Abstract
Significance:
Skin inevitably heals with the formation of a fibrotic scar. Patients affected by skin scarring suffer from long-term psychological and physical burdens.
Recent Advances:
Since the discovery of fetal scarless skin-wound healing, research has hoped to identify and mimic scarless healing for adult skin. Oral mucosa healing in adults provides the closest example to fetal scarless healing. Injuries to the oral mucosa heal with very minimal scarring. Understanding the mechanisms through which this process occurs may bring us closer to achieving scarless healing in adults.
Critical Issues:
In this review, we summarize the current evidence that illustrates distinct mechanisms involved in oral mucosal healing. We discuss the role of the oral niche in contributing to wound repair. The intrinsic properties of immune cells, fibroblasts, and keratinocytes within the oral mucosa that support regenerative repair are provided. We highlight the contribution of cytokines, growth factors, and chemokine secretion in permitting a scarless mucosal environment. Furthermore, we discuss the role of stem cell-like progenitor populations in the mucosa that may contribute to wound healing. We also provide suggestions for future studies that are needed to achieve scarless healing in adults.
Future Directions:
Many characteristics of the oral mucosa have been shown to contribute to decreased scarring, but the specific mechanism(s) is unclear. Advancing our understanding of oral healing may yield therapeutic therapies that can be used to overcome dermal scarring.
Keywords: oral mucosa, dermal fibrosis, scarless healing, wound healing, dermal fibroblast

Derrick C. Wan, MD
SCOPE AND SIGNIFICANCE
Patients acquire scars following many types of injuries, including mechanical trauma, burns, or surgical procedures.1 Scar formation is a consequence of the body's reparative process.2 Unfortunately, scarring causes a profound psychological and physical impact on patients. There is a spectrum of scar formation, and scarless healing and pathological scar formation reside at opposite ends of this spectrum.2 This article focuses on outlining our current understanding of regenerative oral mucosal healing. From this review, clinicians and basic scientists will gain knowledge about the differences in the wound healing process involved in scarring and scarless healing.
TRANSLATIONAL RELEVANCE
Two scenarios have been identified in mammals wherein wounds heal with significantly reduced or absent scarring. These include early gestation fetal tissues and the oral mucosal cavity. Extensive research has been undertaken for deciphering models of regenerative wound healing, but despite the huge clinical burden of scarring, no therapy to date has been shown to effectively mimic this process.3 An improved understanding of the mechanism by which the oral mucosa heals is vital for the development of treatments that can ensure wounds heal with minimal scarring.
CLINICAL RELEVANCE
Scarring has a broad impact, affecting millions of patients.4 Scar prevention and treatment is mainly supportive, creating a challenging problem for patients and surgeons to manage.4 The identification of a drug treatment that may promote/recapitulate the scarless healing of oral mucosa would revolutionize the management of scars and significantly improve the lives of patients.
OVERVIEW
Human skin repairs itself following an injury through a wound healing process, which results in the formation of fibrotic scar tissue.5 Most commonly, the outcome of defect repair is fibrosis, caused by the deposition of abnormally organized connective tissues.2 Fibrosis has been shown to account for 45% of all deaths in the United States, representing a huge medical and economic burden.4 Skin scarring can result in substantial functional and esthetic burdens for patients, especially in the craniofacial region.1 Children are especially prone to the effects of scarring and can develop long-term psychological damage due to surgical or burn scars.
The ability to create skin injuries without scar formation would revolutionize the management of lacerations, incisions, and burns, significantly decreasing patient morbidity. Thus, having access to wound therapies that can result in tissue regeneration without scarring would be of great clinical interest. In adult mammals, the healing of the oral mucosa provides the closest illustration of regenerative healing. It is widely accepted that skin wounds in adults heal with the generation of scar tissue, while oral mucosal wounds heal rapidly and with minimal scar formation.6 Despite the oral mucosa progressing through the same stages of wound healing as skin, the healing of mucosal wounds is akin to that of fetal tissues, with minimal scarring and rapid remodeling.
More recently, fibroblasts have been shown to display functional heterogeneity, with specific subpopulations showing a predisposition for connective tissue deposition, re-epithelization, or hair follicle formation.5 The characterization of specific fibroblast subpopulations has led to advances in our understanding of the wound healing process. Deciphering key fibroblast subpopulations in the oral mucosa that are responsible for regenerative wound healing provides hope for understanding the biology of wound healing and improving aberrant wound repair.
This review provides an update on the evidence underlying the mechanism by which oral mucosa regenerates following a dermal injury. Special attention will be paid to the differences observed between the wound healing phases of mucosa and those of the skin that may account for the “protected environment” of the oral cavity. We will further outline the progress made in overcoming and treating skin scarring through knowledge gained in our understanding oral mucosal healing.
ORAL MUCOSA ANATOMY AND FUNCTION
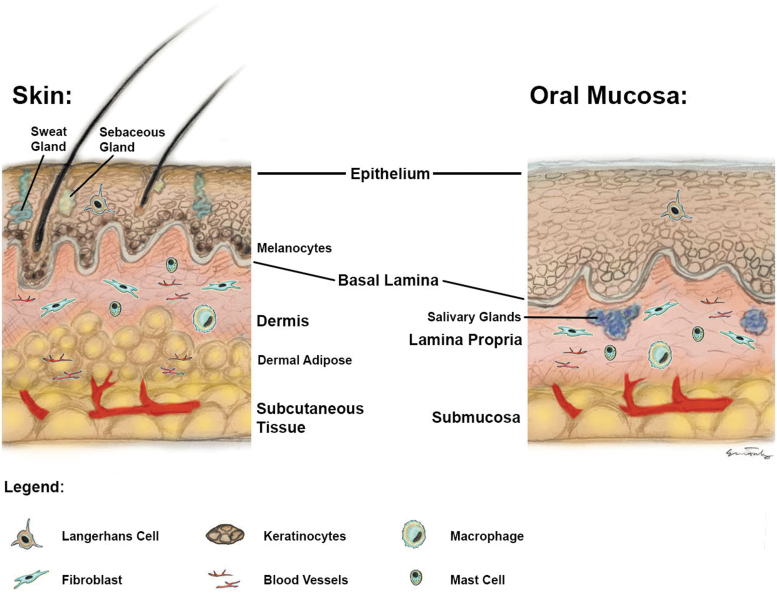
Oral mucosa is the term used to describe the soft lining of the oral cavity. The components of the oral cavity, including the gingivae and the buccal mucosa, consist of many layers which are similar to those of the skin. There are three distinct layers to the oral mucosa, namely the oral epithelium, the lamina propria, and the submucosa.7 The submucosa is attached to the underlying muscle or bone. The oral mucosa is predominantly comprised of nonkeratinized stratified squamous epithelium. However, the areas exposed to frequent mechanical insult are keratinized to resist friction, including the palatal and gingival mucosa.8 The dermis of the skin is equivalent to the lamina propria of the mucosa (Fig. 1).8 This layer provides support to the epidermis and participates in wound remodeling. The epithelium of the mucosa may be thicker than skin. In contrast, skin contains multiple adnexa, whereas the mucosa contains only salivary glands. The mucosa also serves as a protective barrier for mechanical and microbiological insults.9
Figure 1.
Schematic diagram highlighting the similarities and differences in histology between structure of skin and mucosa.
Understanding the mechanism by which mucosa heals with minimal scar
The mechanism by which oral mucosa heals with minimal scar has been intensively studied. Studies examining the difference between mucosa and skin healing can be broadly divided into three avenues of research. First, although mucosa and skin share a similar architecture, there are clear environmental differences that may play a role in wound healing. Notably, the role of saliva has been found to be beneficial for wound repair in the oral cavity.10 In addition, to the lubricating properties of salvia, it contains a diverse mixture of proteins, which influence immune function and cell function. The antimicrobial peptide histatin found within saliva has been linked to accelerated closure of oral wounds.10 The salivary component leptin has also been shown to increase keratinocyte proliferation, thus aiding wound closure.11 Although, an increasing amount of evidence suggests that saliva is important for wound repair, a detailed understanding of its role is still required. Another unique property of the oral cavity is the diverse microbiota that it harbors, being the second most complex after the gut microbiome.12 The saliva provides nutrients for microbial growth. The resident bacteria have both pro- and anti-inflammatory activities to maintain homeostasis in the oral cavity.13 Evidence has shown that if the balance of the oral microbiota is altered, predominant pathogens can lead to oral diseases, including periodontitis and oral mucositis.14 Furthermore, disturbance of the oral microbiome has been shown to decrease oral mesenchymal cell activity and impair normal oral wound repair in mice.15 Despite the importance of the oral microbiome in maintaining oral health, the specific bacteria types responsible for pro- or anti-inflammatory actions are unknown.
In contrast, emerging evidence has suggested that a specific population of cells drives the preferential regenerative outcome within the oral cavity. Transplantation studies have shown that significantly reduced scarring may be more attributable to the innate characteristics of cells and not the environment. Extraoral tissue transplantation into the oral cavity has been shown to produce a scar, and the tissue remains histologically distinct from mucosal tissue.16,17 Furthermore, transplanting mouse dorsal fibroblasts into the oral buccal mucosa cavity of mice creates a scar while oral mucosal fibroblasts placed into dorsal skin wounds results in minimal scar formation.5 These studies suggest that the intrinsic properties of the cells in the oral mucosa may account for reduced scarring seen within the mouth.5
The developmental origin of the oral mucosa may also contribute to the reduced scarring observed in the oral cavity.18 The dermis is derived from different embryonic origins, with the abdominal dermis being derived from the lateral plate mesoderm, the dorsal dermis from the paraxial mesoderm and the face and oral cavity from the neural crest.18 A recent study compared the transcriptome of mouse skin from the abdomen, back, and cheek, representing the three distinct embryonic tissues, during homeostasis and wound repair.19 RNA sequencing highlighted anatomical variation in response to wounding across the regions and upregulation of embryonic precursor markers in the cheek.19 Furthermore, histological analysis of the cheek wounds healed with decreased collagen production than the abdomen. This study highlights that the intrinsic transcriptional activity of neural crest-derived fibroblasts may be an important factor in driving differences in wound healing in the oral cavity that warrants investigation.
Lastly, several studies have concluded that the differences between wound healing in the oral mucosa and that observed in skin may be caused by disparities in the well-described wound healing phases. Wound healing for both skin and oral mucosa involves the four traditional overlapping phases: hemostasias, inflammation, proliferation, and remodeling. Several studies have demonstrated a difference in the mucosa and skin during the inflammation and proliferative phases.20,21
Comparison of the inflammation phase in oral and skin wounds
During the inflammatory phase, the innate immune system is activated by the rapid migration of neutrophils and monocytes. Over the first 2–5 days of wound healing, neutrophils remove pathogens and cell debris by phagocytosis from the wound.22 During this time, monocytes also differentiate into macrophages to support healing and to aid the transition into the proliferative stage. A defect in neutrophil recruitment leads to frequent and repetitive wound infections.23 Conversely, excess neutrophil migration leads to impaired wound healing, as can be seen in conditions such as inflammatory bowel disorders.23 In light of this, Szpaderska et al. compared the inflammatory cell infiltrate and cytokine production in mouse oral mucosa and skin 1 mm punch wounds.20 Oral mucosal wounds on the dorsal surface of the tongue and dorsal skin wounds were compared for 72 h. They observed lower levels of macrophage, neutrophil, and T cell infiltration in the oral wounds with less inflammatory cytokine secretion, including interleukin-6 (IL-6) and other keratinocyte-derived factors. These findings suggest that a diminished inflammatory response is a key feature of oral mucosal wound healing.21
Studies with porcine oral mucosal and skin wounds have also demonstrated significantly improved wound healing in mucosal wounds than corresponding similar-sized skin wounds grossly and histologically.24 Oral full-thickness wounds were created in the mucosal gingiva of the hard palate and compared with dorsal skin wounds. The oral wounds showed a decreased number of macrophages and mast cells (MCs) as compared with dermal wounds. However, the specific subset of macrophages present during wound healing may be as important as the absolute number of macrophages for determining regenerative versus fibrotic outcomes.24
Early macrophage responders, or “M1” macrophages, secrete proinflammatory cytokines, including IL-1, IL-6, and tumor necrosis factor-α during the inflammatory phase. Alternatively, as the proliferation phase continues, macrophages switch to the alternative “M2” phenotype. These cells produce anti-inflammatory cytokines and growth factors, including transforming growth factor-β (TGF-β), IL-10, and vascular endothelial growth factor (VEGF). The persistence of “M1” macrophages, with incomplete switch to the M2 phenotype leads to impaired healing.25 A comparison of human oral and facial skin scars has demonstrated that the contribution to healing by M1 and M2 macrophage subtypes may be different.26 Total macrophages were lower in the oral mucosa than in skin. Although there were no differences observed in the M1 subset, lower levels of M2 macrophages were found in the mucosa compared with the skin.26
MCs are immune cells of the hematopoietic lineage and provide the first line of defense against antigens entering the body due to their location in the skin and mucosa.27 MCs also release proinflammatory mediators and growth factors throughout the inflammatory phase, and an increased number of MCs has been associated with skin scarring. Human MCs have been classified into at least two subtypes, that is, tryptase and chymase-positive MCs.28 However, the precise roles and types of MCs that contribute to the healing of mucosa or skin wounds remain relatively unknown.24 To date, only one study has examined their difference in MC activity in the skin and oral mucosa. Glim et al., observed no significant difference in the MC number from human normal and scared oral palatal mucosal and abdominal skin.26
Beyond these differences in numbers of infiltrating cells, secretion of cytokines, chemokines, and growth factors that are responsible for mediating inflammatory responses might also contribute to the regenerative healing of oral mucosa. In a study by Chen et al., oral mucosal 1 mm wounds were placed on the lateral side of the tongue, and skin wounds were created in the dorsal skin.29 The microarray analysis of paired mouse oral and skin wounds over 10 days further confirmed a notable difference in the expression of proinflammatory and inflammation-related elements.29 Cytokines identified early following wounding, such as IL-23, IL-24, colony-stimulating factor, interferon-α, and interferon-β, were highly expressed in skin wounds but not in mucosal wounds.29 In addition to the differences in these proinflammatory cytokines, several additional factors have been found to be differentially expressed between skin and mucosal wounds. Among these, matrix metalloproteinases (MMPs), a family of zinc-dependent endopeptidases (MMP 1a, MMP 1b, MMP 8, MMP 9, MMP 10, and MMP 13P) that regulates inflammation, degradation of extracellular matrix (ECM), and migration of cells, were observed to be differentially upregulated in early skin wounds but not oral mucosal wounds. These data suggest that cells in the skin respond to wounds with a significant MMP response, which may contribute to the divergent response of the skin and mucosa to dermal injury.29 Furthermore, other chemokines were found to vary between these two sites. In particular, chemokines responsible for neutrophil attraction, such as chemokine (C-X-C motif) ligand (CXCL)3, C-C motif chemokine ligand 20, CXCL7, and CXCL13, were primarily present in skin wounds.29
Finally, TGF-β is a multifunctional growth factor with a critical role in wound healing.30 Due to proinflammatory properties of TGF-β1 signaling, it has also been widely implicated in tissue scarring, and the overexpression of TGF-β1 has been associated with excessive ECM accumulation and scar formation.30 On the other hand, TGF-β3 has been shown in some preclinical studies to be antifibrotic, with topical application shown to improve wound healing outcomes.31 Prolonged expression of TGF-β3 and αvβ6 integrin was also observed in pig oral mucosal wounds compared with skin.32 Therefore, spatial and temporal activity of TGF-β signaling contributed to the enhanced wound repair observed in the oral cavity.32 Of note, clinical studies have not shown any benefit with TGF-β modulation, and the examination of known additional fibrotic signaling pathways will be an important future step for appreciating the mechanism of wound repair in the oral mucosa.
Comparison of the proliferation phase in oral and skin wounds
The proliferative phase of wound repair starts as the inflammation response subsides. An important element of the proliferative phase is the restoration of blood vessels to ensure the delivery of oxygen and nutrients for effective tissue repair. The process of new blood vessel formation is initiated by growth factors, such as the VEGF.33 Early gestation fetal skin wounds heal rapidly and are less vascular than adult wounds. Similarly, oral murine wounds have decreased wound-bed vascularity than comparative skin wounds. Furthermore, oral tongue mucosal mouse wounds have shown lower levels of VEGF expression than dorsal skin wounds by keratinocytes.20 Related to this, skin dorsal mouse wounds have been found to be more hypoxic than oral mucosal tongue wounds and hypoxia-inducible factor1-α, a regulator of hypoxic response in oxygen-deprived tissues, has been shown to be greater in skin.34 However, the manipulation of oxygen levels using hyperbaric oxygen treatment has not shown to ameliorate this difference in murine excisional dorsum skin and tongue wounds. Thus, the differential responses of the skin and oral mucosa to hypoxia may underlie the distinctive wound healing phenotype of the two anatomical regions.34 In summary, the process of angiogenesis in oral mucosal wounds may be differentially regulated as compared with that in the skin.
Melanocytes are melanin-producing cells that originate from the neural crest.35 Melanocytes provide protection from stressors such as ultraviolet radiation, free radicals, and reactive oxygen species.35 These cells have been described to migrate in wound during the proliferative phase and potentially interact with fibroblasts.36 Melanocytes in skin have been shown to express markers such as corticotrophin and proopiomelanocortin, which may mediate local antimicrobial and immune actions.35 However, the contribution of oral melanocytes in response to wound repair requires greater exploration.
PHENOTYPIC DIFFERENCES BETWEEN ORAL AND SKIN FIBROBLASTS
One clear distinction between mucosa and skin is the difference in fibroblasts and keratinocytes within the tissue itself. A comparison of human-derived mucosal and skin fibroblasts has illustrated distinct in vitro characteristics. Lee and Eun compared human skin and oral fibroblasts in collagen gels in vitro for 5 days, observing greater proliferation and weaker contractive ability among oral fibroblasts.37 Human oral mucosal fibroblasts have also shown delayed senescence secondary to long telomere lengths. The embryonic or fetal-like phenotype associated with the long telomere lengths may be responsible for wounds in the mucosa getting repaired in a manner with less scar production.38 Furthermore, the secretome of oral mucosal fibroblasts has been shown to improve wound healing in the skin by promoting migration and proliferation of fibroblasts as well as angiogenesis.3 Murine skin dorsal excisional wounds treated with conditioned media from oral gingival mucosal fibroblasts showed a significant reduction in wound area and histological dermal wound width along with accelerated re-epithelization over 14 days.3
Traditionally, fibroblasts have been considered a static, homogeneous population of cells that maintain the ECM. However, recent studies have now shown that fibroblasts within the skin are composed of functionally heterogeneous subpopulations. Guerrero-Juarez et al. demonstrated that, in large dorsal skin murine wounds, 12 subpopulations of fibroblasts may exist.39 Furthermore, a subpopulation of fibroblasts with myeloid features differentiates into myofibroblasts in wounds.39 In contrast, no study has examined the specific subpopulations within the oral mucosa. Nonetheless, it is likely that there is considerable fibroblast heterogeneity within the oral mucosa along with varied functional roles.
The migration of both fibroblasts and keratinocytes into the wound bed is an important part of regenerative wound healing.39 Several reports have revealed differences in the migratory pattern of oral and skin-derived wound cells. Human buccal fibroblasts have been found to express greater levels of hepatocyte growth factor compared with dermal foreskin fibroblasts, which may account for accelerated wound closure in the oral cavity.40 Molecular profiling of human 3 mm punch biopsies from the cheek of the buccal mucosa and arm skin wounds has further displayed differences in the migratory pattern of oral keratinocytes.41 The sex-determining region Y-box 2 (SOX2) and the paired-like homeodomain 1 (PITX1) transcriptional regulators have been noted to be upregulated in oral wounds as compared with skin wounds. Reprogramming skin keratinocytes with SOX2 and PITX2 increased cell migration and accelerated wound healing in vivo.41 The same study also found that specific transcriptional networks that are activated upon wounding are already present in the unwounded oral mucosa, suggesting that the mucosa may be primed to respond to injury. These findings are consistent with the mucosa undergoing frequent traumatic events (e.g., biting) thereby necessitating adaptations to promote repair. Paralleling this, Uchiyama et al., similarly reported that the overexpression of SOX2 in skin keratinocytes promoted cell migration and the re-epithelization of mouse excisional skin wounds.42
Finally, the ECM provides structural support to cells within the skin and mucosa, allowing for cell adhesion, cell–cell interactions, and migration. Fibroblasts and keratinocytes within the two tissue sites are supported by the ECM and specific composition may significantly contribute to variability in wound healing observed. An analysis of the ECM components of human abdominal skin and palatal oral mucosa demonstrated that mucosal ECM mimics that of fetal skin, with tenascin (TN-C), hyaluronan, fibronectin, and chondroitin sulfate highly expressed, as also seen during embryogenesis.9 A greater level of TN-C has been observed in the palatal mucosa than in the skin for both pigs and humans.43 Hyaluronan acid synthase-1 is also highly expressed in human oral fibroblasts compared with skin fibroblasts.44 These findings suggest that the regenerative wound-healing phenotype in the oral cavity may be partly modulated by the properties of the ECM, which may affect the function of associated fibroblasts and keratinocytes.
STEM CELL POTENTIAL OF THE ORAL MUCOSA
Increasing evidence has shown that an oral progenitor or “stem-like” population of cells may reside within the oral mucosa, which may contribute to the reduced scarring observed during wound repair. To date, these stem cell-like populations have been identified in both the dermal and epithelial layer. The oral progenitor stem cells within the dermal layer have been labeled with numerous names, including oral mucosal lamina propria progenitor cells, human oral mucosa stem cells, and gingiva mesenchymal stem cells. Despite the differences in cell surface marker expression, all populations have shown a high proliferation rate in vitro and the capacity to differentiate down several mesenchymal (bone fat and cartilage) and neuronal lineages.45 Studies have confirmed that oral progenitors express known mesenchymal stem cell markers, including CD90, CD105, and CD73, but remain negative for hematopoietic or fibrocyte cell surface markers (Table 1). Furthermore, the genomic analysis of these progenitor populations has revealed upregulation of several neural crest markers, including Slug, Snail, Twist1, and Sox10.45–50 A more recent study suggested that the progenitor population may exist within the epithelial layer. An oral epithelial mouse progenitor population found within the basal layer, defined by expression of Bmi1, has been shown to divide rapidly and be capable of asymmetrical population divisions and alterations of daughter cell fates in response to exogenous cues.51 Paralleling these findings, Byrd et al. characterized a dividing stem cell population residing within the epithelial junctional zone niche characterized by expression of insulin-like growth factor-binding protein 5 (IGFBP5) that could self-renew, respond to masticatory stress, and promote intraoral wound healing.52 More recently, Caetano et al. profiled the gene transcriptome of the gingiva from four patients with periodontitis and two healthy controls using single-cell RNA sequencing. Although a small data set was analyzed, the study identified an additional basal progenitor cell population expressing homeodomain-only protein homeobox and IGFBP5.53 Moving forward, understanding the specific subpopulation(s) of the cells that are responsible for the regenerative response within the oral mucosa will be of utmost importance.
Table 1.
Properties of “progenitor” stem cell populations identified in the oral mucosa
| Name | Positive markers | Negative markers | Lineage potential | References |
|---|---|---|---|---|
| GMSCs | Stro-1, CD29, CD44, CD73, CD90, CD105, CD146, CD166, SSEA-4. | CD14, CD34, CD45 | Chondrogenic, osteogenic, adipogenic, neuronal | 45 |
| OMLP-PCs | CD44, CD90, CD105, CD166, Sox10, Snail, Slug, Twist | CD34, CD45 | Chondrogenic, osteogenic, adipogenic, and neuronal | 46 |
| GT-MSCs | CD44, CD29, CD73, CD90, CD105. | CD14, CD34, CD45 | Chondrogenic, osteogenic, and adipogenic | 48 |
| GMPCs | CD29, CD44, CD73, CD90, CD104, CD146, Stro1. | CD34, CD45, CD117, CD200, CD271 | Chondrogenic, osteogenic, and adipogenic | 47 |
| pNC-SCs | Nestin, Sox2, p75, ABCG2, Slug, Twist, Sox9, Notch1, c-Myc, Kl4, Oct4. | GFAP | Neuronal | 49 |
| hOMSCs | CD29, CD73, CD90, CD105, CD106 CD166, Stro1, Oct4, Sox2, Nanog, P75, SSEA4, Tra2–54, Tra2–49 | CD34, CD45, nestin, tubulin, SSE1, SSEA1, Tra1–60, Tra1–81 | Chondrogenic, osteogenic, adipogenic, and neuronal | 50 |
Reproduced with permission from Stephens et al.7
GMPCs, gingiva-multipotent progenitor cells; GMSCs, gingiva-derived mesenchymal stem/stromal cells; GT-MSCs, gingiva-derived mesenchymal stem cells; hOMSCs, human oral mucosal stem cells; OMLP-PCs, oral mucosal lamina propria progenitor cells; pNC-SCs, palatal neural crest-related stem cells.
The oral cavity has also been found to harbor cell populations that have the capacity to be reprogrammed into induced pluripotent stem cells (iPSCs). Buccal human mucosal fibroblasts have been shown to contain cell populations that express Oct3/4, Sox2, Nanog, and Kl4.54 Similarly, gingival mouse oral fibroblasts have been shown to express Sox2 and Oct3/4, making both of these cells attractive candidates for generation of iPSCs.55 Further studies are required to understand the effectiveness of specific mucosal subpopulation(s) for iPSC generation to allow for future clinical translation.
THERAPEUTICS THAT MIMIC MUCOSAL REPAIR
Effective scar treatment therapies for the skin are limited. The investigation of novel therapies based on the wound repair process of oral mucosa holds promise for developing effective antifibrotic treatments. Inspired by wound healing characteristics of the oral mucosa, Kong et al.,56 designed a bioinspired hydrogel that simulated the physiological environment of oral mucosa. Epidermal growth factor (EGF) was embedded in chitosan and sodium alginate microcapsules to formulate biomimetic gels. The choice to use EGF was based on their analysis of growth factors that were upregulated in oral wounds of rats as compared with skin wounds. Importantly, treatment of rat skin dermal excisional wounds with EGF hydrogels for 7 days demonstrated a decreased inflammatory response and decreased ECM deposition compared with untreated controls. Similarly, the release of EGF from chitosan scaffolds was described to increase soft tissue regeneration at 3 days in beagle palatal mucosal defects.57 With more detailed molecular profiling of the oral mucosa, expansion of novel biomaterials with integrated growth factors may thus facilitate development of novel treatments for skin scarring.
Alternatively, studies have aimed to identify molecular targets that may be responsible for the regenerative healing of the oral mucosa or scarring of the skin to overcome dermal fibrosis. For example, Simoes et al. examined the role of microRNAs in wound repair by comparing their expression in murine dorsal skin wounds and oral palatal wounds. MicroRNAs have been shown to regulate many physiological and disease processes, although their role in wound healing is not clear.58 At day 5 postwounding, miR-21 became the most abundant microRNA in the skin, suggesting its vital role in skin repair.58 Furthermore, inhibition of miR-21 through a single dose led to significant acceleration of dermal wounds in mice over 10 days, highlighting the therapeutic potential of microRNAs.58 In the future, identification and investigation into the key growth factors or cytokines that may contribute to scarring in the skin that are not highly expressed in the oral mucosa, for example, MMPs,29 may offer additional potential antifibrotic drug targets.
FUTURE OUTLOOK
There is strong evidence to suggest that the oral mucosa displays decreased inflammatory and angiogenic responses following wounding, as compared with skin, with consequent promotion of more regenerative outcomes. The role of macrophages and neutrophils within oral and skin wounds has been thoroughly studied,21 and both immune cell types show reduced presence in healing mucosa relative to skin. In contrast, the exact role of T cells has not yet been understood. However, one study has found a decrease in T cells in oral wounds as compared with those in skin.20 Thus, in-depth studies that delineate inflammatory signaling pathways responsible for the fibrotic response of the skin and antifibrotic behavior of the mucosa are warranted. Several studies have focused on the role of TGF-β in oral wounds.30 However, despite understanding the role of TGF-β signaling in wound repair, effective clinical translation of these findings has to date been limited.
The architectures of both the oral mucosa and cutaneous membrane are very similar and provide a promising comparative tool to identify potential therapeutic targets to overcome skin fibrosis. However, there are some critical differences that should be accounted for in future studies. The palatal and gingival region consist of a keratinized epithelium similarly to the skin, whereas the buccal oral mucosa consists of a nonkeratinized epithelium.59 The buccal mucosa is also distinct from the gingiva and palate as it is not attached to any underlying bone.60 Identification of mechanism accounting for the regenerative healing of the oral mucosa should take into consideration different locations and linings of the oral cavity to enhance clinical translation.61
The identification of a stem-like cell population within the oral mucosa that may facilitate regeneration following an injury is promising for several reasons.7 First, the mucosa offers a reliably accessible site relative to bone marrow, from which cells may be derived for potential regenerative strategies.7 Second, the isolation process would lead to minimal scarring for the donor.7 Lastly, scaling/expansion of these populations may be more straightforward as compared with the heterogeneous population of bone marrow-derived stromal cells.7 A few promising studies56,57 have explored using biomaterials to mimic the regenerative properties of the oral mucosa to improve skin repair, but the optimal material and growth factor require further exploration.
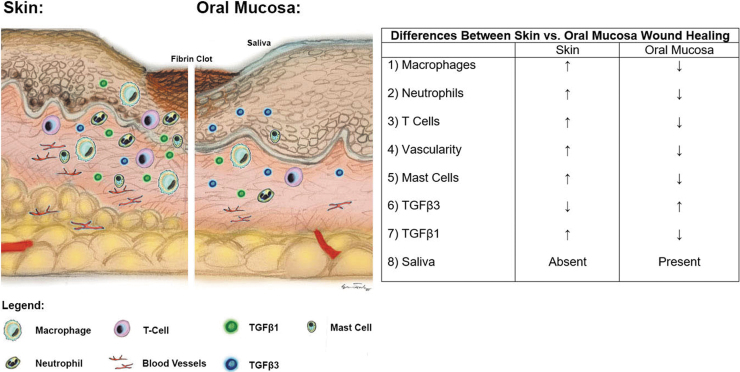
Several mechanisms have been proposed to account for the regenerative healing of the oral mucosa compared with the fibrotic response of skin following injury (Fig. 2). Although some molecular profiling of oral mucosal wound healing has been conducted, a detailed synopsis of the genomic signature during the different wound healing phases, including hemostasis, inflammation, and proliferation, has not been completed. Overall, the precise mechanism by which the oral mucosa drives regeneration, as compared with the scarring behavior of the dermis following an injury, remains unknown, with further investigation required.
Figure 2.
Schematic diagram to highlight healing of mucosa and skin following dermal injury. Oral mucosa heals with minimal scarring compared with skin. Mucosa responds to injury with a reduced inflammatory and angiogenic response compared with skin. There is decreased macrophage, neutrophil, and T cell infiltrate observed in mucosal wounds compared with skin wounds. TGF-β1 signaling is greater in skin compared with mucosal wounds. TGF-β3 signaling is greater in mucosal compared with skin wounds. TGF-β1, transforming growth factor-β1; TGF-β3, transforming growth factor-β3.
SUMMARY
Wound repair within the oral mucosa heals with minimal scarring. Evidence highlights those key differences exist in wound healing between the anatomical sites, such as delayed inflammation and a muted angiogenic response. Further exploration into elucidating the mechanism of mucosal healing may allow for the development of antiscarring therapies to improve the lives of many who are affected by scarring.
TAKE HOME MESSAGES
In adult mammals, the oral mucosa provides a model of regenerative healing, with minimal scar formation following an injury.
The oral mucosa demonstrates a diminished inflammatory response compared with skin, which may contribute to a reduction in scar formation.
Several progenitor-like cell populations have been identified in the oral mucosa, which participate in healing with reduced scarring.
Understanding the mechanism by which the oral mucosa heals may provide therapeutic treatments to prevent or overcome skin scarring.
Abbreviations and Acronyms
- CXCL
chemokine (C-X-C motif) ligand
- ECM
extracellular matrix
- EGF
epidermal growth factor
- GMPCs
gingiva-multipotent progenitor cells
- GMSCs
gingiva-derived mesenchymal stem/stromal cells
- GT-MSCs
gingiva-derived mesenchymal stem cells
- hOMSC
human oral mucosa stem cell
- IGFBP5
insulin-like growth factor-binding protein 5
- IL
interleukin
- iPSC
induced pluripotent stem cell
- MC
mast cell
- MMP
matrix metalloproteinases
- OMLP-PC
oral mucosal lamina propria progenitor cell
- PITX
paired-like homeodomain
- pNC-SCs
palatal neural crest-related stem cells
- SOX2
sex-determining region Y-box 2
- TN-C
tenascin
- TGF-β
transforming growth factor-β
- TGF-β1
transforming growth factor-β1
- TGF-β3
transforming growth factor-β3
- VEGF
vascular endothelial growth factor
AUTHORS' CONTRIBUTIONS
M.F.G. reviewed the literature, designed the review, contributed to writing, and finalized the article. E.J.H., M.K., N.G., K.C., D.B.D., and C.V.L. all contributed to reviewing and writing of the article. M.T.L., H.P.L., and D.C.W. oversaw the design of the review article. All authors approved the final article.
ACKNOWLEDGMENTS AND FUNDING SOURCES
Michelle F. Griffin was supported by the Fulbright Scholar Program. Michael T. Longaker was supported by the NIH grant R01 GM116892, RO1 GM 136659, Gunn/Olivier Research Fund, Stinehart-Reed Fund, and the NIH grant U01 HL099776. Derrick C. Wan was supported by NIH DE027346. H. Peter Lorenz was supported by the NIH grant R01 GM116892.
AUTHOR DISCLOSURE AND GHOSTWRITING
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
ABOUT THE AUTHORS
Michelle F. Griffin, Evan J. Fahy, and Darren B. Abbas are plastic surgery research fellows in Dr. Longaker, Dr. Wan, and Dr. Lorenz's laboratories at Stanford University. Megan King, Nicholas Guardino, and Christopher V. Lavin are research assistants in Dr. Longaker, Dr. Wan, and Dr. Lorenz's laboratories at Stanford University. Dr. Longaker, Dr. Wan, and Dr. Lorenz are Professors of Plastic and Reconstructive Surgery at Stanford University. Dr. Wan and Dr. Lorenz are Professors of Plastic and Reconstructive Surgery at Stanford University.
REFERENCES
- 1. Sen CK, Gordillo GM, Roy S, et al. . Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314–321. [DOI] [PubMed] [Google Scholar]
- 3. Ahangar P, Mills SJ, Smith LE, Gronthos S, Cowin AJ. Human gingival fibroblast secretome accelerates wound healing through anti-inflammatory and pro-angiogenic mechanisms. NPJ Regen Med 2020;5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol 2004;4:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rinkevich Y, Walmsley GG, Hu MS, et al. . Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 2015;348:aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Larjava H, Wiebe C, Gallant-Behm C, Hart DA, Heino J, Hakkinen L. Exploring scarless healing of oral soft tissues. J Can Dent Assoc 2011;77:b18. [PubMed] [Google Scholar]
- 7. Stephens P Davies LC. Oral mucosal progenitor cells. In: Vishwakarma A, Sharpe P, Songtao S, Ramalingam M, eds. Stem Cell Biology and Tissue Engineering in Dental Sciences. Cambridge, MA: Academic Press, 2014, pp. 297–306. [Google Scholar]
- 8. Glim JE, van Egmond M, Niessen FB, Everts V, Beelen RH. Detrimental dermal wound healing: what can we learn from the oral mucosa? Wound Repair Regen 2013;21:648–660. [DOI] [PubMed] [Google Scholar]
- 9. Glim JE, Everts V, Niessen FB, Ulrich MM, Beelen RH. Extracellular matrix components of oral mucosa differ from skin and resemble that of foetal skin. Arch Oral Biol 2014;59:1048–1055. [DOI] [PubMed] [Google Scholar]
- 10. Oudhoff MJ, van den Keijbus PA, Kroeze KL, et al. . Histatins enhance wound closure with oral and non-oral cells. J Dent Res 2009;88:846–850. [DOI] [PubMed] [Google Scholar]
- 11. Frank S, Stallmeyer B, Kampfer H, Kolb N, Pfeilschifter J. Leptin enhances wound re-epithelialization and constitutes a direct function of leptin in skin repair. J Clin Invest 2000;106:501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caselli E, Fabbri C, D'Accolti M, et al. . Defining the oral microbiome by whole-genome sequencing and resistome analysis: the complexity of the healthy picture. BMC Microbiol 2020;20:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van ‘t Hof W, Veerman EC, Nieuw Amerongen AV, Ligtenberg AJ. Antimicrobial defense systems in saliva. Monogr Oral Sci 2014;24:40–51. [DOI] [PubMed] [Google Scholar]
- 14. Struzycka I. The oral microbiome in dental caries. Pol J Microbiol 2014;63:127–135. [PubMed] [Google Scholar]
- 15. Su Y, Chen C, Guo L, Du J, Li X, Liu Y. Ecological balance of oral microbiota is required to maintain oral mesenchymal stem cell homeostasis. Stem Cells 2018;36:551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reilly JS, Behringer WH, Trocki I. Intraoral keloid: complication of forehead flap. Otolaryngol Head Neck Surg (1979) 1980;88:139–141. [DOI] [PubMed] [Google Scholar]
- 17. Bussi M, Valente G, Curato MP, Carlevato MT, Cortesina G. Is transposed skin transformed in major head and neck mucosal reconstruction? Acta Otolaryngol 1995;115:348–351. [DOI] [PubMed] [Google Scholar]
- 18. Griffin MF, desJardins-Park HE, Mascharak S, Borrelli MR, Longaker MT. Understanding the impact of fibroblast heterogeneity on skin fibrosis. Dis Model Mech 2020;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Usansky I, Jaworska P, Asti L, et al. . A developmental basis for the anatomical diversity of dermis in homeostasis and wound repair. J Pathol 2021;253:315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szpaderska AM, Walsh CG, Steinberg MJ, DiPietro LA. Distinct patterns of angiogenesis in oral and skin wounds. J Dent Res 2005;84:309–314. [DOI] [PubMed] [Google Scholar]
- 21. Szpaderska AM, Zuckerman JD, DiPietro LA. Differential injury responses in oral mucosal and cutaneous wounds. J Dent Res 2003;82:621–626. [DOI] [PubMed] [Google Scholar]
- 22. Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res 2012;49:35–43. [DOI] [PubMed] [Google Scholar]
- 23. Leoni G, Neumann PA, Sumagin R, Denning TL, Nusrat A. Wound repair: role of immune-epithelial interactions. Mucosal Immunol 2015;8:959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mak K, Manji A, Gallant-Behm C, et al. . Scarless healing of oral mucosa is characterized by faster resolution of inflammation and control of myofibroblast action compared to skin wounds in the red Duroc pig model. J Dermatol Sci 2009;56:168–180. [DOI] [PubMed] [Google Scholar]
- 25. Sindrilaru A, Peters T, Wieschalka S, et al. . An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest 2011;121:985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Glim JE, Beelen RH, Niessen FB, Everts V, Ulrich MM. The number of immune cells is lower in healthy oral mucosa compared to skin and does not increase after scarring. Arch Oral Biol 2015;60:272–281. [DOI] [PubMed] [Google Scholar]
- 27. Krystel-Whittemore M, Dileepan KN, Wood JG. Mast cell: a multi-functional master cell. Front Immunol 2015;6:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saito H, Matsumoto K, Okumura S, et al. . Gene expression profiling of human mast cell subtypes: an in silico study. Allergol Int 2006;55:173–179. [DOI] [PubMed] [Google Scholar]
- 29. Chen L, Arbieva ZH, Guo S, Marucha PT, Mustoe TA, DiPietro LA. Positional differences in the wound transcriptome of skin and oral mucosa. BMC Genomics 2010;11:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schrementi ME, Ferreira AM, Zender C, DiPietro LA. Site-specific production of TGF-beta in oral mucosal and cutaneous wounds. Wound Repair Regen 2008;16:80–86. [DOI] [PubMed] [Google Scholar]
- 31. Flanders KC, Burmester JK. Medical applications of transforming growth factor-beta. Clin Med Res 2003;1:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eslami A, Gallant-Behm CL, Hart DA, et al. . Expression of integrin alphavbeta6 and TGF-beta in scarless vs scar-forming wound healing. J Histochem Cytochem 2009;57:543–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech 2003;60:107–114. [DOI] [PubMed] [Google Scholar]
- 34. Chen L, Gajendrareddy PK, DiPietro LA. Differential expression of HIF-1alpha in skin and mucosal wounds. J Dent Res 2012;91:871–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peters A. The self-similarity of the melanocortin system. Endocrinology 2005;146:529–531. [DOI] [PubMed] [Google Scholar]
- 36. Gao FL, Jin R, Zhang L, Zhang YG. The contribution of melanocytes to pathological scar formation during wound healing. Int J Clin Exp Med 2013;6:609–613. [PMC free article] [PubMed] [Google Scholar]
- 37. Lee HG, Eun HC. Differences between fibroblasts cultured from oral mucosa and normal skin: implication to wound healing. J Dermatol Sci 1999;21:176–182. [DOI] [PubMed] [Google Scholar]
- 38. Enoch S, Wall I, Peake M, et al. . Increased oral fibroblast lifespan is telomerase-independent. J Dent Res 2009;88:916–921. [DOI] [PubMed] [Google Scholar]
- 39. Guerrero-Juarez CF, Dedhia PH, Jin S, et al. . Single-cell analysis reveals fibroblast heterogeneity and myeloid-derived adipocyte progenitors in murine skin wounds. Nat Commun 2019;10:650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stephens P, Davies KJ, Occleston N, et al. . Skin and oral fibroblasts exhibit phenotypic differences in extracellular matrix reorganization and matrix metalloproteinase activity. Br J Dermatol 2001;144:229–237. [DOI] [PubMed] [Google Scholar]
- 41. Iglesias-Bartolome R, Uchiyama A, Molinolo AA, et al. . Transcriptional signature primes human oral mucosa for rapid wound healing. Sci Transl Med 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Uchiyama A, Nayak S, Graf R, et al. . SOX2 epidermal overexpression promotes cutaneous wound healing via activation of EGFR/MEK/ERK signaling mediated by EGFR ligands. J Invest Dermatol 2019;139:1809–1820.e1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wong JW, Gallant-Behm C, Wiebe C, et al. . Wound healing in oral mucosa results in reduced scar formation as compared with skin: evidence from the red Duroc pig model and humans. Wound Repair Regen 2009;17:717–729. [DOI] [PubMed] [Google Scholar]
- 44. Yamada Y, Itano N, Hata K, Ueda M, Kimata K. Differential regulation by IL-1beta and EGF of expression of three different hyaluronan synthases in oral mucosal epithelial cells and fibroblasts and dermal fibroblasts: quantitative analysis using real-time RT-PCR. J Invest Dermatol 2004;122:631–639. [DOI] [PubMed] [Google Scholar]
- 45. Tang L, Li N, Xie H, Jin Y. Characterization of mesenchymal stem cells from human normal and hyperplastic gingiva. J Cell Physiol 2011;226:832–842. [DOI] [PubMed] [Google Scholar]
- 46. Davies LC, Lonnies H, Locke M, et al. . Oral mucosal progenitor cells are potently immunosuppressive in a dose-independent manner. Stem Cells Dev 2012;21:1478–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fournier BP, Ferre FC, Couty L, et al. . Multipotent progenitor cells in gingival connective tissue. Tissue Eng Part A 2010;16:2891–2899. [DOI] [PubMed] [Google Scholar]
- 48. Tomar GB, Srivastava RK, Gupta N, et al. . Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem Biophys Res Commun 2010;393:377–383. [DOI] [PubMed] [Google Scholar]
- 49. Widera D, Zander C, Heidbreder M, et al. . Adult palatum as a novel source of neural crest-related stem cells. Stem Cells 2009;27:1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Marynka-Kalmani K, Treves S, Yafee M, et al. . The lamina propria of adult human oral mucosa harbors a novel stem cell population. Stem Cells 2010;28:984–995. [DOI] [PubMed] [Google Scholar]
- 51. Jones KB, Furukawa S, Marangoni P, et al. . Quantitative clonal analysis and single-cell transcriptomics reveal division kinetics, hierarchy, and fate of oral epithelial progenitor cells. Cell Stem Cell 2019;24:183–192 e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Byrd KM, Piehl NC, Patel JH, et al. . Heterogeneity within stratified epithelial stem cell populations maintains the oral mucosa in response to physiological stress. Cell Stem Cell 2019;25:814–829 e816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Caetano AJ, Yianni V, Volponi A, Booth V, D'Agostino EM, Sharpe P. Defining human mesenchymal and epithelial heterogeneity in response to oral inflammatory disease. Elife 2021;10:e62810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Miyoshi K, Tsuji D, Kudoh K, et al. . Generation of human induced pluripotent stem cells from oral mucosa. J Biosci Bioeng 2010;110:345–350. [DOI] [PubMed] [Google Scholar]
- 55. Egusa H, Okita K, Kayashima H, et al. . Gingival fibroblasts as a promising source of induced pluripotent stem cells. PLoS One 2010;5:e12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kong X, Fu J, Shao K, Wang L, Lan X, Shi J. Biomimetic hydrogel for rapid and scar-free healing of skin wounds inspired by the healing process of oral mucosa. Acta Biomater 2019;100:255–269. [DOI] [PubMed] [Google Scholar]
- 57. Park KM, Lee HJ, Koo KT, et al. . Oral soft tissue regeneration using nano controlled system inducing sequential release of trichloroacetic acid and epidermal growth factor. Tissue Eng Regen Med 2020;17:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Simoes A, Chen L, Chen Z, et al. . Differential microRNA profile underlies the divergent healing responses in skin and oral mucosal wounds. Sci Rep 2019;9:7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Squier CA, Kremer MJ, Wertz PW. Effect of ethanol on lipid metabolism and epithelial permeability barrier of skin and oral mucosa in the rat. J Oral Pathol Med 2003;32:595–599. [DOI] [PubMed] [Google Scholar]
- 60. Liu J, Bian Z, Kuijpers-Jagtman AM, Von den Hoff JW. Skin and oral mucosa equivalents: construction and performance. Orthod Craniofac Res 2010;13:11–20. [DOI] [PubMed] [Google Scholar]
- 61. Squier CA, Kremer MJ. Biology of oral mucosa and esophagus. J Natl Cancer Inst Monogr 2001;7–15. DOI: 10.1093/oxfordjournals.jncimonographs.a003443. [DOI] [PubMed] [Google Scholar]