treatment: PTFE patch or alternating saphenous vein patch (from ankle) and jugular vein patch Control: primary closure % shunted: routine shunting and general anaesthesia for all Medication: 325 mg daily aspirin started within 24 hours of surgery for all participants | |
| Outcomes | Death, ipsilateral stroke, ipsilateral TIA and ipsilateral RIND within 30 days and 48 months; duplex evidence of restenosis > 50% during follow‐up | |
| Notes | Exclusion criteria: repeat CEA, CEA with concomitant coronary artery bypass grafting, ICA diameter < 4 mm (12 CEAs, 3%) FU: mean 30 months (range 1–62 months) Funding sources: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Computer‐generated (artery randomised). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants not reported. Because of the nature of the intervention (patch or primary closure), the surgeon could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants (3%) lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Study authors published findings on all study outcomes. |
| Other bias | Low risk | None suspected. |
Al‐Rawi 2006.
| Study characteristics | ||
| Methods | Allocation concealment: unmarked envelope Blinding of outcome assessment: duplex blind, clinical FU not blind Cross‐overs: unknown Exclusion during trial: none Participants lost to FU: 7 patch, 8 no patch (4.5%) | |
| Participants | Country: UK Number of participants: 315 Number of operations: 328 Sex: 68% male Mean age: 69 years % asymptomatic carotid disease: 10% % stenosis: unknown Comparability: age, sex, vascular risk factors and % asymptomatic disease similar in each group | |
| Interventions | Treatment: collagen‐coated polyester vascular patch Control: primary closure % shunted: unknown (indication for shunt: any sign of ischaemia from transcranial Doppler, cerebral function monitor, NIRS, Doppler flowmetry) Medication: all participants given rectal aspirin (600 mg) after surgery | |
| Outcomes | Death within 30 days and during FU; stroke within 30 days and during FU; perioperative occlusion; bleeding or evacuation of clot; cardiac event; restenosis > 50% or occlusion at end of FU (ultrasound) | |
| Notes | Exclusion criteria: 10 patients excluded due to poor cerebral blood flow (3 patients), ST depression (1 patient), high tortuosity (6 patients) FU: 12 months The study was stopped at 315 participants (328 operations) on the basis of futility: an interim analysis indicated that a large sample size would be required to demonstrate a difference in carotid occlusion rate and to detect a difference in clinical outcome in favour of the direct closure group. Funding sources: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each month, equal numbers of patch and nonpatch cards were randomised to avoid any influence of operator experience changing over time. |
| Allocation concealment (selection bias) | Low risk | Drawing of an unmarked envelope by a member of the theatre team not directly associated with the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants not reported. Because of the nature of the intervention (patch or primary closure), the surgeon could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants (4.5%) lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether study authors reported the results of all prestated outcomes. |
| Other bias | Low risk | None suspected. |
De Vleeschauwer 1987.
| Study characteristics | ||
| Methods | Allocation concealment: sealed envelopes (not opaque or numbered) Blinding of outcome assessment: duplex and clinical FU blind Cross‐overs: none Exclusions during trial: participants with residual stenosis/occlusion Participants lost to FU: none during perioperative period but unknown at the end of follow‐up | |
| Participants | Country: Germany Number of participants: 126 Number of operations: 174 Sex: 60% male Mean age: 64 years % asymptomatic carotid disease: 30% % stenosis: unknown Comparability: age, sex, vascular risk factors and % asymptomatic disease not reported by treatment group | |
| Interventions | Treatment: autologous vein patch Control: primary closure % shunted: routine shunting for all Medication: postoperative aspirin (1 g) for all | |
| Outcomes | Death within 30 days and during FU; stroke within 30 days and during FU; wound haemorrhage or infection; restenosis > 50% or occlusion at end of FU (duplex) | |
| Notes | Exclusion criteria: recurrent stenosis, kinked ICA FU: 1 year Funding sources: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation not reported. |
| Allocation concealment (selection bias) | High risk | Sealed envelopes but not opaque or numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants not reported. Because of the nature of the intervention (patch or primary closure), the surgeon could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of participants lost to follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether study authors reported the results of all prestated outcomes. |
| Other bias | Low risk | None suspected. |
Eikelboom 1988.
| Study characteristics | ||
| Methods | Allocation concealment: odd/even hospital number (participants randomised) Blinding of outcome assessment: duplex blind, clinical FU not blind Cross‐overs: 3 primary closure to patch, 3 patch to primary closure (all analysed in original group) Exclusion during trial: none Participants lost to FU: 10 patch, 7 no patch (13%) | |
| Participants | Country: The Netherlands Number of participants: 126 Number of operations: 129 Sex: 73% male Mean age: 63 years % asymptomatic carotid disease: 18% % stenosis: all arteries > 60% Comparability: age, sex, vascular risk factors and % asymptomatic disease similar in each group | |
| Interventions | Treatment: saphenous vein patch Control: primary closure % shunted: 20% Medication: postoperative warfarin +/− antiplatelet for all | |
| Outcomes | Death within 30 days and during FU; stroke within 30 days and during FU; perioperative occlusion (intravenous DSA); wound haemorrhage; restenosis > 50% or occlusion at end of FU (duplex and intravenous DSA) | |
| Notes | Exclusion criteria: simultaneous cardiac surgery FU: mean 5 years Funding sources: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Odd/even hospital number (participants randomised). |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants not reported. Because of the nature of the intervention (patch or primary closure), the surgeon could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13% of participants lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether study authors reported the results of all prestated outcomes. |
| Other bias | Low risk | None suspected. |
İyigün 2019.
| Study characteristics | ||
| Methods | Allocation concealment: unknown Blinding of outcome assessment: duplex and clinical FU blinding unknown Cross‐overs: unknown Exclusions during trial: unknown Participants lost to FU: unknown | |
| Participants | Country: Turkey Number of participants: 137 Number of operations: 137 in 2 arms (70 patch angioplasty closure, 67 primary closure) Sex: 69% male Mean age: 64.4 years % asymptomatic carotid disease: unknown % stenosis: all arteries > 70% Comparability: age, sex and vascular risk factors similar in each group | |
| Interventions | Treatment: autologous vein or synthetic patch (distribution unknown) Control: primary closure General anaesthesia with NIRS monitoring routinely used % shunted: unknown Medication: postoperative aspirin for all | |
| Outcomes | Restenosis, defined as > 70% diameter stenosis (measurement method unknown); cranial nerve palsy | |
| Notes | Exclusion criteria: renal failure or dialysis FU: 2–7 years Funding sources: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants not reported. Because of the nature of the intervention (patch or primary closure), the surgeon could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of participants lost to follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether study authors reported the results of all prestated outcomes. |
| Other bias | Low risk | None suspected. |
Katz 1994.
| Study characteristics | ||
| Methods | Allocation concealment: odd/even social security number (participants randomised) Blinding of outcome assessment: neither duplex nor clinical FU blind Cross‐overs: none Exclusions during trial: none Participants lost to FU: 12 (12%) | |
| Participants | Country: USA Number of participants: 87 Number of operations: 100 Sex: 56% male Mean age: 67 years % asymptomatic carotid disease: 40% % stenosis: unknown Comparability: age, sex, vascular risk factors and % asymptomatic disease similar in each group | |
| Interventions | Treatment: PTFE patch Control: primary closure % shunted: routine shunting for all Medication: postoperative aspirin (325 mg) for all | |
| Outcomes | Death within 30 days and during FU; stroke within 30 days and during FU; perioperative occlusion (duplex); wound haemorrhage, infection, or cranial nerve palsy; restenosis > 50% or occlusion at end of FU (duplex) | |
| Notes | Exclusion criteria: previous endarterectomy, simultaneous cardiac surgery, ICA diameter < 3.5 mm FU: mean 29 months Funding sources: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Odd/even social security number (participants randomised). |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants not reported. Because of the nature of the intervention (patch or primary closure), the surgeon could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12% of participants lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether study authors reported the results of all prestated outcomes. |
| Other bias | Low risk | None suspected. |
Lord 1989.
| Study characteristics | ||
| Methods | Allocation concealment: sealed envelopes (arteries randomised) Blinding of outcome assessment: probably neither duplex nor clinical FU blind Cross‐overs: 4 (unclear which group these were in) Exclusions during trial: 4 cross‐overs Participants lost to FU: none | |
| Participants | Country: Australia Number of participants: 123 Number of operations: 140 Sex: 62% male Mean age: 63 years % asymptomatic carotid disease: unknown % stenosis: unknown Comparability: age, sex, vascular risk factors and % symptomatic disease similar in each group | |
| Interventions | Treatment: saphenous vein patch or PTFE patch (random allocation) Control: primary closure % shunted: 17% Medication: postoperative aspirin for all | |
| Outcomes | Ipsilateral stroke within 30 days; perioperative occlusion (intravenous DSA); wound haemorrhage | |
| Notes | Exclusion criteria: unknown FU: until hospital discharge Funding sources: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation not reported. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants not reported. Because of the nature of the intervention (patch or primary closure), the surgeon could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether study authors reported the results of all prestated outcomes. |
| Other bias | Low risk | None suspected. |
Mannheim 2005.
| Study characteristics | ||
| Methods | Allocation concealment: sealed envelopes Blinding of outcome assessment: duplex and clinical FU blinding unknown Cross‐overs: none Exclusions during trial: none Participants lost to FU: unknown | |
| Participants | Country: Israel Number of participants: 404 Number of operations: 422 Sex: 65.6% male Mean age: 69.5 years % asymptomatic carotid disease: 53.7% % stenosis: unknown Comparability: sex, vascular risk factors and % asymptomatic disease similar in each group | |
| Interventions | Treatment: polyester urethane patch angioplasty Control: primary closure Indication for shunt: change in neurological status during carotid clamping or in participants in general anaesthesia with stump pressure < 40 mmHg Medication: unknown | |
| Outcomes | Death within 30 days and during FU; stroke within 30 days and during FU; coronary event; wound haemorrhage, infection or cranial nerve palsy; restenosis > 50% or occlusion at end of FU (duplex) | |
| Notes | Exclusion criteria: small ICA diameter or need for interposition graft FU: 5 years Funding sources: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation not reported. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants not reported. Because of the nature of the intervention (patch or primary closure), the surgeon could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of participants lost to follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether study authors reported the results of all prestated outcomes. |
| Other bias | Low risk | None suspected. |
Myers 1994.
| Study characteristics | ||
| Methods | Allocation concealment: opaque, sequentially numbered sealed envelopes (arteries randomised) Blinding of outcome assessment: duplex blind, clinical FU not blind Cross‐overs: none Exclusions during trial: none Participants lost to FU: 6 patch, 8 no patch (11%) | |
| Participants | Country: USA Number of participants: 109 Number of operations: 126 Sex: 99% male Mean age: 62 years % asymptomatic carotid disease: 23% % stenosis: unknown Comparability: age, sex, vascular risk factors and % asymptomatic disease similar in each group | |
| Interventions | Treatment: saphenous vein patch Control: primary closure % shunted: routine shunt for all Medication: peri‐ and postoperative aspirin (325 mg) for all | |
| Outcomes | Death within 30 days and during FU; stroke within 30 days and during FU; perioperative occlusion (ocular pneumoplethysmography); wound haemorrhage, infection or cranial nerve palsy; restenosis > 50% or occlusion at end of FU (duplex) | |
| Notes | Exclusion criteria: ICA diameter < 5 mm, arteriotomy > 3 cm, looped or kinked ICA FU: 4 to 5 years Funding sources for the study: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequentially numbered (arteries randomised). |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants not reported. Because of the nature of the intervention (patch or primary closure), the surgeon could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11% of participants lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether study authors reported the results of all prestated outcomes. |
| Other bias | Low risk | None suspected. |
Pratesi 1986.
| Study characteristics | ||
| Methods | Allocation concealment: unknown Blinding of outcome assessment: duplex and clinical FU blinding unknown Cross‐overs: unknown Exclusions during trial: unknown Participants lost to FU: unknown | |
| Participants | Country: Italy Number of participants: 90 Number of operations: 100 Sex (male:female ratio): 6:5 Mean age: unknown % asymptomatic carotid disease: 10% % stenosis: unknown Comparability: age, sex and vascular risk factors not reported by treatment group | |
| Interventions | Treatment: autologous vein or synthetic patch (distribution unknown) Control: primary closure % shunted: unknown Medication: aspirin before surgery, unknown after surgery | |
| Outcomes | Death within 30 days and during FU; stroke within 30 days and during FU; restenosis during FU (DSA) | |
| Notes | Exclusion criteria: unknown FU: 2 years Funding sources: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants not reported. Because of the nature of the intervention (patch or primary closure), the surgeon could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of participants lost to follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether study authors reported the results of all prestated outcomes. |
| Other bias | Low risk | None suspected. |
Ranaboldo 1993.
| Study characteristics | ||
| Methods | Allocation concealment: opaque, sequentially numbered, sealed envelopes (arteries randomised) Blinding of outcome assessment: duplex and clinical FU blind Cross‐overs: 4 primary closure to patch (all analysed in original group) Exclusions during trial: none Participants lost to FU: 5 patch, 12 no patch (7.9%) | |
| Participants | Country: UK Number of participants: 199 Number of operations: 213 Sex: 69% male Mean age: 66 years % asymptomatic carotid disease: 8% % stenosis: > 75% stenosis in 60% of arteries Comparability: age, sex and vascular risk factors not reported by treatment group | |
| Interventions | Treatment: autologous vein patches (53 arteries) or Dacron patches (56 arteries); non‐random allocation Control: primary closure % shunted: shunt 'when technically possible' Medication: aspirin before surgery, unknown after surgery | |
| Outcomes | Death within 30 days and during FU; stroke within 30 days and during FU; perioperative occlusion (duplex); wound haemorrhage or infection; restenosis > 50% or occlusion at end of FU (duplex) | |
| Notes | Exclusion criteria: unknown FU: 12 months Funding sources for the study: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequentially numbered (artery randomised). |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of study participants not reported. Because of the nature of the intervention (patch or primary closure), the surgeon could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7.9% of participants lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether study authors reported the results of all prestated outcomes. |
| Other bias | Low risk | None suspected. |
CABG: coronary artery bypass graft; CEA: carotid endarterectomy; DSA: digital subtraction angiography; FU: follow‐up; ICA: internal carotid artery; NIRS: near infrared spectroscopy; PTFE: polytetrafluoroethylene; RIND: reversible ischaemic neurological deficit; TIA: transient ischaemic attack.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gale 1988 |
|
| Hertzer 1987 |
|
| Ignatenko 2019 |
|
CEA: carotid endarterectomy.
Differences between protocol and review
For this review update, we did not plan to carry out sensitivity analyses: the current authors considered them unreasonably critical, as the previous version of this review found no evidence of a difference between the trials with different allocation techniques.
Contributions of authors
SO, TB, KR: refined the protocol, performed searches, selected studies for inclusion, extracted and entered data, and wrote the review.
BS, KR: refined the protocol, co‐ordinated the project, and commented on the design of the protocol and on the final manuscript.
Sources of support
Internal sources
-
Chiang Mai University, Thailand
This study was partially supported by Chiang Mai University
External sources
No sources of support provided
Declarations of interest
SO: none known.
TB: none known.
BS: none known.
KR: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
AbuRahma 1996 {published data only}
- AbuRahma AF, Khan JH, Robinson PA, Saiedy S, Short YS, Boland JP, et al. Prospective randomized trial of carotid endarterectomy with primary closure and patch angioplasty with saphenous vein, jugular vein, and polytetrafluoroethylene: perioperative (30 day) results. Journal of Vascular Surgery 1996;24:998-1007. [DOI] [PubMed] [Google Scholar]
- AbuRahma AF, Robinson PA, Mullins DA, Holt SM, Herzog TA, Mowery NT. Frequency of postoperative carotid duplex surveillance and type of closure: results from a randomized trial. Journal of Vascular Surgery 2000;32:1043-51. [DOI] [PubMed] [Google Scholar]
- AbuRahma AF, Robinson PA, Saiedy S, Kahn JH, Boland JP. Prospective randomized trial of carotid endarterectomy with primary closure and patch angioplasty with saphenous vein, jugular vein, and polytetrafluoroethylene: long-term follow-up. Journal of Vascular Surgery 1998;27(2):222-32. [DOI] [PubMed] [Google Scholar]
- AbuRahma AF, Robinson PA, Saiedy S, Richmond BK, Khan J. Prospective randomized trial of bilateral carotid endarterectomies. Stroke 1999;30:1185-9. [DOI] [PubMed] [Google Scholar]
Al‐Rawi 2006 {published data only}
- Al-Rawi PG, Turner CL, Waran V, Ng I, Kirkpatrick PJ. A randomized trial of synthetic patch versus direct primary closure in carotid endarterectomy. Neurosurgery 2006;59:822-9. [DOI] [PubMed] [Google Scholar]
De Vleeschauwer 1987 {published and unpublished data}
- De Vleeschauwer P, Wirthle W, Holler L, Krause E, Horsch S. Is venous patch grafting after carotid endarterectomy able to reduce the rate of restenosis? Prospective randomized pilot study with stratification. Acta Chirurgica Belgica 1987;87:242-6. [PubMed] [Google Scholar]
Eikelboom 1988 {published and unpublished data}
- De Letter JA, Moll FL, Welten RJ, Eikelboom BC, Ackerstaff RG, Vermeulen FE, et al. Benefits of carotid patching: a prospective randomized study with long-term follow-up. Annals of Vascular Surgery 1994;8:54-8. [DOI] [PubMed] [Google Scholar]
- Eikelboom BC, Ackerstaff RG, Hoeneveld H, Ludwig JW, Teeuwen C, Vermeulen FE, et al. Benefits of carotid patching: a randomized study. Journal of Vascular Surgery 1988;7:240-7. [DOI] [PubMed] [Google Scholar]
İyigün 2019 {published data only}
- İyigün T, Kyaruzi M, Timur B, İyigün M, Aydın Ü. Our midterm restenosis results using patch angioplasty closure versus primary closure in patients undergoing carotid endarterectomy: a comparative study. Turkish Journal of Vascular Surgery 2019;28(1):19-23. [Google Scholar]
Katz 1994 {published and unpublished data}
- Katz D, Snyder SO, Gandhi RH, Wheeler JR, Gregory RT, Gayle RG, et al. Long-term follow up for recurrent stenosis: a prospective randomized study of expanded polytetrafluoroethylene patch angioplasty versus primary closure after carotid endarterectomy. Journal of Vascular Surgery 1994;19:198-205. [DOI] [PubMed] [Google Scholar]
Lord 1989 {published and unpublished data}
- Lord RS, Raj TB, Stary DL, Nash PA, Graham AR, Goh KH. Comparison of saphenous vein patch, polytetrafluoroethylene patch, and direct arteriotomy closure after carotid endarterectomy. Part I: perioperative results. Journal of Vascular Surgery 1989;9:521-9. [PubMed] [Google Scholar]
Mannheim 2005 {published data only}
- Mannheim D, Weller B, Vahadim E, Karmeli R. Carotid endarterectomy with a polyurethane patch versus primary closure: a prospective randomized study. Journal of Vascular Surgery 2005;41:403-8. [DOI] [PubMed] [Google Scholar]
Myers 1994 {published and unpublished data}
- Clagett GP, Patterson CB, Fisher Jr DF, Fry RE, Eidt JF, Humble TH, et al. Vein patch versus primary closure for carotid endarterectomy. A randomized prospective study in a selected group of patients. Journal of Vascular Surgery 1989;9:213-23. [DOI] [PubMed] [Google Scholar]
- Myers SI, Valentine RJ, Chervu A, Bowers BL, Clagett GP. Saphenous vein patch versus primary closure for carotid endarterectomy: long term assessment of a randomized prospective study. Journal of Vascular Surgery 1994;19:15-22. [DOI] [PubMed] [Google Scholar]
Pratesi 1986 {published data only}
- Pratesi C, Frullini A, Rega L, Fonda C, Matticari S, Alessi Innocenta A, et al. The follow-up after carotid TEA. In: Maurer PC, Becker HM, Heidrich H, Hoffmann G, Kriessmann A, Muller-Wiefel H, Pratorius C, editors(s). Trends and Controversies. Vol. 1. Munich: W Zuckschwerdt, 1986:313-5. [Google Scholar]
Ranaboldo 1993 {published and unpublished data}
- Ranaboldo CJ, Barros D'Sa AB, Bell PR, Chant AD, Perry PM, the Joint Vascular Research Group. Randomized controlled trial of patch angioplasty for carotid endarterectomy. British Journal of Surgery 1993;80:1528-30. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Gale 1988 {unpublished data only}
- Gale S. Carotid endarterectomy with and without vein patch: a prospective randomized study. Journal of Vascular Surgery 1988;7:240-7. [Google Scholar]
Hertzer 1987 {published and unpublished data}
- Hertzer NR, Beven EG, O'Hara PJ, Krajewski LP. A prospective study of vein patch angioplasty during carotid endarterectomy. Three-year results for 801 patients and 917 operations. Annals of Surgery 1987;206:628-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ignatenko 2019 {published data only}
- Ignatenko P, Novikova O, Gostev A, Starodubtsev V, Zeidlits G, Kuznetsov K, et al. Carotid endarterectomy with autoarterial remodeling of bifurcation of the common carotid artery and carotid endarterectomy with patch closure: comparison of methods. Journal of Stroke & Cerebrovascular Diseases 2019;28(3):741-50. [DOI] [PubMed] [Google Scholar]
Additional references
Aboyans 2018
- Aboyans V, Ricco JB, Bartelink ML, Björck M, Brodmann M, Cohnert T, et al. Editor’s Choice 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). European Journal of Vascular and Endovascular Surgery 2018;55:305-68. [DOI] [PubMed] [Google Scholar]
AbuRahma 1998
- AbuRahma AF, Robinson PA, Saiedy S, Kahn JH, Boland JP. Prospective randomized trial of carotid endarterectomy with primary closure and patch angioplasty with saphenous vein, jugular vein, and polytetrafluoroethylene: long-term follow-up. Journal of Vascular Surgery 1998;27:222-34. [DOI] [PubMed] [Google Scholar]
AbuRahma 1999
- AbuRahma AF, Robinson PA, Saiedy S, Richmond BK, Khan J. Prospective randomized trial of bilateral carotid endarterectomies. Stroke 1999;30:1185-9. [DOI] [PubMed] [Google Scholar]
AbuRahma 2000
- AbuRahma AF, Robinson PA, Mullins DA, Holt SM, Herzog TA, Mowery NT. Frequency of postoperative carotid duplex surveillance and type of closure: results from a randomized trial. Journal of Vascular Surgery 2000;32:1043-51. [DOI] [PubMed] [Google Scholar]
ACAS 1995
- Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995;273(18):1421-8. [PubMed] [Google Scholar]
Awad 1989
- Awad IA, Little JR. Patch angioplasty in carotid endarterectomy. Advantages, concerns, and controversies. Stroke 1989;20:417-22. [DOI] [PubMed] [Google Scholar]
Barkat 2018
- Barkat M, Roy I, Antoniou S, Torella F, George A. Systematic review and network meta-analysis of treatment strategies for asymptomatic carotid disease. Scientific Reports 2018;8:4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernstein 1990
- Bernstein EF, Torem S, Dilley RB. Does carotid restenosis predict an increased risk of late symptoms, stroke or death? Annals of Surgery 1990;212:629-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernstein 1992
- Bernstein EF. Routine versus selective carotid patching. Journal of Vascular Surgery 1992;15:868-9. [DOI] [PubMed] [Google Scholar]
Bond 2003
- Bond R, Rerkasem K, Naylor AR, Rothwell PM. Patches of different types for carotid patch angioplasty. Cochrane Database of Systematic Reviews 2004, Issue 2. Art. No: CD000071. [DOI: 10.1002/14651858.CD000071] [DOI] [PubMed] [Google Scholar]
Cheng 2021
- Cheng SF, Richards T, Gregson J, Brown MM, Borst GJ, Bonati LH. Long term restenosis rate after carotid endarterectomy: comparison of three surgical techniques and intra-operative shunt use. European Journal of Vascular and Endovascular Surgery 2021;62(4):513-21. [DOI] [PubMed] [Google Scholar]
Chung 2020
- Chung BH, Heo SH, Park YJ, Kim YW, Woo SY, Kim DI. Comparative analysis using propensity score matching analysis: primary closure versus patch angioplasty during carotid endarterectomy. Annals of Vascular Surgery 2020;62:166-72. [DOI] [PubMed] [Google Scholar]
Clagett 1989
- Clagett GP, Patterson CB, Fisher Jr DF, Fry RE, Eidt JF, Humble TH, et al. Vein patch versus primary closure for carotid endarterectomy. A randomized prospective study in a selected group of patients. Journal of Vascular Surgery 1989;9:213-23. [DOI] [PubMed] [Google Scholar]
Counsell 1998
- Counsell C, Salinas R, Warlow C, Naylor R. Patch angioplasty compared to primary closure in carotid endarterectomy. Cochrane Database of Systematic Reviews 1998, Issue 1. Art. No: CD000160. [DOI: 10.1002/14651858.CD000160] [DOI] [PubMed] [Google Scholar]
Das 1985
- Das MB, Hertzer NR, Ratliff NB, O'Hara PJ, Beven EG. Recurrent carotid stenosis. A five-year series of 65 reports. Annals of Surgery 1985;202:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors(s). Systematic Reviews in Health Care. 2nd edition. London (UK): BMJ Books, 2001:200. [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
De Letter 1994
- De Letter JA, Moll FL, Welten RJ, Eikelboom BC, Ackerstaff RG, Vermeulen FE, et al. Benefits of carotid patching: a prospective randomized study with long-term follow-up. Annals of Vascular Surgery 1994;8:54-8. [DOI] [PubMed] [Google Scholar]
ECST 1991
- European Carotid Surgery Trialists' Collaborative Group. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70-99%) or with mild (0-29%) carotid stenosis. Lancet 1991;337:1235-43. [PubMed] [Google Scholar]
Edenfield 2019
- Edenfield L, Blazick E, Healey C, Hawkins R, Bloch P, Eldrup-Jorgensen J, et al. Long-term impact of the Vascular Study Group of New England carotid patch quality initiative. Journal of Vascular Surgery 2019;69(6):1801-6. [DOI] [PubMed] [Google Scholar]
Frericks 1998
- Frericks H, Kievit J, Baalen JM, Bockel JH. Carotid recurrent stenosis and risk of ipsilateral stroke: a systematic review of the literature. Stroke 1998;29:244-50. [DOI] [PubMed] [Google Scholar]
Girn 2008
- Girn HR, Dellagrammaticas D, Laughlan K, Gough MJ, on behalf of the GALA Trial Collaborators. Carotid endarterectomy: technical practices of surgeons participating in the GALA trial. European Journal of Vascular and Endovascular Surgery 2008;36:385-9. [DOI] [PubMed] [Google Scholar]
Golledge 1996
- Golledge J, Cuming R, Davies AH, Greenhalgh RM. Outcome of selective patching following carotid endarterectomy. European Journal of Vascular and Endovascular Surgery 1996;11:458-63. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University, 2015 (accessed 15 January 2022).
Halliday 2004
- Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 2004;363:1491-502. [DOI] [PubMed] [Google Scholar]
Harrison 2012
- Harrison GJ, Brennan JA, Naik JB, Vallabhaneni SR, Fisher RK. Patch variability following carotid endarterectomy: a survey of Great Britain and Ireland. Annals of the Royal College of Surgeons of England 2012;94(6):411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2016
- Higgins JP, Lasserson T, Chandler J, Tovey D, Churchill R. Standards for the conduct of new Cochrane Intervention Reviews 2016. In: Higgins JP, Lasserson T, Chandler J, Tovey D, Churchill R, editors(s). Methodological Expectations of Cochrane Intervention Reviews. London: Cochrane, 2016:Available from methods.cochrane.org/resources. [Google Scholar]
Higgins 2020
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Knudsen 1990
- Knudsen L, Sillesen H, Schroeder T, Hansen HJ. Eight to ten years follow-up after carotid endarterectomy: clinical evaluation and doppler examination of patients operated on between 1978–1980. European Journal of Vascular Surgery 1990;4:259-64. [DOI] [PubMed] [Google Scholar]
Liu 2020
- Liu D, Li ZL, Wang M, Wu RD, Wang JS, Wang SM, et al. Comparative analysis of patch angioplasty versus selective primary closure during carotid endarterectomy performed at a single vascular center in China. Annals of Vascular Surgery 2021;73:344-50. [DOI] [PubMed] [Google Scholar]
Naylor 2018
- Naylor AR, Ricco JB, Borst GJ, Debus S, Haro J, Halliday A, et al. Editor's Choice – Management of atherosclerotic carotid and vertebral artery disease: 2017 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). European Journal of Vascular and Endovascular Surgery 2018;55(1):3-81. [DOI] [PubMed] [Google Scholar]
Ouriel 1987
- Ouriel K, Green RM. Clinical and technical factors influencing recurrent carotid stenosis and occlusion after endarterectomy. Journal of Vascular Surgery 1987;5:702-6. [PubMed] [Google Scholar]
Rerkasem 2009b
- Rerkasem K, Rothwell PM. Temporal trends in the risks of stroke and death due to endarterectomy for symptomatic carotid stenosis: an updated systematic review. European Journal of Vascular and Endovascular Surgery 2009;37:504-11. [DOI] [PubMed] [Google Scholar]
Rerkasem 2011
- Rerkasem K, Rothwell PM. Systematic review of randomized controlled trials of patch angioplasty versus primary closure and different types of patch materials during carotid endarterectomy. Asian Journal of Surgery 2011;34:32-40. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Center, The Cochrane Collaboration, 2020.
Rothwell 1995
- Rothwell P, Warlow C. Is self-audit reliable? Lancet 1995;346:1623. [DOI] [PubMed] [Google Scholar]
Rothwell 2003
- Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003;361:107-16. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Volteas 1994
- Volteas N, Labropoulos N, Leon M, Kalodiki E, Chan P, Nicolaides AN. Risk factors associated with recurrent carotid stenosis. International Angiology 1994;13:143-7. [PubMed] [Google Scholar]
Zierler 1982
- Zierler RE, Bandyk DF, Thiele BL, Strandness Jr DE. Carotid artery stenosis following endarterectomy. Archives of Surgery 1982;117:1408-15. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bond 2004
- Bond R, Rerkasem K, AbuRahma AF, Naylor AR, Rothwell PM. Patch angioplasty versus primary closure for carotid endarterectomy. Cochrane Database of Systematic Reviews 2004, Issue 2. Art. No: CD000160. [DOI: 10.1002/14651858.CD000160] [DOI] [PubMed] [Google Scholar]
Counsell 1996
- Counsell C, Salinas R, Warlow C, Naylor R. Patch angioplasty versus primary closure for carotid endarterectomy. Cochrane Database of Systematic Reviews 1996, Issue 1. Art. No: CD000160. [DOI: 10.1002/14651858.CD000160] [DOI] [PubMed] [Google Scholar]
Rerkasem 2009a
- Rerkasem K, Rothwell PM. Patch angioplasty versus primary closure for carotid endarterectomy. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD000160. [DOI: 10.1002/14651858.CD000160] [DOI] [PMC free article] [PubMed] [Google Scholar]


