Abstract
The udhA gene of Escherichia coli was cloned and expressed in E. coli and found to encode an enzyme with soluble pyridine nucleotide transhydrogenase activity. The N-terminal end of the enzyme contains the fingerprint motif of a dinucleotide binding domain, not present in published E. coli genome sequences due to a sequencing error. E. coli is hereby the first organism reported to possess both a soluble and a membrane-bound pyridine nucleotide transhydrogenase.
Pyridine nucleotide transhydrogenases catalyze the reversible transfer of reducing equivalents between NAD and NADP pools according to the following equation:
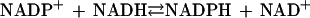 |
1 |
Based on the stereospecificity of the transfer, two groups of transhydrogenases have been defined. The AB transhydrogenases specifically transfer the 4A hydrogen of the nicotinamide ring of NADH to NADP+ and the 4B hydrogen from NADPH to NAD+, while the BB transhydrogenases transfer the 4B hydrogen from both NADPH and NADH (16). The AB transhydrogenases are found in mitochondria and in some bacteria, such as Escherichia coli (6). They are integral membrane proteins bound to the inner mitochondrial membrane or the bacterial membrane where they couple the transfer of hydride from NADH to NADP+ with proton import (12). The genes encoding these enzymes have been cloned from numerous sources (1, 4, 27), facilitating the study of these membrane-bound transhydrogenases, and their physiological role is assumed to be the generation of NADPH, which can be used for reductive biosynthetic reactions.
The BB transhydrogenases are structurally unrelated to the AB transhydrogenases and have been found in Pseudomonas fluorescens, Pseudomonas aeruginosa, and Azotobacter vinelandii (17). They are soluble flavoproteins containing flavin adenine dinucleotide (FAD) and are remarkable for their formation of large polymers. The enzyme from P. aeruginosa has a minimal active form of approximately 1.6 × 106 Da (23), probably composed of four stacked rings of seven or eight monomers (23), and upon isolation filaments exceeding 500 nm in length were observed (13). The P. fluorescens and A. vinelandii enzymes also form polymers (7, 21); however, the structure of the A. vinelandii polymers appears to be different from that of the P. aeruginosa enzyme (22). These soluble transhydrogenases (STH) also display interesting kinetic behavior, with NADPH and 2′-AMP strongly activating the enzyme and NADP+ inhibiting its activity (24). Ca2+ is found to increase the activation and decrease the inhibition effect. The inhibition of STH by NADP+ suggests that its physiological role is the conversion of NADPH, generated by peripheral catabolic pathways, to NADH, which can enter the respiratory chain for energy generation (20).
The first soluble pyridine nucleotide transhydrogenase gene (sth) cloned was from P. fluorescens and revealed that the enzyme was related to the family of flavoprotein disulfide oxidoreductases (7). This family of enzymes includes the well-characterized dihydrolipoamide dehydrogenase, glutathione reductase, and mercuric reductase. These enzymes are active as homodimers and possess a characteristic redox-active disulfide bond. The subunits of these enzymes consist of an N-terminal FAD binding domain, a central NAD(P) binding domain, and a C-terminal dimerization domain (26). However, one of the cysteines involved in the redox-active disulfide bond characteristic of this family of enzymes was lacking in the P. fluorescens sth sequence. The sth sequence was found to be most similar to an E. coli gene of unknown function (udhA), showing 60% sequence identity and 77% similarity (7).
In order to investigate the identity of udhA from E. coli, and because no transhydrogenase activity could be detected in extracts of E. coli grown on rich medium under standard conditions, we cloned and overexpressed this gene in order to study its product.
Cloning and sequence analysis of udhA from E. coli.
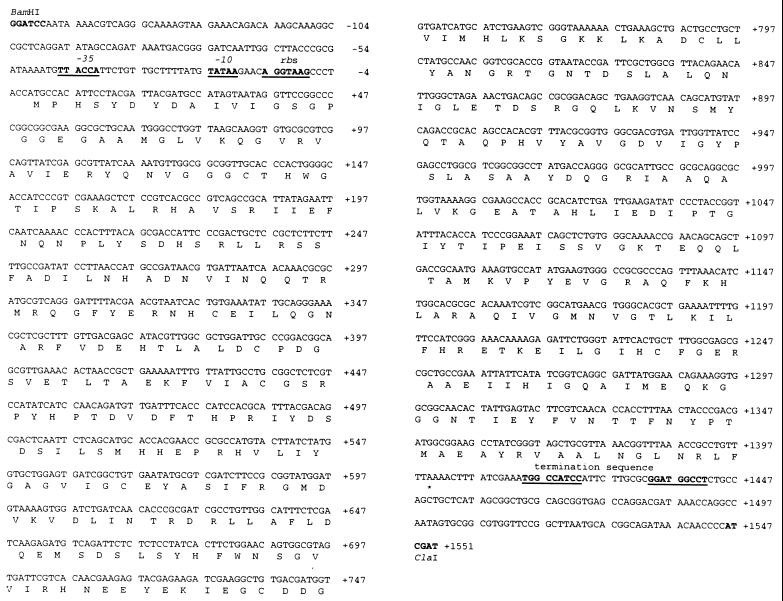
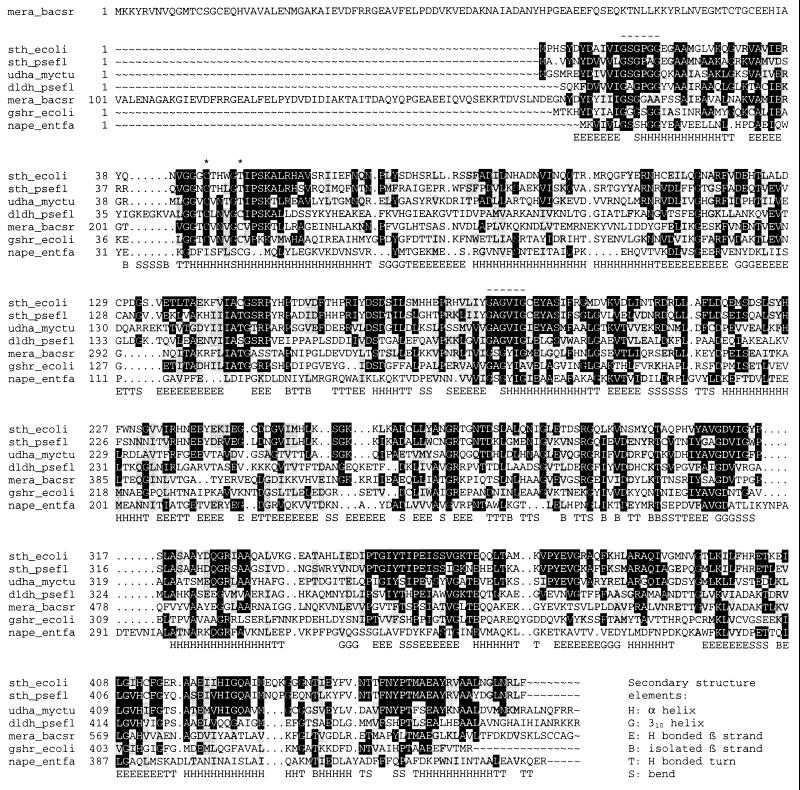
Based on the published E. coli genome sequence (GenBank no. U00006), oligonucleotides were designed in order to amplify by PCR the udhA gene from E. coli JM109 cells. The primers 5′-AGGGATCCAATAAAACGTCAGGGC-3′ and 5′-CCATCGATGGGGTTGTTTATCTGC-3′ (with restriction sites underlined), annealing at positions approximately 150 bp upstream and downstream, respectively, of the potential structural gene, were used. PCR was performed with 30 s of denaturing (94°C), 30 s of annealing (55°C), and 90 s of polymerization (72°C) for 30 cycles, with an additional 90 s of denaturing prior to the first cycle (polymerase added after the 90 s) and 3 min of polymerization after the last cycle. The 1.6-kb PCR product was digested with BamHI and ClaI and cloned into the multiple cloning site of pBluescript SK(+) (no. 52325; Stratagene). The resulting construct was designated pUDHA1 and the insert was sequenced in both orientations. The udhA sequence and the deduced amino acid sequence are given in Fig. 1. An open reading frame encoding a protein of 466 amino acids (including the initiating Met) was identified, with E. coli-like −35 and −10 promoter sequences and a Shine-Dalgarno sequence upstream of the initiating ATG (Fig. 1). The molecular mass of the protein excluding the initiating methionine was determined by the Genetics Computer Group PEPTIDESORT program (8) to be 51,457 Da. The sequence differed from previously published genomic sequences (GenBank no. U00006, X66026, and AE000470) in having a 1-base deletion (a C) after nucleotide 40 (Fig. 1). This extra base in previously published sequences had given rise to a deduced protein sequence (P27306) which started at the methionine 22 amino acids downstream of the methionine presented in this study to be the start of the protein (see below). The N-terminal part of the protein missing in previously deduced sequences contains the amino acid sequence Gly-X-Gly-X-X-Gly/Ala, which is characteristic for a dinucleotide binding domain (Rossman fold). Homology with the disulfide pyridine nucleotide oxidoreductases (see Fig. 4) suggests that this is the FAD binding domain. The protein was found to be 59% identical and 77% similar to P. fluorescens STH.
FIG. 1.
Sequence of udhA and deduced amino acid sequence of STH. Nucleotides are numbered, with 1 being the A of the initiating ATG. Restriction sites indicate the insertion sites in pBluescript SK(+). Putative promoter (−35 and −10) and terminator regions are indicated. rbs, ribosome binding site.
FIG. 4.
Sequence alignment of E. coli STH with related enzymes. The alignment was generated by the CLUSTAL W program of the Genetics Computer Group package (8), and shading was applied by BOXSHADE; the black background shows identical residues and the grey background shows similar residues, both with a threshold of 0.5. The enzymes shown are as follows (with database accession numbers in parentheses): sth_ecoli, STH from E. coli (this study); sth_psefl, STH from P. fluorescens (GenBank U91523); udha_myctu, unknown dehydrogenase from M. tuberculosis (GenBank MTCY05A6; gene name, Rv2713); dldh_psefl, dihydrolipoamide dehydrogenase from P. fluorescens (Swissprot P14128); mera_bacsr, mercuric reductase from Bacillus sp. strain RC607 (Swissprot P16171); gshr_ecoli, glutathione reductase from E. coli (Swissprot P06715); nape_entfa, NADH peroxidase from Enterococcus faecalis (Swissport P37062). *, redox-active cysteine residues; -, Rossman fold Gly-X-Gly-X-X-Gly/Ala motifs forming the FAD and NAD(P) binding sites (14, 25). The bottom line shows the secondary structure elements aligned with dldh_psefl (from 1lpf.pdb).
Expression of recombinant STH in E. coli.
Crude extracts were prepared from JM109/pUDHA1, grown to saturation in SOB medium (Difco) at 37°C, and assessed for STH activity. STH activity was assayed by monitoring the reduction of thionicotinamide adenine dinucleotide (tNAD+) at 400 nm in a reaction mixture consisting of 0.1 mM NADPH and 0.1 mM tNAD+ (Sigma Chemical Co.) in 50 mM Tris-HCl buffer (pH 7.0) at 30°C. The molar extinction coefficient of tNADH at 400 nm was taken as 11,300 liters mol−1 cm−1 (5). One unit of enzyme activity was defined as the amount of activity reducing 1 μmol of tNAD+ per min under these conditions. Protein concentration was measured by using a reagent from Pierce (Rockford, Ill.). Control extracts of JM109 grown under the same conditions did not have measurable STH activity (lowest detectable activity, approximately 0.001 U/mg) while extracts of the recombinant cells, JM109/pUDHA1, possessed about 2.9 U of STH activity per mg. This result implies that the udhA gene of E. coli encodes an enzyme with STH activity, and its putative product was therefore designated STH.
Purification and characterization of recombinant E. coli STH.
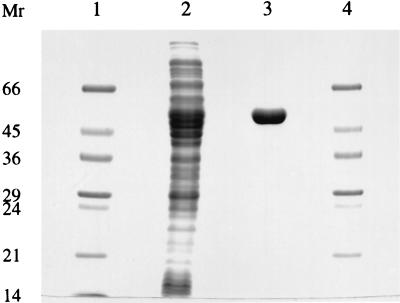
The recombinant STH was purified to apparent homogeneity in a single affinity chromatography step by using adenosine-2′,5′-diphosphate agarose. The protocol used for the purification of P. fluorescens STH was applied except that the 0.7 M NaCl wash step was replaced by 0.5 M NaCl (7). A total of 1,600 U was loaded onto a column with a 6-ml packed volume in the presence of 5 mM CaCl2. The most active eluted fractions, totalling 9.6 ml, were pooled and dialyzed against 50 mM Tris-HCl buffer (pH 7.0) with 5 mM dithiothreitol (DTT) in order to remove salts and reduce the pH. The product contained 1,150 U of STH at a specific activity of 85 U/mg. The specific activity of the purified E. coli STH is about 3.5-fold lower than that of the P. fluorescens STH under standard assay conditions (7). An expression level of about 3.5% of total soluble protein was estimated. The protein appeared to be homogeneous, as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 2), and the subunit Mr determined from SDS-PAGE correlates well with the calculated size and with that reported for P. fluorescens, P. aeruginosa, and A. vinelandii STHs (7, 17, 23). The N-terminal sequence of the recombinant enzyme was determined by automated Edman degradation and was found to be P-H-S-Y-D-Y-D-A-I-V-I-G-S-G-P-G-G-E-(R/G)-A-A-M-G-L-V-K. This is consistent with the deduced amino acid sequence excluding the initiating methionine and confirmed the translation start of the udhA gene and the presence of a Rossman fold fingerprint motif at the N terminus.
FIG. 2.
SDS-PAGE showing purification of STH from E. coli JM109/pUDHA1. Lanes 1 and 4, Mr markers; lane 2, crude extract (20 μg); lane 3, purified STH (5 μg). Numbers on the left are in thousands.
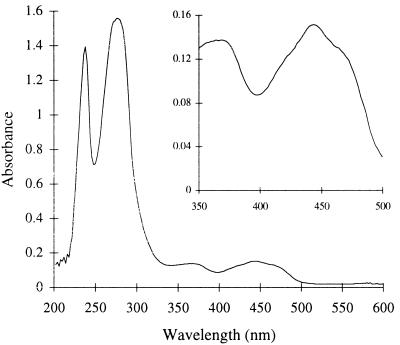
The UV-visible absorption spectrum of the purified STH (Fig. 3) has a peak at 444 nm with a shoulder at 472 nm, and it is characteristic of an oxidized flavoprotein (15). When the enzyme was boiled and the denatured protein was removed by centrifugation, the supernatant retained the yellow coloration and showed the visible absorption spectrum of a free flavin. The flavin liberated in this way was subjected to thin-layer chromatography together with the flavin standards riboflavin, flavin mononucleotide, and FAD. The flavin liberated from STH comigrated with FAD and not with riboflavin or flavin mononucleotide in two different thin-layer chromatography systems (system I, 3% [wt/vol] Na2B4O7 · 10H2O in distilled H2O [dH2O]; system II, n-butanol–dH2O–acetic acid–methanol [14:14:1:6]). This suggests that E. coli STH contains the noncovalently bound prosthetic group FAD. This is also consistent with earlier reports of STH from P. aeruginosa and A. vinelandii (17). Assuming the extinction coefficient of FAD given by Siegel (18), the absorbance spectrum suggests a flavin content of 0.76 mol of FAD per mol of STH subunit.
FIG. 3.
UV-visible spectrum of STH. The spectrum of purified E. coli STH (1.0 mg/ml) in 50 mM Tris-HCl buffer (pH 7.0), with 5 mM DTT and 10% glycerol, was measured against a blank consisting of the same buffer. The inset shows an enlargement of the region characteristic of an oxidized flavoprotein.
In order to determine whether the E. coli STH was capable of forming large polymers, as found for the Pseudomonas (7, 13) and Azotobacter (22) STHs, samples were adsorbed to glow-discharged carbon Formar films from a 1.0-mg/ml solution, negatively stained with 1% (wt/vol) uranyl acetate, and examined by transmission electron microscopy with a Philips CM100 electron microscope operated at 80 kV. These electron microscopy studies showed that the E. coli enzyme differs from the other STHs in that it does not form filaments under these conditions (data not shown).
In order to get an estimate of the native size of the E. coli STH, gel filtration on Superose 6 10/30 (Pharmacia) was performed. The column was equilibrated with 50 mM Tris-HCl (pH 7.0) containing 100 mM NaCl and 5 mM DTT, and the STH was dialyzed against the same buffer prior to application. The column was calibrated with appropriate standards (thyroglobulin, ferritin, catalase, aldolase, albumin, and ovalbumin [from Pharmacia Biotech]; 200 μl of 1- to 5-mg/ml solutions), and STH (200 μl at a concentration of 1.0 mg/ml) was loaded, all at a flow rate of 0.2 ml/min. STH was eluted as a broad peak with an average molecular mass (± standard error) of 386 ± 31 kDa and a shoulder at the monomer size, 51.6 ± 3.5 kDa (data not shown). This shows that E. coli STH does form multimeric structures; however, the enzyme is different from the Pseudomonas and Azotobacter STHs as it is devoid of large polymers. The average molecular weight suggests that the E. coli STH is present in a form consisting of seven or eight monomers. As the A. vinelandii STH is though to have a minimal active form consisting of eight subunits (22), this could suggest that these two STHs have a similar subunit arrangement.
Sequence comparisons.
The deduced amino acid sequence of the E. coli STH was compared to other sequences in protein databases by using the BLAST 2 service of the National Center for Biotechnology Information (NCBI) accessed at http://www.bio.cam.ac.uk (Fig. 4). Various dihydrolipoamide dehydrogenases showed up to 27% identity and 45% similarity to the E. coli STH. The purified E. coli STH did not show significant activity with lipoamide (<0.1 U/mg) in an assay system consisting of 0.2 mM lipoamide and 0.2 mM NADH or NADPH in 50 mM Tris-HCl (pH 7.0) at 30°C. Under the same conditions, dihydrolipoamide dehydrogenase from porcine heart (Sigma) displayed very high activity with NADH (35 U/mg) but no significant activity with NADPH (<0.3 U/mg). Dihydrolipoamide dehydrogenase did not display significant STH activity (<0.1 U/mg). Lack of dihydrolipoamide dehydrogenase activity is consistent with earlier reports on STH (5, 7).
In contrast to the flavoprotein disulfide oxidoreductases (7), both the E. coli and P. fluorescens STHs have a threonine at the position of one of the redox-active cysteines characteristic of this family (amino acids 50 and 49, respectively). The cysteine residue missing in the STH sequences is directly involved in the electron transfer between the nonnicotinamide substrate and the FAD in dihydrolipoamide dehydrogenase, glutathione reductase, and mercuric reductase. The conservation of the threonine residue at this position in STH suggests that this residue might be of importance in the STH reaction. The STH sequences were found to be most similar to an unknown dehydrogenase sequence of Mycobacterium tuberculosis, showing 41% identity and 61% similarity to the E. coli STH. Interestingly, the M. tuberculosis sequence also possesses a threonine at the same position as the STHs.
To the best of our knowledge, the finding of an STH in E. coli makes this organism the first one reported to possess both a soluble and a membrane-bound transhydrogenase. The existence of two types of transhydrogenases in E. coli raises interesting questions about the relative functions of these two enzymes. It is very interesting that the 3′ end of udhA has a 12-nucleotide overlap with the 3′ end of the oxyR gene (10). Overlapping genes often have important regulatory implications, and this unusual overlap suggests that the two genes are possibly not expressed under the same conditions. The oxyR gene encodes the transcription factor OxyR, which regulates the expression of several antioxidant genes (e.g., those for catalase hydroperoxidase I and glutathione reductase) in response to hydrogen peroxide (2, 3). The expression of oxyR is maximal in the exponential phase of aerobic growth, when cells are most likely to encounter oxidative stress (9). It has been demonstrated that it is the OxyR protein itself which is sensitive to hydrogen peroxide, activating the protein through the reversible formation of a disulfide bond (3, 28). The oxidized OxyR binds to promoter elements of the genes it regulates, resulting in the onset of their expression, and the protein also represses its own synthesis (3, 19). Hoek and Rydström proposed that the mitochondrial transhydrogenase is particularly important during oxidative stress (11), and we can in this respect speculate whether the STH and the membrane-bound transhydrogenase of E. coli have complementing functions in the cell. A study of the wild-type expression of udhA in E. coli under various conditions will assist in understanding the physiological role of this enzyme.
Acknowledgments
We thank S. J. Rosser and A. Basran for valuable discussions.
This work was funded in part by a grant from the BBSRC. B.B. was supported by a Ph.D. fellowship from the Norwegian Research Council (grant 115555/410).
REFERENCES
- 1.Arkblad E L, Betsholtz C, Rydström J. The cDNA sequence of proton-pumping nicotinamide nucleotide transhydrogenase from man and mouse. Biochim Biophys Acta. 1996;1273:203–205. doi: 10.1016/0005-2728(95)00159-x. [DOI] [PubMed] [Google Scholar]
- 2.Christman M F, Morgan R W, Jacobsen F S, Ames B N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985;41:753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- 3.Christman M F, Storz G, Ames B N. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proc Natl Acad Sci USA. 1989;86:3484–3488. doi: 10.1073/pnas.86.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke D M, Loo T W, Gillam S, Bragg P D. Nucleotide sequence of the pntA and pntB genes encoding the pyridine nucleotide transhydrogenase of Escherichia coli. Eur J Biochem. 1986;158:647–653. doi: 10.1111/j.1432-1033.1986.tb09802.x. [DOI] [PubMed] [Google Scholar]
- 5.Cohen P T, Kaplan N O. Purification and properties of the pyridine nucleotide transhydrogenase from Pseudomonas aeruginosa. J Biol Chem. 1970;245:2825–2836. [PubMed] [Google Scholar]
- 6.Fisher R R, Earle S R. Membrane-bound pyridine dinucleotide transhydrogenases. In: Everse J, Anderson B, You K S, editors. The pyridine nucleotide coenzymes. New York, N.Y: Academic Press Inc.; 1982. pp. 279–324. [Google Scholar]
- 7.French C E, Boonstra B, Bufton K A J, Bruce N C. Cloning, sequence, and properties of the soluble pyridine nucleotide transhydrogenase of Pseudomonas fluorescens. J Bacteriol. 1997;179:2761–2765. doi: 10.1128/jb.179.8.2761-2765.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genetics Computer Group. Program manual for the Wisconsin package. Version 9.1. September 1997. Madison, Wis: Genetics Computer Group; 1997. [Google Scholar]
- 9.Gonzalez-Flecha B, Demple B. Transcriptional regulation of the Escherichia coli oxyR gene as a function of cell growth. J Bacteriol. 1997;179:6181–6186. doi: 10.1128/jb.179.19.6181-6186.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustafsson C, Warne S R. Physical map of the oxyR-trmA region (minute 89.3) of the Escherichia coli chromosome. J Bacteriol. 1992;174:7878–7879. doi: 10.1128/jb.174.23.7878-7879.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoek J B, Rydström J. Physiological roles of nicotinamide nucleotide transhydrogenase. Biochem J. 1988;254:1–10. doi: 10.1042/bj2540001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson J B, Cotton N P J, Williams R, Bizouarn T, Hutton M N, Sazanov L A, Thomas C M. Proton-translocating transhydrogenase in bacteria. Biochem Soc Trans. 1993;21:1010–1013. doi: 10.1042/bst0211010. [DOI] [PubMed] [Google Scholar]
- 13.Louie D D, Kaplan N O, McLean J D. Allosteric effect of 2′-adenylic acid on the Pseudomonas pyridine nucleotide transhydrogenase. J Mol Biol. 1972;70:651–664. doi: 10.1016/0022-2836(72)90564-5. [DOI] [PubMed] [Google Scholar]
- 14.McKie J H, Douglas K T. Evidence for gene duplication forming similar binding folds for NAD(P)H and FAD in pyridine nucleotide-dependent flavoenzymes. FEBS Lett. 1991;279:5–8. doi: 10.1016/0014-5793(91)80236-v. [DOI] [PubMed] [Google Scholar]
- 15.Palfey B A, Massey V. Flavin-dependent enzymes. In: Sinnott M, editor. Radical reactions and oxidation/reduction. III. London, United Kingdom: Academic Press Limited; 1998. pp. 81–153. [Google Scholar]
- 16.Rydström J, Hoek J B, Ernster L. Nicotinamide nucleotide transhydrogenases. In: Boyer P D, editor. The enzymes. 3rd ed. Vol. 13. New York, N.Y: Academic Press; 1976. pp. 51–89. [Google Scholar]
- 17.Rydström J, Persson B, Carlenor E. Transhydrogenases linked to pyridine nucleotides. In: Dolphin D, Aramovic O, Poulson R, editors. Pyridine nucleotide coenzymes: chemical, biochemical and medical aspects, part B. New York, N.Y: John Wiley & Sons; 1987. pp. 433–461. [Google Scholar]
- 18.Siegel L M. Quantitative determination of noncovalently bound flavins: types and methods of analysis. Methods Enzymol. 1978;53:419–429. doi: 10.1016/s0076-6879(78)53046-2. [DOI] [PubMed] [Google Scholar]
- 19.Toledano M B, Kullik I, Trinh F, Baird P T, Schneider T D, Storz G. Redox-dependent shift of OxyR-DNA contacts along an extended DNA-binding site: a mechanism for differential promoter selection. Cell. 1994;78:897–909. doi: 10.1016/s0092-8674(94)90702-1. [DOI] [PubMed] [Google Scholar]
- 20.Voordouw G, van der Vies S M, Themmen A P N. Why are two different types of pyridine nucleotide transhydrogenase found in living organisms? Eur J Biochem. 1983;131:527–533. doi: 10.1111/j.1432-1033.1983.tb07293.x. [DOI] [PubMed] [Google Scholar]
- 21.Voordouw G, Veeger C, van Breemen J F L, van Bruggen E F J. Structure of pyridine nucleotide transhydrogenase from Azotobacter vinelandii. Eur J Biochem. 1979;98:447–454. doi: 10.1111/j.1432-1033.1979.tb13205.x. [DOI] [PubMed] [Google Scholar]
- 22.Voordouw G, van der Vies S M, Eweg J K, Veeger C, van Breemen J F, van Bruggen E F. Pyridine nucleotide transhydrogenase from Azotobacter vinelandii: improved purification, physical properties and subunit arrangement in purified polymers. Eur J Biochem. 1980;111:347–355. doi: 10.1111/j.1432-1033.1980.tb04948.x. [DOI] [PubMed] [Google Scholar]
- 23.Wermuth B, Kaplan N O. Pyridine nucleotide transhydrogenase from Pseudomonas aeruginosa: purification by affinity chromatography and physiochemical properties. Arch Biochem Biophys. 1976;176:136–143. doi: 10.1016/0003-9861(76)90149-1. [DOI] [PubMed] [Google Scholar]
- 24.Widmer F, Kaplan N O. Pseudomonas aeruginosa transhydrogenase: affinity of substrates for the regulatory site and possible hysteretic behavior. Biochem Biophys Res Commun. 1977;76:1287–1292. doi: 10.1016/0006-291x(77)90995-0. [DOI] [PubMed] [Google Scholar]
- 25.Wierenga R K, Demaeyer M C H, Hol W G J. Interaction of pyrophosphate moieties with alpha-helixes in dinucleotide binding-proteins. Biochemistry. 1985;24:1346–1357. [Google Scholar]
- 26.Williams C H. Lipoamide dehydrogenase, glutathione reductase, thioredoxin reductase, and mercuric ion reductase—a family of flavoenzyme transhydrogenases. In: Müller F, editor. Chemistry and biochemistry of flavoenzymes. III. Boca Raton, Fla: CRC Press Inc.; 1992. pp. 121–211. [Google Scholar]
- 27.Williams R, Cotton N P J, Thomas C M, Jackson J B. Cloning and sequencing of the genes for the proton-translocating nicotinamide nucleotide transhydrogenase from Rhodospirillum rubrum and the implications for the domain-structure of the enzyme. Microbiology—UK. 1994;140:1595–1604. doi: 10.1099/13500872-140-7-1595. [DOI] [PubMed] [Google Scholar]
- 28.Zheng M, Aslund F, Storz G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science. 1998;279:1718–1721. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]