Abstract
Objective:
Spinal cord injury (SCI) causes motor deficits, urinary incontinence, and neuropathic pain. This study was designed to optimize a photobiomodulation therapy (PBMT) protocol using a continuous wave (CW) 660 nm laser in rats with SCI. Specifically, the number of days of irradiation and the daily dose of PBMT were investigated.
Methods:
The study was performed in two steps. In the first step, a comparison between the effects of PBMT (45 sec) daily for 2 and 4 weeks on pain and movement [Basso, Beattie, and Brenham (BBB) score] was made. In the second step, a comparison between different durations of irradiation (27, 45, 90, and 117 sec) was performed. PBMT used a 100 mW laser delivered to 9 points on and around the lesion site. Oxidative stress, fibroblast invasion, and time to achieve spontaneous urination were also assessed.
Results:
The improvement in movement and pain stopped with discontinuation of radiation at week 2 and fibroblast invasion resumed. No improvement was seen in movement and pain in the group receiving PBMT for 27 sec compared with the groups receiving higher doses of laser radiation. Animals receiving 117 sec of photobiomodulation showed a higher BBB score even in the first 3 days.
Conclusions:
The number of days is an important factor for improving mobility; however, the daily dose of radiation is more important for pain relief.
Keywords: photobiomodulation therapy, spinal cord injury, motor function recovery, hyperalgesia, GPX, SOD, MDA
Introduction
Spinal cord injury (SCI) leads to paraplegia and chronic pain, as well as urinary incontinence, and depression, which interfere with patient quality of life.1 Urinary incontinence is a common problem after SCI that affects around half of the affected population, besides impaired mobility, sexual difficulty, and inability to perform personal tasks, all leading to a poor quality of life.2,3
Despite a great deal of research, no gold standard treatment for SCI has yet been established, and little overall success has been achieved in improving functional recovery. Ultra-early surgical interventions, including spinal cord decompression and spine fixation, are now routinely performed.4
It seems that SCI causes such severe damage to the axons and blood vessels that the ability for recovery is limited. The pathophysiology of SCI involves the initial injury (primary phase), followed by a secondary phase in which oxidative stress and inflammation are critical components.5,6 Tissue swelling resulting from hemorrhage and edema, inflammation, cytotoxic free radicals and generation of excitotoxic substances, and excessive gliosis take hours to months to develop.7 One of the best validated secondary injury mechanisms in acute SCI is the post-traumatic generation of reactive oxygen species (ROS) and the resulting free radical-induced oxidative tissue damage that results in axonal degeneration, glia scar, and cavity formation.8
All these phenomena occur in a short time.9 So in addition to selecting the appropriate type of treatment, the initiation time and duration of treatment is also very important. A variety of treatments have been proposed, yet none has been conclusively shown to benefit either the short-term or long-term complications of SCI.10–13
Among several individual or combination treatments, photobiomodulation therapy (PBMT) has been investigated in SCI, as well as for neurotrauma, neurodegenerative diseases, and pain relief.14,15 PBMT accelerates mitochondrial electron transport, oxygen consumption, and membrane potential and increases the synthesis of adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate (NADH).16–18
It has been reported that PBMT is able to reduce neutrophil accumulation and edema after SCI, through mechanisms that involve downregulation of proinflammatory mediators, such as tumor necrosis factor-α and interleukin (IL)-1β, as well as upregulation of anti-inflammatory mediators, such as IL-10 and transforming growth factor-β.19 PBMT is able to stimulate the mitochondria, due to photon absorption by biological chromophores (e.g., cytochrome c oxidase) and nanostructured water.20,21 Mitochondria are involved in the production of ROS and oxidative stress.22
Therefore, the measurement of ROS and biomarkers of antioxidants and oxidative stress, such as glutathione peroxidase (GPx), superoxide dismutase (SOD), and malondialdehyde (MDA) can be used to assess the mechanisms and efficacy of SCI recovery.
The aim of the present study was to answer the question of whether the number of days of radiation or the daily dose of radiation would be important for improving the hyperalgesia and/or movement recovery. Central nervous system (CNS) damage leads to glial scar formation, which acts as a double-edged sword. On the one hand, the glial scar prevents the further spread of the lesion, while on the other hand, it prevents the growth of axons. In fact, the injury site can be classified as either “glial” or “fibrotic.” In animal studies, fibrotic scarring is more often observed in penetrating lesions, which can rupture the dura and allow meningeal fibroblasts to invade into the lesion site.23,24 The entry of fibroblasts into the lesion site follows infiltration of immune cells and inflammation.25,26 This study was designed in such a way as to assess the effect of each treatment protocol on antioxidant status, tissue repair, but also on fibroblast invasion.
Materials and Methods
Experimental design and animals
In this study, all experiments were performed on adult male Wistar rats (170–200 g). Efforts were made to minimize the number of animals used in the experiment. The proposal was approved by the Institutional Animal Care and Use Committee (IACUC) of the Iran University of Medical Science, Tehran, Iran, with the ethical code (IR.IUMS.REC 1397.31954). The animal experiments were also performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996, the U.K. Animals (Scientific Procedures) Act 1986 and associated guidelines, and the European Communities Council Directive of November 24, 1986 (86/609/EEC).
Rats were housed under controlled conditions (23°C ± 2°C, 12 h light/dark cycle) with access to food and water ad libitum. The study was designed in two steps. In the first step, we assessed the effectiveness of 2 and 4 weeks of photobiomodulation (PBM) radiation (45 sec) in improving movement and reducing pain. In the second step, the effect of different PBM radiation doses (27, 45, 90, and 117 sec) daily for 4 weeks on pain and movement was evaluated. Finally, oxidative stress levels and tissue changes were also studied.
Forty-eight rats were randomly divided into eight groups, as shown in Table 1.
Table 1.
Animal Grouping
| Experimental groups | Procedure |
|---|---|
| (1) Control (n = 6) | Intact animals |
| (2) SCI (n = 6) | Animals with SCI (no treatment) |
| (3) SCI +2 weeks of PBMT (n = 6) | PBMT starting 30 min after SCI induction, which underwent laser treatment 45 sec over 9 points daily for 2 weeks |
| (4) SCI +4 weeks of PBMT (n = 6) | PBMT starting 30 min after SCI induction, which underwent laser treatment 45 sec, daily for 4 weeks |
| (5) SCI +27 sec of PBMT (n = 6) | PBMT starting 30 min after SCI, 27 sec (3 sec at each point) daily, daily for 4 weeks |
| (6) SCI +45 sec of PBMT (n = 6) | PBMT starting 30 min after SCI, 45 sec each day (5 sec at each point and continues for 4 weeks) |
| (7) SCI +90 sec of PBMT (n = 6) | PBMT starting 30 min after SCI, 90 sec each day (10 sec at each point and continues for 4 weeks) |
| (8) SCI +117 sec of PBMT (n = 6) | PBMT starting 30 min after SCI, 117 sec each day (10 sec at each point and continues for 4 weeks) |
PBMT, photobiomodulation therapy; SCI, spinal cord injury.
SCI induction
Animals were anesthetized by an intraperitoneal (IP) injection of ketamine (80 mg/kg) and xylazine (10 mg/kg) and the spinal cord surgically exposed. A compression SCI model was performed at the level of T13-L1 of the spinal cord. The compression model of SCI involved a laminectomy, and then, an aneurysm clip (FST Company, Foster City, CA) was applied vertically on the spinal cord for 90 sec to deliver a force equal to 20 g/cm2. The muscles and skin were sutured closed separately.27,28 Postsurgery care included: Ringer's solution for prevention of dehydration (3 mL, IP, after surgery), penicillin G for 3 days after surgery (8 mg/100 g, IP), and bladder massage twice daily until animals could urinate spontaneously.
Photobiomodulation therapy
In the present study, a diode continuous wave (CW) laser was used with the parameters shown in Table 2. Thirty minutes after SCI induction (removal of the aneurysm clip), PBM irradiation was performed on the lesion site, and at 9 points surrounding the injury site 5 mm away in each direction to form a circle.15,28,29
Table 2.
Laser Parameters
| Manufacturer | Heltschl Medizintechnik, Schlusslberg, Austria |
| Model identifier | ME-TL10000-SK |
| Year produced | 2011 |
| Number and type of emitters (laser or LED) | 1 laser diode |
| Wavelength and bandwidth (nm) | 660 and 2 |
| Pulse mode (CW or Hz, duty cycle) | CW |
| Beam spot size at target (cm2) | 0.197 |
| Total power (mW) | 100 |
| Irradiance at target (mW/cm2) | 500 |
| Exposure duration (sec) | 27, 45, 90, and 117 |
| Radiant exposure (J/cm2) | 13.7, 22.8, 45.6, and 60 |
| Radiant energy (J) | 2.7, 4.5, 9, and 11.7 |
| No. of points irradiated | 9 |
| Area irradiated (cm2) | 1.5–2 |
| Application technique | At distance of 1 cm from skin |
| Number and frequency of treatment sessions | 28 sessions (daily for 4 weeks) |
| Total radiant energy over entire treatment course (J) | 27, 126, 252, and 327 |
CW, continuous wave; LED, light-emitting diode.
PBMT was performed using two protocols. In the first protocol, the difference between the effects of 2 and 4 weeks of radiation was investigated and compared. The animals received 45 sec of laser treatment daily (5 sec per point). In group (3) for 2 weeks and in group (4) for 4 weeks.
In the second protocol, the effect of different daily radiation times (3, 5, 10, and 13 sec per point) (total times 27, 45, 90, and 117 sec) for 4 weeks was investigated and compared.
Behavioral assessment
Functional recovery of movement
Following surgery, the Basso, Beattie, and Brenham (BBB) test was carried out weekly to evaluate the motor function in a blinded manner up to 5 weeks. Each animal was allowed to freely move in an open field of 90 cm diameter with a wall height of 24 cm for 4 min. In groups that received different doses of laser daily, evaluation and scoring of movement began 24 h after SCI induction and were repeated every day until the third day. Two independent observers scored separately based on the BBB scoring table scores 0 (complete paralysis) to 21 (normal walking).30 The average score given by two observers was recorded as the main score.
Mechanical hyperalgesia evaluation (pinch test)
For hyperalgesia pain assessment, the Ugo Basile Analgesia Meter (Ugo Basile, Varese, Italy) apparatus was used. Mechanical stimulation was carried out with a weight connected to a lever. The increasing pressure produced by pushing of the lever was determined using a ruler connected to the device. When the animal responded to the applied pressure by removing its hind paw, the pressure increase would stop and the value of pressure that led the animal to respond was recorded. This test was carried out twice on each leg with at least 5-min time intervals, and the mean of the results was recorded.
Spontaneous control of urination
The time for each animal to urinate spontaneously was recorded individually and reported.
Histological evaluation
Hematoxylin and eosin staining was performed for assessment of fibroblast invasion.31 After staining, digital images were captured (magnification 40 × ; Olympus).
Assays for MDA, GPx, and SOD
Five weeks after surgery, three animals from each group were randomly selected. After deep anesthesia as described above, the lesion sites were surgically reexposed, and the injured sections of tissue were carefully removed.
The harvested tissues were rapidly frozen, and the samples were thawed and homogenized in RIPA buffer to prepare a supernatant. Glutathione peroxidase activity in the spinal cord tissues was determined using a GPx ELISA Kit (ZellBio GmbH, Ulm-Germany). The MDA Assay Kit (Zellbio Co, Germany) was used to measure MDA, a lipid peroxidation (LPO) marker. SOD Assay Kit (Zellbio Co) was used to measure the SOD activity by converting superoxide anion to hydrogen peroxide and oxygen with enzymatic reaction.
Statistical analyses
Data were analyzed using the Prism software version 6 and are presented as mean and standard error. To compare the collected data from the behavioral assessments of different groups, two-way analysis of variance (ANOVA) analysis was used with the Tukey follow-up test, and one-way ANOVA analysis was used to evaluate the findings of enzyme-linked immunosorbent assays. In all analyses, p < 0.05 was considered significant.
Results
Comparison between 2 and 4 weeks of PBMT
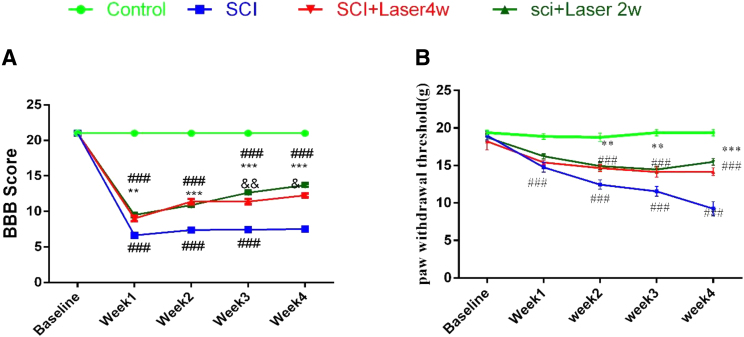
The motor function measured by the BBB score was significantly reduced in all SCI groups compared with the control group (df: 7.319; F = 35; n = 8; p < 0.001). In Fig. 1A, in the comparison between 2 and 4 weeks of PBMT, it was observed that the improvement in the motor function recovery depended on the number of days of irradiation. In the third and fourth week, a significant difference was observed between the two treatment groups, with the 2 weeks of PBMT being superior to the 4 weeks of PBMT, p < 0.01 and p < 0.05, respectively.
FIG. 1.
Effect of PBMT for 2 and 4 weeks. (A) Functional recovery BBB test; (B) mechanical hyperalgesia. Data are expressed as the mean ± SEM. **p < 0.01, ***p < 0.001 versus SCI group; ###p < 0.001 versus control; &p < 0.05, &&p < 0.01 PBMT 2 weeks versus 4 weeks. BBB, Basso, Beattie, and Brenham; PBMT, photobiomodulation therapy; SCI, spinal cord injury.
Induction of SCI reduced the mechanical pain threshold compared with the control group (p < 0.001) (Fig. 1B). PBMT reduced the pain intensity (increased the threshold) for 2- and 4-week PBMT groups, compared with the SCI group (p < 0.001). The pain threshold did not significantly differ between 2- and 4-week PBMT groups (Fig. 1B).
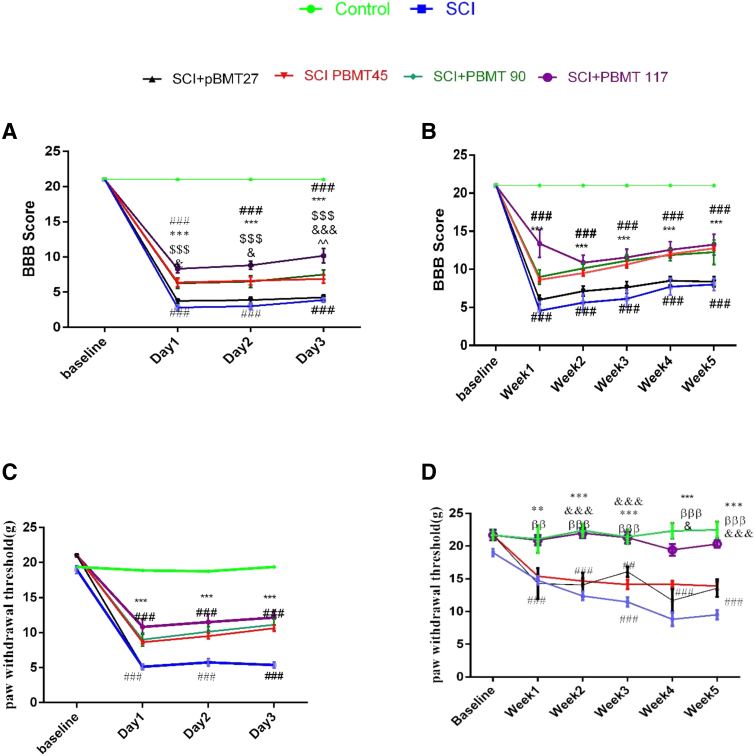
Comparison between different radiation times
As shown in Fig. 2A, during the first 3 days after surgery, the BBB score of the SCI and the 27 sec PBMT groups was almost the same and was significantly lower compared with control animals (p < 0.001). Figure 2B shows that during this 5-week study, no additional motor recovery was observed in animals receiving 4 weeks of 27 sec daily PBMT compared with the SCI group. However, in the other groups (45, 90, and 117 sec), a significant improvement in motor activity was observed. Altogether, a significant difference between the 27 sec PBMT group and the other three PBMT groups was observed (p < 0.001). However, there was no significant difference between the motor activity of the PBMT 45, 90, and 117 sec groups. The BBB scores of the 27 sec group were significantly different from the 45 sec group, on day 2 p < 0.05, and on day 3 p < 0.001.
FIG. 2.
Effect of different irradiation times (27, 45, 90, and 117 sec) of PBMT. (A) Functional recovery BBB at 3 days and (B) at 5 weeks. (C) Mechanical hyperalgesia at 3 days and (D) at 5 weeks. Data are expressed as the mean ± SEM. **p < 0.01, ***p < 0.001 versus SCI group and PBM 27 group; ###p < 0.001 versus control. &p < 0.05, &&&p < 0.001 compression PBMT 45 and 117, ^^p < 0.01 compression PBMT 90 and 117. ββp < 0.01, βββp < 0.001 compression PBMT 45 and 90.
PBM irradiation for 27 sec had no effect on the pain threshold, but the other times of irradiation improved the pain threshold, but no difference between them was observed. However, from the second week, the best pain improvement effect was obtained with PBM for 117 sec, which brought the pain threshold up to that of the control group.
The PBM 45 and 90 sec treatment groups were not different from each other. Both recovered compared with the SCI group (p < 0.001) but were still different from the control group (p < 0.001).
Urination control
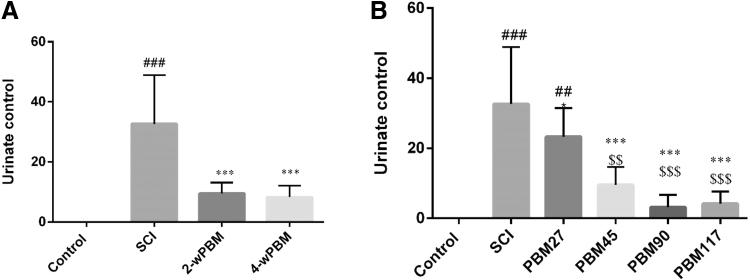
Induction of SCI caused inability to urinate spontaneously (p < 0.01). In rats, it sometimes takes 2 weeks or more to get the ability to urinate spontaneously.32 Urination control is regulated by a complex neural system in the brain and lumbosacral spinal cord.33 Following SCI, the bladder is initially flaccid but later becomes hyperreflexive due to the disturbance of the reflex pathway by SCI. Animal studies have shown that improved bladder function after SCI depends partly on the plasticity of the bladder afferent pathways.33 PBMT significantly reduced the total number of days required for bladder massage compared with the SCI group (p < 0.001). However, this improvement was significantly less pronounced in animals treated with PBM for 27 sec. The difference between PBMT and SCI was p < 0.05 and with the control group was p < 0.01 (Fig. 3).
FIG. 3.
Effect of PBMT on regaining spontaneous urination. (A) 2 and 4 weeks of PBMT; (B) duration of daily PBMT (27, 45, 90, and 117 sec). ***p < 0.001 versus SCI group; $$p < 0.01, $$$p < 0.001 versus PBM 27 sec; ### p < 0.001 versus control group. PBM, photobiomodulation.
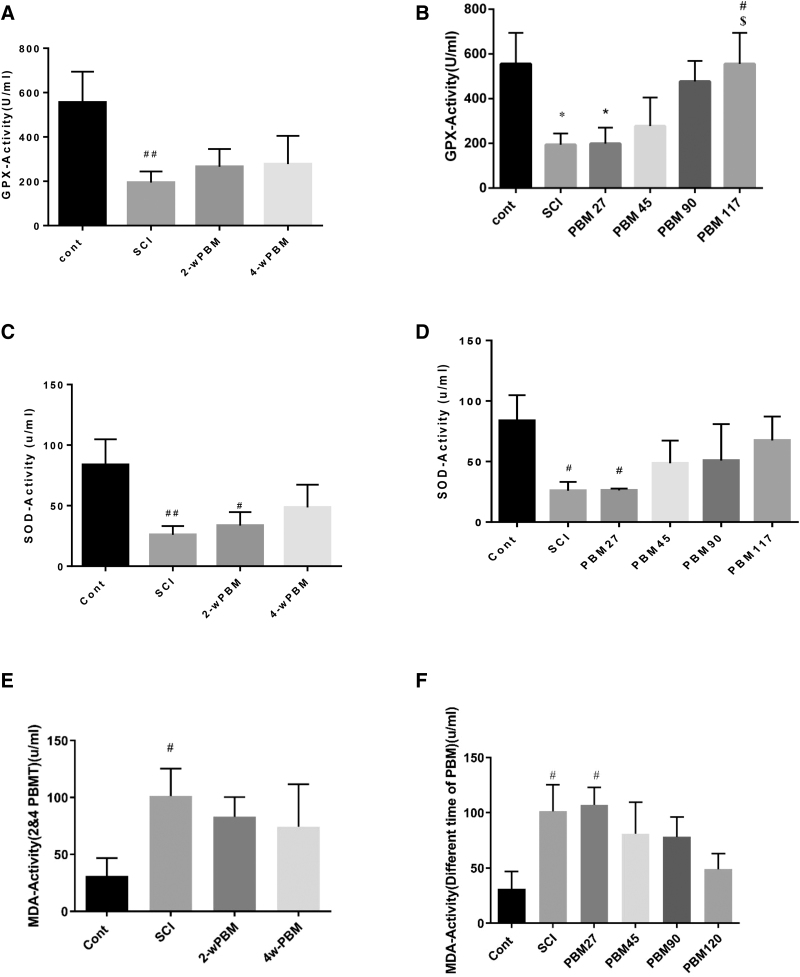
Glutathione peroxidase activity
Glutathione peroxidase (GPx) levels were sharply lower at week 5 after SCI induction (df: 51; F = 12; n = 3; p < 0.01). No difference in glutathione level was observed between the groups receiving 2 and 4 weeks of PBMT. However, the levels were higher compared with the SCI group (p < 0.01) (Fig. 4A). In the PBM 27 sec group, the GPx activity was not significantly different at the end of the study compared with the control group (p < 0.05). An increasing trend was observed in the other groups. The PBM 117 sec group showed a significant difference compared with the SCI and PBM 27 sec groups (Fig. 4A), and the GPx activity was the same as the control value (Fig. 4B).
FIG. 4.
Effect of PBMT on oxidative stress makers after PBMT for SCI. (A) GPX after 2 and 4 weeks PBMT; (B) GPX at PBMT times (27, 45, 90, and 117 sec); (C) SOD after 2 and 4 weeks PBMT; (D) SOD at PBMT times (27, 45, 90, and 117 sec); (E) MDA after 2 and 4 weeks PBMT; (F) MDA at PBMT times (27, 45, 90, and 117 sec). *p < 0.05 versus SCI group; #p < 0.05, ###p < 0.01 versus control group; $versus PBM 27. GPX, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase.
SOD activity
The results showed that the SOD activity decreased significantly after SCI compared with the control group (p < 0.05). There was a difference in the 2-week PBM group compared with the control group) p < 0.05), but at week 4, the difference was less pronounced (Fig. 4C). In the 27 sec group, the SOD level was also significantly lower than the control group (p < 0.05). SOD levels in the 45, 90 and 117 sec PBM groups were not significantly different from the control group (Fig. 4D).
MDA activity
MDA as an indicator of oxidative stress was higher at 5 weeks after SCI (p < 0.05) compared with control. The levels of MDA after PBMT for 2 and 4 weeks were not significantly different from the SCI group. Results in the 27 sec PBMT group showed that MDA was higher compared with the control group (p < 0.05) and was almost the same as the MDA level in the SCI group. MDA levels in the PBMT-treated groups (45, 90, and 117 sec) showed a decreasing trend (Fig. 4E, F), but values were not significantly different from the SCI group. However, these values were also not significantly different from the mean control value.
Fibroblast count
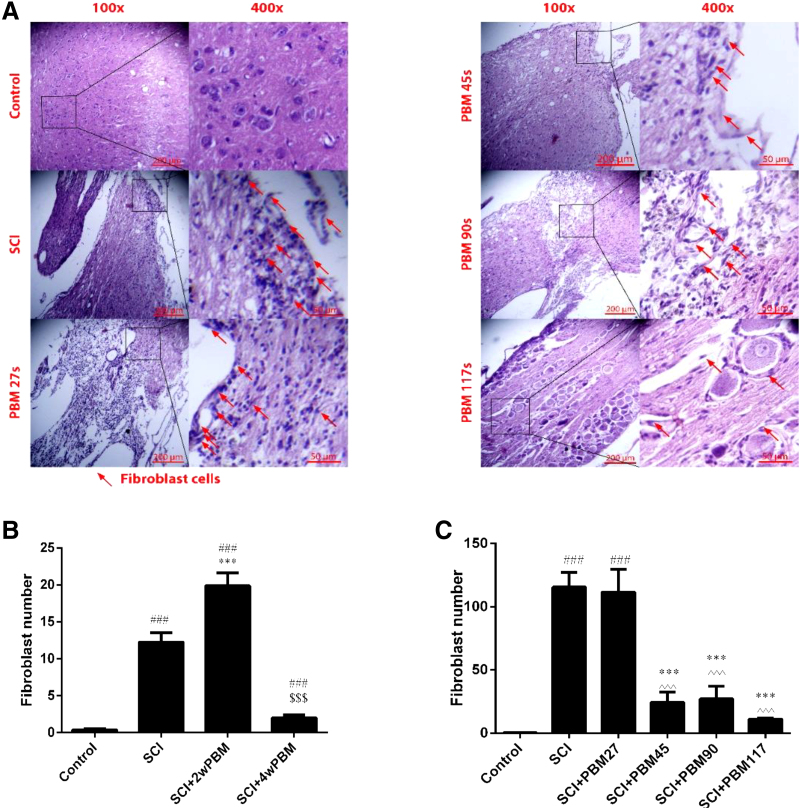
The results showed that 2 weeks of PBM irradiation did not prevent the invasion of fibroblasts into the SCI lesion, and a significant difference was observed with the control group (p < 0.001). Moreover, 4 weeks of PBMT 27 sec per day does not seem to be an adequate to reduce or prevent the invasion (p < 0.001). However, 4 weeks of PBMT 45, 90, and 117 sec per day prevented the invasion of fibroblasts. Differences were observed compared with the SCI and 27 sec groups (p < 0.001) (Fig. 5A–C).
FIG. 5.
Quantification of fibroblasts penetration by hematoxylin and eosin staining, (A) rush of fibroblasts are indicated by red arrows, (B) comparing 2 and 4 weeks PBMT, (C) comparing 27, 45, 90, and 117 sec everyday PBMT until 4 weeks. Data are expressed as the mean ± SEM. ###p < 0.001 versus control group, ***p < 0.001 versus SCI group; ^^^p < 0.001 versus PBM 27 group; $$$comparing 2 and 4 weeks PBMT.
Discussion
The results of this study showed that this compression model of SCI led to a major reduction in motor function, as well as an increase in mechanical hyperalgesia as shown by a decrease in the pain threshold. Moreover, the antioxidant activity (Gpx and SOD) was reduced, and the MDA level (a well-known marker of oxidative stress) was increased. In common with human patients, loss of control of urination was also observed.
PBMT had a noticeable effect on the improvement in functional recovery. Both the variables mentioned earlier, that is, number of days of radiation and the daily dose of radiation, seem to be important for improving movement. No improvement was seen in the group receiving PBMT for only 27 sec compared with the ones receiving higher durations of laser radiation. As shown in Fig. 1B, the discernible improvement in movement in the first 3 days continued, so that animals receiving 117 sec of PBM had a higher BBB score.
A previous study used continuous 810 nm laser PBMT in an SCI model to demonstrate the recovery of motor function, a reduction in lesion cavity size, and an increased number of surviving neurons.34
The relationship between oxidative stress in the injured spinal cord and disrupted motor function has not yet been firmly established. There have been some studies that have used various antioxidant approaches such as tetrahydrocurcumin,35 polydatin,36 or coenzyme Q1037 to improve motor dysfunction in SCI. The experimental data of the present study reinforce the likely association between functional recovery and oxidative stress level after SCI.
In the SCI group, the motor function, pain threshold, and lack of urination control decreased as expected. Moreover, the antioxidant enzymes (Gpx and SOD) that have been shown to function as neuroprotective factors were also decreased.38,39
Lack of urination control is a major problem that affects patients suffering from SCI.33 The present study showed that PBMT is also helpful to return the ability to control urination. However, in case of urination control, the role of the dose (duration of radiation) received per day was more important. Daily administration of PBM radiation for 90 and 117 sec led to a faster regain of spontaneous urination.
A study by Cevik et al. found that SCI causes oxidative stress damage in the bladder because the oxidant products such as MDA and nitric oxide increase, while levels of antioxidants such as Gpx and SOD decrease.40
One way to counter the oxidative stress produced in SCI is to increase GPX, which acts as an antioxidant and is able to remove H2O2, an ROS, by its reduction to water.41 In 2016, Janzadeh et al. showed that GPX was increased after PBMT. This increase possibly indicates the effect of PBMT to improve mitochondrial function.16
To maintain normal cellular function, the equilibrium between pro-oxidants and antioxidants is important.42 Therefore, a successful treatment for SCI should be able to restore this balance as well as being able to improve motor function, pain relief, and urinary control. The pathological processes occurring after SCI are complex and multi-functional. In the secondary phase of SCI, it has already been shown that PBMT can exert pronounced anti-inflammatory effects,15,43,44 as well as after many other diseases and injuries.45
It has been shown that antioxidant enzymes at levels below the normal range (GPX and/or SOD) are well known to reflect inflammation in many physiological situations.41,46,47 These markers have been shown to be improved by PBMT48 delivered to animal models of wound healing,49 muscle injury,50 high-intensity exercise, and traumatic brain injury.51
Consistent with these studies, our results showed that PBMT increased the antioxidant levels of GPX, while the daily dose of radiation was more important than the overall duration. The 2-week PBMT and PBMT 27 sec did not prevent the reduction of antioxidants. The same trend was seen when SOD was evaluated, but the slope was less pronounced.
MDA is a well-known product of LPO.52,53 These reactive lipid by-products can damage cellular fluidity and permeability, alter the function of membrane-associated proteins, and bind to and inactivate various molecules, including amino acids, proteins, and nucleic acids.42,47 LPO and oxidative stress have been proposed to play a role in the production of the glial scar, which is implicated in the failure of neural regeneration, chronic pain, and low BBB score.54–56
The process of excitotoxicity involves excessive release of glutamate from damaged neurons in the spinal cord. The overactivation of glutamate receptors leads to neuronal hyperactivation and therefore chronic pain.57 The hyperalgesia and allodynia pain after SCI were reduced by PBMT in the present study.
The higher doses of PBMT started to become effective in a dose-dependent manner, specifically at the highest dose (117 sec or 11.7 J/day), causing not only the best improvement in motor function and pain but also a significant reduction in oxidative stress biomarkers. The effect of PBMT 117 sec on GPX reached statistical significance compared with SCI or PBMT 27 sec. Intermediate doses of PBMT showed a linear trend in improvement of oxidative stress markers to the extent that they were no longer significantly different from control uninjured rats.
Although no significant reduction was observed in the oxidative stress biomarker MDA after 2 and 4 weeks of PBMT or different durations of PBMT, a decreasing trend was observed as the dose of PBMT increased (45, 90, and 117 sec).
Contradictory results in previous studies have shown that PBM can increase the rate of ROS production in some circumstances, while in other circumstances, ROS and oxidative stress are reduced by PBM and antioxidants are increased.16,58 The SCI process is characterized by the production of a wide range of inhibitory factors that prevent axonal regeneration, as well as the absence of other factors that promote regeneration. This imbalance has been termed “the microenvironment imbalance of SCI.”56,59,60 Fibroblasts play an important role in scar formation preventing axonal regeneration and prolonging motor dysfunction. Therefore, reducing the entry of fibroblasts could be a strategy to help axonal renewal and repair.23,61 PBMT is thought to assist to preserve the dura intact by reducing inflammation and preventing fibroblasts from entering.23,43,60
Our study showed that number of days of radiation was as important as the dose of PBMT. Scar-associated fibroblasts may be a source of stress-induced inflammatory mediators and pain. Inflammatory cytokine, such as IL-1α, NF-kappaB (NF-κB), and the CCL2 signaling pathway within scar-associated fibroblasts, could also be responsible for inflammation and chronic pain in the fibrotic scars.62
In 2-week PBMT, a high influx of fibroblasts, a low threshold of pain, and a low BBB score were observed, probably due to fibrosis in the lesion site and inhibition of axon repair after the PBMT was discontinued. In other words, more days of radiation and higher doses of radiation are needed to prevent fibroblast entry.
In the PBMT 27 sec group, the BBB and urination control were similar to the untreated SCI group, as were the oxidative stress markers. A low dose of PBMT (27 sec or 2.7 J/day) was clearly insufficient to produce any benefit to motor function, pain reduction, urinary control, fibroblast invasion, or to ameliorate oxidative stress.
In conclusion, we aimed to find an appropriate PBMT protocol that could be combined with routine decompression surgery and other new approaches to reach a higher level of recovery if used in medical practice. The results of 660 nm PBMT were in line with our expectation to improve the oxidative balance, restore urinary function, reduce fibroblast entry, and improve movement and pain. Practitioners should consider the use of PBMT in medical and surgical situations. Because humans are much larger that rats, we expect that much higher power lasers (several wavelengths) would be required.
Moreover, near-infrared (NIR) wavelengths (810 or 904 nm) would penetrate much deeper into the human body. In the present study, the laser was applied from a distance, but in human treatment, the laser could be applied in contact with the skin, which would ensure a higher proportion of the light would penetrate into the body because diffuse reflection would be reduced. We speculate that major complications of SCI in patients could be reduced by PBMT if the treatment was started early enough and a sufficient dose was used for several weeks.
Acknowledgments
The authors would like to thank the International Consortium for Personalized Pain Medicine (ICPPM) and Heltschl Company for the gift of the laser.
Authors' Contributions
Made a substantial contribution to the concept or design of the work, acquisition, analysis, or interpretation of data: A.J. and F.N. Drafted the article or revised it critically for important intellectual content: A.N.R., M.R.H., F.R., and A.J. Surgery: A.J., K.K., and S.M.F. Bladder massage, behavioral testing, and photobiomodulation therapy: K.K. and S.M.F. Histology staining: A.M., S.M.F., and M.A.
Author Disclosure Statement
M.R.H. declares the following potential conflicts of interest. Scientific Advisory Boards: Transdermal Cap, Inc., Cleveland, OH; Hologenix, Inc., Santa Monica, CA; Vielight, Toronto, Canada; JOOVV, Inc., Minneapolis-St. Paul, MN. Consulting: USHIO Corp, Japan; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany. Stockholding: Niraxx Light Therapeutics, Inc., Irvine, CA; JelikaLite Corp, New York, NY. The other authors declare no conflicts of interest.
Funding Information
This study was supported by a scientific project grant (No: 96-04-223-31954) financed by Iran University of Medical Science (IUMS). M.R.H. was supported by the U.S. National Institutes of Health Grants R01AI050875 and R21AI121700.
References
- 1. Jörgensen S, Costa Andersson MV, Lexell J. Changes in health-related quality of life among older adults aging with long-term spinal cord injury. Spinal Cord 2021;59:769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akkoç Y, Ersöz M, Yıldız N, et al. Effects of different bladder management methods on the quality of life in patients with traumatic spinal cord injury. Spinal Cord 2013;51:226–231. [DOI] [PubMed] [Google Scholar]
- 3. Elmelund M, Klarskov N, Biering-Sørensen F. Prevalence of urinary incontinence in women with spinal cord injury. Spinal Cord 2018;56:1124–1133. [DOI] [PubMed] [Google Scholar]
- 4. Burke JF, Yue JK, Ngwenya LB, et al. Ultra-early (<12 hours) surgery correlates with higher rate of American Spinal Injury Association impairment scale conversion after cervical spinal cord injury. Neurosurgery 2019;85:199–203. [DOI] [PubMed] [Google Scholar]
- 5. Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord 2012;50:264–274. [DOI] [PubMed] [Google Scholar]
- 6. Ahuja CS, Nori S, Tetreault L, et al. Traumatic spinal cord injury-repair and regeneration. Neurosurgery 2017;80(3S):S9–S22. [DOI] [PubMed] [Google Scholar]
- 7. Qin D, Wang J, Le A, Wang TJ, Chen X, Wang J. Traumatic brain injury: Ultrastructural features in neuronal ferroptosis, glial cell activation and polarization, and blood-brain barrier breakdown. Cells 2021;10:1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hall ED. Antioxidant therapies for acute spinal cord injury. Neurotherapeutics 2011;8:152–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus 2008;25:E2. [DOI] [PubMed] [Google Scholar]
- 10. Huang L, Fu C, Xiong F, He C, Wei Q. Stem cell therapy for spinal cord injury. Cell Transplant 2021;30:963689721989266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chakraborty A, Ciciriello AJ, Dumont CM, Pearson RM. Nanoparticle-based delivery to treat spinal cord injury-a mini-review. AAPS PharmSciTech 2021;22:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huie JR, Ferguson AR, Kyritsis N, et al. Machine intelligence identifies soluble TNFa as a therapeutic target for spinal cord injury. Sci Rep 2021;11:3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu S, Schackel T, Weidner N, Puttagunta R. Biomaterial-supported cell transplantation treatments for spinal cord injury: challenges and perspectives. Front Cell Neurosci. 2018;11:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. da Silva FC, Gomes AO, da Costa Palácio PR, et al. Photobiomodulation improves motor response in patients with spinal cord injury submitted to electromyographic evaluation: randomized clinical trial. Lasers Med Sci 2018;33:883–890. [DOI] [PubMed] [Google Scholar]
- 15. Sarveazad A, Janzadeh A, Taheripak G, Dameni S, Yousefifard M, Nasirinezhad F. Co-administration of human adipose-derived stem cells and low-level laser to alleviate neuropathic pain after experimental spinal cord injury. Stem Cell Res Ther 2019;10:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Janzadeh A, Nasirinezhad F, Masoumipoor M, Jameie SB, Hayat P. Photobiomodulation therapy reduces apoptotic factors and increases glutathione levels in a neuropathic pain model. Lasers Med Sci 2016;31:1863–1869. [DOI] [PubMed] [Google Scholar]
- 17. Oron U. Photoengineering of tissue repair in skeletal and cardiac muscles. Photomed Laser Surg 2006;24:111–120. [DOI] [PubMed] [Google Scholar]
- 18. Ramezani F, Neshasteh-Riz A, Ghadaksaz A, Fazeli SM, Janzadeh A, Hamblin MR. Mechanistic aspects of photobiomodulation therapy in the nervous system. Lasers Med Sci 2021;37:1–8. [DOI] [PubMed] [Google Scholar]
- 19. de Lima FM, Albertini R, Dantas Y, et al. Low-level laser therapy restores the oxidative stress balance in acute lung injury induced by gut ischemia and reperfusion. Photochem Photobiol 2013;89:179–188. [DOI] [PubMed] [Google Scholar]
- 20. Karu TI, Pyatibrat LV, Afanasyeva NI. A novel mitochondrial signaling pathway activated by visible-to-near infrared radiation. Photochem Photobiol 2004;80:366–372. [DOI] [PubMed] [Google Scholar]
- 21. Chung H, Dai T, Sharma SK, Huang Y-Y, Carroll J-D, Hamblin M-R. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 2012;40:516–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pickering RJ, Rosado CJ, Sharma A, Buksh S, Tate M, de Haan JB. Recent novel approaches to limit oxidative stress and inflammation in diabetic complications. Clin Transl Immunology 2018;7:e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soderblom C, Luo X, Blumenthal E, et al. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J Neurosci 2013;33:13882–13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu L, Yao Y. Central nervous system fibroblast-like cells in stroke and other neurological disorders. Stroke 2021;52:2456–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dorrier CE, Aran D, Haenelt EA, et al. CNS fibroblasts form a fibrotic scar in response to immune cell infiltration. Nat Neurosci 2021;24:234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yousefi S, Hojati V, Nasirinezhad F, Ramezani F, Janzadeh A, Vaezi G. The effect of four weeks of low-level laser radiation (660 nm) on movement recovery and fibroblasts invasion. Arch Neurosci 2019;6:e87225. [Google Scholar]
- 27. Behroozi Z, Ramezani F, Janzadeh A, Rahimi B, Nasirinezhad F. Platelet-rich plasma in umbilical cord blood reduces neuropathic pain in spinal cord injury by altering the expression of ATP receptors. Physiol Behav 2021;228:113186. [DOI] [PubMed] [Google Scholar]
- 28. Janzadeh A, Sarveazad A, Hamblin MR, Teheripak G, Kookli K, Nasirinezhad F. The effect of chondroitinase ABC and photobiomodulation therapy on neuropathic pain after spinal cord injury in adult male rats. Physiol Behav 2020;227:113141. [DOI] [PubMed] [Google Scholar]
- 29. Ramezani F, Razmgir M, Tanha K, et al. Photobiomodulation for spinal cord injury: a systematic review and meta-analysis. Physiol Behav 2020;224:112977. [DOI] [PubMed] [Google Scholar]
- 30. Ferguson AR, Hook MA, Garcia G, Bresnahan JC, Beattie MS, Grau JW. A simple post hoc transformation that improves the metric properties of the BBB scale for rats with moderate to severe spinal cord injury. J Neurotrauma 2004;21:1601–1613. [DOI] [PubMed] [Google Scholar]
- 31. Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc 2008;2008:pdb.prot4986. [DOI] [PubMed] [Google Scholar]
- 32. Saito T, Gotoh D, Wada N, et al. Time-dependent progression of neurogenic lower urinary tract dysfunction after spinal cord injury in the mouse model. Am J Physiol Renal Physiol 2021;321:F26–F32. [DOI] [PubMed] [Google Scholar]
- 33. de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res 2006;152:59–84. [DOI] [PubMed] [Google Scholar]
- 34. Wang X, Zhang Z, Zhu Z, et al. Photobiomodulation promotes repair following spinal cord injury by regulating the transformation of A1/A2 reactive astrocytes. Front Neurosci 2021;15:768262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xi J, Luo X, Wang Y, et al. Tetrahydrocurcumin protects against spinal cord injury and inhibits the oxidative stress response by regulating FOXO4 in model rats. Exp Ther Med 2019;18:3681–3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lv R, Du L, Zhang L, Zhang Z. Polydatin attenuates spinal cord injury in rats by inhibiting oxidative stress and microglia apoptosis via Nrf2/HO-1 pathway. Life Sci 2019;217:119–127. [DOI] [PubMed] [Google Scholar]
- 37. Hosseini M, Karami Z, Janzadeh A, Nasirinezhad F. Effect of coenzyme Q10 on neuropathic pain threshold resulting from spinal cord injury in male rats. Physiol Pharmacol. 2014;18:204–214. [Google Scholar]
- 38. Crack PJ, Taylor JM, de Haan JB, Kola I, Hertzog P, Iannello RC. Glutathione peroxidase-1 contributes to the neuroprotection seen in the superoxide dismutase-1 transgenic mouse in response to ischemia/reperfusion injury. J Cereb Blood Flow Metab 2003;23:19–22. [DOI] [PubMed] [Google Scholar]
- 39. Huang Y, Liu C, Song X, et al. Antioxidant and anti-inflammatory properties mediate the neuroprotective effects of hydro-ethanolic extract of tiliacora triandra against cisplatin-induced neurotoxicity. J Inflamm Res 2021;14:6735–6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cevik O, Erşahin M, Sener TE, et al. Beneficial effects of quercetin on rat urinary bladder after spinal cord injury. J Surg Res 2013;183:695–703. [DOI] [PubMed] [Google Scholar]
- 41. Ljubisavljevic S, Stojanovic I, Cvetkovic T, et al. Glutathione homeostasis disruption of erythrocytes, but not glutathione peroxidase activity change, is closely accompanied with neurological and radiological scoring of acute CNS inflammation. Neuroimmunomodulation 2014;21:13–20. [DOI] [PubMed] [Google Scholar]
- 42. Demiroz S, Ur K, Ulucan A, et al. Neuroprotective effects of lacosamide in experimental traumatic spinal cord injury in rats. Turk Neurosurg 2019;29:718–723. [DOI] [PubMed] [Google Scholar]
- 43. Janzadeh A, Sarveazad A, Yousefifard M, et al. Combine effect of Chondroitinase ABC and low level laser (660nm) on spinal cord injury model in adult male rats. Neuropeptides 2017;65:90–99. [DOI] [PubMed] [Google Scholar]
- 44. Mojarad N, Janzadeh A, Yousefifard M, Nasirinezhad F. The role of low level laser therapy on neuropathic pain relief and interleukin-6 expression following spinal cord injury: an experimental study. J Chem Neuroanat 2018;87:60–70. [DOI] [PubMed] [Google Scholar]
- 45. Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys 2017;4:337–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ren X, Zou L, Zhang X, et al. Redox signaling mediated by thioredoxin and glutathione systems in the central nervous system. Antioxid Redox Signal 2017;27:989–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Madrigal JL, Olivenza R, Moro MA, et al. Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharmacology 2001;24:420–429. [DOI] [PubMed] [Google Scholar]
- 48. Hamblin MR. Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem Photobiol 2018;94:199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tatmatsu-Rocha JC, Ferraresi C, Hamblin MR, et al. Low-level laser therapy (904nm) can increase collagen and reduce oxidative and nitrosative stress in diabetic wounded mouse skin. J Photochem Photobiol B 2016;164:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dos Santos SA, Serra AJ, Stancker TG, et al. Effects of photobiomodulation therapy on oxidative stress in muscle injury animal models: a systematic review. Oxid Med Cell Longev 2017;2017:5273403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Quirk BJ, Torbey M, Buchmann E, Verma S, Whelan HT. Near-infrared photobiomodulation in an animal model of traumatic brain injury: improvements at the behavioral and biochemical levels. Photomed Laser Surg 2012;30:523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lucas JH, Wheeler DG, Guan Z, Suntres Z, Stokes BT. Effect of glutathione augmentation on lipid peroxidation after spinal cord injury. J Neurotrauma 2002;19:763–775. [DOI] [PubMed] [Google Scholar]
- 53. Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem 2017;524:13–30. [DOI] [PubMed] [Google Scholar]
- 54. Bradbury EJ, Burnside ER. Moving beyond the glial scar for spinal cord repair. Nat Commun 2019;10:3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang T, Wu L, Wang H, Fang J, Yao N, Xu Y. Inflammation level after decompression surgery for a rat model of chronic severe spinal cord compression and effects on ischemia-reperfusion injury. Neurol Med Chir (Tokyo) 2015;55:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Byrnes KR, Waynant RW, Ilev IK, et al. Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers Surg Med 2005;36:171–185. [DOI] [PubMed] [Google Scholar]
- 57. Tai WL, Sun L, Li H, Gu P, Joosten EA, Cheung CW. Additive effects of environmental enrichment and ketamine on neuropathic pain relief by reducing glutamatergic activation in spinal cord injury in rats. Front Neurosci 2021;15:635187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lubart R, Eichler M, Lavi R, Friedman H, Shainberg A. Low-energy laser irradiation promotes cellular redox activity. Photomed Laser Surg 2005;23:3–9. [DOI] [PubMed] [Google Scholar]
- 59. Fan B, Wei Z, Yao X, et al. Microenvironment imbalance of spinal cord injury. Cell Transplant 2018;27:853–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rochkind S, Shahar A, Amon M, Nevo Z. Transplantation of embryonal spinal cord nerve cells cultured on biodegradable microcarriers followed by low power laser irradiation for the treatment of traumatic paraplegia in rats. Neurol Res 2002;24:355–360. [DOI] [PubMed] [Google Scholar]
- 61. Wang W, Liu R, Su Y, Li H, Xie W, Ning B. MicroRNA-21-5p mediates TGF-β-regulated fibrogenic activation of spinal fibroblasts and the formation of fibrotic scars after spinal cord injury. Int J Biol Sci. 2018;14:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Paish HL, Kalson NS, Smith GR, et al. Fibroblasts promote inflammation and pain via IL-1α induction of the monocyte chemoattractant chemokine (CC motif) ligand 2. Am J Pathol 2018;188:696–714. [DOI] [PMC free article] [PubMed] [Google Scholar]