Salmonella
is a major cause of foodborne disease outbreaks worldwide, mainly through poultry. Recently, there has been an increase in multidrug-resistant (MDR) Salmonella infections globally. The increased drug resistance results in increased costs and poorer health outcomes due to unavailability or delayed treatment. This study aims to determine the prevalence of Salmonella in retail raw chicken meat and identify their antimicrobial resistance profiles. A total of 270 retail raw chicken carcasses (local and imported) were collected from three hypermarket chains in Qatar between November 2017 and April 2018. Thirty carcasses were contaminated with Salmonella (11.11%). The prevalence of Salmonella in locally produced chicken was higher than in imported chicken (OR = 2.56, 95% CI: 1.18–5.53, p = 0.016). No significant differences were found between the prevalence and storage temperature or hypermarket chain. The highest resistance rates in the isolates were reported to tetracycline (73.7%) followed by nitrofurantoin (53.3%), ampicillin (50%), amoxicillin-clavulanic acid, ceftriaxone (26.7%), and ciprofloxacin (23.3%). Eight isolates were potential extended-spectrum β-lactamase-producers, all in imported frozen chicken (p < 0.0001). Additionally, 43.3% of the isolates were MDR and associated with frozen chicken (OR = 16.88, 95% CI: 2.55–111.47, p = 0.002). The findings indicate that while the prevalence of Salmonella in retail chicken in Qatar is moderate, a large proportion of them are MDR.
Keywords: Salmonella, retail chicken, foodborne, AMR
Introduction
Salmonella (S) is one of the most common zoonotic foodborne pathogens that cause outbreaks and sporadic cases of gastroenteritis in humans throughout the world.1 The genus consists of two species, S. bongori and S. enterica. The latter is divided into seven subspecies (arizonae, diarizonae, enterica, houtenae, indica, salamae, and subspecies VII).2 The majority of salmonellosis cases are due to S. enterica subsp. enterica. It contains over 2500 serovars (based on serological typing of the O-and H-antigens), most of which cause diseases in humans.2–5
The main reservoirs for Salmonella are the gastrointestinal tracts of livestock animals, which may lead to the contamination of food products.6 As such, salmonellosis is typically associated with the handling and consuming food of animal origin. Salmonellosis is among the most common foodborne infections worldwide, constituting a significant healthcare and economic burden.7 Chickens are thought to be the leading risk factor for human salmonellosis, as they are frequently asymptomatically colonized by non-typhoidal Salmonella (i.e., serovars other than Typhi and Paratyphi).8
While salmonellosis is typically self-limiting, Salmonella can cause invasive disease, which requires the use of antibiotics. Resistance to ampicillin, chloramphenicol, and sulfamethoxazole-trimethoprim has been reported in Salmonella for many years, which shifted treatment toward fluoroquinolones and extended-spectrum cephalosporins.9 However, recent years have seen an increase in multidrug-resistant (MDR) Salmonella infections, further increasing the significance of this pathogen and its healthcare burden.10
Salmonella is not naturally transformable, meaning that it can only develop antimicrobial resistance (AMR) through mutations or acquisition from both within the genus and with other genera.11–13 Animal housing conditions and antimicrobial use in farms are thought to facilitate AMR dissemination between Salmonella species and the acquisition of genes from other related species.14 Additionally, the conditions in poultry processing facilities can promote the growth of Salmonella biofilms on both biotic and abiotic surfaces, which in turn may promote AMR acquisition.15 Evidence indicates that poultry act as a reservoir for antimicrobial-resistant Salmonella and links to human antimicrobial-resistant salmonellosis.6
Antimicrobial-resistant bacteria, including Salmonella, can be transmitted from animals to humans or vice-versa by direct contact or indirectly through animal products and the environment.16 The interconnectedness of humans, animals, and the environment in AMR transmission increases the need to adopt a one health approach. Food products represent a link between humans and animals. Thus, they are a potential source of transmission and bioaccumulation.17 Moreover, a potential Salmonella transmission link between poultry and humans has been previously reported.18–21 MDR Escherichia coli have been reported previously in Qatar in both humans and animals, including chicken and sheep.22–25 To complete the picture, studies on AMR in food are necessary. In this study, we investigate the prevalence of Salmonella in retail chicken and report its AMR profile.
Materials and Methods
Sample collection
The retail chicken carcasses used in this study were described in our previous study.24 Briefly, a total of 270 chicken carcasses were used in this study were collected from two branches of each of three major hypermarket chains (designated A, B, and C) in Doha and Al-Rayyan municipalities in Qatar from November 2017 to April 2018 using a stratified random sampling method (Table 1). The retail chicken samples were transferred in cooled boxes (4°C–8°C) to the Qatar University Biomedical Research Center (Doha, Qatar) laboratories. Upon arrival, the samples were stored at 4°C and processed within 24 hrs. Information on the store name, location, storage temperature (chilled or frozen), collection date, source (local or imported), producer, and sell-by date (or expiration date) were recorded. Research approval to process samples was obtained from Qatar University's Institutional Biohazard Committee under approval number QU (QU-IBC-2018/034).
Table 1.
Number and Location of the Chicken Meat Samples Collected from Hypermarket Stores by Storage Temperature, Chicken Source, and Municipality in Qatar (n = 270)
| Store | Storage temperature | Sourcea | Number of samples (Doha) | Number of samples (AL-Rayyan) |
|---|---|---|---|---|
| Hypermarket A | Chilled | Local | 15 | 15 |
| Chilled | Imported | 15 | 15 | |
| Frozen | Imported | 15 | 15 | |
| Hypermarket B | Chilled | Local | 15 | 15 |
| Chilled | Imported | 15 | 15 | |
| Frozen | Imported | 15 | 15 | |
| Hypermarket C | Chilled | Local | 15 | 15 |
| Chilled | Imported | 15 | 15 | |
| Frozen | Imported | 15 | 15 | |
| Total | 135 | 135 |
There were no frozen local chicken available in the hypermarkets. All local chicken sold at the time of the study was chilled.
Salmonella isolation and identification
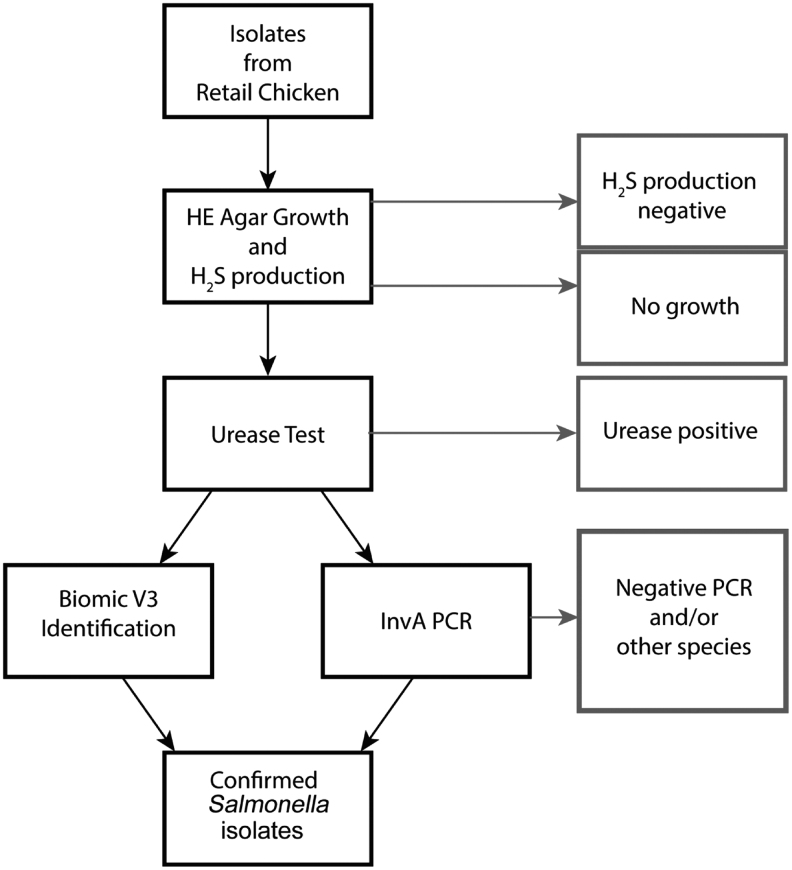
The Salmonella isolation and identification process is summarized in Fig. 1. Each chicken carcass was soaked in 250 mL of sterilized buffered peptone water (HiMedia, Mumbai, India), vigorously shaken for 1 min, and incubated at 37°C for 30 min at 200 rpm. Then, 50 mL of the rinsate was inoculated into 50 mL of sterile selenite cystine broth (HiMedia, Mumbai, India) for selective enrichment of Salmonella. The rinsate-broth mixture was incubated for 24 h at 37°C in a shaking incubator at 100 rpm. A loop-full (20 μL) of broth culture was then streaked onto Hektoen Enteric (HE) Agar (HiMedia) and incubated at 37°C for 16–20 h. HE agar is a selective and differential medium that selects Salmonella and Shigella. Salmonella is detected by the presence of black colonies due to the production of H2S.
FIG. 1.
Flowchart of the Salmonella isolation and identification process. The flow chart describes the sequential process that was used to determine whether a chicken carcass was contaminated with Salmonella. The red boxes on the right detail the exclusion criteria (i.e., sample is not contaminated by Salmonella) at each step of the process. First, the chicken carcasses were homogenized and mixed with SCB to enrich for Salmonella. Second, 20 μL of the SCB broth-homogenate mixture was streaked on HE agar to select for Salmonella and Shigella. HE agar differentiates between the two species through H2S production in Salmonella, which results in black colonies. The samples that did not have growth on HE agar or had growth without H2S production were determined not to be contaminated with Salmonella and excluded from subsequent steps. Third, a urease test was performed on the H2S-producing isolates. Salmonella is urease negative, as such, the urease positive were excluded from the next steps, and the chicken carcasses they came from were determined not to be contaminated with Salmonella. The last two steps in the process were PCR for the invA gene (A conserved gene in Salmonella) and biochemical identification with the Biomic V3 platform. The process resulted in identifying 30 retail chicken carcasses that were positive for Salmonella. SCB, selenite cystine broth; HE, Hektoen Enteric.
Three separate black colonies were picked from each Salmonella-positive plate, subcultured onto nutrient agar (HiMedia), and incubated at 37°C for 16–20 h. The urease test was then performed to confirm the identity. For each nutrient agar plate, two to three colonies were inoculated into a Urea broth tube, such that each tube corresponds to a nutrient agar plate. The broth tubes were then incubated at 35°C for 18–24 h. Salmonella is urease-negative; thus, no change in broth color would be observed. The identities of the urease negative isolates were then further confirmed to be Salmonella through polymerase chain reaction (PCR) of the conserved invasion A (invA) gene and biochemically using the Biomic V3 System (Giles Scientific) with the BBL CRYSTAL™ Enteric/Nonfermenter (E/NF) id KIT (BD).
For PCR, total DNA was extracted from the urease negative isolates using the QIAamp® UCP Pathogen mini kit (Qiagen, Germany) following the manufacturer's protocol. The PCR for invA was performed using the primers and procedure described by Naik et al.26 The PCR was performed on a Biometra TAdvanced thermocycler (Analyticjena, Germany), and the results were visualized through agarose gel electrophoresis.
It should be noted that the two methods used to confirm the Salmonella identity do not differentiate the serovars.
Antibiotic susceptibility testing
All Salmonella isolates obtained from retail chicken carcasses were tested for their susceptibility to a relevant panel of antibiotics. A single pure Salmonella colony from each nutrient agar plate was inoculated into a phosphate buffer solution (Atom Scientific) to achieve a 0.5 McFarland inoculum, measured by DensiCHEK PLUS (bioMérieux). The suspension was then swabbed onto a Mueller–Hinton agar plate (HiMedia) and allowed to dry completely. Next, antibiotic-impregnated discs (Liofilchem®, Roseto degli Abruzzi) were applied to the agar surface (up to 6 per plate) and incubated at 37°C for 24 hrs. The zone of inhibition was measured in mm and interpreted as per the Clinical and Laboratory Standards Institute (CLSI) guidelines.27
The antibiotic susceptibility panel included 13 disks: Ampicillin (AMP, 10 μg), Amoxicillin/Clavulanic acid (AMC, 20/10 μg), Tetracycline (TET, 30 μg), Piperacillin/Tazobactam (TZP, 100/10 μg), Ciprofloxacin (CIP, 5 μg), Trimethoprim/Sulfamethoxazole (SXT, 1.25/23.75 μg), Ceftriaxone (CRO, 30 μg), Cefepime (FEP, 30 μg), Fosfomycin (FOS, 200 μg), Nitrofurantoin (F, 300 μg), Ertapenem (ETP, 10 μg), and Meropenem (MRP, 10 μg). Colistin susceptibility was measured using E-test (Liofilchem) following the manufacturer's instructions to avoid the possible false-negative results with Colistin resistance. E. coli ATCC 25922 was used as a negative control. Non-susceptible isolates to any third-generation cephalosporins were considered potential extended-spectrum β-lactamase (ESBL) producers.
Data analysis
Statistical analysis was performed using R version 4.1.0.28 For statistical analysis, all intermediate resistances were recoded as susceptible. The χ2-test of independence was used to determine whether significant associations existed between the presence of Salmonella or phenotypic antibiotic resistances and storage temperature (chilled or frozen), chicken source (local or imported), hypermarket (A, B, or C), or municipality (Doha or Al-Rayyan). The Binomial test was used to determine whether the difference between the proportions of sensitive and resistant isolates per antibiotic is significant. The Goodman–Kurksal tau was used to measure pair-wise associations between the resistance phenotypes. A p-value <0.05 was considered statistically significant. All plots were generated using the ggplot2 version 3.3.3 and ggpubr version 0.4.0 packages in R.29,30
Results
Prevalence of Salmonella in retail chicken
Thirty retail chicken carcasses were positive for Salmonella as identified by invA PCR and Biomic V3 identification (Supplementary Fig. S1 and Supplementary Table S1). The total prevalence of Salmonella in chicken carcasses was 11.11%. Hypermarket A had the highest prevalence with 13.3% of the samples containing Salmonella, followed by hypermarkets B and C with 11.1% and 8.89%, respectively (Table 2). There was a significant difference between local and imported chicken, with higher odds in local chicken (OR = 2.56, 95% CI: 1.18–5.53, p = 0.016). All local chicken was chilled; however, no significant difference was observed when the storage temperatures were compared (10.6% vs. 12.2%; p = 0.84). The origins of the imported chicken samples included in the study were from Brazil for the frozen chicken and France, The Netherlands, Pakistan, and Turkey for the chilled samples. No Salmonella was isolated from chilled imported chicken from hypermarket B.
Table 2.
The Prevalence of Salmonella in Three Hypermarket Chains (A–C) in Qatar
| Chicken type | Number of samples positive for Salmonella (%)a |
|||
|---|---|---|---|---|
| A | B | C | Total | |
| Local chilled | 5 (5.56) | 7 (7.78) | 4 (4.44) | 16 (17.8) |
| Imported chilled | 1 (1.11) | 0 | 2 (2.22) | 3 (3.33) |
| Imported frozen | 6 (6.67) | 3 (3.33) | 2 (2.22) | 11 (12.2) |
| Total | 12 (13.3) | 10 (11.1) | 8 (8.89) | 30 |
Thirty specimens were collected from each of the three types of chicken from each hypermarket (15 from each municipality), for a total of 270 chicken specimen.
Phenotypic resistance profile of Salmonella in retail chicken
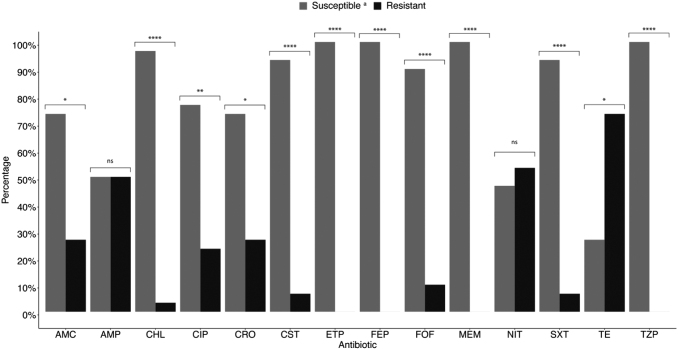
The antibiotic resistance profiles of the Salmonella isolated from the 30 retail chicken carcasses are summarized in Fig. 2 and Table 3. All the Salmonella isolates were susceptible to carbapenems, the 4th-generation cephalosporin cefepime, and the piperacillin-tazobactam. The highest resistance rate was to tetracycline (73.7%), followed by nitrofurantoin (53.3%) and ampicillin (50%). However, the difference between the proportion of chicken carcasses containing nitrofurantoin susceptible and resistant isolates was not statistically significant. Eight samples contained isolates that were resistant to ceftriaxone (26.7%), indicating ESBL producers. The Salmonella in all eight samples were also resistant to amoxicillin-clavulanic acid but susceptible to piperacillin-tazobactam and cefepime.
FIG. 2.
The Antibiotic resistance profiles in the Salmonella isolated from retail chicken carcasses in Qatar (n = 30). aIsolates were classified as susceptible if they were sensitive (S) or intermediate (I) with in vitro antibiotic susceptibility testing. A binomial test was performed to determine whether the difference in proportions between susceptible and resistant isolates is significant. ns: not significant (p > 0.05), *p ≤ 0.05, **p ≤ 0.01, ****p ≤ 0.0001. AMC, amoxicillin-clavulanic acid; AMP, ampicillin; CHL, chloramphenicol; CIP, ciprofloxacin; CRO, ceftriaxone; CST, colistin; ETP, ertapenem; FEP, Cefepime; FOF, Fosfomycin; MEM, meropenem; NIT, nitrofurantoin; SXT, sulfamethoxazole-trimethoprim; TE, tetracycline; TZP, piperacillin-tazobactam.
Table 3.
Antibiotic Resistance Rates in Salmonella Obtained from Retail Chicken Carcasses in Qatar
| Group | Drug | Percentage of chicken samples with resistant Salmonella (n) |
|---|---|---|
| Amphenicols | Chloramphenicol | 3.3% (1) |
| Beta-lactams/penicillins | Ampicillin | 50% (15) |
| Amoxicillin/clavulanic acid | 26.7% (8) | |
| Piperacillin/tazobactam | 0.0% (0) | |
| Cephalosporins (3rd gen.) | Ceftriaxone | 26.7% (8) |
| Cephalosporins (4th gen.) | Cefepime | 0.0% (0) |
| Carbapenems | Ertapenem | 0.0% (0) |
| Meropenem | 0.0% (0) | |
| Polymyxins | Colistin | 6.7% (2) |
| Quinolones | Ciprofloxacin | 23.3% (7) |
| Trimethoprims | Trimethoprim/sulfamethoxazole | 6.7% (2) |
| Tetracyclines | Tetracycline | 73.7% (22) |
| Other antibiotics | Fosfomycin | 10% (3) |
| Nitrofurantoin | 53.3% (16) |
Two samples contained isolates resistant to colistin (6.7%); each was obtained from a different hypermarket. Incidentally, both isolates were resistant to ciprofloxacin, tetracycline, and nitrofurantoin. However, they were susceptible to β-lactams. Approximately 93.3% of the samples contained isolates that were resistant to at least one antibiotic; 16.67%, 20%, 16.67%, 30%, 6.67%, and 3.33% contained isolates that were resistant to 1, 2, 3, 4, 5, and 6 antibiotics, respectively (Fig. 3). Thirteen chicken samples (43.33%) contained MDR Salmonella, defined as resistant to ≥3 antibiotic classes. A Goodman-Kruskal τ was measured for each pair of phenotypic resistances to assess the pair-wise associations (Supplementary Fig. S2). An association was observed between ceftriaxone and amoxicillin-clavulanic acid resistance (τ = 1).
FIG. 3.
The distribution of the number of phenotypic resistances to up to 14 antibiotics among the Salmonella isolates (n = 30) from retail chicken carcasses in Qatar. Multidrug resistance is defined as the resistance to ≥3 antibiotic classes.
Phenotypic resistance profile of Salmonella by storage temperature, hypermarket chain, and source
There was a significant difference between the proportions of resistance to amoxicillin-clavulanic acid and ceftriaxone between chilled and frozen chicken (p = 5 × 10−4) in addition to local and imported chicken (p = 4.7 × 10−5). All the isolates resistant to amoxicillin-clavulanic acid were also resistant to ceftriaxone (isolates aR127, aR215, aR217, aR218, cR104, cR231, cR232, and bD34). Additionally, all of these isolates were obtained from imported frozen chicken. No significant difference was found between the other antibiotics and storage temperature or source (Supplementary Fig. S3). Moreover, no significant difference was observed between the three hypermarket chains. As for MDR Salmonella, there was a significant difference between chilled and frozen chicken, with MDR being more likely in frozen chicken (OR = 16.88, 95% CI: 2.55–111.47, p = 0.002). No significant difference was observed between local and imported chicken and the hypermarkets.
Discussion
Antimicrobial overuse and misuse are the main contributing drivers in the development and the global spread of AMR.16 Many of the antimicrobials used to treat infections in humans are also used for treatment and prophylaxis in farm animals.16 Antimicrobial overuse may increase AMR in livestock, creating a farm to fork transmission path. Studies that compared AMR pathogens in animals, food, and humans found significant similarities in AMR genes and plasmids and, to a lesser extent, between the pathogens, indicating a route from animal to human through food (particularly poultry).18–21 Thus, adopting a one health approach in AMR surveillance is necessary. While various studies have been conducted to investigate the epidemiology of AMR in the human population in Qatar, data on livestock, food, and the environment is still limited.
Antimicrobial-resistant Salmonella is classified as a serious threat by the Centers for Disease Control (CDC) in the United States. Fluoroquinolone-resistant Salmonella is classified as a high-priority pathogen by the World Health Organization (WHO).31,32 Additionally, the main route for Salmonella infections is through contaminated food, particularly poultry products. To that extent, this study aimed to investigate the prevalence of Salmonella in retail chicken carcasses and their AMR profiles. We report on the prevalence, and AMR profiles of Salmonella isolates from retail chicken carcasses. These chicken samples were obtained from three hypermarket chains across the two most population-dense municipalities, Doha and Al-Rayyan.
The prevalence of Salmonella in retail chicken varies widely across the globe, ranging from as low as 2.7% in Brazil to 97.9% in Myanmar, with most recent reports in the range 25%–55%.33–41 In the present study, the prevalence of Salmonella was found to be 11.11%. No significant difference in the prevalence of Salmonella was found between the two municipalities or the three hypermarket chains. In contrast, the prevalence was significantly higher in local compared to imported chicken (OR = 2.56, 95% CI: 1.18–5.53, p = 0.016). All the local chicken in the study was chilled; however, no significant difference was found between the storage temperatures (chilled vs. frozen). This finding is in contrast to other studies that found a significantly higher prevalence in chilled chicken.36,42
The higher prevalence in chilled chicken in those studies could be attributed to the fluctuating storage temperatures associated with chilled chicken transport. The relation with temperature fluctuation is further supported by the higher prevalence of Salmonella in retail chicken in summer than winter, likely due to the considerable temperature difference between the environment and storage conditions.37 Another reason is that thawing frozen chicken may reduce the viability of Salmonella.36 Additionally, multiple studies found an association between the type of chicken production company and Salmonella prevalence. A study in Colombia found that integrated companies (those that own and control all stages of production) have a lower prevalence in the final chicken product than non-integrated companies.36 The difference is likely related to fluctuations in temperature during transport between the various involved entities and differences in quality and safety standards.
Twenty-eight of the 30 Salmonella contaminated retail chicken carcasses in this study (93.3%) had isolates resistant to at least one antibiotic. The highest resistance rate observed was to tetracycline (73.3%), commonly used in animal feed as prophylaxis and a growth promoter.16 The high rate of tetracycline-resistance is consistent with other reports that found an increase in resistance rates over the past few years.43 Other frequently overused antibiotics in animal feed and veterinary medicine include fluoroquinolones, cephalosporins, and colistin.16 Overuse of these antibiotics in animals is a key driver in the increase and spread of AMR. Of those antibiotics, fluoroquinolones, ampicillin, and amoxicillin-clavulanic acid are first-line drugs in treating salmonellosis.43
In this study, the resistance rates for the three antibiotics in contaminated chicken were 50%, 26.7%, and 23.3% for ampicillin, amoxicillin-clavulanic acid, and ciprofloxacin, respectively. The resistance rates for these antibiotics vary between regions.37,39,40 All the amoxicillin-clavulanic acid-resistant isolates are likely ESBL-producers, as indicated by their resistance to the third generation cephalosporin ceftriaxone. Notably, all eight ESBL-producers were isolated from imported frozen chicken. Of greater concern is that all the potential ESBL-producers, and the majority of ciprofloxacin-resistant isolates, are MDR (Supplementary Table S1). Thirteen chicken carcasses were contaminated with MDR Salmonella (43.3%). The MDR prevalence was significantly higher in frozen chicken (OR = 16.88, 95% CI: 2.55–111.47, p = 0.002). The prevalence of MDR found in our study is similar to a study performed in Brazil, which found that 53.2% of the Salmonella isolated from retail chicken were MDR.35 However, the same study also reported a 2.7% prevalence of Salmonella.
Nevertheless, several studies in Europe reported high rates of MDR Salmonella in chicken imported from Brazil, particularly serovar Heidelberg.44–46 These studies suggest that the food hygiene regulations need to be revised to curb the spread of MDR Salmonella. A more detailed genomic investigation is necessary to characterize the isolates better and elucidate whether a similar trend is occurring in Qatar.
Conclusions
Our findings revealed a moderate prevalence of Salmonella in retail chicken carcasses in Qatar, with 11.1% of retail chicken carcasses contaminated with Salmonella. However, the high rate of MDR (43.3% of the Salmonella isolates) and resistance to first-line drugs are of significant concern. These isolates can potentially be transmitted along the food chain to humans and eventually back into the environment and other animals.16 This highlights the importance of incorporating antimicrobial susceptibility testing in food hygiene monitoring. Interestingly, the locally produced chicken was associated with a higher prevalence of Salmonella. In contrast, the imported chicken was associated with a higher rate of MDR Salmonella. The association between imported chicken and MDR indicates a possible route of the global dissemination of AMR that emphasizes the need for an interdisciplinary and multicountry effort to tackle the issue on a global scale.
Supplementary Material
Acknowledgment
The authors would like to acknowledge the administrative staff for assisting in all the paperwork.
Authors' Contributions
All authors certify that they have participated sufficiently to take public responsibility for the content, including in the manuscript's concept, design, analysis, writing, or revision.
Conceptualization, N.O.E.; methodology, N.O.E., H.A., S.H.A., S.Z.A.; formal analysis, H.A., N.O.E., S.H.A.; resources, N.O.E., T.E.; data curation, N.O.E., H.A.,W.Q.A.; writing original draft preparation, H.A., S.H.A.; writing review and editing, N.O.E., W.Q.A., T.E.; project administration, N.O.E.; funding acquisition, N.O.E. All authors have read and agreed to the published version of the article.
Disclosure Statement
The authors declare no conflict of interest.
Funding Information
This research was funded by, Biomedical Research Centre, Qatar University, grant number “BRC-2018-ID-01 to Nahla O. Eltai.”
Supplementary Materials
References
- 1. Borges, K.A., T.Q. Furian, Borsoi A., H.L.S. Moraes, C.T.P. Salle, and V.P. Nascimento. 2013. Detection of virulence-associated genes in Salmonella enteritidis isolates from chicken in South of Brazil. Pesqui. Vet. Bras. 33:1416–1422. [Google Scholar]
- 2. Schoch, C.L., Ciufo S., Domrachev M., et al. 2020. NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database 2020:baaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Porwollik, S., E.F. Boyd, Choy C., et al. 2004. Characterization of Salmonella enterica Subspecies I Genovars by Use of Microarrays. J. Bacteriol. 186:5883–5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andino, A., and Hanning I.. 2015. Salmonella enterica: survival, colonization, and virulence differences among Serovars. Sci. World J. 2015:520179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention (CDC). 2013. An Atlas of Salmonella in the United States, 1968–2011: Laboratory-Based Enteric Disease Surveillance. US Department of Health and Human Services, CDC, Atlanta, GA. [Google Scholar]
- 6. Antunes, P., Mourão J., Campos J., and Peixe L.. 2016. Salmonellosis: the role of poultry meat. Clin. Microbiol. Infect. 22:110–121. [DOI] [PubMed] [Google Scholar]
- 7. Chlebicz, A., and K. Śliżewska. 2018. Campylobacteriosis, salmonellosis, yersiniosis, and listeriosis as zoonotic foodborne diseases: a review. Int. J. Environ. Res. Public. Health 15:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hugas, M., and Beloeil P.A.. 2014. Controlling Salmonella along the food chain in the European Union - progress over the last ten years. Eurosurveillance. 19:20804. [DOI] [PubMed] [Google Scholar]
- 9. Parry, C.M., and Threlfall E.J.. 2008. Antimicrobial resistance in typhoidal and nontyphoidal salmonellae. Curr. Opin. Infect. Dis. 21:531–538. [DOI] [PubMed] [Google Scholar]
- 10. Parisi, A., J.A. Crump, Glass K., et al. 2018. Health Outcomes from multidrug-resistant Salmonella infections in high-income countries: a systematic review and meta-analysis. Foodborne Pathog. Dis. 15:428–436. [DOI] [PubMed] [Google Scholar]
- 11. Lorenz, M.G., and Wackernagel W.. 1994. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol. Rev. 58:563–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bythwood, T.N., Soni V., Lyons K., et al. 2019. Antimicrobial Resistant Salmonella enterica Typhimurium Colonizing Chickens: the impact of plasmids, genotype, bacterial communities, and antibiotic administration on resistance. Front. Sustain. Food Syst. 3:20. [Google Scholar]
- 13. Sieckmann, D.G., N.D. Reed, and Georgi C.E.. 1969. Transferable drug resistance among Enterobacteriaceae isolated from human urinary tract infections. Appl. Microbiol. 17:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Noyes, N.R., K.M. Benedict, S.P. Gow, et al. 2016. Modelling considerations in the analysis of associations between antimicrobial use and resistance in beef feedlot cattle. Epidemiol. Infect. 144:1313–1329. [DOI] [PubMed] [Google Scholar]
- 15. Cadena, M., Kelman T., M.L. Marco, and Pitesky M.. 2019. Understanding antimicrobial resistance (AMR) profiles of Salmonella biofilm and planktonic bacteria challenged with disinfectants commonly used during poultry processing. Foods 8:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McEwen, S.A., and Collignon P.J.. 2018. Antimicrobial resistance: a one health perspective. Microbiol. Spectr. 6. DOI: 10.1128/microbiolspec.ARBA-0009-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Badau, E. 2021. A One Health perspective on the issue of the antibiotic resistance. Parasite. 28:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lazarus, B., D.L. Paterson, J.L. Mollinger, and Rogers B.A.. 2015. Do human extraintestinal Escherichia coli infections resistant to expanded-spectrum cephalosporins originate from food-producing animals? A systematic review. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 60:439–452. [DOI] [PubMed] [Google Scholar]
- 19. Johnson, J.R., M.R. Sannes, Croy C., et al. 2007. Antimicrobial drug-resistant Escherichia coli from humans and poultry products, Minnesota and Wisconsin, 2002–2004. Emerg. Infect. Dis. 13:838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jakobsen, L., Kurbasic A., Skjøt-Rasmussen L., et al. 2010. Escherichia coli isolates from broiler chicken meat, broiler chickens, pork, and pigs share phylogroups and antimicrobial resistance with community-dwelling humans and patients with urinary tract infection. Foodborne Pathog. Dis. 7:537–547. [DOI] [PubMed] [Google Scholar]
- 21. Willemsen, I., Oome S., Verhulst C., Pettersson A., Verduin K., and Kluytmans J.. 2015. Trends in Extended Spectrum Beta-Lactamase (ESBL) Producing Enterobacteriaceae and ESBL Genes in a Dutch Teaching Hospital, Measured in 5 Yearly Point Prevalence Surveys (2010–2014). PLoS One 10:e0141765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eltai, N.O., H.M. Yassine, A.A. Al Thani, et al. 2018. Prevalence of antibiotic resistant Escherichia coli isolates from fecal samples of food handlers in Qatar. Antimicrob. Resist. Infect. Control 7:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eltai, N.O., E.A. Abdfarag, Al-Romaihi H., et al. 2018. Antibiotic resistance profile of commensal Escherichia coli Isolated from broiler chickens in Qatar. J. Food Prot. 81:302–307. [DOI] [PubMed] [Google Scholar]
- 24. Eltai, N.O., H.M. Yassine, El-Obeid T., S.H. Al-Hadidi, A.A. Al Thani, and Alali W.Q.. 2020. Prevalence of antibiotic-resistant Escherichia coli isolates from local and imported retail chicken carcasses. J. Food Prot. 83:2200–2208. [DOI] [PubMed] [Google Scholar]
- 25. Eltai, N., A.A. Al Thani, S.H. Al-Hadidi, et al. 2020. Antibiotic resistance profile of commensal Escherichia coli isolated from healthy sheep in Qatar. J. Infect. Dev. Ctries. 14:138–145. [DOI] [PubMed] [Google Scholar]
- 26. Naik, V.K., Shakya S., Patyal A., N.E. Gade, and Bhoomika null. 2015. Isolation and molecular characterization of Salmonella spp. from chevon and chicken meat collected from different districts of Chhattisgarh, India.. Vet. World 8:702–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. CLSI. 2021. Performance Standards for Antimicrobial Susceptibility Testing (M100). Clinical and Laboratory Standards Institute, Wayne, PA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Core Team R. 2011. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 29. Wickham, H. 2016. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York. [Google Scholar]
- 30. Kassambara, A. 2020. ggpubr: “ggplot2” Based Publication Ready Plots [Computer Software]. Available at https://CRAN.R-project.org/package=ggpubr (accessed January 11, 2022).
- 31. Centers for Disease Control and Prevention. 2019. Antibiotic Resistance Threats in the United States. U.S Department of Health and human Cervices, CDC, Atlanta, GA. [Google Scholar]
- 32. Shrivastava, S.R., P.S. Shrivastava, and Ramasamy J.. 2018. World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. J. Med. Soc. 32:76. [Google Scholar]
- 33. Wang, Y., Chen Q., Cui S., et al. 2014. Enumeration and characterization of Salmonella isolates from retail chicken carcasses in Beijing, China. Foodborne Pathog. Dis. 11:126–132. [DOI] [PubMed] [Google Scholar]
- 34. Ta, Y.T., T.T. Nguyen, P.B. To, et al. 2012. Prevalence of Salmonella on chicken carcasses from retail markets in Vietnam. J. Food Prot. 75:1851–1854. [DOI] [PubMed] [Google Scholar]
- 35. Medeiros, M.A.N., D.C.N. de Oliveira, dos P D.. Rodrigues, and D.R.C. de Freitas. 2011. Prevalence and antimicrobial resistance of Salmonella in chicken carcasses at retail in 15 Brazilian cities. Rev. Panam. Salud Publica. 30:555–560. [DOI] [PubMed] [Google Scholar]
- 36. Donado-Godoy, P., Clavijo V., León M., et al. 2012. Prevalence of Salmonella on retail broiler chicken meat carcasses in Colombia. J. Food Prot. 75:1134–1138. [DOI] [PubMed] [Google Scholar]
- 37. Zdragas, A., Mazaraki K., Vafeas G., Giantzi V., Papadopoulos T., and Ekateriniadou L.. 2012. Prevalence, seasonal occurrence and antimicrobial resistance of Salmonella in poultry retail products in Greece. Lett. Appl. Microbiol. 55:308–313. [DOI] [PubMed] [Google Scholar]
- 38. Alali, W.Q., Gaydashov R., Petrova E., et al. 2012. Prevalence of Salmonella on retail chicken meat in Russian Federation. J. Food Prot. 75:1469–1473. [DOI] [PubMed] [Google Scholar]
- 39. Fall-Niang, N.K., Sambe-Ba B., Seck A., et al. 2019. Antimicrobial resistance profile of Salmonella isolates in chicken carcasses in Dakar, Senegal. Foodborne Pathog. Dis. 16:130–136. [DOI] [PubMed] [Google Scholar]
- 40. Moe, A.Z., Paulsen P., Pichpol D., et al. 2017. Prevalence and antimicrobial resistance of Salmonella isolates from chicken carcasses in retail markets in Yangon, Myanmar. J. Food Prot. 80:947–951. [DOI] [PubMed] [Google Scholar]
- 41. Alarjani, K.M., M.F. Elkhadragy, A.H. Al-Masoud, and Yehia H.M.. 2021. Detection of Campylobacter jejuni and Salmonella typhimurium in chicken using PCR for virulence factor hipO and invA genes (Saudi Arabia). Biosci. Rep. 41:BSR20211790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oscar, T.P. 2004. A quantitative risk assessment model for Salmonella and whole chickens. Int. J. Food Microbiol. 93:231–247. [DOI] [PubMed] [Google Scholar]
- 43. Marchello, C.S., S.D. Carr, and Crump J.A.. 2020. A systematic review on antimicrobial resistance among Salmonella Typhi Worldwide. Am. J. Trop. Med. Hyg. 103:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Campos, J., Mourão J., Silveira L., et al. 2018. Imported poultry meat as a source of extended-spectrum cephalosporin-resistant CMY-2-producing Salmonella Heidelberg and Salmonella Minnesota in the European Union, 2014–2015. Int. J. Antimicrob. Agents 51:151–154. [DOI] [PubMed] [Google Scholar]
- 45. van den Berg, R.R., Dissel S., M.L.B.A. Rapallini, C.C. van der Weijden, Wit B., and Heymans R.. 2019. Characterization and whole genome sequencing of closely related multidrug-resistant Salmonella enterica serovar Heidelberg isolates from imported poultry meat in the Netherlands. PLoS One 14:e0219795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Silveira, L., Nunes A., Â. Pista, et al. 2021. Characterization of multidrug-resistant isolates of Salmonella enterica Serovars Heidelberg and Minnesota from fresh poultry meat imported to portugal. Microb. Drug Resist. 27:87–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.