Abstract
The extracellular matrix (ECM) mechanical properties regulate key cellular processes in tissue development and regeneration. The majority of scientific investigation has focused on ECM elasticity as the primary mechanical regulator of cell and tissue behavior. However, all living tissues are viscoelastic, exhibiting both solid- and liquid-like mechanical behavior. Despite increasing evidence regarding the role of ECM viscoelasticity in directing cellular behavior, this aspect is still largely overlooked in the design of biomaterials for tissue regeneration. Recently, with the emergence of various bottom-up material design strategies, new approaches can deliver unprecedented control over biomaterial properties at multiple length scales, thus enabling the design of viscoelastic biomaterials that mimic various aspects of the native tissue ECM microenvironment. This review describes key considerations for the design of viscoelastic biomaterials for tissue regeneration. We provide an overview of the role of matrix viscoelasticity in directing cell behavior toward regenerative outcomes, highlight recent strategies utilizing viscoelastic hydrogels for regenerative therapies, and outline remaining challenges, potential solutions, and emerging applications for viscoelastic biomaterials in tissue engineering and regenerative medicine.
Impact statement
All living tissues are viscoelastic. As we design viscoelastic biomaterials for tissue engineering and regenerative medicine, we must understand the effect of matrix viscoelasticity on in vitro cell behavior and in vivo regenerative outcomes. Engineering the next generation of biomaterials with tunable viscoelasticity to direct cell and tissue behavior will contribute to the development of in vitro tissue models and in vivo regenerative therapies to address unmet clinical needs.
Keywords: viscoelasticity, biomaterials, hydrogels, extracellular matrix, mechanotransduction, regenerative medicine
Introduction
Historically, the mechanical properties of biomaterials have been one of the most fundamental determinants of their suitability for biomedical use. At earlier times, biomaterials were employed mainly in the form of implants aiming to structurally replace missing tissues or body parts. Subsequently, in addition to the requirement to be “bioinert,” biomaterials were designed with a key aim of being mechanically strong to withstand mechanical loadings in patients.1,2
Over time, with our improved understanding of in vivo biomaterials function, more complex design parameters for optimal biomaterials were identified. For instance, bone loss around metallic implants that were stiffer than adjacent bone tissue was observed and the stress shielding phenomenon was identified, highlighting the importance of matching the mechanical properties of surrounding bone in biomaterial design for bone replacement.2
With the emergence of tissue engineering in the 1990s, biomaterials scientists shifted their focus largely toward the design of biomaterials for tissue regeneration rather than replacement.3 In these strategies, biomaterials commonly serve as temporary scaffolds that mimic the native extracellular matrix (ECM), aiming to provide a favorable environment for desired cellular activities and the formation of tissue(s) of interest.3
In native tissues, resident cells exist within a complex and physically confining three-dimensional (3D) ECM, which provides key signals directing cellular behavior. In the ECM, in addition to biochemical signals such as growth factors and chemokines, cells sense mechanical cues within their microenvironment. The cell-ECM mechanotransduction is facilitated through binding between cell surface receptors, such as integrins, to ECM adhesion motifs, such as arginine–glycine–aspartate (RGD) ligands.4
Early research identified substrate elasticity (or stiffness) as a key factor directing cell spreading and migration.5 Subsequently, several research groups demonstrated the role of substrate stiffness on a wider range of cellular behavior such as differentiation of mesenchymal stem cells (MSCs).6,7 Accordingly, design strategies evolved aiming at developing biomaterials with superior regenerative capacity by tuning the biomaterial elasticity.8–10
Despite this focus on elasticity of biomaterials for tissue engineering applications, natural tissues are viscoelastic, not purely elastic materials. While some materials such as metals typically exhibit purely elastic responses under loading before undergoing plastic deformation, tissues exhibit a viscoelastic behavior as they respond to mechanical deformation or force in a time-dependent manner.7
Indeed, recent findings have shown that even hard tissues such as bone are viscoelastic, and temporary structures involved in bone healing such as blood clots or fracture hematomas in particular are highly viscoelastic.7,11–14 Significant research has globally been devoted to altering chemical composition, morphology/architecture, and stiffness of biomaterials to facilitate tissue regeneration.2 Nevertheless, despite the increasing evidence regarding the role of ECM viscoelasticity in directing cellular behavior, this aspect is still largely overlooked in the design of regenerative biomaterials.
Previous reviews highlighted approaches to tune viscoelasticity of hydrogels for 3D cell culture,15 summarized hydrogel cross-linking and characterization strategies,16 and discussed the biological impact of matrix viscoelasticity on cell and tissue behavior.17,18 However, a focused review on key considerations in the design of viscoelastic biomaterials for applications in tissue regeneration and the underlying cellular mechanobiology mechanisms for sensing and responding to viscoelasticity is lacking.
Therefore, since hydrogels are the most commonly used type of viscoelastic biomaterials, here we provide an overview of viscoelastic hydrogels for tissue regeneration, and discuss the role of matrix viscoelasticity in directing cell behavior toward regenerative outcomes, and molecular mechanisms of viscoelasticity sensing. Subsequently, we outline recent strategies that have successfully employed viscoelastic hydrogels for regenerative therapies and highlight important insights gained from these investigations. Finally, we describe challenges, potential solutions, and emerging applications for viscoelastic hydrogels in tissue engineering and regenerative medicine.
Viscoelastic Properties of Biomaterials
Elasticity versus viscoelasticity
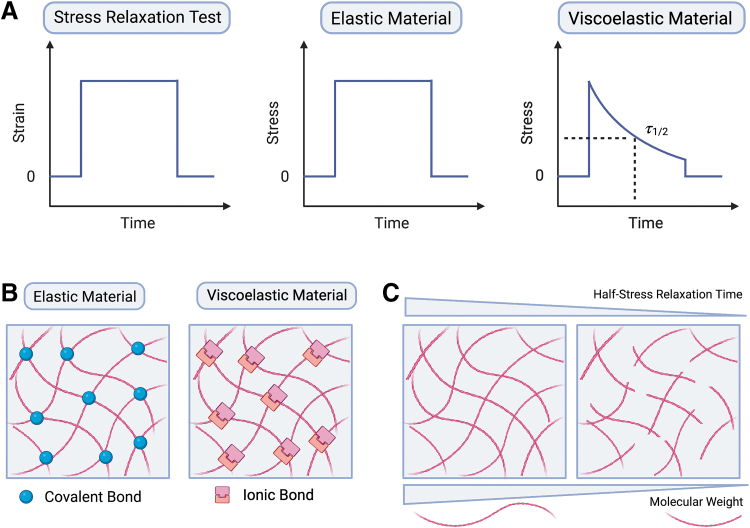
The terms stiffness and elasticity are commonly used interchangeably to refer to the ability of a material to resist elastic deformation.19 Elastic materials have long been used to understand how the stiffness of the ECM affects cellular behavior.11,20 These materials include hydrogel and elastomer systems such as polyacrylamide (PAM) and polydimethylsiloxane (PDMS).20 When subjected to a force, purely elastic materials maintain a constant deformation as long as the force is constant, and immediately return to their original shape upon removal of the force (Fig. 1A).18 While energy is stored in purely elastic materials, it is dissipated in viscous materials as they flow.
FIG. 1.
Mechanical behavior and design of viscoelastic biomaterials. (A) Stress relaxation test is performed to determine the decrease in stress in a material in response to constant strain. The half-stress relaxation time (τ1/2), defined as the time taken for relaxing half of the initial stress value generated under a constant deformation. The viscoelasticity of biomaterials can be regulated by (B) the types and strength of the bonds cross-linking the matrix, and (C) molecular weight of the polymer.
Living tissues and organs are viscoelastic, as they exhibit aspects of both elastic and viscous materials.7 When a force is applied to biological tissues or their ECMs, they exhibit an instantaneous solid-like elastic response. Over time, however, these forces are dissipated, and stresses are relaxed following a time-dependent liquid-like viscous behavior (Fig. 1A).
Tissue viscoelasticity is mainly derived from the dynamic mechanics of cells, ECM, and extracellular fluids.7,17 The ECM consists of a complex 3D network of fibrous structural proteins, adhesive proteins, and polysaccharides forming a space-filling nanoporous gel. The interactions between these ECM components, for instance, the release of polymer entanglements, structural protein unfolding, and breaking of weak non-covalent bonds between collagen fibers all contribute to viscoelasticity.21 In addition, cell cytoskeletal network and nuclear dynamic rearrangements, and interstitial fluid movement within the ECM in response to mechanical forces also result in viscoelastic responses.
Strategies for viscoelastic hydrogel design
Viscoelastic biomaterials have been developed to recapitulate the structure and mechanical properties of living tissues and elucidate the effects of viscoelasticity on cell behavior and function. While different types of biomaterials can be designed to exhibit viscoelastic behavior, available literature in this area is largely focused on hydrogel-based biomaterials.
Hydrogels are 3D interconnected networks within an aqueous phase and are typically composed of natural or synthetic hydrophilic polymers that are cross-linked. These materials are widely used as tissue engineering scaffolds and cell delivery vehicles for regenerative medicine.22 The mechanical properties of hydrogels are determined by factor such as polymer structure and composition, cross-link type and density, and conditions of their aqueous phase (e.g., ionic strength).23 Accordingly, these determinants can be exploited to engineer hydrogels and tune their viscoelasticity.
Hydrogel viscoelasticity specifically can be tuned through various methods including (1) changing the molecular weight or length of polymer chain, (2) varying polymer concentration or cross-linking density, (3) altering the strength and dynamics of non-covalent and/or covalent bonds (Fig. 1B, C).11,16,20,24–27
Viscoelastic hydrogels are based on two primary cross-linking approaches: non-covalent physical cross-linking and/or dynamic covalent interactions. Non-covalent interactions used for viscoelastic hydrogel design include ionic bonding/electrostatic interactions,11,25,28–31 hydrogen bonding,32–37 hydrophobic interactions,38–41 and metal-ligand coordination.42,43 Furthermore, thioester exchange, hydrazone adaptable covalent networks, and reversible boronate bonds are among the dynamic covalent interactions employed for viscoelastic hydrogel design.44–47
Moreover, static covalent bonds (e.g., through Michael addition reaction) have been also employed together with non-covalent interactions to tune the viscoelasticity of hydrogel materials.48 Cross-linking strategies used to tune the properties of viscoelastic hydrogels were recently reviewed in depth by Ma et al.16
The design of hydrogels with tunable viscoelasticity requires appropriate measurements and methodologies for in-depth analysis of their viscoelastic behavior at relevant length-scales. One parameter often used is the quantification of stress relaxation behavior. In particular, the half-stress relaxation time (τ1/2), defined as the time taken for relaxing half of the initial stress value generated under a constant deformation, is often used to characterize hydrogels (Fig. 1A).11,16,24 An overview of viscoelastic hydrogel systems applicable to tissue regeneration along with their stress relaxation behavior is provided in Table 1.
Table 1.
Overview of Viscoelastic Hydrogel Systems Relevant to Tissue Engineering and Regeneration, and Their Stress Relaxation Behavior
| Material system | Cross-linking strategy | Stress relaxation behavior |
|
|---|---|---|---|
| Qualitative features | Quantitative features | ||
| Alg11 | Ionic cross-linking | Stress relaxation tuned by varying molecular weights of alginate leading to decreasing network connectivity and increasing chain mobility | τ½: ∼2 × 102 s to ∼5 × 103 s Quantified using compression test at a fixed strain of 15% with an initial deformation rate of 1 mm/min |
| Alg-PEG11,28 | Ionic cross-linking | Hydrogels made of alginate grafted with increasing PEG concentrations exhibited faster stress relaxation | τ½: 102–103 s Quantified using compression stress relaxation test at strain of 20% at a deformation rate of 1 mm/min |
| Click-functionalized alginate and fibrillar collagen type I30 | Sequential ionic and covalent cross-linking | Combination of alginate polymers, each with covalently conjugated click partner, underwent ionic cross-linking of constituent G-blocks followed by collagen self-assembly and covalent cross-linking of spatially confined click groups | τ½: ∼3 × 103 s to 1.0 × 104 s Quantified using oscillatory shear rheology (1 Hz, 1% strain at 25°C and at 37°C) with cone-plate geometry |
| PEO-based triblock co-polymer39 | Hydrophobic interactions | Viscoelasticity tuned by changes in the length of hydrophobic end-blocks (alkyl side-chains) | τ½: 10−1–105 s Quantified using oscillatory shear rheology at 5% strain at 25°C |
| PEG-hydrophobic DFA40 | Hydrophobic interactions | Viscoelasticity tuned by adding surfactant SDS or urea | τ½: 47–270 s Quantified using oscillatory shear rheology at 0.1% strain, 25°C |
| 8-Arm PEG star macromers end-functionalized with thiols (PEG-8SH)44 | Dynamic covalent bonding based on thioester exchange | Viscoelasticity tuned with varying concentration of thiols and pH (ranging from 6.0 to 9.0) Stress relaxation increased upon alteration of cross-linker structure by uncaging of thioester exchange catalyst MPAA |
τ: 1.1 × 104 s to 6.5 × 106 s (calculated values) Quantified using oscillatory shear rheology at 1 Hz, 1% strain at 1 Hz followed by 10% strain over 5 s and monitoring over 20 s |
| HA-collagen27 | Dynamic covalent hydrazone cross-linking | Stress relaxation tuned by modulating HA cross-linker affinity, molecular weight of HA, or HA concentration | τ½: ∼2.3 × 102 s to 1.8 × 104 s Quantified using oscillatory shear rheology at 1 Hz, 10% strain, 37°C with cone-plate geometry |
| PEG45 | Dynamic covalent bonding with hydrazone bonds | Viscoelasticity tuned by changing the relative percentage of aHz bHz cross-links | τ½: 4 × 103 s (h) to 2.78 × 106 s (months) Quantified using oscillatory shear rheology with 10% shear strain applied over a 10 s ramp at 37°C |
| cis-1,2-diols functionalized octa-arm PEGs117 | Dynamic covalent bonding with adaptable boronate bonds | Viscoelasticity tuned using different boronic acid derivates (Wulff-type-like boronic acid, m-boroxole, 2-fluorophenyl boronic acid) | τ½: tuned on the order of 1 s or below Relaxation dynamics characterized by frequency sweep instead of direct relaxation tests (inferred from G′-G″ cross over frequency) |
| UPy-functionalized PEG chains37 | Hierarchical assembly of supramolecular fibers based on hydrogen bonding | Viscoelastic behavior of the hydrogels tuned by altering the ratio of UPy-based building blocks used | τ½: ∼5 s to >10 min Quantified using oscillatory shear rheology at a fixed strain of 1% |
| Colloidal hydrogels assembled from gelatin nanoparticles31 | Attractive colloidal particles aggregate and form an arrested network of percolated particles | Viscoelascity modulated via interparticle interactions | τ½: 10−1–102 s Quantified using oscillatory shear rheology with step strain and subsequent relaxation within 10 min at 25°C |
τ1/2: Half relaxation time (defined as time taken for relaxing half of the initial stress value generated under a constant deformation). τ: Calculated mean relaxation time (calculated by fitting the normalized stress curves using a method reported by Brown et al.44).
aHz, alkyl-hydrazone; Alg, alginate; bHz, benzyl-hydrazone; DFA, dimer fatty acid; HA, hyaluronic acid; MPAA, 4-mercaptophenylacetic acid; PEG, poly(ethylene glycol); PEO, poly(ethylene oxide); SDS, sodium dodecyl sulfate; UPy, ureido-pyrimidinone.
Another indication of viscous behavior of hydrogels is their creep behavior, defined as change in strain over time under the influence of a constant stress.7,16,49 A creep parameter often used to compare materials is the time required to reach a strain level that is 150% of the initial, elastic response value (τ3/2).16 The loss moduli of biomaterials is another parameter related to viscous response that is often determined for viscoelastic materials.50 To obtain these various measures, a variety techniques have been developed to characterize the viscoelastic properties both at the macroscale and microscale.16
For the design of regenerative biomaterials, it is important to consider the mechanical properties of tissues at multiple length scales and their differences arising from the hierarchical structure of native tissues.51 One well-known example is bone tissue, which is a composite material consisting of collagen and mineral building blocks. The hierarchical assembly of these building blocks in bone from nano- to macro-scale can result in cortical or cancellous bone, which exhibit different mechanical properties.52 Historically, the viscoelasticity of hydrogels could only be evaluated at the macroscale with static mechanical testing (i.e., stress relaxation under constant deformation and creep tests under constant stress), and dynamic mechanical testing (i.e., frequency-dependent [oscillatory] rheology tests and cyclic loading tests).15,17,49,53
Although macroscale mechanical testing methods, such as compression testing, and oscillatory rheology, provide insight into the mechanical properties of bulk hydrogel and biomaterials-based devices, cells experience biomaterial mechanical properties at a much lower length scale. Recently, advances in nanomechanics (i.e., depth-sensing nanoindentation, atomic force microscopy (AFM)-based nanoindentation) and micromechanics (i.e., particle-based microrheology) have enabled the characterization of viscoelasticity at the nano- and micro-scale.54–59
In addition to the length scale of the mechanical cues, it is important to also consider the types (i.e., compressive, tensile, and shear forces) and the magnitude (i.e., values of stress and strain) of mechanical cues that are experienced and/or exerted by the cells embedded within the ECM.
For instance, experimental evidence indicate that cells typically experience strain of up to 3–4% in two-dimensional (2D) culture and 20–30% in 3D culture.60–63 Living tissues also experience deformation during physiological function. Previous studies have reported strains of 5–15% in alveoli during respiration, 30% in contracting muscles, and up to 40% in skin of knees during movement.64–66
In addition, traction stresses generated by cells in 2D and 3D cultures fall within the range of 100 to >1000 Pa.61,62 In comparison, peak stresses generated during the stress relaxation tests of various tissues are within the range of stresses generated by cells. More specifically, stress relaxation tests on tissues measured peak stresses of ∼70 Pa for coagulated bone marrow, ∼150 Pa for adipose tissue, ∼310 Pa for brain, ∼380 Pa for liver, and ∼1 kPa for fracture hematoma.11 Overall, methods including particle-based microrheology, depth-sensing nanoindentation, and AFM-based indentation that can provide a high spatial resolution are valuable tools for in-depth local characterization of viscoelastic hydrogels.17,26,67,68
Role of Viscoelastic Biomaterials on In Vitro Cell Behavior
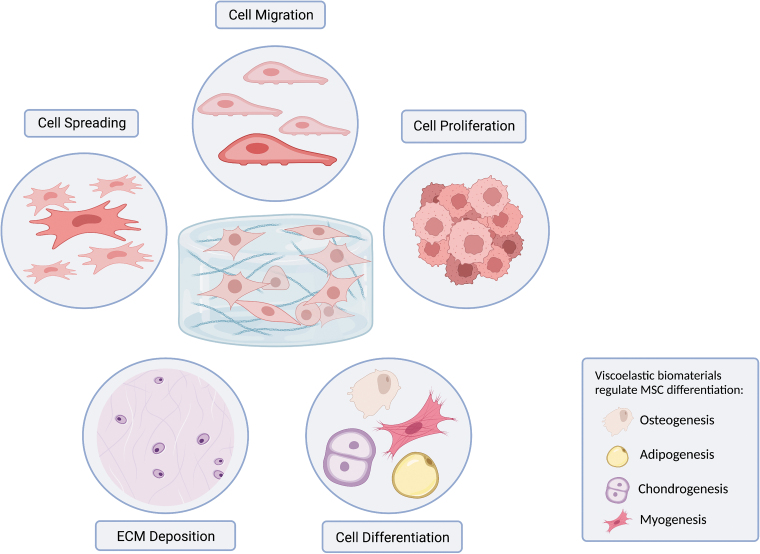
An increasing number of investigations have recognized the viscous behavior of hydrogels as an important mechanical cue that dictates behavior of embedded cells such as spreading. migration, proliferation, differentiation, and matrix deposition (Fig. 2).7,16,17
FIG. 2.
Viscoelastic biomaterials regulate various aspects of cell behavior. Viscoelastic biomaterials, notably hydrogels, provide time-dependent mechanical cues (i.e., stress relaxation) that affect cell behavior including cell spreading, migration, proliferation, differentiation, and ECM deposition. MSCs have osteogenic, adipogenic, chondrogenic, and myogenic potential, which can be enhanced by appropriate tuning of biomaterial viscoelasticity. ECM, extracellular matrix; MSCs, mesenchymal stem cells.
Cell spreading and migration
The viscoelasticity of biomaterials controls cell spreading behavior in both 2D and 3D cell culture systems. In 2D, modulating the loss modulus of PAM hydrogels independently of elastic modulus enabled the creation of substrates with varying viscoelasticity and similar storage modulus. Studies with these gels demonstrated that increased creep under cell-generated stresses promoted spreading of MSCs in 2D culture.69
Prior studies using viscoelastic RGD-modified alginate hydrogels with similar initial moduli demonstrated substrates with rapid stress relaxation enhanced U2OS cell and myoblast spreading.24,70 Similar findings were observed in 3D cultures, with fibroblasts and MSCs exhibiting greater spreading within fast relaxing ionically cross-linked alginate hydrogels (τ1/2 ∼1 min) compared to slow relaxing gels (τ1/2 ∼1 h) and covalently cross-linked gels.11 In addition, myoblast spreading in 3D with extended lamellipodia and filopodia occurred in fast relaxing, reversibly cross-linked poly(ethylene glycol) (PEG) hydrogels.71 Several mechanistic models have been proposed to explain enhanced cell spreading on viscoelastic biomaterials, including variations on the molecular clutch model.72–76
Originally proposed by Mitchison and Kirschner, the molecular clutch model is a leading concept in the field of mechanobiology to explain the coupling between the actin cytoskeleton and ECM through a macromolecular complex of focal adhesion molecules (FAs) including integrin, talin, and vinculin.77–80 Biophysical cues provided by the ECM can alter the dynamics and interactions of FA proteins to change FA composition, morphology, or signaling, all of which result in downstream changes in FA-dependent gene expression and cellular functions.79 The dynamics of this model has been explored in depth by Gong et al.75
While viscoelasticity plays a regulatory role in cell migration, the mechanisms underlying its effects remain unclear. Mechanical cues from viscoelastic substrates can initiate and guide collective cell migration,81 and substrate stress relaxation can enhance cell migration on soft substrates.82 MSCs migrate robustly on substrates with fast stress relaxation, but migrating cells did not extend lamellipodial protrusions. Instead, migrating MSCs exhibited rounded morphology with filopodia protrusions extending at the leading edge.82
Moreover, MSCs have been shown to migrate rapidly in nanoporous, physically confining viscoelastic hydrogels with rapid stress relaxation.7 Mechanistically, MSCs may use the nucleus as a piston to activate mechanosensitive ion channels in confining viscoelastic microenvironments.83 In this model, the resulting increase in osmotic pressure outcompetes hydrostatic pressure to drive protrusion expansion.83
Cell proliferation
Viscoelastic matrices have been demonstrated to promote and regulate the proliferation of several cell types. For instance, studies have shown that alginate and PAM hydrogels with fast stress relaxation promoted MSC proliferation.11,69 In addition, both myoblasts and fibroblasts exhibited higher proliferation rates in fast relaxing alginate hydrogels compared to elastic hydrogels with the same initial modulus.11,70 Similarly, when encapsulated in viscoelastic chitosan-modified poly(l-lactide-co-ɛ-caprolactone) scaffolds with comparable stress relaxation time as that of native cartilage, chondrocyte proliferation was promoted.84
It is currently unclear whether nuclear translocation of Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ), a key mechanotransducer in cellular responses to mechanical cues, is involved in the promotion of cell proliferation in response to viscoelasticity.11,24 Further studies are needed to understand the cellular mechanism of viscoelasticity sensing leading to cell proliferation.
Cell differentiation and ECM deposition
Matrix viscoelasticity can regulate the differentiation of various cell types used in tissue engineering and regenerative medicine. For example, hydrogels with rapid stress relaxation (τ1/2 < 100 s) led to greater osteogenic differentiation of MSCs in 3D culture than gels with slow relaxation rates.11 Interestingly, the optimal stress relaxation rate for osteogenesis matched that of human fracture hematomas isolated from patients.11,12 In contrast, adipogenic differentiation was found to be enhanced in gels with slow relaxation.11 Viscoelastic hydrogels have also been successfully applied to regulate cell-to-cell, and cell–matrix interactions for regeneration of bone and cartilage defects with MSC spheroids.85–87
ECM deposition is often a key aspect of cell differentiation in the engineering and regeneration of various connective tissues, including bone and cartilage. Viscoelastic alginate hydrogels with fast stress relaxation time (τ1/2 ∼1 min) that promoted MSC osteogenic differentiation also increased production of bone-like matrix with mineralized collagen I.11 Similarly, chondrocytes encapsulated in scaffolds with similar viscoelasticity as native cartilage tissue had greater deposition of cartilage-like matrix composed of type 2 collagen and aggrecan.84 Similarly, hydrogels with faster stress relaxation promoted an increase in matrix formation, while slow relaxing gels impeded chondrocyte volume expansion and activated inflammatory IL-1B signaling associated with cartilage degradation and cell death.88
The immune system plays a critical role in tissue repair and regeneration outcomes,89 and promoting tissue regeneration through immune modulation is an active area of research.90 Interestingly, ECM viscoelasticity has been recently found to play a role in inflammation and immunomodulation.91 Elastic ECM with slow stress relaxation drive pro-inflammatory polarization of human bone marrow-derived monocytes and differentiation into antigen-presenting cells.91 MSCs encapsulated in a viscoelastic hydrogel consisting of an interpenetrating network of alginate and fibrillar collagen type I with interferon γ (IFN-γ) loaded heparin-coated beads suppressed proliferation of human T cells.92
Cellular mechanosensing of viscoelasticity
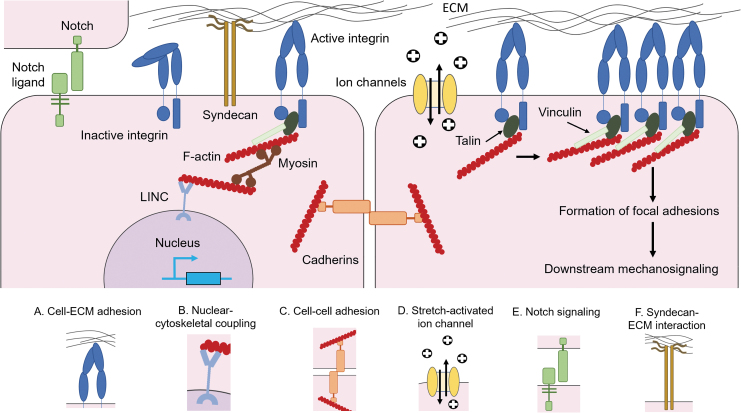
Mechanotransduction within viscoelastic biomaterials is an active field of investigation. There are a myriad of canonical mechanosensitive signal transduction pathways that enable cells to transmit mechanical cues of their microenvironment into biochemical signals (Fig. 3).93–95
FIG. 3.
Cellular mechanosensing of ECM viscoelasticity. (A) Cells directly sense mechanical resistance to ECM viscoelasticity through cell-ECM adhesions (e.g., integrin binding) and the formation of FACs. (B) Cell-ECM bonds directly link ECM mechanosensing to gene expression via nuclear-cytoskeletal interactions. (C) Adjacent cells are mechanically coupled by cadherin complexes to facilitate cell–cell adhesion. (D) Mechanosensitive SACs regulate ion transport across the cell membrane to regulate downstream mechanosignaling. (E) Notch signaling requires mechanical force to induce proteolytic activation. (F) Syndecan-ECM interactions maintain cell homeostasis and can interact with focal adhesion associated molecules. FACs, focal adhesion complexes; SACs, stretch-activated ion channels.
Integrins are well-characterized mechanosensors that bind to ECM ligands like RGD-containing peptides, and activate focal adhesion kinases (FAK), thus regulating cell adhesion to their respective substrates.96,97 During processes such as spreading, cells can maintain mechanical homeostasis by modifying focal adhesion ligand affinity, regulating focal adhesion assembly and disassembly, and by regulating intracellular machinery such as the actin cytoskeleton through actomyosin contractility in a mechanical feedback control loop that responds to ECM mechanics.98–101
Focal adhesion complexes and their mechanical coupling to cytoskeletal components activate mechanosensitive signaling, such as ras homolog family member A (RhoA) and mitogen-activated protein kinase (MAPK), leading to the activation of downstream effectors of mechanotransduction pathways like Rac family small GTPase 1 (Rac1) and RhoA signaling.102,103 Focal adhesion assemblies can also lead to the activation of YAP/TAZ, resulting in nuclear translocation and downstream transcriptional activities.104
An important aspect of mechanotransduction is the direct linkage of the actin cytoskeleton to the nucleus, allowing changes in the physical properties of cell-ECM and cell-cell adhesions to directly alter gene expression via molecular complexes such as the Linker of Nucleoskeleton and Cytoskeleton (LINC).105 These signaling pathways are strongly implicated in a myriad of biological processes ranging from cell spreading to stem cell differentiation.11,24
Cell-extrinsic mechanical forces also regulate cell function through ECM receptors, cadherin complexes, and stretch activated ion channels (SACs).106 Mechanosensing of ECM stiffness and viscoelasticity through SACs such as Transient receptor potential vanilloid-type 4 (TRPV4) are implicated in processes such as cartilage homeostasis and osteogenesis by MSCs.107,108 SACs such as Piezo1 and Piezo2 are implicated in processes such as stem cell lineage specification by neural stem cells.109
The focus of mechanotransduction has largely been on a limited set of mechanosensors such as integrins and SACs. However, there exists other mechanosensitive pathways in which viscoelasticity has potential to be exploited to both understand and control biological processes (Fig. 3). For example, the notch signaling pathway requires mechanical force to be proteolytically activated and is involved in various developmental processes ranging from angiogenesis to embryogenesis.110–112
Cadherins facilitate mechanical coupling between cells and are important in cell migration, stem cell differentiation, and the epithelial-to-mesenchymal transition.113–115 Syndecans are proteoglycans that can bind to ECM and interact with cytoskeletal and focal adhesion associated molecules that maintain cell homeostasis (e.g., nucleus pulposus cell phenotype in intervertebral disc regeneration).116,117 Exploring the effects of viscoelasticity on these and other signaling pathways could lead to new fundamental insights on how cells and tissues mechanically interpret their microenvironments. This could further lead to translational applications for design of new biomaterials for tissue engineering and regenerative medicine.
Exploiting Viscoelastic Biomaterials for In Vivo Tissue Regeneration
In regenerative medicine, biomaterials are typically used to deliver cells or are applied as acellular systems that exploit endogenous mechanisms employing host cells in vivo for in situ tissue repair.1 Although there are few studies demonstrating the impact of viscoelastic mechanical stimuli in vivo, there exist both direct evidence and indirect correlations demonstrating the importance of viscoelasticity as a biomaterial design parameter for regenerative medicine.
Applications in regenerative medicine
Matrix viscoelasticity has been demonstrated to impact in vivo tissue regeneration. Application of ionically cross-linked, viscoelastic chitosan hydrogels in osteochondral defects in rabbits led to enhanced cartilage matrix formation and woven bone deposition for defects filled with the gels.118 In addition, implantation of alginate hydrogels with rapid stress relaxation carrying human MSCs into rat calvarial defects led to greater new bone formation than slow relaxing gels, and extensive matrix remodeling.12 Interestingly, fast relaxing hydrogels without encapsulated human MSCs also significantly enhanced new bone formation, suggesting the mechanical environment alone provided by these gels enhanced progenitor cell invasion into the scaffold to promote bone regeneration.12
Viscoelastic hydrogels have also enhanced the therapeutic potential of MSC spheroids for bone formation and repair in vivo.87 MSC spheroids encapsulated in ionically cross-linked fast relaxing viscoelastic hydrogels in combination with the use of bone morphogenetic protein-2 (BMP-2) and hyaluronic acid (HA) nanoparticles led to spatially uniform osteogenic differentiation and greater bone formation than slow relaxing gels.87 Together, these investigations suggest that viscoelastic properties of biomaterials can serve as a powerful regulator of cell behavior in vivo, which can be harnessed for regenerative medicine.
The hidden effect of viscoelasticity
One may speculate that viscoelasticity could also be a hidden factor that may explain the regenerative potential of various biomaterials presented in past work in the field of tissue engineering. First, some of the most widely used biomaterials are hydrogels made of collagen, hyaluronic acid, and reconstituted basement membrane matrix, which are typically viscoelastic materials. These have been successfully applied to promote the formation of liver, neural, and skeletal muscle organoids.119–122
Several studies have also demonstrated that hydrogels with rapid degradation enhance tissue regeneration, but in certain of these studies matrix degradation/dissolution was controlled by varying the polymer molecular weight, which will impact viscoelastic properties.123–126 Similarly, synthetic hydrogels with tunable degradability have been demonstrated to support organoid formation and enhance colonic wound,127,128 but the cell activities leading to gel degradation might have transitioned these biomaterials locally to a viscoelastic state with rapid stress relaxation.7
The Future of Viscoelastic Biomaterials
While significant advances have been made, several materials-related challenges remain in the design and application of viscoelastic hydrogels, including the mechanical stability under physiological condition, full decoupling of materials properties (e.g., viscoelasticity, elasticity, swelling, degradation), processability and handling for clinical applications. More specifically, high-stiffness hydrogels can suffer from inferior processability (e.g., through extrusion-based bioprinting) and clinical handling (e.g., through minimally invasive injection) due to limited injectability.
While application of uncross-linked hydrogel precursors can overcome this limitation, excessively rapid or slow gelation kinetics can result in issues such as non-uniform gelation or lack of cohesion of resulting hydrogels, respectively. Finally, in terms of development of precision medicine for regenerative therapies utilizing viscoelastic biomaterials, a potential challenge is the increasing sophistication of biomaterial development creating manufacturing challenges and/or driving up costs of these novel therapeutics.
Viscoelastic biomaterials play an important role in the development of in vitro organoid models. These promise to reduce in vivo animal experiments that often do not reliably recapitulate the intricacies of human biology, fail to predict therapeutics responses in humans, and have ethical and financial considerations. The recent developments of human organ-on-a-chip technologies have demonstrated the ability to recapitulate human physiology and disease states besides human patient responses to therapeutics with higher fidelity compared to other in vitro models and animal studies.129
With increasing awareness regarding the role of matrix viscoelasticity in cellular function and tissue and organ development, significant research efforts is now focused on the design of viscoelastic hydrogels as tunable ECM mimics.2,130 Subsequently, the development of in vitro models utilizing viscoelastic hydrogels could enable large-scale experiments combined with computational biology to make fundamental discoveries in developmental biology and organ regeneration for applications in regenerative medicine.
Advances in the design of viscoelastic biomaterials will enable researchers to further explore the relationship between viscoelasticity and higher-order biological behaviors of living organisms ranging from development, organogenesis, tissue regeneration, and disease progression.7 Viscoelastic biomaterials may be useful to reconstruct complex and highly organized tissues. Biofabrication of tissue engineering scaffolds with different layers of distinct viscoelastic properties may further enhance regeneration of complex tissues with precise anatomical organization. For example, utilizing strategies such as intra-operative 3D bioprinting of different tissues in a stratified arrangement with controlled spatial bioink deposition allows the simultaneous reconstruction of various tissues including bone, skin, and composite hard/soft tissues.131,132
The design of viscoelastic biomaterials may also benefit from merging expertise from various disciplines including computational biology and biomechanics, mathematical modeling, and 3D printing.133 The application of machine learning combined with high-throughput theoretical predictions and experiments could further enhance biomaterials scientists' capability to generate and analyze vast amount of data leading to discovery of new biomaterial formulations, optimization of biomaterial properties toward regenerative outcomes, and better understanding of cell–matrix interactions.134
Although the viscoelastic properties of a biomaterial play a significant role in directing cellular behavior toward tissue regeneration, it is only one of many key parameters that should be taken into consideration for the design of tissue regenerative biomaterials. These parameters include biochemical properties (e.g., inclusion of ligands for cell receptors, biodegradability), the interior morphology of scaffolds (e.g., porosity and pore size), and exterior architecture of the final tissue engineered construct/device.135 Altogether, these add to the complexity of designing matrices for applications in regenerative therapies. Exploring the interrelationship between these design parameters and uncovering their potential synergism is an important area for future research.
In conclusion, viscoelasticity is a key design parameter of biomaterials for tissue engineering and regenerative medicine. Developing the next generation of biomaterials with tunable viscoelasticity to control cell and tissue behavior has promising applications in both in vitro tissue models and clinical applications in regenerative medicine. Additional investigations are needed to expand the mechanical tunability of viscoelastic biomaterials and to understand the role of matrix viscoelasticity in guiding complex interactions at the tissue and organ level. These will enable us to harness the regenerative potential of viscoelastic biomaterials in vivo in order to address unmet clinical needs and improve human health.
Acknowledgment
Authors' Contributions
All authors contributed to the conceptualization and writing of this review.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work is financially supported by the National Institutes of Health Grants (R01-CA223255-04 and R01-DE013349-19) and the Osteology Foundation Young Researcher Grant (21-032).
References
- 1. Huebsch, N., and Mooney, D.J.. Inspiration and application in the evolution of biomaterials. Nature 462, 426, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koons, G.L., Diba, M., and Mikos, A.G.. Materials design for bone-tissue engineering. Nat Rev Mater 5, 584, 2020. [Google Scholar]
- 3. Langer, R., and Vacanti, J.P.. Tissue engineering. Science 260, 920, 1993. [DOI] [PubMed] [Google Scholar]
- 4. Sun, Z., Guo, S.S., and Fässler, R.. Integrin-mediated mechanotransduction. J Cell Biol 215, 445, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pelham, R.J., and Wang, Y.. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A 94, 13661, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Engler, A.J., Sen, S., Sweeney, H.L., et al. . Matrix elasticity directs stem cell lineage specification. Cell 126, 677, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Chaudhuri, O., Cooper-White, J., Janmey, P.A., et al. . Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gu, Y., Ji, Y., Zhao, Y., et al. . The influence of substrate stiffness on the behavior and functions of Schwann cells in culture. Biomaterials 33, 6672, 2012. [DOI] [PubMed] [Google Scholar]
- 9. Keogh, M.B., O'Brien, F.J., and Daly, J.S.. Substrate stiffness and contractile behaviour modulate the functional maturation of osteoblasts on a collagen-GAG scaffold. Acta Biomater 6, 4305, 2010. [DOI] [PubMed] [Google Scholar]
- 10. Hu, X., Park, S.-H., Gil, E.S., et al. . The influence of elasticity and surface roughness on myogenic and osteogenic-differentiation of cells on silk-elastin biomaterials. Biomaterials 32, 8979, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chaudhuri, O., Gu, L., Klumpers, D., et al. . Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater 15, 326, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Darnell, M., Young, S., Gu, L., et al. Substrate stress-relaxation regulates scaffold remodeling and bone formation in vivo. Adv Healthc Mater 6, 2017. DOI: 10.1002/adhm.201601185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gersh, K.C., Nagaswami, C., and Weisel, J.W.. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb Haemost 102, 1169, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu, S., Bao, G., Ma, Z., et al. . Fracture mechanics of blood clots: measurements of toughness and critical length scales. Extreme Mech Lett 48, 101444, 2021. [Google Scholar]
- 15. Chaudhuri, O. Viscoelastic hydrogels for 3D cell culture. Biomater Sci 5, 1480, 2017. [DOI] [PubMed] [Google Scholar]
- 16. Ma, Y., Han, T., Yang, Q., et al. . Viscoelastic cell microenvironment: hydrogel-based strategy for recapitulating dynamic ECM mechanics. Adv Funct Mater 31, 2100848, 2021. [Google Scholar]
- 17. Huang, D., Huang, Y., Xiao, Y., et al. . Viscoelasticity in natural tissues and engineered scaffolds for tissue reconstruction. Acta Biomater 97, 74, 2019. [DOI] [PubMed] [Google Scholar]
- 18. Elosegui-Artola, A. The extracellular matrix viscoelasticity as a regulator of cell and tissue dynamics. Curr Opin Cell Biol 72, 10, 2021. [DOI] [PubMed] [Google Scholar]
- 19. Baumgart, E. Stiffness—an unknown world of mechanical science? Injury 31(Suppl 2), S-B14, 2000. [PubMed] [Google Scholar]
- 20. Vining, K.H., and Mooney, D.J.. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol 18, 728, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sauer, F., Oswald, L., de Schellenberger, A.A., et al. . Collagen networks determine viscoelastic properties of connective tissues yet do not hinder diffusion of the aqueous solvent. Soft Matter 15, 3055, 2019. [DOI] [PubMed] [Google Scholar]
- 22. Lee, K.Y., and Mooney, D.J.. Hydrogels for tissue engineering. Chem Rev 101, 1869, 2001. [DOI] [PubMed] [Google Scholar]
- 23. Anseth, K.S., Bowman, C.N., and Brannon-Peppas, L.. Mechanical properties of hydrogels and their experimental determination. Biomaterials 17, 1647, 1996. [DOI] [PubMed] [Google Scholar]
- 24. Chaudhuri, O., Gu, L., Darnell, M., et al. . Substrate stress relaxation regulates cell spreading. Nat Commun 6, 6365, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Han, B., Ma, T., Vergara, J.H., et al. . Non-additive impacts of covalent cross-linking on the viscoelastic nanomechanics of ionic polyelectrolyte complexes. RSC Adv 7, 53334, 2017. [Google Scholar]
- 26. Dey, K., Agnelli, S., and Sartore, L.. Dynamic freedom: substrate stress relaxation stimulates cell responses. Biomater Sci 7, 836, 2019. [DOI] [PubMed] [Google Scholar]
- 27. Lou, J., Stowers, R., Nam, S., et al. . Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials 154, 213, 2018. [DOI] [PubMed] [Google Scholar]
- 28. Nam, S., Stowers, R., Lou, J., et al. . Varying PEG density to control stress relaxation in alginate-PEG hydrogels for 3D cell culture studies. Biomaterials 200, 15, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun, T.L., Kurokawa, T., Kuroda, S., et al. . Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat Mater 12, 932, 2013. [DOI] [PubMed] [Google Scholar]
- 30. Vining, K.H., Stafford, A., and Mooney, D.J.. Sequential modes of crosslinking tune viscoelasticity of cell-instructive hydrogels. Biomaterials 188, 187, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bertsch, P., Andrée, L., Besheli, N.H., et al. . Colloidal hydrogels made of gelatin nanoparticles exhibit fast stress relaxation at strains relevant for cell activity. Acta Biomater 138, 124, 2022. [DOI] [PubMed] [Google Scholar]
- 32. Bercea, M., Morariu, S., and Teodorescu, M.. Rheological investigation of poly(vinyl alcohol)/poly(N-vinyl pyrrolidone) mixtures in aqueous solution and hydrogel state. J Polym Res 23, 142, 2016. [Google Scholar]
- 33. Xu, C., Tang, Q., Yang, H., et al. . High-strength, thermally activated shape memory hydrogels based on hydrogen bonding between MAAc and NVP. Macromol Chem Phys 219, 1700636, 2018. [Google Scholar]
- 34. Ding, Q., Xu, X., Yue, Y., et al. . Nanocellulose-mediated electroconductive self-healing hydrogels with high strength, plasticity, viscoelasticity, stretchability, and biocompatibility toward multifunctional applications. ACS Appl Mater Interfaces 10, 27987, 2018. [DOI] [PubMed] [Google Scholar]
- 35. Liu, H., Xiong, C., Tao, Z., et al. . Zwitterionic copolymer-based and hydrogen bonding-strengthened self-healing hydrogel. RSC Adv 5, 33083, 2015. [Google Scholar]
- 36. Bao, G., Jiang, T., Ravanbakhsh, H., et al. . Triggered micropore-forming bioprinting of porous viscoelastic hydrogels. Mater Horiz 7, 2336, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Diba, M., Spaans, S., Hendrikse, S.I.S., et al. Engineering the dynamics of cell adhesion cues in supramolecular hydrogels for facile control over cell encapsulation and behavior. Adv Mater 33, 2008111, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaewprasit, K., Kobayashi, T., and Damrongsakkul, S.. Thai silk fibroin gelation process enhancing by monohydric and polyhydric alcohols. Int J Biol Macromol 118, 1726, 2018. [DOI] [PubMed] [Google Scholar]
- 39. Murakami, T., Kawamori, T., Gopez, J.D., et al. . Synthesis of PEO-based physical gels with tunable viscoelastic properties. J Polym Sci A Polym Chem 56, 1033, 2018. [Google Scholar]
- 40. Mihajlovic, M., Wyss, H.M., and Sijbesma, R.P.. Effects of surfactant and urea on dynamics and viscoelastic properties of hydrophobically assembled supramolecular hydrogel. Macromolecules 51, 4813, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cui, S., Chen, L., Yu, L., et al. . Synergism among polydispersed amphiphilic block copolymers leading to spontaneous physical hydrogelation upon heating. Macromolecules 53, 7726, 2020. [Google Scholar]
- 42. Hu, X., Zhou, J., Daniel, W.F.M., et al. Dynamics of dual networks: strain rate and temperature effects in hydrogels with reversible H-bonds. Macromolecules 50, 652, 2017. [Google Scholar]
- 43. Zou, X., Kui, X., Zhang, R., et al. . Viscoelasticity and structures in chemically and physically dual-cross-linked hydrogels: insights from rheology and proton multiple-quantum NMR spectroscopy. Macromolecules 50, 9340, 2017. [Google Scholar]
- 44. Brown, T.E., Carberry, B.J., Worrell, B.T., et al. . Photopolymerized dynamic hydrogels with tunable viscoelastic properties through thioester exchange. Biomaterials 178, 496, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Richardson, B.M., Wilcox, D.G., Randolph, M.A., et al. . Hydrazone covalent adaptable networks modulate extracellular matrix deposition for cartilage tissue engineering. Acta Biomater 83, 71, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tang, S., Ma, H., Tu, H.-C., et al. . Adaptable fast relaxing boronate-based hydrogels for probing cell–matrix interactions. Adv Sci 5, 1800638, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Accardo, J.V., and Kalow, J.A.. Reversibly tuning hydrogel stiffness through photocontrolled dynamic covalent crosslinks. Chem Sci 9, 5987, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chrisnandy, A., Blondel, D., Rezakhani, S., et al. . Synthetic dynamic hydrogels promote degradation-independent in vitro organogenesis. Nat Mater 21, 479, 2022. [DOI] [PubMed] [Google Scholar]
- 49. Nam, S., Hu, K.H., Butte, M.J., et al. . Strain-enhanced stress relaxation impacts nonlinear elasticity in collagen gels. Proc Natl Acad Sci U S A 113, 5492, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Charrier, E.E., Pogoda, K., Wells, R.G., et al. . Control of cell morphology and differentiation by substrates with independently tunable elasticity and viscous dissipation. Nat Commun 9, 449, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Qian, L., and Zhao, H.. Nanoindentation of soft biological materials. Micromachines 9, 654, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sabet, F.A., Raeisi Najafi, A., Hamed, E., et al. . Modelling of bone fracture and strength at different length scales: a review. Interface Focus 6, 20150055, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Charbonier, F., Indana, D., and Chaudhuri, O.. Tuning viscoelasticity in alginate hydrogels for 3D cell culture studies. Curr Protoc 1, e124, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rubiano, A., Delitto, D., Han, S., et al. . Viscoelastic properties of human pancreatic tumors and in vitro constructs to mimic mechanical properties. Acta Biomater 67, 331, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hoorn, H. van, Kurniawan, N.A., Koenderink, G.H., et al. Local dynamic mechanical analysis for heterogeneous soft matter using ferrule-top indentation. Soft Matter 12, 3066, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Canovic, E.P., Qing, B., Mijailovic, A.S., et al. . Characterizing multiscale mechanical properties of brain tissue using atomic force microscopy, impact indentation, and rheometry. J Vis Exp 115, e54201, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Caluori, G., Pribyl, J., Pesl, M., et al. . Advanced and rationalized atomic force microscopy analysis unveils specific properties of controlled cell mechanics. Front Physiol 9, 1121, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wirtz, D. Particle-tracking microrheology of living cells: principles and applications. Annu Rev Biophys 38, 301, 2009. [DOI] [PubMed] [Google Scholar]
- 59. Bonakdar, N., Gerum, R., Kuhn, M., et al. . Mechanical plasticity of cells. Nat Mater 15, 1090, 2016. [DOI] [PubMed] [Google Scholar]
- 60. Discher, D.E., Janmey, P., and Wang, Y.-L.. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139, 2005. [DOI] [PubMed] [Google Scholar]
- 61. Legant, W.R., Miller, J.S., Blakely, B.L., et al. . Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nat Methods 7, 969, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Legant, W.R., Pathak, A., Yang, M.T., et al. . Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues. Proc Natl Acad Sci U S A 106, 10097, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vogel, V., and Sheetz, M.. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol 7, 265, 2006. [DOI] [PubMed] [Google Scholar]
- 64. Huh, D., Matthews, B.D., Mammoto, A., et al. . Reconstituting organ-level lung functions on a chip. Science 328, 1662, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gordon, A.M., Huxley, A.F., and Julian, F.J.. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 184, 170, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wessendorf, A.M., and Newman, D.J.. Dynamic understanding of human-skin movement and strain-field analysis. IEEE Trans Biomed Eng 59, 3432, 2012. [DOI] [PubMed] [Google Scholar]
- 67. Cohen, S.R., and Kalfon-Cohen, E.. Dynamic nanoindentation by instrumented nanoindentation and force microscopy: a comparative review. Beilstein J Nanotechnol 4, 815, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhu, X., Cirovic, S., Shaheen, A., et al. . Investigation of fullerenol-induced changes in poroelasticity of human hepatocellular carcinoma by AFM-based creep tests. Biomech Model Mechanobiol 17, 665, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cameron, A.R., Frith, J.E., and Cooper-White, J.J.. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials 32, 5979, 2011. [DOI] [PubMed] [Google Scholar]
- 70. Bauer, A., Gu, L., Kwee, B., et al. . Hydrogel substrate stress-relaxation regulates the spreading and proliferation of mouse myoblasts. Acta Biomater 62, 82, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McKinnon, D.D., Domaille, D.W., Cha, J.N., et al. . Biophysically defined and cytocompatible covalently adaptable networks as viscoelastic 3D cell culture systems. Adv Mater 26, 865, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ingber, D.E. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci 104, 613, 1993. [DOI] [PubMed] [Google Scholar]
- 73. Ingber, D.E. Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci 116, 1157, 2003. [DOI] [PubMed] [Google Scholar]
- 74. Bennett, M., Cantini, M., Reboud, J., et al. . Molecular clutch drives cell response to surface viscosity. Proc Natl Acad Sci U S A 115, 1192, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gong, Z., Szczesny, S.E., Caliari, S.R., et al. . Matching material and cellular timescales maximizes cell spreading on viscoelastic substrates. Proc Natl Acad Sci U S A 115, E2686, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chan, C.E., and Odde, D.J.. Traction dynamics of filopodia on compliant substrates. Science 322, 1687, 2008. [DOI] [PubMed] [Google Scholar]
- 77. Case, L.B., and Waterman, C.M.. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat Cell Biol 17, 955, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Elosegui-Artola, A., Trepat, X., and Roca-Cusachs, P.. Control of mechanotransduction by molecular clutch dynamics. Trends Cell Biol 28, 356, 2018. [DOI] [PubMed] [Google Scholar]
- 79. Swaminathan, V., and Waterman, C.M.. The molecular clutch model for mechanotransduction evolves. Nat Cell Biol 18, 459, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mitchison, T., and Kirschner, M.. Cytoskeletal dynamics and nerve growth. Neuron 1, 761, 1988. [DOI] [PubMed] [Google Scholar]
- 81. Barriga, E.H., and Mayor, R.. Adjustable viscoelasticity allows for efficient collective cell migration. Semin Cell Dev Biol 93, 55, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Adebowale, K., Gong, Z., Hou, J.C., et al. . Enhanced substrate stress relaxation promotes filopodia-mediated cell migration. Nat Mater 20, 1290, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lee, H.-P., Alisafaei, F., Adebawale, K., et al. . The nuclear piston activates mechanosensitive ion channels to generate cell migration paths in confining microenvironments. Sci Adv 7, eabd4058, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Li, C., Wang, L., Yang, Z., et al. . A viscoelastic chitosan-modified three-dimensional porous poly(L-lactide-co-ɛ-caprolactone) scaffold for cartilage tissue engineering. J Biomater Sci Polym Ed 23, 405, 2012. [DOI] [PubMed] [Google Scholar]
- 85. Lee, N.-H., Bayaraa, O., Zechu, Z., et al. . Biomaterials-assisted spheroid engineering for regenerative therapy. BMB Rep 54, 356, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ho, S.S., Keown, A.T., Addison, B., et al. . Cell migration and bone formation from mesenchymal stem cell spheroids in alginate hydrogels are regulated by adhesive ligand density. Biomacromolecules 18, 4331, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Whitehead, J., Griffin, K.H., Gionet-Gonzales, M., et al. . Hydrogel mechanics are a key driver of bone formation by mesenchymal stromal cell spheroids. Biomaterials 269, 120607, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lee, H.-P., Gu, L., Mooney, D.J., et al. . Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat Mater 16, 1243, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Moore, E.M., Maestas, D.R., Comeau, H.Y., et al. . The immune system and its contribution to variability in regenerative medicine. Tissue Eng Part B Rev 27, 39, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Piotto, C., Julier, Z., and Martino, M.M.. Immune regulation of tissue repair and regeneration via miRNAs—new therapeutic target. Front Bioeng Biotechnol 6, 98, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Vining, K.H., Marneth, A.E., Adu-Berchie, K., et al. . Mechanical checkpoint regulates monocyte differentiation in fibrotic matrix. Blood 138, 2539, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gonzalez-Pujana, A., Vining, K.H., Zhang, D.K.Y., et al. Multifunctional biomimetic hydrogel systems to boost the immunomodulatory potential of mesenchymal stromal cells. Biomaterials 257, 120266, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rübsam, M., Mertz, A.F., Kubo, A., et al. . E-cadherin integrates mechanotransduction and EGFR signaling to control junctional tissue polarization and tight junction positioning. Nat Commun 8, 1250, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ghajar, C.M., and Bissell, M.J.. Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: insights from imaging. Histochem Cell Biol 130, 1105, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nonomura, K., Lukacs, V., Sweet, D.T., et al. . Mechanically activated ion channel PIEZO1 is required for lymphatic valve formation. Proc Natl Acad Sci U S A 115, 12817, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Seong, J., Tajik, A., Sun, J., et al. . Distinct biophysical mechanisms of focal adhesion kinase mechanoactivation by different extracellular matrix proteins. Proc Natl Acad Sci U S A 110, 19372, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Alexandrova, A.Y., Arnold, K., Schaub, S., et al. . Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow. PLoS One 3, e3234, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhou, D.W., Lee, T.T., Weng, S., et al. . Effects of substrate stiffness and actomyosin contractility on coupling between force transmission and vinculin-paxillin recruitment at single focal adhesions. Mol Biol Cell 28, 1901, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Elosegui-Artola, A., Bazellières, E., Allen, M.D., et al. . Rigidity sensing and adaptation through regulation of integrin types. Nat Mater 13, 631, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Huebsch, N., Arany, P.R., Mao, A.S., et al. . Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater 9, 518, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rezania, A., and Healy, K.E.. Biomimetic peptide surfaces that regulate adhesion, spreading, cytoskeletal organization, and mineralization of the matrix deposited by osteoblast-like cells. Biotechnol Prog 15, 19, 1999. [DOI] [PubMed] [Google Scholar]
- 102. Kunschmann, T., Puder, S., Fischer, T., et al. . The small GTPase Rac1 increases cell surface stiffness and enhances 3D migration into extracellular matrices. Sci Rep 9, 7675, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Connelly, J.T., Gautrot, J.E., Trappmann, B., et al. . Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat Cell Biol 12, 711, 2010. [DOI] [PubMed] [Google Scholar]
- 104. Elosegui-Artola, A., Andreu, I., Beedle, A.E.M., et al. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171, 1397, 2017. [DOI] [PubMed] [Google Scholar]
- 105. Jahed, Z., Domkam, N., Ornowski, J., et al. . Molecular models of LINC complex assembly at the nuclear envelope. J Cell Sci 134, jcs258194, 2021. [DOI] [PubMed] [Google Scholar]
- 106. Sachs, F. Stretch-activated ion channels: what are they? Physiology (Bethesda) 25, 50, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Agarwal, P., Lee, H.-P., Smeriglio, P., et al. . A dysfunctional TRPV4-GSK3β pathway prevents osteoarthritic chondrocytes from sensing changes in extracellular matrix viscoelasticity. Nat Biomed Eng 5, 1472, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lee, H., Stowers, R., and Chaudhuri, O.. Volume expansion and TRPV4 activation regulate stem cell fate in three-dimensional microenvironments. Nat Commun 10, 529, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pathak, M.M., Nourse, J.L., Tran, T., et al. . Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci U S A 111, 16148, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gordon, W.R., Zimmerman, B., He, L., et al. . Mechanical allostery: evidence for a force requirement in the proteolytic activation of Notch. Dev Cell 33, 729, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cao, L., Arany, P., Kim, J., et al. . Modulating Notch signaling to enhance neovascularization and reperfusion in diabetic mice. Biomaterials 31, 9048, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Contakos, S.P., Gaydos, C.M., Pfeil, E.C., et al. . Subdividing the embryo: a role for Notch signaling during germ layer patterning in Xenopus laevis. Dev Biol 288, 294, 2005. [DOI] [PubMed] [Google Scholar]
- 113. Shih, W., and Yamada, S.. N-cadherin as a key regulator of collective cell migration in a 3D environment. Cell Adh Migr 6, 513, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bian, L., Guvendiren, M., Mauck, R.L., et al. . Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc Natl Acad Sci U S A 110, 10117, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Loh, C.-Y., Chai, J.Y., Tang, T.F., et al. . The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: signaling, Therapeutic implications, and challenges. Cells 8, E1118, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Chronopoulos, A., Thorpe, S.D., Cortes, E., et al. . Syndecan-4 tunes cell mechanics by activating the kindlin-integrin-RhoA pathway. Nat Mater 19, 669, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Tan, X., Jain, E., Barcellona, M.N., et al. . Integrin and syndecan binding peptide-conjugated alginate hydrogel for modulation of nucleus pulposus cell phenotype. Biomaterials 277, 121113, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ghosh, P., Rameshbabu, A.P., and Dhara, S.. Citrate cross-linked gels with strain reversibility and viscoelastic behavior accelerate healing of osteochondral defects in a rabbit model. Langmuir 30, 8442, 2014. [DOI] [PubMed] [Google Scholar]
- 119. Sato, T., and Clevers, H.. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340, 1190, 2013. [DOI] [PubMed] [Google Scholar]
- 120. Shansky, J., Del Tatto, M., Chromiak, J., et al. . A simplified method for tissue engineering skeletal muscle organoids in vitro. In Vitro Cell Dev Biol Anim 33, 659, 1997. [DOI] [PubMed] [Google Scholar]
- 121. Balikov, D.A., Neal, E.H., and Lippmann, E.S.. Organotypic neurovascular models: past results and future directions. Trends Mol Med 26, 273, 2020. [DOI] [PubMed] [Google Scholar]
- 122. Prior, N., Inacio, P., and Huch, M.. Liver organoids: from basic research to therapeutic applications. Gut 68, 2228, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Alsberg, E., Kong, H.J., Hirano, Y., et al. . Regulating bone formation via controlled scaffold degradation. J Dent Res 82, 903, 2003. [DOI] [PubMed] [Google Scholar]
- 124. Simmons, C.A., Alsberg, E., Hsiong, S., et al. . Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone 35, 562, 2004. [DOI] [PubMed] [Google Scholar]
- 125. Bryant, S.J., and Anseth, K.S.. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res 59, 63, 2002. [DOI] [PubMed] [Google Scholar]
- 126. Khetan, S., Guvendiren, M., Legant, W.R., et al. . Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater 12, 458, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cruz-Acuña, R., Quirós, M., Farkas, A.E., et al. . Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat Cell Biol 19, 1326, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gjorevski, N., Sachs, N., Manfrin, A., et al. . Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560, 2016. [DOI] [PubMed] [Google Scholar]
- 129. Ingber, D.E. Is it time for reviewer 3 to request human organ chip experiments instead of animal validation studies? Adv Sci 7, 2002030, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Aisenbrey, E.A., and Murphy, W.L.. Synthetic alternatives to Matrigel. Nat Rev Mater 5, 539, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Moncal, K.K., Gudapati, H., Godzik, K.P., et al. . Intra-operative bioprinting of hard, soft, and hard/soft composite tissues for craniomaxillofacial reconstruction. Adv Funct Mater 31, 2010858, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Tibbitt, M.W. Bioprinting within live animals. Nat Biomed Eng 4, 851, 2020. [DOI] [PubMed] [Google Scholar]
- 133. Elosegui-Artola, A., Gupta, A., Najibi, A.J., et al. Matrix viscoelasticity controls spatio-temporal tissue organization. bioRxiv 10.1101/2022.01.19.476771v1, 2022. [DOI] [Google Scholar]
- 134. Suwardi, A., Wang, F., Xue, K., et al. . Machine learning-driven biomaterials evolution. Adv Mater 34, e2102703, 2022. [DOI] [PubMed] [Google Scholar]
- 135. Echeverria Molina, M.I., Malollari, K.G., and Komvopoulos, K.. Design challenges in polymeric scaffolds for tissue engineering. Front Bioeng Biotechnol 9, 617141, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]