Abstract
Coordinating the four limbs is critical for terrestrial mammalian locomotion. Thoracic spinal transection abolishes neural communication between the brain and spinal networks controlling hindlimb/leg movements. Several studies have shown that animal models of spinal transection (spinalization), such as mice, rats, cats, and dogs recover hindlimb locomotion with the forelimbs stationary or suspended. We know less on the ability to generate quadrupedal locomotion after spinal transection, however. We collected kinematic and electromyography data in four adult cats during quadrupedal locomotion at five treadmill speeds before (intact cats) and after low-thoracic spinal transection (spinal cats). We show that adult spinal cats performed quadrupedal treadmill locomotion and modulated their speed from 0.4 m/sec to 0.8 m/sec but required perineal stimulation. During quadrupedal locomotion, several compensatory strategies occurred, such as postural adjustments of the head and neck and the appearance of new coordination patterns between the forelimbs and hindlimbs, where the hindlimbs took more steps than the forelimbs. We also observed temporal changes, such as shorter forelimb cycle/swing durations and shorter hindlimb cycle/stance durations in the spinal state. Forelimb double support periods occupied a greater proportion of the cycle in the spinal state, and hindlimb stride length was shorter. Coordination between the forelimbs and hindlimbs was weakened and more variable in the spinal state. Changes in muscle activity reflected spatiotemporal changes in the locomotor pattern. Despite important changes in the pattern, our results indicate that biomechanical properties of the musculoskeletal system play an important role in quadrupedal locomotion and offset some of the loss in neural communication between networks controlling the forelimbs and hindlimbs after spinal transection.
Keywords: central pattern generator, interlimb coordination, locomotion, somatosensory feedback, spinal transection
Introduction
Coordinating movements of the four limbs is an essential feature of terrestrial locomotion in quadrupedal mammals and plays an important role in human locomotion.1–4 Interlimb coordination involves complex dynamic interactions between the spinal circuits that generate the basic pattern of locomotion, somatosensory feedback that informs the central nervous system of changes within the body and the environment, supraspinal structures that regulate posture and volitional aspects of locomotion, and properties of the musculoskeletal system that stabilize the locomotor pattern.5,6
Transection of the spinal cord at low thoracic levels completely and permanently abolishes neural communication between the brain and spinal networks controlling hindlimb movements. Despite this disruption, several studies have shown that pre-clinical models of spinal transection, such as mice, rats, cats, and dogs, recover hindlimb locomotion with the forelimbs suspended or on a stationary platform after a few weeks of treadmill locomotor training7–15 or even without task-specific training.16 This remarkable recovery is because of neural circuits located in the spinal cord and capable of generating the basic locomotor pattern without inputs from the brain and sensory feedback from peripheral receptors—the so-called central pattern generator (CPG).6,17–22
After a thoracic spinal transection, the spinal locomotor CPG that controls the hindlimbs, located at lumbar levels, retains the ability to interact with somatosensory feedback from muscles, tendons, joints, and skin that send afferents to spinal neurons located below the lesion. This remaining sensory feedback adjusts the hindlimb pattern to locomotor task demands, such as treadmill speed,16,23,24 different speeds for the left and right hindlimbs on a split-belt treadmill,4,25–28 stepping on a slope,29 and stepping backward when the treadmill is reversed.30 After a thoracic spinal transection, left-right coordination between the forelimbs remains unaffected, and the hindlimbs maintain coordination over a range of speeds and with large differences in speed between the hindlimbs during split-belt locomotion.4
Much less is known, however, about the coordination between the forelimbs and hindlimbs after a thoracic spinal transection. Most studies in spinal mammals (animals with a spinal transection) have been performed with the forelimbs suspended, mainly in rats,15,31–33 or placed on a stationary platform in cats.11,16,23,27,28,30 Evidently, after a thoracic spinal transection, no neural communication exists between the spinal locomotor CPGs controlling the forelimbs, located at low cervical/upper thoracic segments,31,32 and those of the hindlimbs, located at upper to midlumbar spinal segments.33–37
Although no neural communication exists between spinal circuits located at cervicothoracic and lumbar levels, biomechanical properties of the musculoskeletal system remain and are known to play an important role in stabilizing the locomotor pattern in intact animals during locomotion.38–41 These biomechanical properties could help offset the loss of neural communication to coordinate the forelimbs and hindlimbs, as highlighted in the design of quadruped42,43 and hexapod robots.44
Studies have shown that puppies/dogs,45–50 kittens/cats,7,51,52 and neonatal rats53,54 can produce quadrupedal locomotion because of mechanical interactions between the limbs after spinal transection at thoracic levels. While some authors have described that some of their spinal mammals could walk and run short distances quadrupedally with some degree of forelimb-hindlimb coordination,7 others have reported a lack of forelimb-hindlimb coordination, with the forelimbs adopting a faster and seemingly independent cadence.51,52 Moreover, despite weeks of treadmill locomotor training, adult cats did not perform quadrupedal locomotion as well as kittens.52 Indeed, eight weeks after spinal transection, adult cats required a strong stimulation below the level of the spinal transection, such as pinching the skin at the base of the tail, to provide transient weight support of their hindquarters during quadrupedal locomotion.52
A detailed investigation of interlimb coordination in adult spinal cats during quadrupedal locomotion with analyses of both kinematics and electromyography (EMG), however, has not been specifically performed. The purpose of this study was to characterize the pattern of quadrupedal locomotion before and after a low-thoracic spinal transection that completely abolishes the neural communication between spinal networks controlling the forelimbs and hindlimbs. We performed our investigation on a treadmill across different speeds (0.4–0.8 m/sec) allowing us to determine how the quadrupedal pattern adjusts to an increase in speed in spinal cats. Our results show that adult spinal cats can perform quadrupedal treadmill locomotion and modulate their speed using several compensatory strategies.
Methods
Animals and ethical information
All procedures were approved by the Animal Care Committee of the Université de Sherbrooke in accordance with policies and directives of the Canadian Council on Animal Care (Protocol no. 442-18). We obtained the current data set from four adult cats (>1 year of age at the time of experimentation), two females and two males, weighing between 3.5 kg and 6.5 kg (4.9 ± 1.6). Before and after the experiments, cats were housed and fed in a dedicated room within the animal care facility of the Faculty of Medicine and Health Sciences at the Université de Sherbrooke. Our study followed ARRIVE guidelines for animal studies.55 As part of our effort to reduce the number of animals used in research, some of the cats (Cats 1 and 3) were used in another study to answer a different scientific question.56
General surgical procedures
Surgical procedures were performed under aseptic conditions with sterilized equipment in an operating room, as described previously.16,23,28,30,57,58 Before surgery, cats were sedated with an intramuscular injection of butorphanol (0.4 mg/kg), acepromazine (0.1 mg/kg), and glycopyrrolate (0.01 mg/kg). Ketamine/diazepam (0.05 mL/kg) was then administered with an intramuscular injection for induction. Cats were anesthetized with isoflurane (1.5–3%) delivered in O2, first with a mask and then with an endotracheal tube. During surgery, we adjusted isoflurane concentration by monitoring cardiac and respiratory rates, by applying pressure to the paw (to detect limb withdrawal), by assessing the size and reactivity of pupils, and by evaluating jaw tone.
We shaved the animal's fur (back, stomach, forelimbs and hindlimbs) using electric clippers and cleaned the skin with chlorhexidine soap. Cats received a continuous infusion of lactated Ringers solution (3 mL/kg/h) during surgery through a catheter placed in a cephalic vein. A rectal thermometer monitored body temperature, which was maintained within physiological range (37 ± 0.5°C) using a water-filled heating pad placed under the animal and an infrared lamp ∼50 cm over it.
At the end of surgery, we injected an antibiotic (cefovecin, 0.1 mL/kg) subcutaneously and taped a transdermal fentanyl patch (25 mcg/h) to the back of the animal 2-3 cm rostral to the base of the tail to provide prolonged analgesia (4-5–day period). We also injected buprenorphine (0.01 mg/kg), a fast-acting analgesic, subcutaneously at the end of the surgery and a second dose ∼7 h later. After surgery, we placed the cat in an incubator until it regained consciousness. We removed the fentanyl patch 5–7 days after surgery.
Implantation of electrodes
Cats were implanted with electrodes to chronically record the activity (EMG) of several forelimb and hindlimb muscles. We directed pairs of Teflon-insulated multi-strain fine wires (AS633; Cooner Wire, Chatsworth, CA) subcutaneously from two head-mounted 34-pin connectors (Omnetics, Minneapolis, MN,). Electrodes were sewn into the belly of selected forelimb and hindlimb muscles for bipolar recordings, with 1-2 mm of insulation stripped from each wire. We verified electrode placement during surgery by electrically stimulating each muscle through the matching head connector channel. The head connector was secured to the skull using dental acrylic.
In the present study, we used recordings from the following muscles: biceps brachii (BB, elbow flexor), biceps femoris anterior (BFA, hip extensor), biceps femoris posterior (BFP, knee flexor/hip extensor), the long head of the triceps brachii (TRI, elbow and shoulder extensor), lateral gastrocnemius (LG, ankle extensor/knee flexor), sartorius anterior (SRT, hip flexor/knee extensor), soleus (SOL, ankle extensor), and vastus lateralis (VL, knee extensor).
Spinal transection
After collecting data in the intact state (see below), a complete spinal transection (i.e., spinalization) was made at low thoracic levels. General surgical procedures were the same as described above. The skin was incised over the 12th and 13th thoracic vertebrae and, after carefully setting aside muscle and connective tissue, a small laminectomy of the dorsal bone was made. After exposing the spinal cord, we applied xylocaine (lidocaine hydrochloride, 2%) topically and made two to three injections within the spinal cord. We then completely transected the spinal cord with surgical scissors between the 12th and 13th thoracic vertebrae. We then cleaned the ∼0.5-cm gap between the two cut ends of the spinal cord and stopped any residual bleeding.
We verified that no spinal cord tissue remained connecting rostral and caudal ends, which we later confirmed histologically. A hemostatic material (Spongostan) was inserted within the gap, and muscles and skin were sewn back to close the opening in anatomical layers. After spinalization, we manually expressed the cat's bladder and large intestine one to two times daily, or as needed. Cats were monitored daily by experienced personnel. The hindlimbs were cleaned as needed to prevent infection.
Experimental protocol
We collected kinematic and EMG data before (intact state) and after cats recovered spontaneous quadrupedal locomotion after spinal transection (spinal state) during tied-belt (equal left-right speeds) locomotion from 0.4 m/s to 0.8 m/sec in 0.1 m/sec increments. The treadmill consisted of two independently controlled running surfaces 120 cm long and 30 cm wide (Bertec, Columbus, OH). A Plexiglas separator (120 cm long, 3 cm high, and 0.5 cm wide) was placed between the left and right belts to prevent the limbs from impeding each other. Data collection in the spinal state began between the 10th and 14th weeks after spinalization, depending on the locomotor recovery of the animals. During the recovery period after spinal transection, however, no task-specific training for quadrupedal locomotion was performed on a treadmill on any of the four cats.
We collected data from five to 30 consecutive step cycles using positive reinforcement (food, affection) in both intact and spinal states. To avoid fatigue, ∼20 sec of rest was given between episodes of locomotion. In the spinal state, all data were collected with manual stimulation of the skin of the perineal region. For perineal stimulation, the same experimenter (J. Harnie) manually pinched the perineal region (the skin under the tail) with the index finger and thumb. The strength of perineal stimulation is difficult to quantify but we adjusted the pressure applied to the perineal region on a case-by-case basis (light/strong, tonic/rhythmic) to achieve the best rhythmic behavior possible and to maximize support during stance.59
Without perineal stimulation, the hindlimbs simply dragged behind the body. If the perineal stimulation was too strong, we observed exaggerated flexion of the hindlimbs (hip, knee, and ankle) and/or improper left-right alternation, which impaired quadrupedal treadmill locomotion. In other words, too much excitability to spinal locomotor networks was detrimental. Perineal stimulation increases spinal excitability and facilitates hindlimb locomotion in spinal mammals through an undefined mechanism.58 During data collection, the same experimenter (J. Harnie) held the tail of the animal to provide balance but did not provide weight support.
Data collection and analysis
We collected kinematic data as described previously.16,24,30,60 Reflective markers were placed on the skin over bony landmarks: the scapula, minor tubercle of the humerus, elbow, wrist, metacarpophalangeal (MCP) joint, and at the tip of the toes for the forelimbs and over the iliac crest, greater trochanter, lateral malleolus, metatarsophalangeal (MTP) joint, and at the tip of the toes for the hindlimbs. Videos of the left and right sides were obtained with two cameras (Basler AcA640-100 g) at 60 frames/sec with a spatial resolution of 640 × 480 pixels. A custom-made program (Labview) acquired the images and synchronized acquisition with EMG data. EMG signals were pre-amplified (10 × , custom-made system), bandpass filtered (30–1000 Hz), and amplified (100–5000 × ) using a 16- channel amplifier (model 3500; A-M Systems).
We implanted more than 16 muscles per cat, so we obtained data in each locomotor condition twice, one for each connector, because our data acquisition system limits us to recording a maximum of 16 channels simultaneously. The EMG data were digitized (2000 Hz) with a National Instruments card (NI 6032E, Austin, TX), acquired with custom-made acquisition software and stored on computer.
Temporal variables
By visual detection, the same experimenter (J. Audet) determined, for all four limbs, paw contact as the first frame where the paw made visible contact with the treadmill surface and limb liftoff as the most caudal displacement of the toes.
We measured cycle duration from successive paw contacts, while stance duration corresponded to the interval of time from foot contact to the most caudal displacement of the toe/finger relative to the hip/shoulder.61 We calculated swing duration as cycle duration minus stance duration. We measured double support periods between homologous limbs (forelimbs and hindlimbs) from contact of the ipsilateral limb to liftoff (stance offset) of the contralateral limb (DS1) and from contact of the contralateral limb to liftoff of the ipsilateral limb (DS2).24 The DS1 and DS2 were then summed and divided by the cycle period of the reference limb.
We evaluated temporal interlimb coordination by measuring phase intervals between four pairs of limbs62: (1) left and right forelimbs (forelimb coupling), (2) left and right hindlimbs (hindlimb coupling), (3) left forelimb and left hindlimb (homolateral coupling), and (4) left forelimb and right hindlimb (diagonal coupling). Phase intervals were calculated as the absolute amount of time between contacts of two limbs divided by the cycle duration of the reference limb.62–67 The reference limb was always the forelimb, with the exception of hindlimb coupling. These values were then multiplied by 360 and expressed in degrees to illustrate their continuous nature and possible distributions.62,66
To determine whether spinal cats displayed greater variations in limb couplings, we calculated the coefficient of variation, a statistical measure of the relative dispersion of data points around the mean, by dividing the standard deviation by the mean. These values were then multiplied by 100 and expressed as a percentage.
Postural characterization
By visual inspection of the videos obtained on the right side, we traced the silhouette of each animal from a representative cycle at the same scale at three key events of the locomotor cycle (liftoff, midswing, and contact of the right forelimb). To characterize the postural adjustments of the head and neck of individual cats during locomotion, the resulting silhouettes were then superimposed before (intact state) and after the recovery of spontaneous quadrupedal locomotion after spinal transection (spinal state). We then qualitatively described the differences between the two states.
Spatial variables
The following spatial variables were analyzed using DeepLabCutTM, an open-source transfer learning program with deep neural network,68 as we recently described in the cat.60 Step length, defined as the anterior-posterior distance between the leading and trailing limbs at stance onset of the leading limb was measured for the forelimbs and hindlimbs.24,27,63,69 The left and right limbs were the leading and trailing limbs, respectively. Stride length was measured for the right forelimbs and right hindlimbs as the distance between contact and liftoff added to the distance traveled by the treadmill during the swing phase, obtained by multiplying swing duration by treadmill speed.60,63,70–72.
We measured the relative distance of the paw at contact and liftoff as the horizontal distance between the toe and shoulder or hip markers at stance onset and offset, respectively, for the right forelimb and right hindlimb, respectively. We measured limb interference as the horizontal distance between the toe markers of the forelimbs and hindlimbs on the same side at stance onset and offset of each of the four limbs of the animals.
Electromyography. The same experimenter (J. Audet) determined burst onsets and offsets by visual inspection from the raw EMG waveforms using a custom-made program. Burst duration was measured from onset to offset. The onset and offset of some muscles, particularly those displaying two bursts per cycles, became clearer at faster speeds. As such, a few passes were made to accurately define the onsets and offsets of EMG bursts. We also measured the phasing of EMG burst onsets and offsets by calculating their timing in a normalized cycle.
Euthanasia and histology
At the end of the experiments, cats were anesthetized with isoflurane before receiving a lethal dose (100 mg/kg) of pentobarbital through the left or right cephalic vein. To confirm that spinal transection was complete in four cats, we performed histological analysis. Briefly, we removed a 2 cm long segment of the spinal cord around the spinal lesion immediately after euthanasia. We then placed the spinal segment in 25 mL of a 4% paraformaldehyde solution in 0.1 M phosphate-buffered saline (PBS) solution at 4°C. After 5 days, the spinal segment was cryoprotected in PBS with 30% sucrose for 72 h at 4°C. Coronal sections of 50 μm of the spinal cord were mounted on slides and stained with 1% cresyl violet. We then performed qualitative and quantitative evaluations of the injury site.
Statistical analysis
We performed statistical analyses using IBM SPSS Statistics 20.0 software. To determine the effects of locomotor state and speed on spatiotemporal and interlimb coordination dependent variables during quadrupedal tied-belt locomotion, we performed a two-factor repeated measures analysis of variance (ANOVA) [(states: Intact, Spinal) × (speeds: from 0.4 m/sec to 0.8 m/sec)]. Significance level was set at p < 0.05.
The Rayleigh test was performed to determine whether phase intervals shown in circular plots were randomly distributed, as described.62,63,73,74 Briefly, we calculated the r value to measure the dispersion of phase interval values around the mean, with a value of 1 indicating a perfect concentration in one direction, and a value of 0 indicating uniform dispersion. To test the significance of the directional mean, we performed the Rayleigh z test: z = nr2, where n is the sample size (number of steps). The z value was then compared with a critical z value on the Rayleigh table to determine whether there was a significant concentration around the mean (p value).
Results
Quadrupedal locomotion after spinal transection
In the present study, we show that adult spinal cats performed quadrupedal treadmill locomotion and modulated their speed from 0.4 m/sec to 0.8 m/sec when an experimenter assisted with balance by holding the tail. As stated in Methods, quadrupedal locomotion in these spinal cats required perineal stimulation. In a recent study, we also showed that all four of these spinal cats required perineal stimulation to perform hindlimb-only locomotion with the forelimbs placed on a stationary platform.56
Postural adjustments during quadrupedal locomotion in adult spinal cats
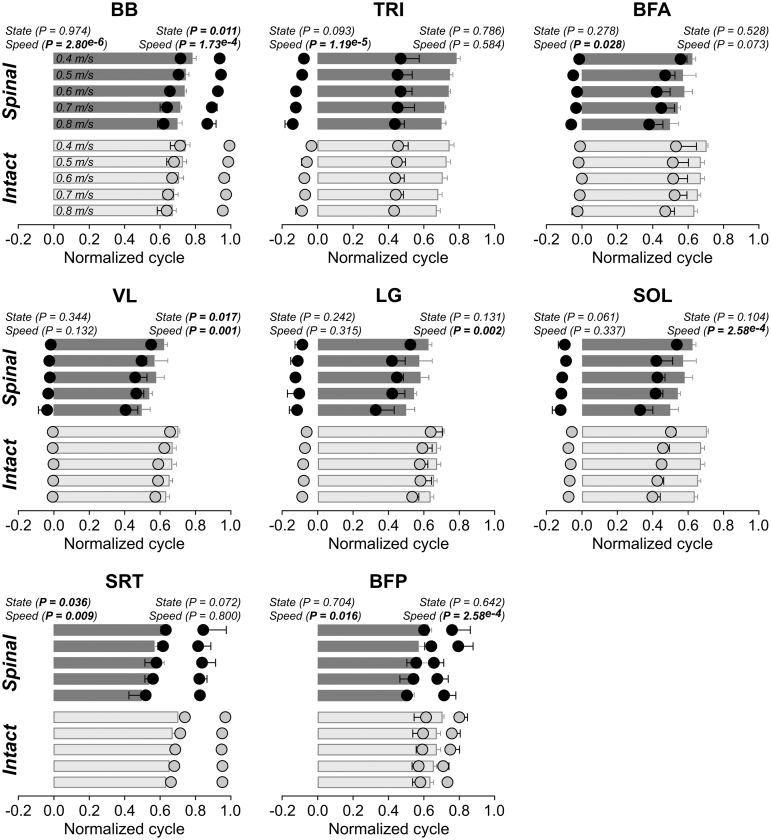
Previous studies have shown that postural orientation of the head influences hindlimb weight support in spinal dogs and cats.7,49,50 Having this in mind, we characterized the posture of individual spinal cats during quadrupedal locomotion. To visualize postural differences, we superimposed the silhouette of each cat in the spinal state (black) with the one obtained in the intact state (gray) at three key events of the gait cycle: (1) right forelimb liftoff, (2) right forelimb midswing, and (3) right forelimb liftoff. This is illustrated in Fig. 1A, showing representative cycles for each state.
FIG. 1.
Postural adjustments during quadrupedal locomotion in adult spinal cats. (A) Superimposed silhouettes for each cat in the intact and spinal states at (1) right forelimb liftoff, right forelimb midswing and (3) right forelimb liftoff. (B) Stance phases during quadrupedal locomotion in the intact and spinal states at 0.4 m/sec for Cat 3 and Cat 1. RFL, right forelimb; RHL, right hindlimb; LFL, left forelimb; LHL, left hindlimb.
Individual cats adopted different head and neck postures during quadrupedal treadmill locomotion. During quadrupedal locomotion at 0.4 m/sec, Cat 1 lowered its head considerably with a convexity in the thoracic spine. Cat 2 lowered its head slightly but maintained a relatively straight back. Cat 3 protracted its head instead of lowering it, with a slight forward tilt of the back. Cat 4 protracted its head and tilted its back slightly forward. The postural strategies adopted by spinal cats during locomotion were similar across treadmill speeds.
Adult spinal cats perform 1:1 and 1:2 fore-hind coordination during quadrupedal locomotion
During tied-belt treadmill locomotion, intact quadrupedal animals perform 1:1 fore-hind coordination, indicating an equal number of coordinated steps at the shoulder and hip girdles.62,75,76 After a spinal transection in kittens51 and adult cats,52 however, studies have reported an apparent loss of coordination between the forelimbs and hindlimbs, with the forelimbs taking more steps than the hindlimbs—or a 2:1 fore-hind step ratio.
In the present study, two spinal cats (Cats 3 and 4) maintained a 1:1 coordination between the forelimbs and hindlimbs (Fig. 1B, left panel), whereas in the other two spinal cats (Cats 1 and 2), the 1:1 coordination broke down. Contrary to previous studies that showed the forelimbs taking more steps than the hindlimbs,51,52 however, we observed the hindlimbs taking more steps than the forelimbs, or a 1:2 fore-hind coordination (Fig. 1B, right panel). In these cats, cycles with 1:2 coordination were interspersed with 1:1 coordination (Fig. 1B, right panel) and represented 32% (Cat 1) and 35% (Cat 2) of cycles at 0.4 m/sec.
As treadmill speed increased to 0.6 m/sec and above, the proportion of cycles with 1:2 coordination decreased, particularly in Cat 1: 56% (Cat 1) and 29% (Cat 2) of cycles at 0.5 m/sec; 19% (Cat 1) and 18% (Cat 2) of cycles at 0.6 m/sec; 6% (Cat 1) and 30% (Cat 2) of cycles at 0.7 m/sec; and 9% (Cat 1) and 22% (Cat 2) of cycles at 0.8 m/sec. It is possible that perineal stimulation played a role in the emergence of 1:2 coordination.
Temporal adjustments of the forelimbs and hindlimbs during quadrupedal locomotion in spinal cats with an increase in speed
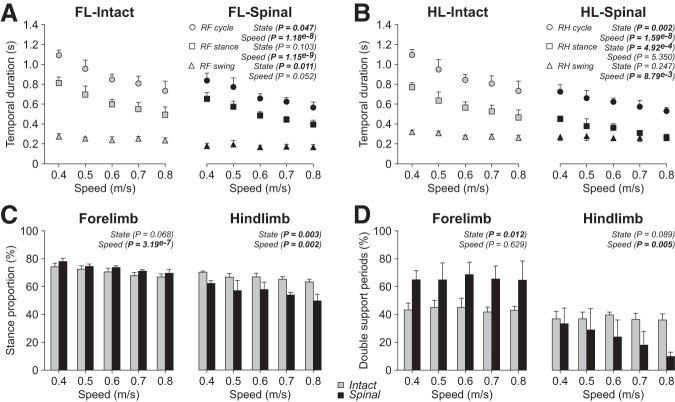
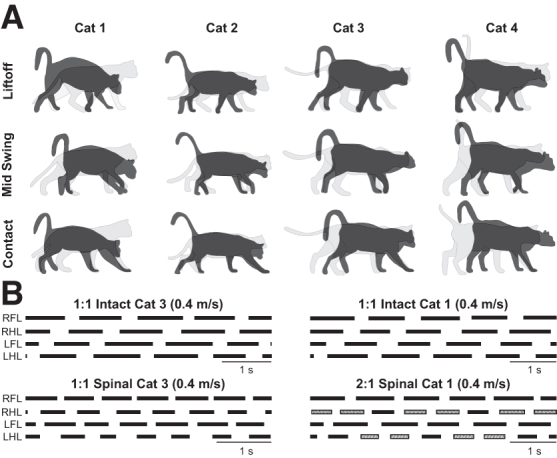
To determine how animals adjust movements of both the forelimbs and hindlimbs to treadmill speed during quadrupedal locomotion, we measured various spatiotemporal variables from 0.4 m/sec to 0.8 m/sec in the intact and spinal states. For these measurements, we only report cycles with 1:1 fore-hind coordination. In the right forelimb, cycle and stance durations significantly decreased during quadrupedal locomotion with increasing speed while swing did not change significantly (Fig. 2A). On average, forelimb cycle duration was significantly longer (+22%) in the intact state compared with the spinal state. Forelimb stance duration did not differ significantly between states while swing duration was significantly longer (+30%) in the intact state. In the right hindlimb, cycle, stance, and swing durations during quadrupedal locomotion decreased significantly with increasing speed (Fig. 2B).
FIG. 2.
Temporal adjustments during quadrupedal locomotion in the intact and spinal states across speeds. Each panel shows cycle, stance, and swing durations during quadrupedal locomotion in the intact and spinal states at five treadmill speeds for the forelimbs (FL) (A) and the hindlimbs (HL) (B). Stance proportions (C) and double support periods (D) for the forelimbs and hindlimbs in the intact and spinal states at five treadmill speeds. At each speed, we averaged 4–42 (14.53 ± 7.74) cycles per cat. Each data point or bar is the mean ± standard deviation for the group (n = 4 cats). The p values comparing state and speeds are indicated (main effects of repeated measures analysis of variance.
On average, hindlimb cycle duration was significantly longer (+30%) in the intact state compared with the spinal state, as was stance duration (+41%). On the other hand, hindlimb swing duration did not significantly differ between states.
To determine whether the forelimbs remained in stance relatively longer in the spinal state to provide more propulsion and stability, we measured the proportion of stance and double support periods from 0.4 m/sec to 0.8 m/sec in intact and spinal cats. For stance proportion, we only used cycles with 1:1 coordination, while for double support periods, we used all cycles (i.e., cycles with 1:1 and 1:2 coordination).
In the right forelimb, stance proportion decreased during quadrupedal locomotion with increasing speed but did not significantly differ between states (Fig. 2C, left panel). In the right hindlimb, stance proportion decreased during quadrupedal locomotion with increasing speed and, on average, was significantly longer in the intact state compared with the spinal state (+16%) (Fig. 2C, right panel). Forelimb double support periods were significantly longer (+34%) in the spinal state compared with the intact state while hindlimb double support periods did not significantly differ between states (Fig. 2D). Forelimb double support periods did not significantly change with increasing speed while hindlimb double support periods decreased visibly because of a decrease in the spinal state.
Spatial adjustments of the forelimbs and hindlimbs during quadrupedal locomotion in spinal cats with an increase in speed
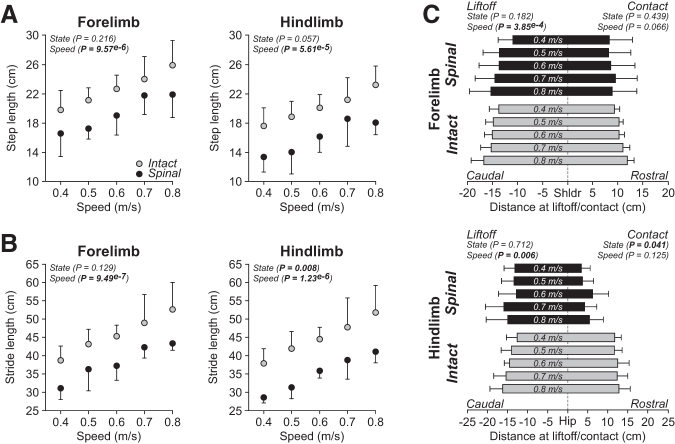
To comparatively investigate spatial adjustments of limb movements in intact and spinal cats with increasing speed, we measured the distances traveled by the limbs and their relation to each other. In the right forelimb, step and stride lengths significantly increased with increasing speed during quadrupedal locomotion, with no significant effect of state for both measures (Figs. 3A, 3B, left panels). In intact and spinal cats, the distance of the forepaw relative to the shoulder at liftoff was significantly more caudal to the shoulder with increasing speed (Fig. 3C, top panel). In contrast, speed did not significantly affect the distance of the forepaw relative to the shoulder at contact. In addition, state did not significantly affect the distance between the toe of the forepaw and the shoulder at liftoff and contact.
FIG. 3.
Spatial adjustments during quadrupedal locomotion in the intact and spinal states across speeds. Step length (A), stride length (B), and the horizontal distances at liftoff/contact (C) during quadrupedal locomotion in the intact and spinal states at five treadmill speeds for the forelimbs (top panels) and hindlimbs (bottom panels). At each speed, we averaged 4–42 (14.53 ± 7.74) cycles per cat. Each data point or bar is the mean ± standard deviation for the group (n = 4 cats). The p values comparing state and speeds are indicated (main effects of repeated measures analysis of variance). Vertical dashed lines in C indicate the zero or shoulder/hip position.
In the right hindlimb, step and stride lengths significantly increased with increasing speed during quadrupedal locomotion in intact and spinal cats (Figs. 3A, 3B, right panels). On average, stride length was significantly longer (+22%) in the intact state while step length did not significantly differ between states. In intact and spinal cats, the distance of the hindpaw relative to the hip at liftoff was significantly more caudal with increasing speed while the distance at contact was unaffected (Fig. 3C). State did not significantly affect the distance of the toe relative to the hip at liftoff, but it was significantly more rostral (+63%) in intact cats at contact (i.e., further from the hip).
We also measured the horizontal distance between the toe markers of the right forelimbs and hindlimbs at contact and liftoff of each limb to assess limb interference. Cats often adopt a pacing-like gait on the treadmill, where the forelimbs and hindlimbs perform approximately simultaneous directional movements, supposedly to prevent the forelimbs and hindlimbs from interfering with one another at hindlimb contact.77 Treadmill speed did not significantly affect the horizontal distance between the forelimbs and hindlimbs at forelimb contact, forelimb liftoff, hindlimb contact, and hindlimb liftoff (Fig. 4). When comparing states, however, the distance between the forelimbs and hindlimbs was significantly longer in spinal cats at forelimb contact (+24%), forelimb liftoff (+63%), and hindlimb contact (+52%) but not at hindlimb liftoff.
FIG. 4.
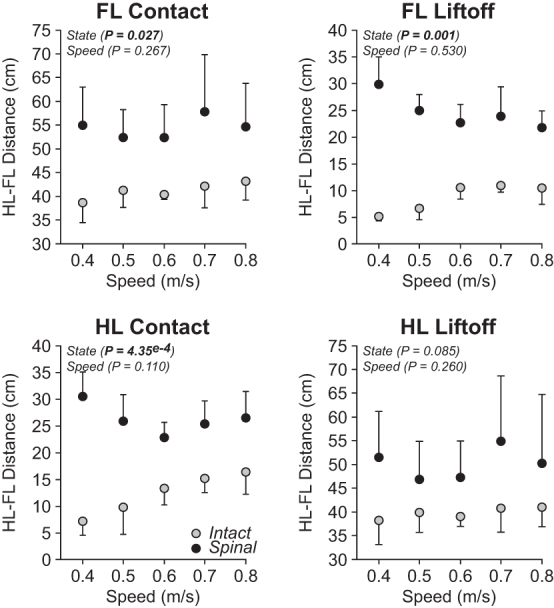
Homolateral limb interference during quadrupedal locomotion in the intact and spinal states across speeds. Each panel shows horizontal distances between the homolateral forelimbs (FL) and hindlimbs (HL) at contact (left) and liftoff (right) of the forelimbs (top panels) and hindlimbs (bottom panels) during quadrupedal locomotion in the intact and spinal state at five treadmill speeds. At each speed, we averaged 4–42 (14.53 ± 7.74) cycles per cat. Each data point is the mean ± standard deviation for the group (n = 4 cats). The p values comparing state and speeds are indicated (main effects of repeated measures analysis of variance).
Modulation of muscle activity in the forelimbs and hindlimbs during quadrupedal locomotion in spinal cats with an increase in speed
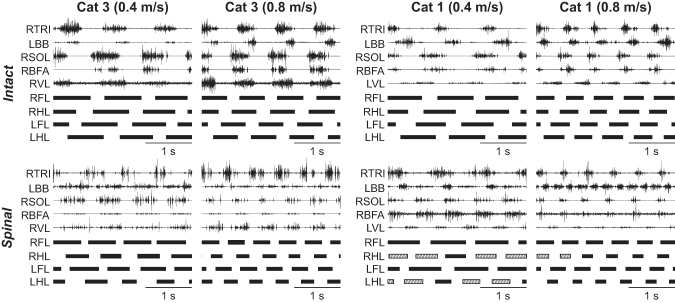
Figure 5 illustrates EMG activity in selected forelimb and hindlimb muscles during quadrupedal locomotion in the intact and spinal states for Cats 1 and 3. Cat 3 maintained a 1:1 coordination while Cat 1 performed some steps with a 1:2 fore-hind coordination. In the intact state for both cats, the burst duration of forelimb and hindlimb extensor muscles (TRI; SOL; BFA; VL) decreased from 0.4 m/sec to 0.8 m/sec. Similarly, in the spinal state for both cats, the burst duration of these extensor muscles decreased with increasing speed. For the same speed, however, the burst duration is slightly longer in the intact state compared with the spinal state. In both states, the burst onsets of TRI and SOL muscles occurred before paw contact during quadrupedal locomotion while those of BFA and VL muscles occurred at or around contact.
FIG. 5.
Quadrupedal locomotion in the intact and spinal states at 0.4 m/sec and 0.8 m/sec speed. Top and bottom panels show electromyography activity from selected forelimb and hindlimb muscles along with stance phases (thick horizontal lines) of the left (L) and right (R) limbs in two cats in the intact and spinal states during quadrupedal locomotion. TRI, triceps brachii; BB, biceps brachii; SOL, soleus; BFA, biceps femoris anterior; VL, vastus lateralis.
In contrast, burst onsets of the BB muscle differed between states. In both intact cats, BB burst onset occurred at the stance to swing transition while the burst offset occurred at the swing to stance transition. In both spinal cats, the burst duration of the BB muscle was prolonged. For instance, in both spinal cats at 0.4 m/sec, BB burst onsets occurred right before the swing phase and continued until the middle of the stance phase. At the faster speed of 0.8 m/sec, it became easier to distinguish two bursts of muscle activity, one during swing and another during stance (especially in Cat 1 in the spinal state).
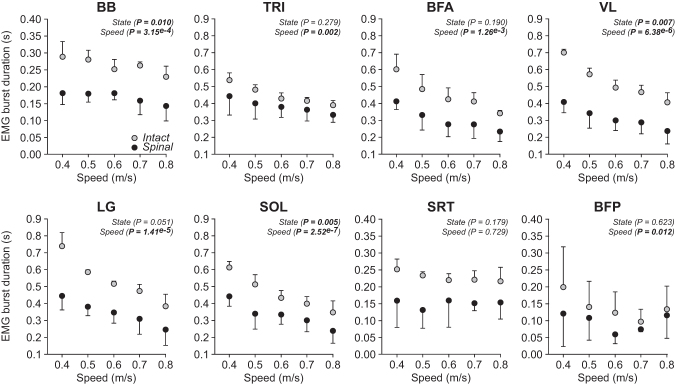
Burst duration
Figure 6 shows EMG burst durations of selected forelimb and hindlimb muscles during quadrupedal locomotion from 0.4 m/sec to 0.8 m/sec in intact and spinal cats for the group. The EMG burst duration of the BB muscle, an elbow flexor, significantly changed (slight decrease) with increasing speed in intact and spinal cats and, on average, was significantly longer (+36%) in intact cats.
FIG. 6.
Modulation of electromyography burst durations during quadrupedal locomotion in the intact and spinal states across speeds. The figure shows burst durations in selected muscles during quadrupedal locomotion in the intact and spinal states at five treadmill speeds. At each speed, we averaged 4–42 (14.53 ± 7.74) cycles per cat. Each data point is the mean ± standard deviation for the group. The p values comparing state and speeds are indicated (main effects of repeated measures analysis of variance). BB, biceps brachii (n = 3–4 cats); TRI, triceps brachii (n = 3 cats); BFA, biceps femoris (n = 3 cats); VL, vastus lateralis (n = 3 cats); LG, lateral gastrocnemius (n = 3 cats); SOL, soleus (n = 4 cats); SRT, sartorius anterior (n = 3 cats); BFP, biceps femoris posterior (n = 3 cats).
In intact and spinal cats, the EMG burst duration of the TRI muscle, an elbow and shoulder extensor, significantly decreased with increasing speed during quadrupedal locomotion, with no significant effect of state. In intact and spinal cats, EMG burst durations of hindlimb extensor muscles, BFA, VL, LG, and SOL significantly decreased with increasing speed. On average, extensor burst duration was significantly longer in intact cats compared with spinal cats for VL (+40%) and SOL (+28%) but not LG and BFA.
The EMG burst duration of the BFP muscle, a knee flexor/hip extensor significantly changed with speed, albeit not in a constant direction with increasing speed, while the burst duration of the SRT muscle, a hip flexor/knee extensor, did not change significantly. On average, BFP and SRT bursts durations did not significantly differ between intact and spinal cats.
Burst phasing
Figure 7 shows the phasing of EMG bursts for selected forelimb and hindlimb muscles in a cycle normalized to stance onset of the corresponding limb (i.e., BB and TRI muscles were normalized to stance onset of the forelimb while VL, LG, SOL, SRT, and BFP muscles were normalized to stance onset of the hindlimb) during quadrupedal locomotion from 0.4 m/sec to 0.8 m/sec in intact and spinal cats for the group. For the phasing of forelimb and hindlimb EMG bursts, we found a significant shift in onsets and offsets with increasing speed for some muscles. In the normalized cycle, onsets occurred significantly earlier with increasing speed for BB, TRI, BFA, SRT, and BFP. Speed did not affect the burst onsets of VL, LG, and SOL.
FIG. 7.
Modulation of electromyography phasing during quadrupedal locomotion in the intact and spinal states across speeds. The figure shows burst onsets and offsets from selected muscles in a normalized cycle during quadrupedal locomotion in the intact and spinal states at five treadmill speeds. At each speed, we averaged 4–42 (14.53 ± 7.74) cycles per cat. The bars represent stance durations while the circles represent bursts onsets and offsets for the group. Each data point is the mean ± standard deviation for the group (n = 3–4 cats). P values comparing state and speeds are indicated (main effects of repeated measures analysis of variance). BB, biceps brachii (n = 4 cats); TRI, triceps brachii (n = 3 cats); BFA, biceps femoris (n = 3 cats); VL, vastus lateralis (n = 3 cats); LG, lateral gastrocnemius (n = 3 cats); SOL, soleus (n = 4 cats); SRT, sartorius anterior (n = 3 cats); BFP, biceps femoris posterior (n = 3 cats).
On average, we observed an earlier burst onset of the SRT in spinal cats, whereas state had no significant effect on the burst onsets of the other muscles. Burst offsets occurred significantly earlier at faster speeds for BB, VL, LG, SOL, and BFP but not for TRI and BFA. We observed earlier offsets in spinal cats compared with intact cats for BB and VL but not for the other muscles.
Interlimb coordination during quadrupedal locomotion in intact and spinal cats
To determine whether spinal cats coordinated their limbs during quadrupedal locomotion, we measured the phase interval between contacts of four limb pairs (see Methods). Values of 0 degrees or 360 degrees indicate a strict in-phase coupling, while a value of 180 degrees indicates a strict out-of-phase coupling. Previous studies have used values between 270 degrees and 90 degrees to denote an in-phase coupling and values between 90 degrees and 270 degrees for out-of-phase coupling.63,66
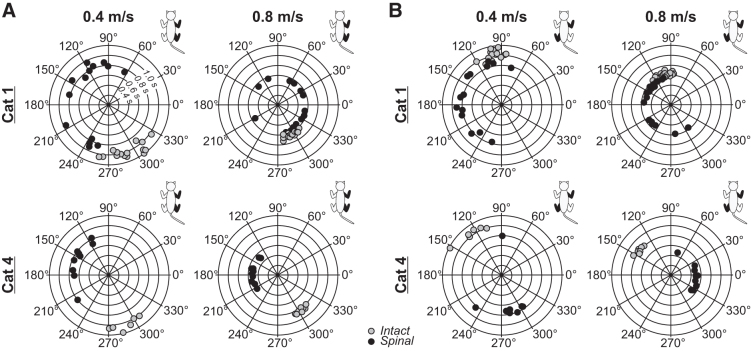
Figure 8 shows circular plots for homolateral and diagonal couplings for Cats 1 and 4 during quadrupedal locomotion in the intact and spinal states at 0.4 m/sec and 0.8 m/sec. We performed the Rayleigh test and calculated the r value, a measure of angular dispersion around the mean. In the intact state, r values for homolateral (Table 1) and diagonal (Table 2) couplings were high, with a mean of 0.97 ± 0.03 and a range of 0.86 to 1.00. In the spinal state, r values were almost always consistently lower, with a mean of 0.60 ± 0.24 and a range of 0.14 to 0.93, indicating a less consistent coordination between the forelimbs and hindlimbs.
FIG. 8.
Step-by-step phasing of homolateral and diagonal limb pairs during tied-belt quadrupedal locomotion in two cats in the intact and spinal states at 0.4 m/sec and 0.8 m/sec. In the circular plots, phase intervals are expressed in degrees around the circumference, whereas cycle durations are plotted in radii. Each data point represents a locomotor cycle from one session in a single cat in the intact and spinal states. Only cycles with 1:1 fore-hind coordination are shown.
Table 1.
Homolateral Couplings during Quadrupedal Locomotion in the Intact and Spinal States
| State | Speed (m/sec) | Range (°) | r | p | |
|---|---|---|---|---|---|
| Cat 1 | Intact | 0.4 | 259 - 325 | 0.95 | < 0.05 |
| 0.8 | 237 - 310 | 0.97 | < 0.05 | ||
| Spinal | 0.4 | 65 - 254 | 0.46 | Not significant | |
| 0.8 | 24 - 348 | 0.43 | < 0.05 | ||
| Cat 2 | Intact | 0.4 | 244 - 308 | 0.97 | < 0.05 |
| 0.8 | 257 - 307 | 0.98 | < 0.05 | ||
| Spinal | 0.4 | 96 - 328 | 0.51 | < 0.05 | |
| 0.8 | 76 - 252 | 0.55 | < 0.05 | ||
| Cat 3 | Intact | 0.4 | 185 - 300 | 0.86 | < 0.05 |
| 0.8 | 278 - 299 | 1.00 | < 0.05 | ||
| Spinal | 0.4 | 106 - 204 | 0.89 | < 0.05 | |
| 0.8 | 120 - 349 | 0.40 | < 0.05 | ||
| Cat 4 | Intact | 0.4 | 265 - 313 | 0.96 | < 0.05 |
| 0.8 | 295 - 315 | 0.99 | < 0.05 | ||
| Spinal | 0.4 | 115 - 217 | 0.87 | < 0.05 | |
| 0.8 | 132 - 211 | 0.93 | < 0.05 |
The table shows the range of homolateral coupling values in degrees for individual cats during episodes at 0.4 m/sec and 0.8 m/sec and the r value, a measure of angular dispersion around the mean, along with p values of the Rayleigh test. We only used cycles with 1:1 coordination.
Table 2.
Diagonal Couplings during Quadrupedal Locomotion in the Intact and Spinal States
| State | Speed (m/sec) | Range (°) | r | p | |
|---|---|---|---|---|---|
| Cat 1 | Intact | 0.4 | 87 - 122 | 0.99 | < 0.05 |
| 0.8 | 85 - 127 | 0.98 | < 0.05 | ||
| Spinal | 0.4 | 78 - 253 | 0.66 | < 0.05 | |
| 0.8 | 106 - 307 | 0.56 | < 0.05 | ||
| Cat 2 | Intact | 0.4 | 73 - 132 | 0.97 | < 0.05 |
| 0.8 | 85 - 136 | 0.98 | < 0.05 | ||
| Spinal | 0.4 | 92 - 281 | 0.54 | < 0.05 | |
| 0.8 | 27 - 295 | 0.23 | < 0.05 | ||
| Cat 3 | Intact | 0.4 | 48 - 125 | 0.94 | < 0.05 |
| 0.8 | 92 - 123 | 0.99 | < 0.05 | ||
| Spinal | 0.4 | 7 - 360 | 0.87 | < 0.05 | |
| 0.8 | 10 - 350 | 0.54 | < 0.05 | ||
| Cat 4 | Intact | 0.4 | 95 - 152 | 0.96 | < 0.05 |
| 0.8 | 127 - 152 | 0.99 | < 0.05 | ||
| Spinal | 0.4 | 90 - 304 | 0.69 | < 0.05 | |
| 0.8 | 13 - 348 | 0.82 | < 0.05 | ||
The table shows the range of diagonal coupling values in degrees for individual cats during episodes at 0.4 m/sec and 0.8 m/sec and the r value, a measure of angular dispersion around the mean, along with p values of the Rayleigh test. We only used cycles with 1:1 coordination.
For the group, speed and state did not significantly affect forelimb and hindlimb couplings, because cats maintained values around 180 degrees, close to a strict out-of-phase coupling (Figs. 9A and 9B). Eidelberg and coworkers52 had reported that mean intervals and standard deviations of the homolateral coupling were altered in spinal adult cats. Here, we confirm and extend these findings in a larger sample.
FIG. 9.
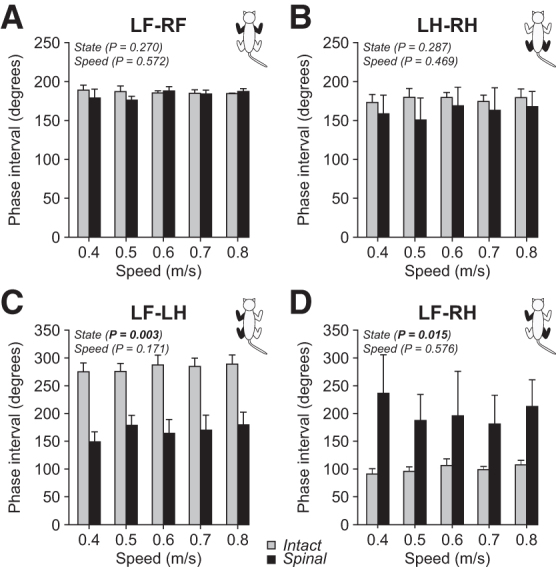
Interlimb coordination during quadrupedal locomotion in the intact and spinal states across speeds. Forelimb (A), hindlimb (B), homolateral (C), and diagonal (D) couplings are shown during quadrupedal locomotion in the intact and spinal states at five treadmill speeds. At each speed, we averaged 4–42 (14.53 ± 7.74) cycles per cat. Each data point is the mean ± standard deviation for the group (n = 4 cats). The p values comparing state and speeds are indicated (main effects of repeated measures analysis of variance).
Although speed did not significantly affect homolateral and diagonal couplings, state had a significant effect because of an earlier contact of the hindlimbs in the normalized cycle (Figs. 9C and 9D). In the intact state, homolateral coupling values ranged from 220 degrees to 338 degrees (282 ± 16), with in-phase or out-of-phase couplings. Spinal cats, however, had values in a larger range between 10 degrees and 342 degrees (168 ± 22). Diagonal coupling values ranged from 61 degrees and 149 degrees (100 ± 9) in the intact state, with an increased and more variable range in the spinal state, with values ranging from 9 degrees to 360 degrees (207 ± 62).
To determine whether spinal cats displayed greater variations in limb couplings, we measured the coefficient of variation (Fig. 10). Coefficients of variation were low for forelimb couplings and not significantly affected by state. We observed a small but significant decrease in forelimb coupling variations with speed. In contrast, hindlimb coupling variations were significantly greater in spinal cats with no significant effect of speed. We observed the most striking changes in variations for homolateral and diagonal couplings, both significantly increasing in spinal cats, with no effect of speed. Again, these results suggest a less consistent coordination between the forelimbs and hindlimbs during quadrupedal locomotion in spinal cats.
FIG. 10.
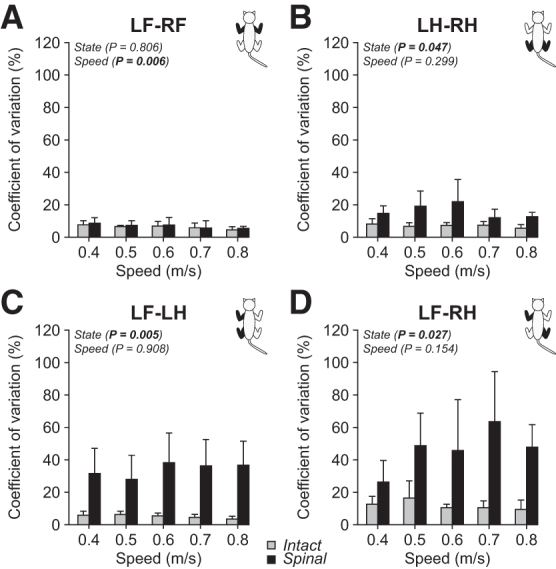
Variations in interlimb coordination during quadrupedal locomotion in the intact and spinal states across speeds. Coefficients of variation of forelimb (A), hindlimb (B), homolateral (C), and diagonal (D) couplings are shown during quadrupedal locomotion in the intact and spinal states at five treadmill speeds. At each speed, we averaged 4–42 (14.53 ± 7.74) cycles per cat. Each data point is the mean ± standard deviation for the group (n = 4 cats). The p values comparing state and speeds are indicated (main effects of repeated measures analysis of variance).
Discussion
The novelty of the present study was the comparative investigation of quadrupedal locomotion (when all four limbs are moving) before and after thoracic spinal transection to highlight the role of forelimb movements and forelimb-hindlimb coordination. We extend the results of our recent study that compared quadrupedal locomotion in intact cats with hindlimb-only locomotion (forelimbs stationary) in the intact and spinal states.56
Quadrupedal locomotion after spinal transection required an increase in spinal neuronal excitability
When evaluating treadmill quadrupedal locomotion in spinal kittens, Howland and associates51 reported that four of their five animals could perform spontaneous quadrupedal treadmill locomotion when an experimenter held the tail to provide weight support and balance. In contrast, Eidelberg and coworkers52 reported that a strong stimulation below the level of the lesion, such as pinching the skin at the base of the tail, was required to elicit weight support and quadrupedal locomotion in adult spinal cats.
Here, we confirm that adult spinal cats required an increase in spinal excitability, which we provided with perineal stimulation. Thus, neonatal or young animals appear to have a greater capacity to compensate for the initial loss of spinal excitability. Several studies have reported that stimulating the perineal region facilitates weight bearing and hindlimb locomotion in spinal-transected animals through a non-specific increase in spinal excitability mediated by an undefined mechanism.58 None of the cats of the present study, however, could perform hindlimb-only locomotion without perineal stimulation.
In the past, we have had spinal cats that performed hindlimb-only locomotion with no perineal stimulation but, unfortunately, we did not characterize their capacity for quadrupedal locomotion, with one exception, where we investigated quadrupedal locomotor capacity for proof-of-concept. This spinal cat could perform quadrupedal locomotion without perineal stimulation (data not shown), indicating that perineal stimulation or an increase in spinal excitability is not necessarily required if a robust locomotor pattern recovers after spinal transection.
From a functional and clinical perspective, the potential benefits of stimulating the perineal region on locomotor recovery in humans have received little attention. To date, clinical studies have shown that after complete or incomplete spinal cord injury, dorsal penile or clitoral nerve stimulation is effective for managing neurogenic detrusor hyperactivity and increasing cystometric bladder capacity.78–85 Interestingly, other clinical studies have suggested that in persons with complete or incomplete spinal cord injury, the spinal networks retain a flexible state of excitability and functionality that can be facilitated by exogenous and invasive methods of neuromodulation, such as epidural electrical stimulation, to facilitate the recovery of standing and/or locomotion.86–93
The potential effects of tonic and/or phasic stimulation of perineal afferents on other sensorimotor functions, such as standing and/or locomotion, remain to be investigated, however. Overall, further investigations are required in pre-clinical models to understand how somatosensory feedback from the perineal region interacts with spinal sensorimotor circuits to guide the development of clinical research of a potentially powerful and promising endogenous method to facilitate locomotion after spinal cord injury.
Factors limiting quadrupedal locomotion after spinal transection
Studies in spinal cats7,94 and rats53,54,95 have shown that the recovery of locomotion was inversely related to age at the time of spinal transection. Howland and colleagues51 reported that 15 weeks after spinal transection at birth, two of their five kittens performed some full weight-supported steps during independent quadrupedal overground locomotion.51 Interestingly, the two spinal kittens that recovered this capacity were the smallest animals of the group. At 20 weeks post-spinalization, however, all locomotor characteristics previously acquired, such as full weight support and balance, deteriorated in both kittens.
The authors proposed that when the weight of the animal reaches a threshold, full weight support and balance of the hindquarters becomes difficult because of inadequate muscle strength and/or joint stability. It is also possible that the higher center of gravity of adult cats and the greater need for balance plays a role in the deterioration of locomotion.
Consistent with this study, none of our four adult spinal cats demonstrated weight-supported steps and/or hindquarter balance during independent quadrupedal overground (not shown) or treadmill locomotion. When moving overground, we found that the forelimbs pulled the cats' body forward while the hindlimbs were positioned together either laterally (Cats 1, 3, and 4) or caudally (Cat 2) to the body. Future studies, however, should investigate the biomechanical factors and constraints that limit the recovery of balance and, therefore, functional locomotion in spinal mammals.
Does treadmill locomotor training facilitate the reexpression of locomotion or does it allow for the development of compensatory strategies?
In the last decades, task-specific training was advocated for functional recovery after spinal cord injury in both pre-clinical research areas7,11–15,51,52 and clinical settings.96–100 Consequently, most studies investigating the recovery of quadrupedal locomotion in spinal puppies/kittens, spinal neonatal rats, and adult spinal cats included exercise regimens,7 weeks of treadmill locomotor training,51,52 or animals were tested frequently in situations that enhanced the opportunity for locomotion.53 Recent studies, however, showed that the recovery of forward16 and backward30 hindlimb locomotion in spinal cats, with the forelimbs on a stationary platform, does not require task-specific training.
In the present study, we extend these results by demonstrating that the recovery of quadrupedal locomotion also does not require task-specific training, because none of our cats were trained to perform quadrupedal locomotion after spinal transection. It is important to mention, however, that all our spinal cats required an increase in spinal excitability in the form of perineal stimulation to induce the expression of hindlimb-only56 and quadrupedal locomotion.
Postural adjustments during quadrupedal locomotion in adult spinal cats
In the present study, we reported that adult spinal cats adopted different head and neck postures during quadrupedal treadmill locomotion, showing interanimal variability. An important question is: why do spinal mammals adopt different head and neck postures during locomotion? At the end of the 19th century, studies described spinal dogs that could stand after low thoracic spinal transection.45–47 Decades later, the arrival of the chronophotographic method made it possible to analyze this phenomenon in spinal dogs and compare it with intact dogs.48
It was proposed that the ability of spinal dogs to maintain their hindquarters in a standing posture was possible because of the positioning of the head.7,49,50 By lowering their head and contracting the musculature of the cephalic region and the shoulder girdle, spinal dogs could hold their trunk nearly horizontal. In other words, to stand and maintain balance, spinal mammals lower their head so the forelimbs act as a fulcrum and the head as a counterweight.7 Simply lowering the head and neck decreases the height of the body's center of mass, which may be useful for balance control.
To unload the hindlimbs and put more weight on the forelimbs, quadrupeds can protract the head and neck forward, as seen in some cats in Figure 1. The importance of head positioning has also been highlighted in mammals with amputations just below the knee, where lowering the head keeps the trunk horizontal.7,101 Moreover, a study in horses with unilateral forelimb lameness reported reduced amplitude of vertical head movements during the stance phase of the lame forelimb and an increase during stance of the intact forelimb.102 We propose that lowering the head could be a compensatory strategy used by spinal cats to stabilize the trunk musculature and lower their center of mass to improve dynamic stability during locomotion.
Adult spinal cats perform 1:1 and 1:2 fore-hind coordination during quadrupedal locomotion
In the present study, we reported that two spinal cats maintained a 1:1 coordination (Cats 3 and 4) between the forelimbs and hindlimbs while in the other two spinal cats, the hindlimbs performed more steps than the forelimbs (Cats 1 and 2). Interestingly, the two large males (Cats 3 and 4) maintained 1:1 coordination, while the two small females adopted 1:2 coordination. Studies after spinal transection in kittens51 and adult cats52 reported an apparent loss of coordination between the forelimbs and hindlimbs, with the forelimbs taking more steps than the hindlimbs (2:1 fore-hind coordination).
Similar findings have been reported after a variety of incomplete thoracic spinal cord injuries, such as lateral hemisection,62,103 compression injury,104 and lesions of the ventral/ventrolateral105 or dorsal/dorsolateral quadrant of the spinal cord.75,106 A recent study also showed that blocking serotonergic receptors in the lumbar spinal cord of intact rats generated 2:1 fore-hind coordination patterns.107
Although the precise reason for the appearance of 2:1 forelimb-hindlimb coordination remains unclear, several hypotheses have been proposed. First, a study proposed that this compensatory strategy (faster forelimb cadence) resulted from a reduction in inhibitory influence from hindlimb CPGs to forelimb CPGs or a shift in the center of gravity rostrally so that the forelimbs have to bear a greater proportion of body weight after lesions of the spinal cord.75 Second, a study proposed that this compensatory strategy was a way to maximize static stability.62 Indeed, performing smaller steps could be a strategy to keep the center of gravity within the support polygon, the surface obtained by joining the different points of contact of the animal.76 Studies have reported that the posture of the animal with a minimum of three limbs on the ground is stable during locomotion if the center of gravity falls within the support polygon.39,108–110
Spatiotemporal and kinematic variables in intact and spinal cats
The majority of studies have compared quadrupedal locomotion in the intact state to hindlimb-only locomotion in the spinal state.11,25,27,28,94,103,111,112 In the present study, we show that spatiotemporal adjustments of the forelimbs and hindlimbs differ between the intact and spinal states during quadrupedal locomotion. For the forelimb, cycle and swing durations were longer in the intact state compared with the spinal state. For the hindlimb, cycle and stance durations were longer in the intact state compared with the spinal state. We did not observe a difference in the duration of the stance phase for the forelimb in spinal cats.
To determine whether the forelimb remained in stance proportionally longer, we measured stance proportion and double support periods from 0.4 m/sec to 0.8 m/sec in both states. Although forelimb stance proportion was unaffected after spinal transection, we show that forelimb double support periods were longer in spinal cats while hindlimb double support periods did not differ between states. Hindlimb stance proportion was longer in the intact state compared with the spinal state with no state-dependent difference in double support periods. The increase in forelimb double support periods is consistent with a greater need for forelimb support during quadrupedal locomotion in spinal cats. Cats might do this voluntarily.
A notable difference between the intact and spinal states was the horizontal distance between the toe markers of the right forelimbs and hindlimbs at contact and liftoff, our measure of limb interference. As stated, some intact cats adopt a pacing-like gait on the treadmill, where the forelimbs and hindlimbs perform approximately simultaneous directional movements, proposed to prevent the forelimbs and hindlimbs from interfering with one another at hindlimb contact.77 We found that the distance between the homolateral forelimbs and hindlimbs increased in spinal cats at contact and liftoff of the forelimb. This increased distance might be a voluntary strategy adopted by spinal cats to keep their forelimb away from their hindlimbs to avoid interference.
Interlimb coordination in intact and spinal cats
Consistent with previous studies in spinal kittens51 and spinal adult cats,52 we show that hindlimb coupling was maintained during quadrupedal treadmill locomotion while homolateral coupling was impaired (Figs. 9 and 10). It was reported that homolateral coordination cannot be restored by assisting with weight support and/or with treadmill locomotor training.51 Here, we show that speed and state did not affect forelimb coupling, because adult spinal cats maintained values around 180 degrees, close to a strict out-of-phase coupling.
The lack of change in forelimb and hindlimb couplings is not surprising, because this coordination is mediated by segmental commissural interneurons, neurons with crossed projections.4 In contrast, the spinal state altered homolateral and diagonal coordination because of an earlier and more variable contact of the hindlimbs. In the intact state, homolateral and diagonal coupling values were consistent, whereas spinal cats had considerably larger variations, consistent with impaired coordination between the forelimbs and hindlimbs.
Spinal transection abolishes short and long propriospinal pathways that project homolaterally or diagonally, as well as supraspinal signals to lumbar levels that coordinate forelimb and hindlimb movements.4 Interestingly, by silencing long ascending propriospinal neurons in rats, a recent study reported a disruption of forelimb and hindlimb couplings during overground but not treadmill locomotion.113 This study highlights that changes in interlimb coordination during locomotion after the loss of communication between cervical and lumbar levels are context-dependent.
Forelimb and hindlimb muscle activity in intact and spinal cats
Several studies have compared EMG activity obtained during quadrupedal locomotion in intact cats and hindlimb-only locomotion with the forelimbs on a stationary platform in spinal cats.11,111 Although a few studies have compared quadrupedal locomotion in the intact and spinal states, they did not investigate changes in forelimb and hindlimb muscle activity.51,52 In the present study, we observed shorter burst durations in VL and SOL in spinal cats. This decreased extensor burst duration can possibly be explained by a reduction in load on the hindlimbs during quadrupedal locomotion in spinal cats. For the other extensor muscles (BFA, LG) and flexor (SRT, BFP) hindlimb muscles, however, no differences between states were observed in burst duration. Therefore, adjustments in muscle activity appear to be muscle specific.
Another important issue is whether the positioning of the head modulates the activity of forelimb and hindlimb muscles. Gottschall and Nichols114 investigated the consequences of head pitch nose down on hindlimb muscle activity during treadmill locomotion in decerebrate cats. They reported that the modulation of head pitch nose down produced immediate but transient changes in hindlimb muscle activity, similar to those observed during downhill locomotion in intact cats. For instance, head pitch nose down mimicked uphill walking, with BFA muscle activity starting just after hindpaw contact and remaining active throughout stance with a higher magnitude than with the head in a neutral position. In contrast, head pitch nose up mimicked downhill walking, with absent BFA muscle activity during the entire step.114–116
In the present study, we observed an important interanimal variability for the BFA muscle (Fig. 5). For instance, for spinal Cat 3, which protracted its head instead of lowering it, with a slight forward tilt of the back (Fig. 1A), we observed weak BFA activity, while for spinal Cat 1, which lowered its head considerably and had a convexity in the thoracic spine, we observed larger magnitude BFA activity that was greater than in the intact state. Thus, even though neural inputs from the head and neck no longer reach lumbar levels after spinal transection, postural adjustments still influence hindlimb muscle activity.
In the forelimbs, we did not detect a difference in burst duration for the TRI muscle between intact and spinal cats. The BB muscle also displayed a prominent second burst of activity during the stance phase in the spinal state, particularly at faster speeds. This stance-related activity could be a way to increase limb stiffness by coactivating forelimb flexor and extensor muscles during the support period. During downslope locomotion, previous studies have reported a second burst of activity in some hindlimb flexor muscles during the stance phase in the spinal state to better control descent.115,116
Functional and clinical considerations
Although we observed clear differences between the intact and spinal states, including impaired fore-hind coordination, adult spinal cats retained the ability to perform quadrupedal locomotion. These results clearly show that biomechanical properties of the musculoskeletal system play an important role in generating quadrupedal locomotion and adjusting it to an increase in speed despite the loss of neural communication between cervical and lumbar levels.
Similar to quadrupedal mammals, injury at any level of the spinal cord disrupts neuronal circuits and/or pathways involved in coordinating the arms and legs in humans.96,117,118 Despite the importance of arm-leg coordination, most rehabilitation strategies, such as task-specific locomotor training and/or the prescription of assistive devices focus solely on the lower limbs, which is likely not optimal to promote the recovery of a functional locomotor pattern. During locomotion, the primary mechanical effect of upper limb swing is to reduce body angular momentum generated by the contralateral lower limbs about the vertical axis.119 Moreover, studies have suggested that during locomotion, upper limb swing may facilitate lower limb muscle activation via neural coupling.120,121
Bidirectional interactions between neural mechanisms and properties of the musculoskeletal system, however, remain poorly understood. A better understanding of these interactions during complex movements such as locomotion would improve clinical rehabilitation strategies and the design of assistive devices.
Acknowledgments
We thank Philippe Drapeau for providing data acquisition and analysis software, developed in the Rossignol and Drew laboratories at the Université de Montréal.
Funding Information
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC RGPIN-2016-03790) to A.F., and the National Institutes of Health: R01 NS110550 to A.F, IAR and BIP. A.F. is a Fonds de Recherche-Santé Quebec (FRQS) Senior Research Scholar. J.H. and A.N.M. were supported by FRQS doctoral and postdoctoral scholarships, respectively. J.A. was supported by master's scholarships from NSERC and FRQS.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Dietz, V. (2002). Do human bipeds use quadrupedal coordination? Trends Neurosci. 25, 462–467. [DOI] [PubMed] [Google Scholar]
- 2. Dietz, V., and Michel, J. (2009). Human bipeds use quadrupedal coordination during locomotion. Ann. N. Y. Acad. Sci. 1164, 97–103. [DOI] [PubMed] [Google Scholar]
- 3. Zehr, E.P., Barss, T.S., Dragert, K., Frigon, A., Vasudevan, E.V., Haridas, C., Hundza, S., Kaupp, C., Klarner, T., Klimstra, M., Komiyama, T., Loadman, P.M., Mezzarane, R.A., Nakajima, T., Pearcey, G.E.P., and Sun, Y. (2016). Neuromechanical interactions between the limbs during human locomotion: an evolutionary perspective with translation to rehabilitation. Exp. Brain Res. 234, 3059–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frigon, A. (2017). The neural control of interlimb coordination during mammalian locomotion. J. Neurophysiol. 117, 2224–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rossignol, S., Dubuc, R., and Gossard, J.-P. (2006). Dynamic sensorimotor interactions in locomotion. Physiol. Rev. 86, 89–154. [DOI] [PubMed] [Google Scholar]
- 6. Frigon, A., Akay, T., and Prilutsky, B.I. (2021). Control of mammalian locomotion by somatosensory feedback. Compr. Physiol. 12, 2877–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shurrager, P.S., and Dykman, R.A. (1951). Walking spinal carnivores. J. Comp. Physiol. Psychol. 44, 252–262. [DOI] [PubMed] [Google Scholar]
- 8. Lovely, R.G., Gregor, R.J., Roy, R.R., and Edgerton, V.R. (1986). Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp. Neurol. 92, 421–435. [DOI] [PubMed] [Google Scholar]
- 9. Barbeau, H., and Rossignol, S. (1987). Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 412, 84–95. [DOI] [PubMed] [Google Scholar]
- 10. Lovely, R.G., Gregor, R.J., Roy, R.R., and Edgerton, V.R. (1990). Weight-bearing hindlimb stepping in treadmill-exercised adult spinal cats. Brain Res. 514, 206–218. [DOI] [PubMed] [Google Scholar]
- 11. Bélanger, M., Drew, T., Provencher, J., and Rossignol, S. (1996). A comparison of treadmill locomotion in adult cats before and after spinal transection. J. Neurophysiol. 76, 471–491. [DOI] [PubMed] [Google Scholar]
- 12. De Leon, R.D., Hodgson, J.A., Roy, R.R., and Edgerton, V.R. (1998). Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J. Neurophysiol. 80, 83–91. [DOI] [PubMed] [Google Scholar]
- 13. De Leon, R.D., Hodgson, J.A., Roy, R.R., and Edgerton, V.R. (1999). Retention of hindlimb stepping ability in adult spinal cats after the cessation of step training. J. Neurophysiol. 81, 85–94. [DOI] [PubMed] [Google Scholar]
- 14. Leblond, H., L'Esperance, M., Orsal, D., and Rossignol, S. (2003). Treadmill locomotion in the intact and spinal mouse. J. Neurosci. 23, 11411–11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cha, J., Heng, C., Reinkensmeyer, D.J., Roy, R.R., Edgerton, V.R., and De Leon, R.D. (2007). Locomotor ability in spinal rats is dependent on the amount of activity imposed on the hindlimbs during treadmill training. J. Neurotrauma 24, 1000–1012. [DOI] [PubMed] [Google Scholar]
- 16. Harnie, J., Doelman, A., de Vette, E., Audet, J., Desrochers, E., Gaudreault, N., and Frigon, A. (2019). The recovery of standing and locomotion after spinal cord injury does not require task-specific training. Elife 8, e50134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown, T.G., and Sherrington, C.S. (1911). The intrinsic factors in the act of progression in the mammal. Proc. R. Soc. Lond. B. Biol. 84, 308–319. [Google Scholar]
- 18. Grillner, S., McClellan, A., Sigvardt, K., Wallén, P., and Wilén, M. (1981). Activation of NMDA-receptors elicits “fictive locomotion” in lamprey spinal cord in vitro. Acta Physiol. Scand. 113, 549–551. [DOI] [PubMed] [Google Scholar]
- 19. Grillner, S., and El Manira, A. (2020). Current principles of motor control, with special reference to vertebrate locomotion. Physiol. Rev. 100, 271–320. [DOI] [PubMed] [Google Scholar]
- 20. McCrea, D.A., and Rybak, I.A. (2008). Organization of mammalian locomotor rhythm and pattern generation. Brain Res. Rev. 57, 134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rossignol, S., and Frigon, A. (2011). Recovery of locomotion after spinal cord injury: some facts and mechanisms. Annu. Rev. Neurosci. 34, 413–440. [DOI] [PubMed] [Google Scholar]
- 22. Kiehn, O. (2016). Decoding the organization of spinal circuits that control locomotion. Nat. Rev. Neurosci. 17, 224–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hurteau, M.F., Thibaudier, Y., Dambreville, C., Chraibi, A., Desrochers, E., Telonio, A., and Frigon, A. (2017). Nonlinear modulation of cutaneous reflexes with increasing speed of locomotion in spinal cats. J. Neurosci. 37, 3896–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harnie, J., Côté-Sarrazin, C., Hurteau, M.-F., Desrochers, E., Doelman, A., Amhis, N., and Frigon, A. (2018). The modulation of locomotor speed is maintained following partial denervation of ankle extensors in spinal cats. J. Neurophysiol. 120, 1274–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Forssberg, H., Grillner, S., Halbertsma, J., and Rossignol, S. (1980). The locomotion of the low spinal cat. II. Interlimb coordination. Acta Physiol. Scand. 108, 283–295. [DOI] [PubMed] [Google Scholar]
- 26. Frigon, A., Hurteau, M.F., Thibaudier, Y., Leblond, H., Telonio, A., and D'Angelo, G. (2013). Split-belt walking alters the relationship between locomotor phases and cycle duration across speeds in intact and chronic spinalized adult cats. J. Neurosci. 33, 8559–8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuczynski, V., Telonio, A., Thibaudier, Y., Hurteau, M.F., Dambreville, C., Desrochers, E., Doelman, A., Ross, D., and Frigon, A. (2017). Lack of adaptation during prolonged split-belt locomotion in the intact and spinal cat. J. Physiol. 595, 5987–6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Desrochers, E., Harnie, J., Doelman, A., Hurteau, M.F., and Frigon, A. (2019). Spinal control of muscle synergies for adult mammalian locomotion. J. Physiol. 597, 333–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Higgin, D., Krupka, A., Maghsoudi, O.H., Klishko, A.N., Nichols, T.R., Lyle, M.A., Prilutsky, B.I., and Lemay, M.A. (2020). Adaptation to slope in locomotor-trained spinal cats with intact and self-reinnervated lateral gastrocnemius and soleus muscles. J. Neurophysiol. 123, 70–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harnie, J., Audet, J., Klishko, A.N., Doelman, A., Prilutsky, B.I., and Frigon, A. (2021). The spinal control of backward locomotion. J. Neurosci. 41, 630–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ballion, B., Morin, D., and Viala, D. (2001). Forelimb locomotor generators and quadrupedal locomotion in the neonatal rat. Eur. J. Neurosci. 14, 1727–1738. [DOI] [PubMed] [Google Scholar]
- 32. Yamaguchi, T. (2004). The central pattern generator for forelimb locomotion in the cat. Progress in Brain Research. 143, 115–122. [DOI] [PubMed] [Google Scholar]
- 33. Cazalets, J.R., Borde, M., and Clarac, F. (1995). Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J. Neurosci. 15, 4943–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Langlet, C., Leblond, H., and Rossignol, S. (2005). Mid-lumbar segments are needed for the expression of locomotion in chronic spinal cats. J. Neurophysiol. 93, 2474–2488. [DOI] [PubMed] [Google Scholar]
- 35. Marcoux, J., and Rossignol, S. (2000). Initiating or blocking locomotion in spinal cats by applying noradrenergic drugs to restricted lumbar spinal segments. J. Neurosci. 20, 8577–8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kiehn, O., and Butt, S.J.B. (2003). Physiological, anatomical and genetic identification of CPG neurons in the developing mammalian spinal cord. Prog. Neurobiol. 70, 347–361. [DOI] [PubMed] [Google Scholar]
- 37. Kiehn, O., and Kjaerulff, O. (1998). Distribution of central pattern generators for rhythmic motor outputs in the spinal cord of limbed vertebrates. Ann. N. Y. Acad. Sci. 860, 110–129. [DOI] [PubMed] [Google Scholar]
- 38. Chiel, H.J., and Beer, R.D. (1997). The brain has a body: adaptive behavior emerges from interactions of nervous system, body and environment. Trends Neurosci. 20, 553–557. [DOI] [PubMed] [Google Scholar]
- 39. Kubow, T.M. (1999). The role of the mechanical system in control: a hypothesis of self-stabilization in hexapedal runners. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 354, 849–861. [Google Scholar]
- 40. Dürr, V., Arena, P.P., Cruse, H., Dallmann, C.J., Drimus, A., Hoinville, T., Krause, T., Mátéfi-Tempfli, S., Paskarbeit, J., Patanè, L., Schäffersmann, M., Schilling, M., Schmitz, J., Strauss, R., Theunissen, L., Vitanza, A., and Schneider, A. (2019). Integrative biomimetics of autonomous hexapedal locomotion. Front. Neurorobot. 13, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dickinson, M.H., Farley, C.T., Full, R.J., Koehl, M.A., Kram, R., and Lehman, S. (2000). How animals move: an integrative view. Science 288, 100–106. [DOI] [PubMed] [Google Scholar]
- 42. Kimura, H., Fukuoka, Y., and Cohen, A.H. (2007). Adaptive dynamic walking of a quadruped robot on natural ground based on biological concepts. Int. J. Robot. Res. 26, 475–490. [Google Scholar]
- 43. Semini, C., Tsagarakis, N.G., Guglielmino, E., Focchi, M., Cannella, F., and Caldwell, D.G. (2011). Design of HyQ – a hydraulically and electrically actuated quadruped robot. Proc.Inst. Mech. Eng., Part I: Journal of Systems and Control Engineering 225, 831–849. [Google Scholar]
- 44. Ijspeert, A.J. (2014). Biorobotics: using robots to emulate and investigate agile locomotion. Science 346, 196–203. [DOI] [PubMed] [Google Scholar]
- 45. Eichhorst, H., and Naunyn, B. (1874). Uber die Regeneration and Veranderungen im Ruckenmarke nach Streckenweiser totaler Zerstorung desselben. Naunyn Schmiedebergs Arch. Pharmacol. 2, 225–253. [Google Scholar]
- 46. Freusberg, A. (1874). Reflexbewegungen beim Hunde. Pflueger Archiv. fuer die gesamte Physiologie 9, 358–391. [Google Scholar]
- 47. Goltz, Fr., and Freusberg, A. (1874). Ueber gefässerweiternde Nerven. Pflüger Archiv. 9, 174–197. [Google Scholar]
- 48. Philippson, M. (1905). L'autonomie et la centralisation dans le système nerveux des animaux: étude de physiologie expérimentale et comparée. Falk: Bruxelles, 258 p. [Google Scholar]
- 49. Magnus, R. (1924). The experimental basis for theories on vestibular function. J. Laryngol. Otol. 39, 677–685. [Google Scholar]
- 50. Cate, J. (1940). Quelques observations sur la locomotion des chiens dont la moelle épinière est sectionnée transversalement. Arch. néerl Physiol. 24, 476–485. [Google Scholar]
- 51. Howland, D.R., Bregman, B.S., Tessler, A., and Goldberger, M.E. (1995). Development of locomotor behavior in the spinal kitten. Exp. Neurol. 135, 108–122. [DOI] [PubMed] [Google Scholar]
- 52. Eidelberg, E., Story, J.L., Meyer, B.L., and Nystel, J. (1980). Stepping by chronic spinal cats. Exp. Brain Res. 40, 241–246. [DOI] [PubMed] [Google Scholar]
- 53. Stelzner, D.J., Ershler, W.B., and Weber, E.D. (1975). Effects of spinal transection in neonatal and weanling rats: survival of function. Exp Neurol 46, 156–177. [DOI] [PubMed] [Google Scholar]
- 54. Weber, E.D., and Stelzner, D.J. (1977). Behavioral effects of spinal cord transection in the developing rat. Brain Res. 125, 241–255. [DOI] [PubMed] [Google Scholar]
- 55. Percie du Sert, N., Hurst, V., Ahluwalia A., et al. (2020). The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br. J. Pharmacol. 177, 3617–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Harnie, J., Audet, J., Mari, S., Lecomte, C.G., Merlet, A.N., Genois, G., Rybak, I.A., Prilutsky, B.I., and Frigon, A. (2022). State-and condition-dependent modulation of the hindlimb locomotor pattern in intact and spinal cats across speeds. Front. Syst. Neurosci. 16, 814028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Merlet, A.N., Harnie, J., Macovei, M., Doelman, A., Gaudreault, N., and Frigon, A. (2020). Mechanically stimulating the lumbar region inhibits locomotor-like activity and increases the gain of cutaneous reflexes from the paws in spinal cats. J. Neurophysiol 123, 1026–1041. [DOI] [PubMed] [Google Scholar]
- 58. Merlet, A.N., Harnie, J., and Frigon, A. (2021). Inhibition and facilitation of the spinal locomotor central pattern generator and reflex circuits by somatosensory feedback from the lumbar and perineal regions after spinal cord injury. Front. Neurosci. 15, 720542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Caron, G., Bilchak, J.N., and Côté, M.P. (2020). Direct evidence for decreased presynaptic inhibition evoked by PBSt group I muscle afferents after chronic SCI and recovery with step-training in rats. J. Physiol. 598, 4621–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lecomte, C.G., Audet, J., Harnie, J., and Frigon, A. (2021). A validation of supervised deep learning for gait analysis in the cat. Front. Neuroinform. 15, 712623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Halbertsma, J.M. (1983). The stride cycle of the cat: the modelling of locomotion by computerized analysis of automatic recordings. Acta Physiol. Scand. Suppl 521, 1–75. [PubMed] [Google Scholar]
- 62. Thibaudier, Y., Hurteau, M.F., Dambreville, C., Chraibi, A., Goetz, L., and Frigon, A. (2017). Interlimb coordination during tied-belt and transverse split-belt locomotion before and after an incomplete spinal cord injury. J. Neurotrauma 34, 1751–1765. [DOI] [PubMed] [Google Scholar]
- 63. Thibaudier, Y., and Frigon, A. (2014). Spatiotemporal control of interlimb coordination during transverse split-belt locomotion with 1:1 or 2:1 coupling patterns in intact adult cats. J. Neurophysiol. 112, 2006–2018. [DOI] [PubMed] [Google Scholar]
- 64. Frigon, A., D'Angelo, G., Thibaudier, Y., Hurteau, M.-F., Telonio, A., Kuczynski, V., and Dambreville, C. (2014). Speed-dependent modulation of phase variations on a step-by-step basis and its impact on the consistency of interlimb coordination during quadrupedal locomotion in intact adult cats. J. Neurophysiol. 111, 1885–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. English, A.W. (1979). Interlimb coordination during stepping in the cat: an electromyographic analysis. J. Neurophysiol. 42, 229–243. [DOI] [PubMed] [Google Scholar]
- 66. English, A.W., and Lennard, P.R. (1982). Interlimb coordination during stepping in the cat: in-phase stepping and gait transitions. Brain Res. 245, 353–364. [DOI] [PubMed] [Google Scholar]
- 67. Orsal, D., Cabelguen, J.M., and Perret, C. (1990). Interlimb coordination during fictive locomotion in the thalamic cat. Exp. Brain Res. 82, 536–546. [DOI] [PubMed] [Google Scholar]
- 68. Mathis, A., Mamidanna, P., Cury, K.M., Abe, T., Murthy, V.N., Mathis, M.W., and Bethge, M. (2018). DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289. [DOI] [PubMed] [Google Scholar]
- 69. Hoogkamer, W., Meyns, P., and Duysens, J. (2014). Steps forward in understanding backward gait: from basic circuits to rehabilitation. Exerc. Sport Sci. Rev. 42, 23–29. [DOI] [PubMed] [Google Scholar]
- 70. Courtine, G., Roy, R.R., Raven, J., Hodgson, J., McKay, H., Yang, H., Zhong, H., Tuszynski, M.H., and Edgerton, V.R. (2005). Performance of locomotion and foot grasping following a unilateral thoracic corticospinal tract lesion in monkeys (Macaca mulatta). Brain 128, 2338–2358. [DOI] [PubMed] [Google Scholar]
- 71. Goetz, L., Piallat, B., Thibaudier, Y., Montigon, O., David, O., and Chabardès, S. (2012). A non-human primate model of bipedal locomotion under restrained condition allowing gait studies and single unit brain recordings. J. Neurosci. Methods 204, 306–317. [DOI] [PubMed] [Google Scholar]
- 72. Dambreville, C., Labarre, A., Thibaudier, Y., Hurteau, M.F., and Frigon, A. (2015). The spinal control of locomotion and step-to-step variability in left-right symmetry from slow to moderate speeds. J. Neurophysiol. 114, 1119–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kjaerulff, O., and Kiehn, O. (1996). Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J. Neurosci. 16, 5777–5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zar, J.H. (1974). Probabilities of rayleigh's test statistics for circular data. Behav. Res. Methods Instrument. 6, 450. [Google Scholar]
- 75. Górska, T., Chojnicka-Gittins, B., Majczyński, H., and Zmysłowski, W. (2013). Changes in forelimb-hindlimb coordination after partial spinal lesions of different extent in the rat. Behav. Brain Res. 239, 121–138. [DOI] [PubMed] [Google Scholar]
- 76. Cartmill, M., Lemelin, P., and Schmitt, D. (2002). Support polygons and symmetrical gaits in mammals. Zoologic. J. Linnean Soc. 136, 401–420. [Google Scholar]
- 77. Blaszczyk, J., and Loeb, G.E. (1993). Why cats pace on the treadmill. Physiol. Behav. 53, 501–507. [DOI] [PubMed] [Google Scholar]
- 78. Wheeler, J.S., Walter, J.S., and Zaszczurynski, P.J. (1992). Bladder inhibition by penile nerve stimulation in spinal cord injury patients. J. Urol. 147, 100–103. [DOI] [PubMed] [Google Scholar]
- 79. Prévinaire, J.G., Soler, J.M., Perrigot, M., Boileau, G., Delahaye, H., Schumacker, P., Vanvelcenaher, J., and Vanhée, J.L. (1996). Short-term effect of pudendal nerve electrical stimulation on detrusor hyperreflexia in spinal cord injury patients: importance of current strength. Paraplegia 34, 95–99. [DOI] [PubMed] [Google Scholar]
- 80. Prévinaire, J.G., Soler, J.M., and Perrigot, M. (1998). Is there a place for pudendal nerve maximal electrical stimulation for the treatment of detrusor hyperreflexia in spinal cord injury patients? Spinal Cord 36, 100–103. [DOI] [PubMed] [Google Scholar]
- 81. Kirkham, A.P., Shah, N.C., Knight, S.L., Shah, P.J., and Craggs, M.D. (2001). The acute effects of continuous and conditional neuromodulation on the bladder in spinal cord injury. Spinal Cord 39, 420–428. [DOI] [PubMed] [Google Scholar]
- 82. Dalmose, A.L., Rijkhoff, N.J.M., Kirkeby, H.J., Nohr, M., Sinkjaer, T., and Djurhuus, J.C. (2003). Conditional stimulation of the dorsal penile/clitoral nerve may increase cystometric capacity in patients with spinal cord injury. Neurourol. Urodyn. 22, 130–137. [DOI] [PubMed] [Google Scholar]
- 83. Hansen, J., Media, S., Nøhr, M., Biering-Sørensen, F., Sinkjær, T., and Rijkhoff, N.J.M. (2005). Treatment of neurogenic detrusor overactivity in spinal cord injured patients by conditional electrical stimulation. J. Urol. 173, 2035–2039. [DOI] [PubMed] [Google Scholar]
- 84. Yoo, P.B., Klein, S.M., Grafstein, N.H., Horvath, E.E., Amundsen, C.L., Webster, G.D., and Grill, W.M. (2007). Pudendal nerve stimulation evokes reflex bladder contractions in persons with chronic spinal cord injury. Neurourol. Urodyn. 26, 1020–1023. [DOI] [PubMed] [Google Scholar]
- 85. Horvath, E.E., Yoo, P.B., Amundsen, C.L., Webster, G.D., and Grill, W.M. (2010). Conditional and continuous electrical stimulation increase cystometric capacity in persons with spinal cord injury. Neurourol. Urodyn. 29, 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Herman, R., He, J., D'Luzansky, S., Willis, W., and Dilli, S. (2002). Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord 40, 65–68. [DOI] [PubMed] [Google Scholar]
- 87. Carhart, M.R., He, J., Herman, R., D'Luzansky, S., and Willis, W.T. (2004). Epidural spinal-cord stimulation facilitates recovery of functional walking following incomplete spinal-cord injury. IEEE Trans. Neural Syst. Rehabil. Eng. 12, 32–42. [DOI] [PubMed] [Google Scholar]
- 88. Harkema, S., Gerasimenko, Y., Hodes, J., Burdick, J., Angeli, C., Chen, Y., Ferreira, C., Willhite, A., Rejc, E., Grossman, R.G., and Edgerton, V.R. (2011). Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377, 1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Angeli, C.A., Edgerton, V.R., Gerasimenko, Y.P., and Harkema, S.J. (2014). Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137, 1394–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rejc, E., Angeli, C.A., Atkinson, D., and Harkema, S.J. (2017). Motor recovery after activity-based training with spinal cord epidural stimulation in a chronic motor complete paraplegic. Sci. Rep. 7, 13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rejc, E., Angeli, C., and Harkema, S. (2015). Effects of lumbosacral spinal cord epidural stimulation for standing after chronic complete paralysis in humans. PLoS One 10, e0133998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Grahn, P.J., Lavrov, I.A., Sayenko, D.G., Van Straaten, M.G., Gill, M.L., Strommen, J.A., Calvert, J.S., Drubach, D.I., Beck, L.A., Linde, M.B., Thoreson, A.R., Lopez, C., Mendez, A.A., Gad, P.N., Gerasimenko, Y.P., Edgerton, V.R., Zhao, K.D., and Lee, K.H. (2017). Enabling task-specific volitional motor functions via spinal cord neuromodulation in a human with paraplegia. Mayo Clin. Proc. 92, 544–554. [DOI] [PubMed] [Google Scholar]
- 93. Gill, M.L., Grahn, P.J., Calvert, J.S., Linde, M.B., Lavrov, I.A., Strommen, J.A., Beck, L.A., Sayenko, D.G., Van Straaten, M.G., Drubach, D.I., Veith, D.D., Thoreson, A.R., Lopez, C., Gerasimenko, Y.P., Edgerton, V.R., Lee, K.H., and Zhao, K.D. (2018). Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat. Med. 24, 1677–1682. [DOI] [PubMed] [Google Scholar]
- 94. Smith, J.L., Smith, L.A., Zernicke, R.F., and Hoy, M. (1982). Locomotion in exercised and nonexercised cats cordotomized at two or twelve weeks of age. Exp. Neurol. 76, 393–413. [DOI] [PubMed] [Google Scholar]
- 95. Commissiong, J.W., and Toffano, G. (1989). Complete spinal cord transection at different postnatal ages: recovery of motor coordination correlated with spinal cord catecholamines. Exp. Brain Res. 78, 597–603. [DOI] [PubMed] [Google Scholar]
- 96. Visintin, M., and Barbeau, H. (1994). The effects of parallel bars, body weight support and speed on the modulation of the locomotor pattern of spastic paretic gait. A preliminary communication. Paraplegia 32, 540–553. [DOI] [PubMed] [Google Scholar]
- 97. Wernig, A., Müller, S., Nanassy, A., and Cagol, E. (1995). Laufband therapy based on “rules of spinal locomotion” is effective in spinal cord injured persons. Eur. J. Neurosci. 7, 823–829. [DOI] [PubMed] [Google Scholar]
- 98. Harkema, S.J., Hurley, S.L., Patel, U.K., Requejo, P.S., Dobkin, B.H., and Edgerton, V.R. (1997). Human lumbosacral spinal cord interprets loading during stepping. J. Neurophysiol. 77, 797–811. [DOI] [PubMed] [Google Scholar]
- 99. Colombo, G., Joerg, M., Schreier, R., and Dietz, V. (2000). Treadmill training of paraplegic patients using a robotic orthosis. J. Rehabil. Res. Dev. 37, 693–700. [PubMed] [Google Scholar]
- 100. Dobkin, B., Apple, D., Barbeau, H., Basso, M., Behrman, A., Deforge, D., Ditunno, J., Dudley, G., Elashoff, R., Fugate, L., Harkema, S., Saulino, M., Scott, M., and Spinal Cord Injury Locomotor Trial Group. (2006). Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology 66, 484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bethe, A., and Fischer, E. (1931). Die Anpassungsfähigkeit (Plastizität) des Nervensystems., in: Arbeitsphysiologie II Orientierung · Plastizität Stimme und Sprache. Springer: Berlin, Heidelberg, pps. 1045–1130. [Google Scholar]
- 102. Buchner, H.H., Savelberg, H.H., Schamhardt, H.C., and Barneveld, A. (1996). Head and trunk movement adaptations in horses with experimentally induced fore- or hindlimb lameness. Equine Vet. J. 28, 71–76. [DOI] [PubMed] [Google Scholar]
- 103. Barrière, G., Frigon, A., Leblond, H., Provencher, J., and Rossignol, S. (2010). Dual spinal lesion paradigm in the cat: evolution of the kinematic locomotor pattern. J. Neurophysiol. 104, 1119–1133. [DOI] [PubMed] [Google Scholar]
- 104. Alluin, O., Karimi-Abdolrezaee, S., Delivet-Mongrain, H., Leblond, H., Fehlings, M.G., and Rossignol, S. (2011). Kinematic study of locomotor recovery after spinal cord clip compression injury in rats. J. Neurotrauma 28, 1963–1981. [DOI] [PubMed] [Google Scholar]
- 105. Brustein, E., and Rossignol, S. (1998). Recovery of locomotion after ventral and ventrolateral spinal lesions in the cat. I. Deficits and adaptive mechanisms. J. Neurophysiol. 80, 1245–1267. [DOI] [PubMed] [Google Scholar]
- 106. Jiang, W., and Drew, T. (1996). Effects of bilateral lesions of the dorsolateral funiculi and dorsal columns at the level of the low thoracic spinal cord on the control of locomotion in the adult cat. I. Treadmill walking. J. Neurophysiol. 76, 849–866. [DOI] [PubMed] [Google Scholar]
- 107. Sławińska, U., Majczyński, H., Kwaśniewska, A., Miazga, K., Cabaj, A.M., Bekisz, M., Jordan, L.M., and Zawadzka, M. (2021). Unusual quadrupedal locomotion in rat during recovery from lumbar spinal blockade of 5-HT7 receptors. Int. J. Mol. Sci. 22, 6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gray, J. (1944). Studies in the mechanics of the tetrapod skeleton. J. Exp. Biol. 20, 88–116. [DOI] [PubMed] [Google Scholar]
- 109. Ting, L.H., Blickhan, R., and Full, R.J. (1994). Dynamic and static stability in hexapedal runners. J. Exp. Biol. 197, 251–269. [DOI] [PubMed] [Google Scholar]
- 110. Farrell, B.J., Bulgakova, M.A., Beloozerova, I.N., Sirota, M.G., and Prilutsky, B.I. (2014). Body stability and muscle and motor cortex activity during walking with wide stance. J. Neurophysiol. 112, 504–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Frigon, A., and Rossignol, S. (2008). Adaptive changes of the locomotor pattern and cutaneous reflexes during locomotion studied in the same cats before and after spinalization. J. Physiol. 586, 2927–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Rossignol, S., Giroux, N., Chau, C., Marcoux, J., Brustein, E., and Reader, T.A. (2001). Pharmacological aids to locomotor training after spinal injury in the cat. J. Physiol. 533, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Pocratsky, A.M., Shepard, C.T., Morehouse, J.R., Burke, D.A., Riegler, A.S., Hardin, J.T., Beare, J.E., Hainline, C., States, G.J., Brown, B.L., Whittemore, S.R., and Magnuson, D.S. (2020). Long ascending propriospinal neurons provide flexible, context-specific control of interlimb coordination. Elife 9, e53565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gottschall, J.S., and Nichols, T.R. (2007). Head pitch affects muscle activity in the decerebrate cat hindlimb during walking. Exp. Brain Res. 182, 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Smith, J.L., Carlson-Kuhta, P., and Trank, T.V. (1998). Forms of forward quadrupedal locomotion. III. A comparison of posture, hindlimb kinematics, and motor patterns for downslope and level walking. J. Neurophysiol. 79, 1702–1716. [DOI] [PubMed] [Google Scholar]
- 116. Klishko, A.N., Akyildiz, A., Mehta-Desai, R., and Prilutsky, B.I. (2021). Common and distinct muscle synergies during level and slope locomotion in the cat. J. Neurophysiol. 126, 493–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Tester, N.J., Howland, D.R., Day, K.V., Suter, S.P., Cantrell, A., and Behrman, A.L. (2011). Device use, locomotor training and the presence of arm swing during treadmill walking after spinal cord injury. Spinal Cord 49, 451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tester, N.J., Barbeau, H., Howland, D.R., Cantrell, A., and Behrman, A.L. (2012). Arm and leg coordination during treadmill walking in individuals with motor incomplete spinal cord injury: a preliminary study. Gait Posture 36, 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Herr, H., and Popovic, M. (2008). Angular momentum in human walking. J. Exp. Biol. 211, 467–481. [DOI] [PubMed] [Google Scholar]
- 120. Ferris, D.P., Huang, H.J., and Kao, P.C. (2006). Moving the arms to activate the legs. Exerc. Sport Sci. Rev. 34, 113–120. [DOI] [PubMed] [Google Scholar]
- 121. Frigon, A., Collins, D.F., and Zehr, E.P. (2004). Effect of rhythmic arm movement on reflexes in the legs: modulation of soleus H-reflexes and somatosensory conditioning. J. Neurophysiol. 91, 1516–1523. [DOI] [PubMed] [Google Scholar]