Abstract
COVID‐19 morbidity and mortality are driven by poor immune regulation. Narrowband ultraviolet B (NB‐UVB) phototherapy is standard of care in a number of immune‐dysregulated diseases. To assess the efficacy of NB‐UVB phototherapy for improving COVID‐19 outcomes in high‐risk, hospitalized, we developed the Adaptive Photo‐Protection Trial. This is a multi‐center, prospective, double‐blinded, randomized, placebo‐controlled trial. The pilot phase results are reported here. Consecutive patients admitted with a positive COVID‐19 PCR were screened for eligibility. Enrolled subjects were computer randomized 1:1 to NB‐UVB or placebo phototherapy. Subjects were treated daily with escalating doses on 27% of their body surface area for up to 8 consecutive days. Primary outcomes were safety and efficacy, defined as persistent or painful erythema and 28‐day mortality. Comparisons were made via non‐parametric exact tests. Patients in treatment (n = 15) and placebo (n = 15) arms had similar demographics. No adverse events occurred. Twenty eight‐day mortality was 13.3% in treatment vs. 33.3% in placebo arms (p = 0.39). NB‐UVB phototherapy in hospitalized COVID‐19 patients was safe. Decreased mortality was observed in treated patients but this was statistically non‐significant. Given its low‐cost, scalability, and adjunctive nature, NB‐UVB has the potential to improve COVID‐19 outcomes. Continuation of this trial is warranted.
Keywords: 28‐day mortality, COVID‐19 outcomes, narrowband ultraviolet B band, phototherapy, randomized trial
1. INTRODUCTION
1.1. Translational phototherapy for COVID‐19
Novel SARS‐CoV‐2 variants continue to emerge, highlighting the persistent need for therapies that address the systemic immune dysregulation underlying COVID‐19 mortality. In population research, environmental UVB is strongly correlated with COVID‐19 morbidity and mortality. 1 Narrowband ultraviolet B band (NB‐UVB) phototherapy is a standard‐of‐care treatment that modulates immune dysfunction in several diseases including graft‐vs.‐host disease and cutaneous T‐cell lymphoma. 2 This clinical benefit of NB‐UVB phototherapy is not disease‐specific but based on systemic improvement in immune regulation. 3 Applying acute levels of NB‐UVB to counter the acute immune imbalance driving outcomes in high‐risk COVID‐19 patients was hypothesized to provide an affordable, scalable option to healthcare providers and their patients.
1.2. Vitamin D insufficiency as a marker for low UV exposure
Vitamin D insufficiency (VDI) has also been associated with poor COVID‐19 outcomes. 4 However, oral supplementation has not shown a consistent benefit in numerous randomized trials. Since UVB light synthesizes vitamin D, there must be a parallel or complementary NB‐UVB pathway to support immunity upstream of vitamin D supplements. 5 To test this hypothesis, we developed the first Adaptive Photo‐Protection Trial (NCT04818970) to quantify the effects of NB‐UVB phototherapy on COVID‐19 outcomes at 28‐day. By association, the trial would subsequently classify VDI as a biomarker of insufficient UVB exposure. The pilot phase results are reported here.
2. QUESTIONS ADDRESSED
Does NB‐UVB phototherapy stabilize immunity sufficiently to improve COVID‐19 mortality at 28 days in hospitalized, high‐risk subjects?
3. EXPERIMENTAL DESIGN
3.1. Adaptive design and target patients
This prospective, multi‐center, randomized, placebo‐controlled, clinical trial with an adaptive design (n = 500) was approved by the WCG Institutional Review Board (#1305616 dated 04/08/2021) and registered with ClinicalTrials.gov (NCT04818970). A pilot phase (n = 30) was conducted at a single academic hospital. Patients signed written informed consents and were not provided with incentive or compensation to participate. Consecutive patients admitted to the hospital with a positive COVID‐19 polymerase chain reaction (PCR) test between May 24, 2021, and August 16, 2021, were screened for eligibility. Enrollment criteria included age between 50 and 95 years, peripheral oxygen saturation <94% on room air, at least one comorbidity (obesity, hypertension, diabetes), admission for <3 days, and the absence of exclusion criteria. Enrolled subjects were randomized 1:1 to NB‐UVB (311 nm) or placebo phototherapy. The randomization list was created using a computer‐generated code.
3.2. Randomization, treatment, and placebo
The treatment and placebo lights were identical FDA cleared phototherapy lights with NB‐UVB bulbs (Daavlin Series 1, Daavlin International, Bryan, Ohio). The Placebo lights had their standard plexiglass replaced with a visually identical UV‐absorbing plexiglass. Lights were calibrated weekly by blinded non‐study staff; placebo lights were confirmed to have zero NB‐UVB output.
3.2.1. Bringing phototherapy to the patient
The lights were mounted on a customized rolling stand to provide therapy to bedridden patients. The stands also carried a battery pack. Together these designs eliminated the need and risk associated with asking acutely ill patients to stand. The battery pack eliminated the potential risk of tripping for the care provider.
3.2.2. The Meridian Regimen – acute NB‐UVB for acute covid‐19
While hospitalized and not requiring positive‐pressure oxygen supplementation or critical care, subjects were treated every 24 h ± 1 h with escalating doses of NB‐UVB or placebo. Initial dose was calculated following American Academy of Dermatology guidelines (according to skin phototype). 6 Previous literature had identified differential level levels of NB‐UVB‐induced serum vitamin D by anatomical region. Face, hands, torso, and arms provided substantially higher levels of endogenous serum vitamin D. 7 Assuming the photo‐immune pathway operated in parallel, a novel Meridian Regimen was proposed that would prioritize 27% of the body surface area (BSA), the upper torso, arms, and hands as calculated by the clinically accepted Wallace rule of nines. 8 This eliminates the need for patients to roll‐over or get out of bed.
3.2.3. A daily escalating dose algorithm
A novel algorithm to maximize daily delivery of NB‐UVB with a net increase of 10% had previously been safely and effectively trialled in a clinical trial for psoriasis. 9 The algorithm accounts for the residual dose and photohardening of the prior day's treatment to maximize dose without erythema. This is the first application of such an algorithm outside of inflammatory skin conditions. The treatment and placebo devices were equipped with an internal UV sensor and operated under Dosimetric Control to increase dose accuracy, allowing clinicians to enter the appropriate daily dose for each patient's escalating treatment (Table S1). The treatment was provided for a maximum of 8 days or until discharge or transfer to the ICU. Minimal erythemal dose calculations were not performed due to the acute clinical nature of these patients.
3.3. Primary endpoints and statistical analysis
The primary endpoints were safety and efficacy at 28 days. Safety was defined as frequency of painful erythema in treated areas. Efficacy was quantified as 28‐day mortality and World Health Organization Ordinal Scale for Clinical Improvement (OSCI) scores. Secondary outcomes included changes in clinical laboratory values. Statistical analysis was performed by a biostatistician using SAS/STAT software, version 9.4. Quality assurance was performed by the study sponsor (Cytokind, Inc.)
4. RESULTS
4.1. Enrolled patients and treatment course
Among 206 consecutive, hospitalized but not critically ill COVID‐19 patients, 53 (25.7%) were eligible (Figure 1). Of those eligible, 30 (56.6%) were enrolled and randomized 1:1 to treatment (n = 15) vs. placebo (n = 15) arms. Demographics, comorbidities, vaccination status, admission vital signs, baseline clinical laboratory values, and COVID‐19 treatment were similar between groups (Table 1). Fitzpatrick skin type was similar between groups (Table 1). The median number of treatments in the treatment arm was n = 3 compared to n = 5 in the placebo (p = 0.13). The average cumulative dose per treated patient ranged from 300 to 2434 mJ/cm2, with a median of 1045 mJ/cm2.
FIGURE 1.
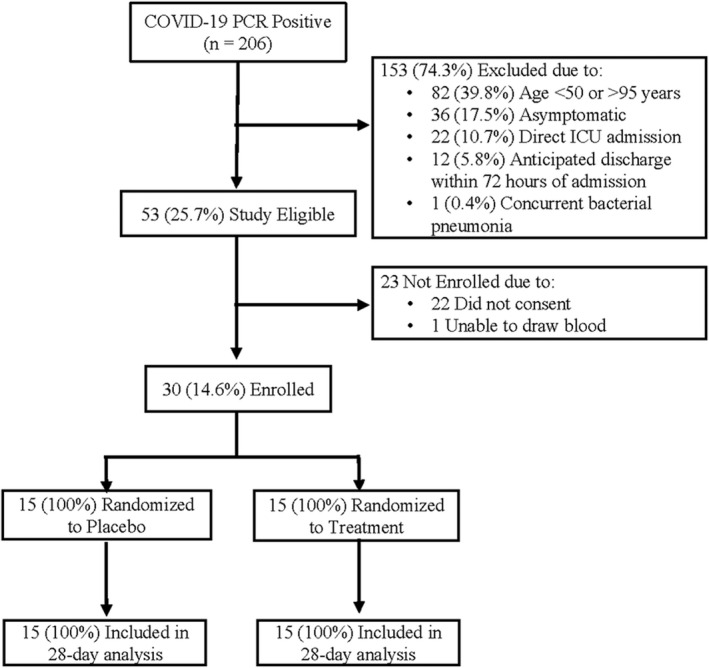
CONSORT diagram for the pilot phase of the adaptive photo‐protection trial of the impact of narrowband ultraviolet B band (NB‐UVB) Phototherapy in high‐risk hospitalized COVID‐19 patients
TABLE 1.
Baseline, treatment and clinical endpoints by group in a randomized trial of the effect of daily narrowband ultraviolet b (nb‐uvb) on the outcomes of high‐risk hospitalized COVID‐19 patients
| Demographics | Placebo (n = 15) | Treatment (n = 15) | p‐value |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 7 (46.7) | 5 (33.3) | 0.7104 |
| Male | 8 (53.3) | 10 (66.7) | |
| Race/Ethnicity, n (%) | |||
| White | 7 (46.7) | 12 (80) | 0.1966 |
| Black | 5 (33.3) | 3 (20) | |
| Hispanic | 2 (13.3) | 0 (0) | |
| Asian | 1 (6.7) | 0 (0) | |
| Age, median (range), years | 69.7 (50.3–82.5) | 64 (52.2–92.4) | 0.9669 |
| Risk factors/comorbidities | |||
| Body Mass Index, median (range), kg/m2 | 32.2 (22.9–44.8) | 30.6 (23.5–61) | 1.0000 |
| Hypertension, n (%) | 5 (33.3) | 10 (66.7) | 0.1431 |
| Diabetes, n (%) | 2 (13.3) | 3 (20) | 1.0000 |
| Vaccination status, n (%) | |||
| Unvaccinated | 6 (40.0) | 10 (66.7) | 0.3139 |
| Partially or fully vaccinated | 7 (46.7) | 3 (20.0) | |
| Unknown | 2 (13.3) | 2 (13.3) | |
| Admission vital signs, median (range) | |||
| Temperature, F | 98.4 (97.7–101.6) | 98.7 (97.9–100.7) | 0.9172 |
| Pulse, bpm | 84 (66–113) | 93 (63–117) | 0.6934 |
| Respiratory rate, breaths/min | 20 (16–38) | 22 (16–50) | 0.6763 |
| Systolic blood pressure | 122 (98–166) | 125 (107–174) | 0.3395 |
| Diastolic blood pressure | 65 (56–75) | 70 (58–95) | 0.0112 |
| Peripheral O2 saturation, % | 0.95 (0.73–1) | 0.93 (0.89–1) | 0.2425 |
| Day 1 laboratory values, median (range) | |||
| Absolute Neutrophil Count, x10e3/μl | 7 (1.5–11.9) | 6 (3–14.6) | 0.9795 |
| Lactate Dehydrogenase, IU/L | 503 (286–1118) | 532 (241–987) | 0.6784 |
| C‐Reactive Protein, mg/dl | 78 (5–280) | 44 (1–188) | 0.4335 |
| Ferritin, mg/L | 1424 (506–3125) | 1161 (135–4525) | 0.2894 |
| D‐Dimer, mg/L | 0.6 (0.25–1.09) | 0.96 (0.33–3.27) | 0.0493 |
| 25‐OHD Vitamin D, ng/ml | 22.9 (8.1–50) | 29.8 (8.4–45.5) | 0.3412 |
| Calcitriol, pg/ml | 108 (54.6–306) | 121 (45–166) | 0.7779 |
| COVID‐19 treatment, n (%) | |||
| Remdesivir | 12 (80.0) | 11 (73.3) | 1.0000 |
| Dexamethasone | 15 (100.0) | 13 (86.7) | 0.4828 |
| Heparin / Enoxaparin | 10 (66.7) | 11 (73.3) | 1.0000 |
| Aspirin | 2 (13.3) | 3 (20) | 1.0000 |
| Apixaban | 3 (20.0) | 2 (13.3) | 1.0000 |
| Rivaroxaban | 2 (13.3) | 0 (0) | 0.4828 |
| Clopidogrel | 2 (13.3) | 0 (0) | 0.4828 |
| Tocilizumab | 4 (26.7) | 5 (33.3) | 1.0000 |
| Casirivimab‐Imdevimab | 0 (0) | 1 (6.7) | 1.0000 |
| Treatment: NB‐UVB phototherapy | |||
| Fitzpatrick skin type, n (%) | |||
| I–II | 8 (53.3) | 12 (80) | 0.3057 |
| III–IV | 2 (13.3) | 0 (0) | |
| V–VI | 5 (33.3) | 3 (20) | |
| Number of treatments, median (range) | 5 (1–8) | 3 (1–8) | 0.1113 |
| Cumulative dose, median (range), mJ/cm2 | 0 (0–0) | 1045 (300–2434) | <0.0001 |
| Primary endpoints | |||
| Adverse events, n (%) | 0 (0) | 0 (0) | ‐‐ |
| 14‐day mortality, n (%) | |||
| Overall | 4/15 (26.7) | 1/15 (6.7) | 0.3295 |
| Unvaccinated | 1/6 (16.7) | 0/10 (0) | 0.3750 |
| Partial or full | 3/7 (42.9) | 0/3 (0) | 0.4750 |
| Unknown | 0/2 (0) | 1/2 (50) | 1.0000 |
| 28‐day mortality, n (%) | |||
| Overall | 5/15 (33.3) | 2/15 (13.3) | 0.3898 |
| Unvaccinated | 2/6 (33.3) | 1/10 (10) | 0.5179 |
| Partial or Full | 3/7 (42.9) | 0/3 (0) | 0.4750 |
| Unknown | 0/2 (0) | 1/2 (50) | 1.0000 |
| WHO OSCI Scores, median (range) | 0 (0–8) | 0 (0–8) | 1.0000 |
| Critical Care, n (%) | |||
| Intensive care unit | 4 (26.7) | 5 (33.3) | 1.0000 |
| Mechanical ventilation | 4 (26.7) | 4 (26.7) | 1.0000 |
| Secondary Endpoints, median (range), n | |||
| Day 3 Deltas (Day 3 ‐ Day 1) | |||
| Absolute Neutrophil Count, x10e3/μl | −0.4 (−4.8–6.2), n = 9 | −0.4 (−2.2–3.7), n = 7 | 0.7911 |
| Lactate Dehydrogenase, IU/L | −35 (−895–77), n = 11 | −31 (−296–267), n = 10 | 0.6472 |
| C‐Reactive Protein, mg/dl | −52 (−132–11), n = 11 | −22 (−136–30), n = 10 | 0.6722 |
| Ferritin, mg/L | −77 (−621–681), n = 11 | −197 (−1136–5), n = 10 | 0.5260 |
| D‐Dimer, mg/L | 0.29 (−0.38–2.28), n = 11 | 0.22 (−0.08–2.44), n = 9 | 0.8792 |
| 25‐OHD Vitamin D, ng/ml | 0.25 (−5–4), n = 10 | −1.5 (−11.9–3.9), n = 10 | 0.3075 |
| Calcitriol, pg/Ml | −10.2 (−47–24.3), n = 9 | −34 (−74.6–21), n = 9 | 0.1853 |
| Day 5 Deltas (Day 5 ‐ Day 1) | |||
| Absolute neutrophil count, x10e3/μl | 2.65 (−5.3–11.4), n = 6 | 5 (3.7–7.3), n = 3 | 0.6985 |
| Lactate dehydrogenase, IU/L | 92 (−210–236), n = 7 | −135 (−218–71), n = 3 | 0.1715 |
| C‐reactive protein, mg/dl | −68 (−182–20), n = 7 | −19 (−165‐−13), n = 3 | 1.0000 |
| Ferritin, mg/L | −409 (−720–879), n = 8 | −239 (−1368–−161), n = 3 | 0.7595 |
| D‐Dimer, mg/L | 0.63 (−0.06–3.2), n = 8 | 2.07 (0.14–2.16), n = 3 | 0.8379 |
| 25‐OHD vitamin D, ng/ml | 1.7 (−2.1–7), n = 7 | −12.2 (−19.7–−4.7), n = 2 | 0.0570 |
| Calcitriol, pg/ml | 9 (−49.5–36.6), n = 4 | −78.1 (−112.5–−43.7), n = 2 | 0.2472 |
Note: Statistical comparisons of study arms were conducted via non‐parametric tests, Fisher's exact test for categorical variables and Wilcoxon Rank Sum tests for continuous or ordinal variables.
Abbreviations: NB‐UVB, narrowband ultraviolet B; OHD, hydroxyvitamin; DWHO OSCI, World Health Organization Ordinal Scale for Clinical Improvement.
4.2. Primary endpoint
Twenty eight‐day follow‐up was complete in 30/30 (100%) subjects. At 28 days, 5 (33.3%) placebo patients died of COVID‐19 hypoxia, vs. 2 (13.3%) treatment patients (p = 0.39) (Figure 2). World Health Organization Ordinal Scale Improvement Scores (OSCI) were similar, with a median score of 0 in both arms (p = 1.00). COVID‐19 outcomes reported by baseline characteristics did not significantly differ between study arms (Table S2).
FIGURE 2.
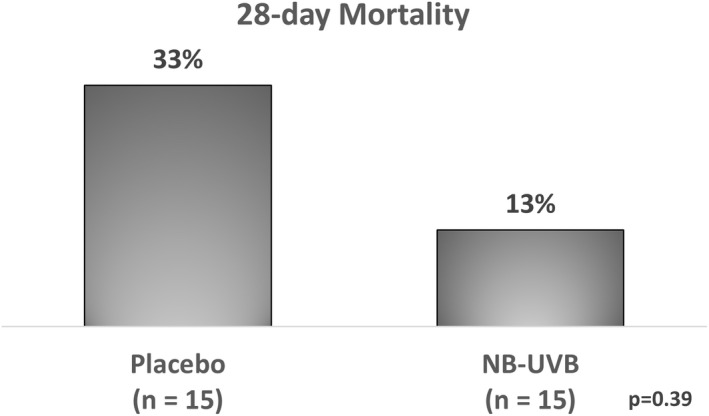
Mortality at 28 days in the pilot phase of the adaptive photo‐protection trial of the impact of narrowband ultraviolet B band (NB‐UVB) Phototherapy in high‐risk hospitalized COVID‐19 patients
No phototherapy‐related adverse events occurred in either arm (Table 1). Erythema did occur in one patient, which required pausing treatment for two consecutive days. This did not reach the trial's definition for an adverse event, which was painful erythema, burns, dermatitis or blisters (as determined by investigator) that require more than 3 days to heal or require concomitant therapy.
4.3. Secondary endpoints
Secondary endpoints revealed no significant differences in laboratory values between day 1 and days 3 and 5 (Table 1). The median change in serum 25‐OHD vitamin D in the treatment group at day 5 was −12.2 ng/ml, compared to +1.7 ng/ml in the placebo arm (p = 0.057).
5. DISCUSSION
5.1. Safety confirmation and adaptive continuation of trial
In this prospective, randomized, double‐blinded, placebo‐controlled clinical trial, daily high‐dose NB‐UVB was safely delivered to high‐risk hospitalized COVID‐19 patients. To our knowledge, this is the first test of NB‐UVB phototherapy in COVID‐19. To respond to the high‐risk patients, this trial prescribed an accelerated dose regimen over 8 days that would normally be provided over 3–4 weeks in chronic skin conditions. 6 At the predetermined assessment of the first 30 patients, the results demonstrated a trend in the treatment arm towards improved 28‐day mortality. Reduced mortality was observed in both unvaccinated and fully or partially vaccinated patients. Given its low‐cost, scalable, and adjunctive nature, NB‐UVB has the potential to improve COVID‐19 outcomes worldwide. Continuation of this clinical trial is warranted: if the observed magnitude of effect holds in the continued trial, with a properly powered sample size to test/validate the benefit of this therapy. It is noted that the number of treatments and total dosage were both below the intended targets. The escalating daily dose did provide patients a median total dose over 3 treatments of 1045 mJ, which is equivalent to 6 treatments delivered over the Dundee psoriasis protocol over 2–3 weeks.
5.2. Divergence of NB‐UVB and vitamin D supplementation on immunity
Multiple randomized trials of oral vitamin D supplementation have not shown benefit in COVID‐19. 5 Administration of NB‐UVB and vitamin D supplementation are not the same in terms of immune regulation. A randomized controlled trial treated VDI patients with either NB‐UVB vs. oral vitamin D supplementation. 10 The transcriptional profile of peripheral blood of each treatment was analysed by VDR and hallmark immune‐pathway gene sets. VDR gene sets were directional consistent while the hallmark demonstrated opposite effects on the immune transcriptome to oral supplements. Specifically, interferon‐α and interferon‐γ response gene sets were significantly upregulated with oral vitamin D3 and were significantly downregulated with NB‐UVB.
Furthermore, there is broad evidence that UVB has vitamin‐D dependent and independent effects on the immune system. 11 A recent meta‐analysis reported that decreased ambient UVB levels were strongly correlated with increased COVID‐19 morbidity and mortality. 1
Despite this distinction, the hypothesis that oral vitamin D supplementation could improve COVID‐19 outcomes arose from the fact that NB‐UVB also initiates cutaneous vitamin D synthesis. VDI has been also associated with worse outcomes in COVID‐19 and was shown to be an independent risk factor for COVID‐19 infection in a study of 14 000 healthcare workers. 3 , 12 A parallel and independent UV‐driven immune modulation pathway would address the paradox of vitamin D as a prognostic indicator and the absence of clear benefit of oral vitamin D supplementation in COVID patients.
5.3. Daily NB‐UVB and serum vitamin D levels
Our pilot phase data suggest that VDI is a biomarker for low environmental UVB exposure. This hypothesis explains the lack of benefit from oral supplementation, which does not confer the immune stabilizing benefits of NB‐UVB. Intriguingly, in our trial, serum vitamin D levels were lower in the treatment group after 3‐ and 5‐day of NB‐UVB treatment but stayed unchanged in the placebo group. This is the opposite of what was observed in healthy patients, where serum vitamin D increased within 10 h of UVB exposure. 13 While our sample size was small by design, our data suggest that 25‐OHD may be consumed by a NB‐UVB driven response to COVID‐19. This fits with recently published pre‐clinical data suggesting that several novel vitamin D and related lumisterol hydroxymetabolites that are produced in response to NB‐UVB exposure have high binding affinities to SARS‐CoV‐2 main protease and RNA‐dependent RNA polymerase, two enzymes that are central to viral replication. 14 Additionally, these metabolites demonstrate broad anti‐inflammatory activity, suggesting overlap with the likely mechanisms whereby systemic corticosteroids improve COVID‐19 outcomes. 15 Intriguingly, these anti‐viral and anti‐inflammatory metabolites are produced only from the vitamin D precursor 7‐dehydrocholesterol and not from 25(OH)D3. 15 They are produced by the enzyme CYP11A1, which is expressed in immune cells. 16 Our data are also consistent with prior reports that 25‐OHD is consumed to produce antiviral molecules such as cathelicidin. 17
5.4. Potential mechanism of action of NB‐UVB in COVID‐19
Decades of clinical experience with NB‐UVB in GvHD and other autoimmune, dermatologic diseases have shown that NB‐UVB stabilizes the immune system. Mechanistically, the skin is a complex organ containing a reservoir of photo‐reactive cells that communicate extensively with our systemic immune systems. 2 , 3 , 18 , 19 Exposure to UV radiation triggers numerous molecular responses in the skin, activating biological cascades that push immune responses towards homeostasis typified by reduced Th1 and Th17 and increased Th2 and circulating regulatory T cells and ameliorates symptoms in acute GVHD by expansion of CD4+ CD25+ Foxp3+. 19 , 20 Downstream effects include decreased pathologic inflammation and improved intracellular killing of pathogens. Improvements in hemostatic regulation may also be driving patient benefit as UVB increases the cytokine IL‐10 that can inhibit both LPS‐induced activation of coagulation and fibrinolysis. 21 Future directions for this trial include continuation of the study to confirm the clinical benefit of NB‐UVB. Additionally, serum samples from this trial's subjects have been banked and will be studied. By analysing 1000s of immunologic markers at multiple timepoints, we aim to uncover NB‐UVB's immunologic mechanisms of action that stabilizes immunity sufficiently enough to improve outcomes in high‐risk COVID‐19 patients.
5.5. Limitations of this study
As the pilot phase of a larger clinical trial, this study was underpowered to detect statistically significant differences in clinical outcomes between treatment arms. The statistical power for a Fisher's exact test with 15 patients per group given the rates of 28‐day mortality observed in this pilot is 14.1%. This power calculation will be used to refine the biostatistical considerations for the planned, larger clinical trial.
AUTHOR CONTRIBUTIONS
FHL, AD, CC, PW, RC contributed to the protocol and trial design. FHL, CP, and GA provided the clinical management. CC designed the treatment regimen and provided phototherapy support. DD provided statistical analysis. All authors contributed equally to the manuscript. Benjamin Wang, PhD and John MacMahon, MS, co‐founders of sponsor Cytokind, Inc., provided the initial hypothesis for this trial.
CONFLICT OF INTEREST
Carmen F. Castilla, MD is a consultant to the sponsor. No other authors have a conflict to disclose. The sponsor provided the initial design of the trial and provided clinical and technical support on the phototherapy treatment equipment and regimen. The sponsor had no role in the conduct of the study; collection, management, analysis, and interpretation of the data.
ACKNOWLEDGEMENT
None.
Supporting information
Table S1. Cytokind meridien treatment regimen. Provided initial clinical dose in accordance with the patient's Fitzpatrick Skin Type. The escalating daily dose allows for a net increase of 10% dose by accounting for the residual dose from the previous treatments.
Table S2. Baseline characteristics and prescriptions reported by clinical outcomes in a randomized trial of the effect of daily narrowband ultraviolet B (NB‐UVB) on the outcomes of high‐risk hospitalized COVID‐19 patients.
Lau FH, Powell CE, Adonecchi G, et al.. Pilot phase results of a prospective, randomized controlled trial of narrowband ultraviolet B phototherapy in hospitalized COVID‐19 patients. Exp Dermatol. 2022;31:1109‐1115. doi: 10.1111/exd.14617
Funding information
This work was supported by the sponsor Cytokind, Inc. Intervale, NH.
Registration: ClinicalTrials.gov (NCT04818970).
IRB Approval: WCG Institutional Review Board (#1305616); IRB Action Date: 04/08/2021.
Catherine E. Powell, Giacomo Adonecchi equally contributed.
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Gorman S, Weller RB. Investigating the potential for ultraviolet light to modulate morbidity and mortality from COVID‐19: A narrative review and update. Front Cardiovasc Med. 2020;7:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vieyra‐Garcia PA, Wolf P. A deep dive into UV‐based phototherapy: mechanisms of action and emerging molecular targets in inflammation and cancer. Pharmacol Therap. 2021;222:107 784. [DOI] [PubMed] [Google Scholar]
- 3. Yu Z, Wolf P. How it works: the immunology underlying phototherapy. Dermatol Clin. 2020;38:37‐53. [DOI] [PubMed] [Google Scholar]
- 4. Lau FH, Majumder R, Torabi R, et al. Vitamin D insufficiency is prevalent in severe COVID‐19. medRxiv. 2020; 2020.04.24.20075838. [Google Scholar]
- 5. Stroehlein JK, Wallqvist J, Iannizzi C, et al. Vitamin D supplementation for the treatment of COVID‐19: a living systematic review. Cochrane Database Syst Rev. 2021;5:CD015043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology‐National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775‐804. [DOI] [PubMed] [Google Scholar]
- 7. Jager N, Schöpe J, Wagenpfeil S, et al. The impact of UV‐dose, body surface area exposed and other factors on cutaneous vitamin D synthesis measured as serum 25(OH)D concentration: systematic review and meta‐analysis. Anticancer Res. 2018;38(2):1165‐1171. [DOI] [PubMed] [Google Scholar]
- 8. Moore RA, Waheed A, Burns B. Rule of nines. StatPearls.. StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 9. Evaluation of efficacy, duration of remission and safety of a light and occlusive patch. therapy for plaque psoriasis. March 15, 2022. https://clinicaltrials.gov/ct2/show/NCT03180866
- 10. Ponda MP, Liang Y, Kim J, et al. A randomized clinical trial in vitamin D–deficient adults comparing replenishment with oral vitamin D3 with narrow‐band UV type B light: effects on cholesterol and the transcriptional profiles of skin and blood. Am J Clin Nutr. 2017;105:1230‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schweintzger NA, Gruber‐Wackernagel A, Shirsath N, Quehenberger F, Obermayer‐Pietsch B, Wolf P. Influence of the season on vitamin D levels and regulatory T cells in patients with polymorphic light eruption. Photochem Photobiol Sci. 2016;15:440‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Merzon E, Tworowski D, Gorohovski A, et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID‐19 infection: an Israeli population‐based study. FEBS J. 2020;287(17):3693‐3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haddad JG, Matsuoka LY, Hollis BW, Hu YZ, Wortsman J. Human plasma transport of vitamin D after its endogenous synthesis. J Clin Invest. 1993;91:2552‐2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qayyum S, Mohammad T, Slominski RM, et al. Vitamin D and lumisterol novel metabolites can inhibit SARS‐CoV‐2 replication machinery enzymes. Am J Physiol Endocrinol Metab. 2021;321(2):E246‐E251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Slominski AT, Chaiprasongsuk A, Janjetovic Z, et al. Photoprotective properties of vitamin D and Lumisterol hydroxyderivatives. Cell Biochem Biophys. 2020;78(2):165‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Slominski RM, Tuckey RC, Manna PR, et al. Extra‐adrenal glucocorticoid biosynthesis: implications for autoimmune and inflammatory disorders. Genes Immun. 2020;21(3):150‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abhimanyu X, Coussens AK. The role of UV radiation and vitamin D in the seasonality and outcomes of infectious disease. Photochem Photobiol Sci. 2017;16(3):314‐338. [DOI] [PubMed] [Google Scholar]
- 18. Slominski AT, Zmijewski MA, Plonka PM, Szaflarski JP, Paus R. How UV light touches the brain and endocrine system through skin, and why. Endocrinology. 2018;159(5):1992‐2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hart PH, Norval M. More than effects in skin: ultraviolet radiation‐induced changes in immune cells in human blood. Front Immunol. 2021;12:2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iyama S, Murase K, Sato T, et al. Narrowband ultraviolet B phototherapy ameliorates acute graft‐versus‐host disease by a mechanism involving in vivo expansion of CD4 + CD25 + Foxp3+ regulatory T cells. Int J Hematol. 2014;99(4):471‐476. [DOI] [PubMed] [Google Scholar]
- 21. van der Poll T, de Jonge E, ten Cate HA. Cytokines as regulators of coagulation. Madame curie Bioscience Database. Landes Bioscience; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cytokind meridien treatment regimen. Provided initial clinical dose in accordance with the patient's Fitzpatrick Skin Type. The escalating daily dose allows for a net increase of 10% dose by accounting for the residual dose from the previous treatments.
Table S2. Baseline characteristics and prescriptions reported by clinical outcomes in a randomized trial of the effect of daily narrowband ultraviolet B (NB‐UVB) on the outcomes of high‐risk hospitalized COVID‐19 patients.
Data Availability Statement
Research data are not shared.


