Abstract
C‐reactive protein (CRP), fibrinogen, and d‐dimer are determined in the human plasma of 2745 hospitalized patients with and without coronavirus disease 2019 (COVID‐19) by automated‐latex enhanced immunoassay and immuno‐turbidimetric assay. SARS‐COV‐2 RNA qualitative test, real time polymerase chain reaction (RT‐PCR) based, is performed in nasopharyngeal swabs to confirm those with SARS‐COV‐2 positivity. Furthermore, serum proteins are separated and quantified in all the patients by serum protein electrophoresis (SPE).
A new SPE parameter, inflammatory protein ratio (IPR), is elaborated for the first time by a mathematical equation that considers the albumin, α1‐globulin, and α2‐globulin. IPR normal reference range (10.7%–28.3%) is calculated considering the normal reference range of albumin, α1‐globulin, and α2‐globulin obtained for controls. Analysis of variance (ANOVA), Pearson's, Kruskal–Wallis, and Spearman's tests application show that IPR significantly correlates with direct proportionality with d‐dimer, CRP, and fibrinogen. Significant (p < 0.001) increase of these parameters, IPR included, is detected in COVID‐19 patients only. Our results show that IPR is more specific for monitoring inflammatory status thanks to its correlation with the only three serum proteins involved in inflammation: albumin, α1‐globulin, and α2‐globulin. Furthermore, IPR can simplify the interpretation of SPE results about inflammatory status, being of unique value compared to the six‐serum protein classes separately presented in the typical SPE clinical reports.
Keywords: albumin, COVID‐19, inflammatory state, SARS‐COV‐2, serum protein electrophoresis
Abbreviations
- CRP
C‐reactive‐protein
- IPR
inflammatory protein ratio
- SPE
serum protein electrophoresis
1. INTRODUCTION
During the coronavirus disease‐2019 (COVID‐19) pandemic, the admissions to the hospitals are significantly increased, stressing out the capacity of public health systems and resulting in a high mortality rate. This situation has been complicated further due to COVID‐19 highly variable clinical course; for instance, some individuals manifest severe inflammatory disease or death, and others remain asymptomatic. Even if genetic and clinical risk factors justify some of these outcomes [1, 2, 3, 4, 5], most of the host biological causes of these adverse COVID‐19 outcomes remain unknown.
Many biomarkers monitor the inflammatory severity of patients with COVID‐19 [6, 7, 8]; furthermore, several prognostic models have been built up with these biomarkers [9, 10, 11].
In recent studies, the role of laboratory findings in the prognosis of COVID‐19 [7, 12] has been assessed; in many of them, it has been shown that the severe inflammatory cases of COVID‐19 are associated with high levels of D‐dimer, fibrinogen, and C reactive protein (CRP) [12, 13, 14, 15]. d‐dimer is assessed because its levels are commonly high in most patients, who are associated with a worse prognosis [13], and because its concentration is highly correlated with the blood clot product in the disseminated intravascular coagulation, which typically characterizes the vast majority of patients died from COVID‐19 [14].
Fibrinogen is considered because it is helpful to promote the early diagnostics of hypercoagulability [15]; for instance, it has been observed that fibrinogen is significantly high in patients with severe disease than in healthy patients [13].
CRP is one of the most used biomarkers to evaluate the COVID‐19 inflammatory status [15], even though its use is limited by a low sensitivity for community‐acquired pneumonia (CAP). While a high CRP value can indicate a severe bacterial infection, lower values are shared in viral infectious diseases and other noninfectious diseases [16].
Despite the consideration of biochemical parameters (d‐dimer, fibrinogen, and CRP) in the definition of the COVID‐19 inflammatory status, there is a limitation related to the interpretation of these proteins; they need to be analyzed altogether for proper evaluation of the inflammatory status; this is because the special assessment of each of them does not provide enough information of that status. This limitation increases the complexity of monitoring the inflammatory status in COVID‐19 patients.
Limited research has been done on simplifying the routine techniques for assessing the inflammatory status in COVID‐19 patients [17]. Based on this limitation, serum protein electrophoresis (SPE) has been considered for the inflammatory status assessment [18, 19]. SPE is a fast laboratory technique that examines specific serum proteins into six main classes: albumin, α1‐, α2‐, β1‐, β2‐, and γ‐globulins. Although SPE analyses all these six serum protein classes, it is well known that only three of them undergo quantitative variation during inflammatory status respect to the normal values [20, 21]: albumin, α1, and α2‐globulins; for instance, in a COVID‐19 patient, a substantial increase of α1‐ and α2‐globulins and a drop of the albumin is observed during the early phase of the inflammation [22, 23]. Instead, no variation is observed for the other classes of serum proteins during this phase: β1‐, β2‐, and γ‐globulins [22, 23].
This study reassesses the SPE methodology and elaborates on a novel SPE parameter, inflammatory protein ratio (IPR), focusing only on the three serum proteins classes directly involved in the inflammatory status.
2. MATHEMATICAL EQUATIONS
It is well known that no variation is observed during the inflammatory phase for β1‐, β2‐, and γ‐globulins [22, 23]; based on this, the IPR is created in this study to try to describe more precisely the inflammation by focusing only on the three serum proteins that change in this status: albumin, α1‐, and α2‐globulins. The mathematical equation for the IPR is empirically deducted considering the increase of α1‐ and α2‐globulins and the decrease of albumin observed during the inflammatory process [22]; in fact, there is inverse proportionality among these three serum proteins during the inflammation (α1‐ and α2‐ increase and albumin decrease). Based on this, the IPR formula is:
| (1) |
For the calculation of the IPR normal reference range that will be used as a cut‐off to define if a patient's IPR value is normal or not, normal reference values of the albumin (54.7–69.6), α1‐, and α2‐globulins (α1 = 2.6–5.0; α2 = 4.9–10.5) reported in the V8 Serum Protein SPE Kit (Helena Laboratories, Product N 800500) are used in the IPR formula; this is applied for both the lower normal limit (LNL) and higher normal limit (HNL) of the IPR range:
| (2) |
| (3) |
The IPR normal reference range is between 10.7% and 28.3%. All the IPR values below LNL or above HNL are not considered normal for the patient.
3. MATERIALS AND METHODS
3.1. Study population
The present study has an observational, retrospective single‐center design. Data from 2745 patients hospitalized in different wards of the University Hospital “Ospedali Riuniti” of Foggia (Italy) in May 2021 are analyzed in this study.
The wards are selected by considering the re‐organization of the university hospital in response to the COVID‐19 pandemic; 18 wards are part of the COVID‐19 area and 18 of the Non‐COVID‐19 area (Table 1).
TABLE 1.
The number of patients in every ward of COVID‐19 and non‐COVID‐19 areas
| COVID‐19 area | Non‐COVID‐19 area | ||
|---|---|---|---|
| Ward | No. of patients | Ward | No. of patients |
| Post‐Surgery Resuscitation Centre | 23 | Resuscitation Centre | 11 |
| Cardiology | 39 | Thalassemia Centre | 25 |
| General Surgery | 28 | Haematology‐DH | 97 |
| Vascular Surgery | 10 | Gastroenterology‐DH | 18 |
| Thoracic Surgery | 30 | Nephrology‐DH | 30 |
| Emergency Surgery | 24 | Neurology‐DH | 24 |
| Haematology | 20 | Paediatrics | 25 |
| Hepatology | 93 | Outpatients | 531 |
| Gastroenterology | 70 | Gynaecology | 74 |
| Infectious Diseases | 66 | University Medicine | 55 |
| Respiratory Tract Diseases | 45 | Central Laboratory | 142 |
| General Medicine | 44 | Preventive Medicine | 103 |
| Nephrology | 52 | Neurosurgery | 21 |
| Neurology | 120 | Ophthalmology | 16 |
| Orthopaedics | 90 | Psychiatry | 21 |
| Pneumology | 27 | Urology | 36 |
| Emergency Room | 20 | SAUB Dialysis | 139 |
| Intensive Care Unit | 26 | Transfusion Medicine | 550 |
| Total | 827 | Total | 1918 |
Abbreviation: DH, Day Hospital.
Patients from the non‐COVID‐19 area are considered in this study as group control to assess the IPR parameter and compare it between COVID‐19 and non‐COVID‐19 patients.
The laboratory tests performed for the selected patients are SPE, SARS‐COV‐2 RNA detection by real‐time polymerase chain reaction (RT‐PCR), and quantification of d‐dimer, fibrinogen, and CRP.
3.2. Serum protein electrophoresis
SPE is performed using the V8 Capillary Electrophoresis analyzer (Helena Biosciences Europe, UK), an automated, quantitative, high‐throughput instrument. This system is based on a capillary isoelectric focusing technology that allows the separation of the serum proteins into six main classes (albumin, α1 globulin, α2 globulin, β1 globulin, β2 globulin, and γ globulin) by the use of the V8 Serum Protein SPE kit (Helena Laboratories, Product N 800500). The analyzer in six respective bands represents the six main classes; the quantization of the electrophoretic bands is performed by automatic programs that calculate the area under each every band and determine the respective serum protein concentration by comparison with specific reference intervals in percentage, which are albumin 54.7–69.6; α1 globulin 2.6–5.0; α2 globulin 4.9–10.5, β1 globulin 5.4–9.2; β2 globulin 2.4–7.1 and γ globulin 9.7–18.9; albumin/globulin ratio > 1 (Helena Laboratories, Product N 800500).
3.3. SARS‐COV‐2 RNA detection in nasopharyngeal swabs
For SARS‐COV‐2 RNA detection in nasopharyngeal swabs, viral RNA is extracted using the Qiamp viral RNA mini kit following the manufacturer's instructions (Qiagen GmbH, Product N 1048147). Specifically, the nasopharyngeal sample is lysed under highly denaturing conditions to inactivate RNases and ensure intact viral RNA isolation. Afterward, the isolated viral RNA is purified by loading the sample onto the QIAamp Mini spin column; during this step, the RNA binds to the membrane, and contaminants are efficiently washed away. High‐quality RNA is eluted in an RNase‐free buffer and stored in –20°C freezers. RT‐PCR of specific RNA genes (RNA‐dependent RNA polymerase, envelope, and nucleocapsid regions) is performed using the commercially available kit Allplex SARS‐COV‐2 Assay (Seegene, Product N RP10250X/RP10252W) with the CFX96 instrument (Bio‐Rad Laboratories, Inc., Hercules CA, USA). The Seegene Viewer software is used to interpret the amplified PCR products.
3.4. d‐dimer, fibrinogen and CRP quantification
For the determination of the d‐dimer, the HemosIL D‐Dimer HS is used (Instrumentation Laboratory, Product N 0020301700), an automated latex enhanced immunoassay coated with particular monoclonal antibodies, for the quantitative determination of d‐dimer in human citrated plasma on the ACL TOP 550 system (Instrumentation Laboratory, Lexington, MA, USA). When plasma containing d‐dimer is mixed with the latex reagent and the reaction buffer included in the D‐Dimer HS kit, the coated latex particles agglutinate. The degree of agglutination is directly proportional to the concentration of d‐dimer in the sample and is determined by measuring the decrease of the transmitted light caused by the aggregates (turbidimetric immunoassay).
The fibrinogen is quantified by the Clauss method [24] in human citrated plasma; this method is based on thrombin usage. This procedure adds excess thrombin to the diluted plasma, and the clot formation time is measured. A reference fibrinogen curve is also determined through the coagulation times determined with repeated fibrinogen measurements from a known plasma titer. The fibrinogen concentration in patient plasma samples is determined by comparing clotting time values to the reference curve.
An immune‐turbidimetric assay quantifies CRP on the Beckman Coulter 5800 Analyser (Beckman Coulter Inc., Brea CA, USA). During the sample preparation, the immune complexes are formed between CRP and goat anti‐CRP antibodies. The analyzer measures the reduction of incidence light due to reflection, absorption, or scatter, which is proportional to the CRP size, shape, and concentration in the serum sample. In this procedure, the measurement of the light intensity rate transmitted (increase in absorbance) through particles results from immune complexes formed during the antigen–antibody reaction.
3.5. Dataset creation
All the patients' SPE, d‐dimer, fibrinogen, CRP data, and the SARS‐COV‐2 nasopharyngeal swab results are extracted in sequential mode from the LIS program Winlab (Tesi Group, Italy). This program manages all the patient's related data from the laboratory analysis through structured query language (SQL) created by technicians considering the Laboratory Manager; the produced queries are created to select specific data according to parameters like period, department, and laboratory test. Afterward, data are retrieved by these queries, saved in Excel (v.14.2.2) as a text file to perform statistical analysis in the R statistical environment (v.4.1.0).
3.6. Statistical analysis
All analyses are performed using the R statistical environment (v.4.1.0). The threshold for the statistical significance applied for the statistical tests is 0.05 (5%).
Potential correlations between IPR and d‐dimer, fibrinogen, and CRP are evaluated using Pearson's or Spearman's correlation test.
A potential association between IPR and several wards is carried out using appropriate ANOVA or Kruskal–Wallis test. We also investigate the association between IPR and COVID19/Non‐COVID19 wards by using t‐test or Mann–Whitney test as appropriate.
Finally, the potential association between the outcome of the SARS‐COV‐2 nasopharyngeal swab, IPR, d‐dimer, fibrinogen, and CRP are evaluated by t‐test or Mann–Whitney test as appropriate.
4. RESULTS
Among 2745 patients enrolled in May 2021 as a whole, 2601 (94.8%) resulted in being negative for the SARS‐COV‐2 RNA detection in the nasopharyngeal swab, 144 (5.3%) resulted in being positive.
The distribution of all the IPR values per ward of COVID‐19 and non‐COVID‐19 areas is represented in Figure 1.
FIGURE 1.
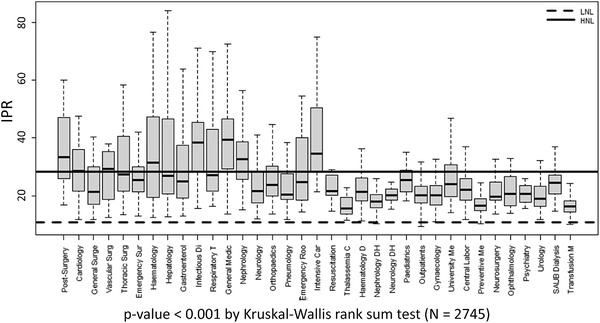
Distribution of IPR means per every department. Significantly high IPR values have been detected in patients located in the COVID‐19 wards compared to non‐COVID‐19 wards (27.26 [20.66–38.92] vs. 19.01 [16.10–22.83], respectively). LNL, lower normal limit (10.7%); HNL, higher normal limit (28.3%)
Considering the IPR of the patients resulted positive to the SARS‐COV‐2 RNA detection in nasopharyngeal swab: one out of 144 patients (0.7%) have the IPR value slightly below the LNL due to the slight decrease of α2‐globulin (Figure 2B), 131 (90.9%) have the IPR above the HNL, and 12 (8.4%) have the IPR within the normal range.
FIGURE 2.
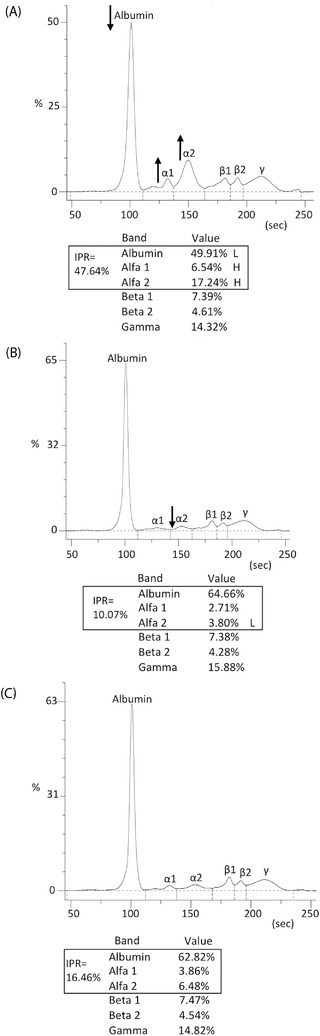
SPE results of three different hospitalized patients. (A) Patient in the intensive care unit with a consistent alteration of the serum proteins specifically decreased albumin and increased α1‐ and α2‐globulins. (B) Patient in infectious diseases ward with most serum proteins within the regular intervals, but α2‐globulin slightly low. (C) Patient in ophthalmology with all serum proteins within the regular intervals. The IPR value resulting from every patient is consistently different among them, in parallel with the related inflammatory status. The IPR shown in (A) is above HNL (HNL = 28.3%); the one shown in (B) is slightly below the LNL (LNL = 10.7%) due to the slight decrease of α2‐globulin; the IPR shown in (C) is within the regular intervals (between 10.7% and 28.3%). None of the three patients reported an alteration of the remaining three serum proteins, β1‐, β2‐, and γ‐globulin, all within the regular intervals. IPR, inflammatory protein ratio
The patients resulted negative to the SARS‐COV‐2 RNA detection in nasopharyngeal swab: 529 out of 2601 (20.3%) have the IPR value above the HNL, and 2072 (79.7%) have the IPR value within the normal range.
A representative example of the different IPR values obtained among all the patients with different inflammatory severity is shown in Figure 2; specifically, the SPE of three patients in three different wards (Intensive Care Unit, Infectious Diseases, and Ophthalmology) are reported in this figure.
Comparing the novel IPR with the largely used parameters CRP, d‐dimer, and fibrinogen, a correlation with direct proportionality is found between IPR and CRP (rho = 0.740, p < 0.001 by Spearman's correlation test, Figure 3A), IPR and d‐dimer (rho = 0.315, p < 0.001 by Spearman's correlation test, Figure 3B), IPR and fibrinogen (rho = 0.636, p < 0.001 by Spearman's correlation test, Figure 3C). The median [first quartile, Q1 – third quartile, Q3] of IPR, d‐dimer, fibrinogen, and CRP is significantly higher in COVID‐19 area wards compared to non‐COVID‐19 area (IPR: 27.26 [20.66–38.92] vs. 19.01 [16.1–22.83], p < 0.001 by Mann–Whitney test, Figure 4A; d‐dimer: 982 [454.8–2098.8] vs. 803 [371–1702], p = 0.036 by Mann–Whitney test, Figure 4B; fibrinogen: 336 [262–463] vs. 294 [241–345], p < 0.001, by Mann–Whitney test, Figure 4C; CRP: 10.70 [1.8–50.95] vs. 2 [0.8–5.4], p < 0.001 by Mann–Whitney test, Figure 4D).
FIGURE 3.
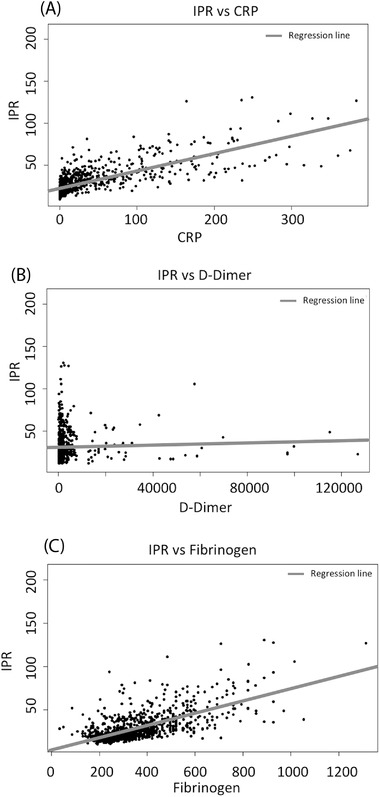
The correlation between IPR with the biomarker was analyzed in this study by performing Spearman's correlation test. (A) Direct proportionality between IPR and CRP with rho = 0.740 and p < 0.001. (B) Direct proportionality between IPR and d‐dimer with rho = 0.315 and p < 0.001. (C) Direct proportionality between IPR and fibrinogen with rho = 0.636 and p < 0.001. IPR, inflammatory protein ratio; CRP, C‐reactive protein
FIGURE 4.
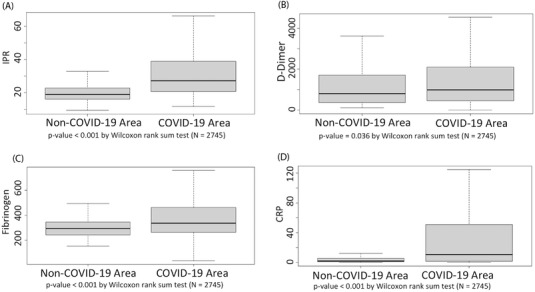
Variation of laboratory findings values of (A) IPR, (B) d‐dimer, (C) fibrinogen, and (D) CRP in all the wards of COVID‐19 area versus non‐COVID‐19 area; IPR, inflammatory protein ratio; CRP, C‐reactive protein
ANOVA test has not revealed any significant variation of the other serum proteins (β1‐, β2‐, and γ‐globulin) in all the 2745 patients, as shown in the electrophoretic traces in Figure 2.
5. DISCUSSION
The progression to severe inflammatory status of patients with COVID‐19 is problematic to monitor [25, 26, 27].
Several laboratory parameters have been considered to monitor the inflammatory status in COVID‐19 patients, such as CRP, d‐dimer, fibrinogen, and serum proteins [12, 18, 19].
The largely proven involvement of serum proteins in the inflammatory progression during COVID‐19 [28, 29] has brought SPE into one of the main biochemical techniques used to monitor the inflammatory status in COVID‐19 patients. For instance, healthcare specialists perform the SPE technique in many hospital laboratories and analyze reports containing electrophoretic traces of COVID‐19 and non‐COVID‐19 patients. Most of these electrophoretic traces of COVID‐19 patients with an altered inflammatory status are characterized by only an albumin drop and increase of α1‐ and α2‐globulins, without the interest of the other three classes of the serum proteins separated by SPE (β1, β2, and γ‐globulins), which result to be within the normal intervals. Based on this consideration, in our study, we have analyzed the SPE results of hospitalized patients and have created, for the first time to our knowledge, a novel parameter, IPR, with the purpose to monitor, more specifically, the inflammatory status of the patients by using a standard technique, which is the SPE.
The first thing to notice about the obtained IPR results is that IPR significantly correlates with the inflammatory conditions of the patient; in fact, most of the obtained IPR values of COVID‐19 patients (131 out of 144, 90.9%) are higher than the normal reference limit, differently to the IPR values of Non‐COVID‐19 patients that are within the normal reference range (between 13.7% and 22.3%). This correlation is also confirmed by the parallel and significant increase, for COVID‐19 patients, of the other parameters primarily used in the biochemical laboratory routine: CRP, fibrinogen, and d‐dimer. The IPR increase, along with the other three parameters, in COVID‐19 patients could represent an indicator of clinical complications compared to non‐COVID‐19 patients. However, this aspect was not investigated in this study due to the inability to access clinical information relating to these patients.
The correlation with direct proportionality of IPR with CRP, fibrinogen, and d‐dimer proves that IPR changes when there is an alteration of the inflammatory status, just like the other three parameters. These findings bring interest in considering IPR, together with the already used parameters, for monitoring the inflammatory status of COVID‐19 patients, thanks to its reliability in responding to the inflammatory alterations.
It is also important to note that the SPE technique used to obtain the IPR is less expensive than the other techniques used for the CRP, fibrinogen, and d‐dimer quantification: immune‐turbidimetric assay, coagulation test, and immunoassay, respectively.
The implementation of the IPR parameter in the SPE report could contribute to monitoring the inflammatory process; this is due to the mathematical equation used to obtain the IPR, which is based only on the three main serum proteins involved in the inflammation (albumin, α1‐, and α2‐globulins) without the involvement of those which are not (β1‐, β2‐, and γ‐globulins).
The limitation of our study is that the SARS‐COV‐2 RNA tests, used to confirm the COVID‐19 positivity, have been performed at a different time compared to SPE; we believe that this time difference has impacted the incongruence observed in some patients (9.1%), in which, despite the confirmed SARS‐COV‐2 positivity, the IPR values are within the normal reference range (between 10.7% and 28.3%).
6. CONCLUDING REMARKS
Our study shows that the IPR represents a more precise way to monitor the inflammatory status of COVID‐19 and non‐COVID‐19 patients through the SPE technique, thanks to its correlation with only those serum proteins directly involved in the inflammatory process: albumin, α1‐, and α2‐globulins. In conclusion, IPR could be added alongside the singular serum protein values in the SPE reports, improving the inflammatory monitoring that electrophoretic trace provides, together with the other biochemical parameters, without any added cost. Further studies would contribute additional information to this field of research.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
Antonucci F, Di Carlo D, Falcone M. Evaluation of a potential prognostic parameter for the inflammatory status in COVID‐19 patients: the inflammatory protein ratio. Electrophoresis. 2022;43:1647–1654. 10.1002/elps.202100396
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Niemi MEK, Karjalainen J, Liao RG, Neale BM, Daly M, Ganna A, et al. Mapping the human genetic architecture of COVID‐19. Nature. 2021;600(7889):472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou S, Butler‐Laporte G, Nakanishi T, Morrison DR, Afilalo J, Afilalo M, et al. A neanderthal OAS1 isoform protects individuals of European ancestry against COVID‐19 susceptibility and severity. Nat Med. 2021;27(4):659–67. [DOI] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nakanishi T, Pigazzini S, Degenhardt F, Cordioli M, Butler‐Laporte G, Maya‐Miles D, et al. Age‐dependent impact of the major common genetic risk factor for COVID‐19 on severity and mortality. J Clin Invest. 2021;131(23):e152386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584(7821):430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yan L, Zhang HT, Goncalves J, Xiao Y, Wang M, Guo Y, et al. An interpretable mortality prediction model for COVID‐19 patients. Nat Mach Intell. 2020;2:283–8. [Google Scholar]
- 7. Samprathi M, Jayashree M. Biomarkers in COVID‐19: an up‐to‐date review. Front Pediatr. 2021;8:607647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li X, To KKW. Biomarkers for severe COVID‐19. EBioMedicine. 2021;68:103405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Letelier P, Encina N, Morales P, Riffo A, Silva H, Riquelme I, et al. Role of biochemical markers in the monitoring of COVID‐19 patients. J Med Biochem. 2021;40(2):115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gemcioglu E, Davutoglu M, Catalbas R, Karabuga B, Kaptan E, Aypak A, et al. Predictive values of biochemical markers as early indicators for severe COVID‐19 cases in admission. Future Virol. 2021;16(5):353–67. [Google Scholar]
- 11. Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, et al. Prediction models for diagnosis and prognosis of covid‐19: systematic review and critical appraisal. BMJ. 2020;369:m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao Y, Li T, Han M, Li X, Wu D, Xu Y, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID‐19. J Med Virol. 2020;92(7):791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spiezia L, Boscolo A, Poletto F, Cerruti L, Tiberio I, Campello E, et al. COVID‐19‐related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120(6):998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang L. C‐reactive protein levels in the early stage of COVID‐19. Med Mal Infect. 2020;50:332–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Melbye H, Stocks N. Point of care testing for C‐reactive protein—a new path for Australian GPs? Aust Fam Physician. 2006;35(7):513–7. [PubMed] [Google Scholar]
- 17. Zhou R, Li F, Chen F, Liu H, Zheng J, Lei C, et al. Viral dynamics in asymptomatic patients with COVID‐19. Int J Infect Dis. 2020;96:288–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen C, Zhang Y, Zhao X, Tao M, Yan W, Fu Y. Hypoalbuminemia—an indicator of the severity and prognosis of COVID‐19 patients: a multicentre retrospective analysis. Infect Drug Resist. 2021;14:3699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garg S, Singh VK, Sonkar SC, Kelkar H, Singh S, Garg S, et al. Pattern of serum protein capillary electrophoretogram in SARS‐CoV‐2 infection. Clin Chim Acta. 2022;527:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henok JN, Okeleye BI, Omodanisi EI, Ntwampe SKO, Aboua YG. Analysis of reference ranges of total serum protein in Namibia: clinical implications. Proteomes. 2020;8(2):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lippi G, Battistelli L, Vernocchi A, Mussap M. Analytical evaluation of the novel Helena v8 capillary electrophoresis system. J Med Biochem. 2013;32:245–9. [Google Scholar]
- 22. Vavricka SR, Burri E, Beglinger C, Degen L, Manz M. Serum protein electrophoresis: an underused but very useful test. Digestion. 2009;79(4):203–10. [DOI] [PubMed] [Google Scholar]
- 23. Feketea GM, Vlacha V. The diagnostic significance of usual biochemical parameters in coronavirus disease 19 (COVID‐19): albumin to globulin ratio and CRP to albumin ratio. Front Med (Lausanne). 2020;7:566591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stang LJ, Mitchell LG. Fibrinogen. Methods Mol Biol. 2013;992:181–92. [DOI] [PubMed] [Google Scholar]
- 25. Gallo Marin B, Aghagoli G, Lavine K, Yang L, Siff EJ, Chiang SS, et al. Predictors of COVID‐19 severity: a literature review. Rev Med Virol. 2021;31(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang J, Zheng X, Chen J. Clinical progression and outcomes of 260 patients with severe COVID‐19: an observational study. Sci Rep. 2021;11(1):3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Modrák M, Bürkner PC, Sieger T, Slisz T, Vašáková M, Mesežnikov G, et al. Disease progression of 213 patients hospitalized with Covid‐19 in the Czech Republic in March‐October 2020: an exploratory analysis. PLoS One. 2021;16(10):e0245103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang J, Wang X, Jia X, Li J, Hu K, Chen G, et al. Risk factors for disease severity, unimprovement, and mortality in COVID‐19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26(6):767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kheir M, Saleem F, Wang C, Mann A, Chua J. Higher albumin levels on admission predict better prognosis in patients with confirmed COVID‐19. PLoS One. 2021;16(3):e0248358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


