Abstract
Aim
To identify, appraise and synthesize the available evidence on the impact of the coronavirus disease 2019 (COVID‐19) pandemic and lockdown (LD) on glycaemic control in people with diabetes.
Materials and Methods
We searched multiple databases up to 2 February 2021 for studies reporting HbA1c, time in range (TIR), average or fasting glucose, severe hypoglycaemia and diabetic ketoacidosis. Data were pooled using random effects meta‐analysis and are presented as mean difference (MD) with 95% confidence intervals (CI). This review was preregistered on PROSPERO (CRD42020179319).
Results
We include 59 studies; 44 (n = 15 464) were included in quantitative syntheses and 15 were narratively synthesized. Pooled data were grouped by diabetes type. Results from 28 studies (n = 5048 type 1 diabetes [T1D] and combined diabetes participants) showed that TIR increased during LD compared with before LD (MD 2.74%, 95% CI 1.80% to 3.69%). Data from 10 studies (n = 1294 T1D participants) showed that TIR increased after LD compared with before LD (MD 5.14%, 95% CI 3.12% to 7.16%). Pooled results from 12 studies (n = 4810 T1D and type 2 diabetes participants) resulted in average glucose decreasing after LD compared with before LD (MD –6.86 mg/dl, 95% CI –8.54 to –5.18). Results for other outcomes, including HbA1c, were not statistically significantly different.
Conclusions
The COVID‐19 pandemic was associated with small improvements across multiple outcomes of glycaemic control, although there was insufficient evidence to suggest that this led to changes in HbA1c. Most evidence came from people with access to diabetes technologies in high‐income countries; more research is needed in less advantaged populations.
Keywords: continuous glucose monitoring, systematic review, type 1 diabetes, type 2 diabetes
1. INTRODUCTION
On 11 March 2020, the World Health Organization declared the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) outbreak a pandemic. 1 Early into the first wave of the pandemic it became apparent that certain patient demographics and co‐morbidities were associated with a higher risk of adverse outcomes following initial infection, including in people living with diabetes. 2 , 3 Compared with individuals without diabetes, the risk of fatal or critical care unit‐treated coronavirus disease 2019 (COVID‐19) is significantly higher in people with diabetes. 4 Not only have individuals with diabetes been disproportionately affected by the initial infection, but also by the changes made to their diabetes care following restrictions imposed during lockdowns (LDs), with usual, regular face‐to‐face access to healthcare providers often severely disrupted. 5
These disruptions may conceivably impact glycaemic control, but to what extent the ‘lockdown effect’ has impacted the management of diabetes and subsequent glucose control remains unclear. Key limitations hamper the utility of existing systematic reviews in this area. For example, one existing review reports that LD improved glycaemic control; however, the review only included adults with well‐controlled type 1 diabetes who had access to continuous or flash glucose monitoring. 6 This selection bias limits the generalizability of the findings, as globally comparatively few people have access to these devices, and individual studies have shown that socioeconomic deprivation could increase the risk of decline in glycaemic control. 7 Another review concluded LD may have improved glycaemic control in those with type 1 diabetes but not in those with type 2 diabetes. 8 However, this review did not consider the impact of many key variables on these estimates, including geographical location.
The impact of COVID‐19 on glycaemic control has important implications, both for targeting care to those whose control has been most affected by the pandemic, and, where glycaemic control has improved, for learning lessons to maintain these improvements as disruptions slow or if LD restrictions are imposed again. Therefore, to facilitate evidence‐informed policy and practice, in this review we set out to identify, appraise and synthesize all the available relevant evidence on the impact of the COVID‐19 pandemic on glycaemic control in people with diabetes, including people with any type of diabetes. We further sought to synthesize evidence on the differential impact of geographical location.
2. MATERIALS AND METHODS
2.1. Data sources and searches
This systematic review and meta‐analysis was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. A protocol was prospectively registered at https://www.crd.york.ac.uk/prospero/ as CRD42020179319. The volume of literature returned following searches led us to specifically focus on glycaemic control outcomes as related to COVID‐19 in this paper (other outcomes will be covered in separate pieces of work), hence following data extraction but prior to data analysis a more detailed analysis plan was also prespecified and registered (https://osf.io/kd2wf). Databases were searched up to 2 February 2021 and included Medline, EMBASE, TRIP, Google Scholar and LitCOVID. Predetermined search terms are provided in Table S1. No language or date of publication restrictions were applied, and reference lists of eligible studies and relevant reviews were also searched to identify additional relevant studies.
2.2. Study selection
We included all empirical studies investigating the effect of the COVID‐19 pandemic on glycaemic control in people with diabetes. Case reports and animal studies were excluded. Studies must have reported at least one of the following glycaemic outcomes: HbA1c (including estimated); time in range (TIR); average glucose; fasting glucose; severe hypoglycaemia; or diabetic ketoacidosis (DKA). Exclusion criteria included: unclear time frames; individuals with newly diagnosed diabetes (<12 months); those without diabetes or where results were not reported in a subgroup of people with diabetes; or not in the context of COVID‐19. Where it was not possible to extract means and SDs for relevant outcomes, they were excluded from the meta‐analyses and narratively synthesized. When SE or 95% CI were the only measures of variance reported they were converted according to Cochrane guidelines. 9
2.3. Data extraction and quality assessment
Titles, abstracts and full‐text articles were evaluated independently by two reviewers to determine whether they met the eligibility criteria using Covidence online software (Melbourne, Australia). Disagreements were resolved by consensus or a third reviewer. Data extraction was performed by two independent reviewers and followed Cochrane methods using a prespecified and piloted data extraction form. Fields included: study dates; study type; funding source; authors’ conflicts of interest; country; setting; COVID‐19 context; population characteristics; intervention details (where relevant); and outcomes as specified above. The quality of included studies was evaluated by the Newcastle‐Ottawa Scale 10 ; scores of 3 or less were considered as having a high risk of bias, as previously reported. 11
2.4. Data synthesis and analysis
Random effects meta‐analyses were performed using Review Manager Software (version 5.3; RevMan, Cochrane Collaboration, Copenhagen, Denmark). Results are grouped by outcome and time period of comparison, and subgrouped by diabetes type and geographical location. All meta‐analysable outcomes were continuous and effect size is presented as weighted mean difference (95% CI). Heterogeneity between studies was assessed using the I 2 statistic, where 0%‐40% suggested heterogeneity may be trivial, 30%‐60% represents moderate heterogeneity, 50%‐90% represent substantial heterogeneity, and 75% and above represents considerable heterogeneity. 9 Where possible, sensitivity analysis was performed excluding studies with a potential high risk of bias (Newcastle‐Ottawa Scale ≤3). Where individual studies analysed between‐group differences, these are synthesized narratively.
3. RESULTS
In total, 7372 titles were identified through database searches, of which 59 were included in this review; 44 (n = 15 464) were included in the quantitative synthesis and 15 were narratively synthesized (Figure 1). Of the 44 studies that we were able to meta‐analyse, the mean age of patients ranged from 7 to 63 years. All patients were diagnosed with diabetes: type 1 diabetes (n = 33 studies), type 2 diabetes (n = 8 studies), a combination of various diabetes types (n = 2), or diabetes type not specified (n = 1). Overall, we judged four studies to be at a high risk of bias (Newcastle‐Ottawa Scale ≤3) (Table S2). 12 , 13 , 14 , 15 Studies were conducted in Europe (n = 33) and Asia (n = 11). Specific countries included Italy (n = 15), India (n = 5), Spain (n = 6), Greece (n = 3), Saudi Arabia (n = 2), Turkey (n = 2), the UK (n = 4) and China, France, Israel, Jordan, Korea, the Netherlands and Slovenia (one study each). Descriptive and raw data of studies included in the quantitative synthesis are provided (Table S3).
FIGURE 1.

Flowchart of trial selection
3.1. HbA1c
3.1.1. Before versus during LD
Pooled results from 16 studies including 22 076 participants showed that HbA1c before LD and during LD did not differ significantly overall (mean difference −0.10%, 95% CI −0.22% to 0.01%; P = .09) (Figure S1). Overall, the degree of heterogeneity between studies was substantial (I 2 = 72%). This heterogeneity was not explained by diabetes type (test for subgroup differences, P = .87; I 2 = 0%) or geographical location (test for subgroup differences, P = .14, I 2 = 55.0%). Although not statistically significant, heterogeneity was reduced in the subgroup of studies conducted in Europe with the pooled effect estimate favouring HbA1c during LD (mean difference −0.17%, 95% CI −0.24% to −0.10%; P < .001; I 2 = 13%), but not in Asia (mean difference 0.08%, 95% CI −0.24% to 0.40%; P = .63; I 2 = 82%). Removing studies at high risk of bias (n = 3) did not change the statistical interpretation of the test (mean difference −0.07%, 95% CI −0.22% to 0.08%, I 2 = 70%; P = .34). One study was a visible outlier, 16 and in a post hoc sensitivity analysis removing this study, I 2 was reduced to 21%, moderating the statistical interpretation of the results from non‐significant to significant (mean difference −0.17%, 95% CI −0.25% to −0.09%; P <.001) (Figure S2). Verma et al. 16 (n = 52) was the only study to report significant disruptions to diabetes care in its participants: it found a statistically significant increase in HbA1c during compared with before LD (mean difference 1.20%, 95% CI 0.66% to 1.74%).
3.1.2. Before versus after LD
Pooled results from nine studies including 2686 participants showed that HbA1c before and after LD did not differ significantly overall (mean difference −0.15%, 95% CI −0.32% to 0.03%; P = .10); again, substantial heterogeneity between studies was observed (I 2 = 70%) (Figure S3). The test for subgroup differences did not indicate a statistically significant subgroup effect for diabetes type (test for subgroup differences, P = .08, I 2 = 66.5%), although the pooled effect estimate favoured HbA1c after LD in type 1 diabetes (P = .01), but not type 2 diabetes (P = .95). However, there was substantial unexplained heterogeneity between the trials within each of these subgroups (type 1 diabetes: I 2 = 56%; type 2 diabetes: I 2 = 62%). The test for subgroup differences indicated no statistically significant subgroup difference for geographical location (P = .49, I 2 = 0%).
3.1.3. Narrative analysis
Nine studies could not be included in the quantitative synthesis for the following reasons: studies compared HbA1c values during LD with the same time period(s) before LD (n = 4), absence of mean values (n = 1), HbA1c assessed in response to an intervention (n = 2), only range was reported as a measure of variance (n = 1), or diabetes type was not specified (n = 1). These studies are narratively synthesized in Table 1 or described below.
TABLE 1.
Effect direction plot for studies synthesized without meta‐analysis
| Authors | COVID‐19 context | Diabetes type | Population (sample size) | Risk of bias | DKA | Fasting glucose | Severe hypoglycaemia | Mean glucose | HbA1c (if not in MA) |
|---|---|---|---|---|---|---|---|---|---|
| Al Agha et al. (2021) 12 | Before and during LD | Type 1 diabetes (100%) | Children (n = 150) | High (3) | ◄► | — | — | — | — |
| Barchetta et al. (2020) 17 | Before and during LD | Type 1 diabetes (100%) | Adults (n = 50) | Low (4) | — | — | ▼ | — | — |
| Biancalana et al. (2020) 18 | Before and during LD | Type 1 diabetes (100%) | Adults (n = 114) | Low (5) | — | ◄► | — | — | — |
| Dovc et al. (2020) 19 | Before and during LD | Type 1 diabetes (100%) |
Children, adolescents, and young adults (n = 234) |
Low (6) | ◄►, ** | — | ◄►, ** | — | — |
| Khare et al. (2020) 20 | Before and during LD | Type 2 diabetes (100%) | Adults (n = 143) | Low (4) | — | ◄► | — | — | — |
| Pla et al. (2020) 21 | Before and during LD | Type 1 diabetes (100%) | Adults (n = 50) | Low (7) | — | — | ◄► | — | — |
| Khader et al. (2020) 22 | Subjective comparison with prepandemic |
Type 2 diabetes (87.01%) Type 1 diabetes (1.39%) Gestational diabetes (6.82%) Other (4.76%) |
Adults (n = 1510) | High (3) | ▼, ** | ||||
| Kamrath et al. (2020) 23 | Compared with the same period pre‐LD | Newly diagnosed type 1 diabetes (100%) | Children (n = 1491) | Low (7) | ▼ 2020 vs. 2018 ▼ 2020 vs. 2019 | — | — | — | — |
| Lawrence et al. (2021) 24 | Compared with the same period pre‐LD (Mar to May of the previous 5 y [prepandemic periods]) | Type 1 diabetes (100%) | Children (n = 11) | Low (7) | ▼ | — | — | ▼ | ◄► |
| Ludvigsson et al. (2020) 25 | Compared with the same period pre‐LD (Jan–Jul 2018, 2019 and 2020) | Type 1 diabetes (100%) | Children (NS) § | Low (5) | ◄►, ** | ||||
| Lui et al. (2020) 26 | Compared with the same period pre‐LD (25 Jan–24 Apr 2019 [inter‐year control] and 25 Oct 2019–24 Jan 2020 [intra‐year control]) | Type 2 diabetes (*) | Adults (n = 1503) | Low (5) | ◄► | — | ▲ | — | ◄► |
| Misra et al. (2021) 27 | Compared with the same period pre‐LD (comparison of DKA in adults during the SARS‐CoV‐2 outbreak and over the same time period for the preceding 3 years) |
Type 1 diabetes (68%) Type 2 diabetes (17%) Other/new (15%) |
Adults (n = 19) | Low (8) | ◄► | — | — | — | — |
| Odeh et al. (2020) 28 | Before and after LD | Type 1 diabetes (100%) | Children (n = 235) | Low (4) | ◄►, ** | — | — | — | — |
| Önmez et al. (2020) 29 | Before and after LD | Type 2 diabetes (100%) | Adults (n = 101) | Low (4) | — | ◄► | — | ||
| Khare et al. (2020) 20 | Before and after LD | Type 2 diabetes (100%) | Adults (n = 143) | Low (4) | — | ▼ | — | — | *** |
Abbreviations: DKA, diabetic ketoacidosis, LD, lockdown; MA, meta‐analysis; NS, not significant; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
, statistically significant (P < .05) positive health impact.
, statistically significant (P < .05) negative health impact.
, no statistically significant change observed (P ≥ .05).
Risk of bias assessed using the Newcastle‐Ottawa scale: high risk of bias ≤3; low risk of bias >3.
*The majority had long‐standing type 2 diabetes (median 18.0 y).
**Only report no change/deterioration (P values were not provided).
***Not included in MA as only the range was reported for variance.
Children aged <10 y. ‡Adolescents aged >10 y.
Nationwide information on about 7000 children aged <18 y is available.
Data from a study including 161 and seven type 2 and type 1 diabetes patients, respectively, reported the number of patients for whom HbA1c had improved (n = 51,) worsened (n = 57) or not changed (n = 60), but did not report mean values or statistical evaluation. 30 In a retrospective cohort study of pregnant women with diabetes managed either during the COVID‐19 pandemic or 1 year earlier, no statistically significant differences in change in HbA1c were observed. 31 Two studies assessed the impact of telehealth during LD on HbA1c, both of which reported significant improvements. People with type 1 diabetes scheduled for an in‐clinic visit during LD were offered to participate in an alternative telehealth visit. Glucose management data were recorded 2 weeks prior and 2 weeks after the telehealth visit in paediatrics and young adults and a significant improvement in estimated HbA1c (glycaemic metabolic index) was observed following the single telehealth visit. 32 Over a period of 4 months, statistically significant improvements in HbA1c were also observed among adults with type 2 diabetes who had uncontrolled diabetes that attended a virtual clinic with frequent appointments every 1‐2 weeks during LD. 33 In 307 type 2 diabetes patients, a study that only reported range as a measure of variance found that HbA1c was significantly higher and therefore worsened immediately post‐LD (before LD = mean [range] 7.85% [6.1%‐13.0%], after LD = 8.37% [6.0%‐15.0%]), 34 whereas in a study where diabetes type was not specified (n = 1927 participants), HbA1c was found to be significantly lower during LD (before LD: 8.2% ± 1.47% [66 ± 16.1 mmol/mol], during LD: 8.1% ± 1.59% [65 ± 17.4 mmol/mol]; P = .005). 35
3.2. Time in range
3.2.1. Before versus during LD
Pooled results from 28 studies including 5048 type 1 diabetes and combined diabetes patients showed that TIR statistically significantly increased during LD when compared with before LD (mean difference 2.74%, 95% CI 1.80% to 3.69%; P <.001), with no heterogeneity observed between studies (I 2 = 0%) (Figure 2). The test for subgroup differences indicated no statistically significant subgroup difference for diabetes type (P = .60, I 2 = 0%) or geographical location (P = .55, I 2 = 0%). Only one study was considered at high risk of bias and removal of this study did not change the statistical interpretation of the test (mean difference 2.72%, 95% CI 1.76% to 3.68%; P <.001).
FIGURE 2.

Forest plot of effect sizes (means ± 95% confidence intervals) for studies evaluating time in range (TIR) before and during lockdown (LD). T1D, type 1 diabetes; T2D, type 2 diabetes
3.2.2. Before versus after LD
Data from 10 studies, comprising 1294 type 1 diabetes participants, resulted in TIR significantly increasing after LD compared with before LD (mean difference 5.14%, 95% CI 3.12% to 7.16%; P < .001), with moderate heterogeneity observed between studies (I 2 = 37%) (Figure 3). The test for subgroup differences suggests that there is a statistically significant subgroup effect for TIR based on geographical location (P = .01). TIR was significantly higher after LD among studies conducted in Europe (mean difference 6.35%, 95% CI 4.91% to 7.80%; P < .001), but not in Asia (mean difference −0.68%, 95% CI −5.96% to 4.60%; P = .80). However, a far smaller number of trials and participants contributed data to the Asian subgroup (one study, 43 participants) than to the Europe subgroup (nine studies, 604 participants), meaning that the analysis is unlikely to produce meaningful conclusions based on geographical location. All studies were conducted in type 1 diabetes, and none were considered to be at a high risk of bias (Newcastle‐Ottawa Scale >3), therefore subgroup analysis by diabetes type and removing studies at a high risk of bias was not applicable.
FIGURE 3.
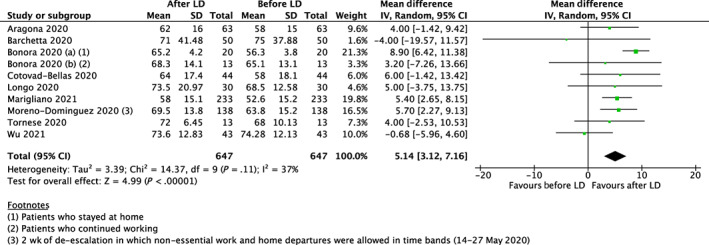
Forest plot of effect sizes (means ± 95% confidence intervals) for studies evaluating time in range (TIR) before and after lockdown (LD)
3.2.3. Intervention studies
A randomized controlled trial investigated the effect of remote management during LD on TIR in 160 patients (intervention group n = 80; control group n = 80). Compared with the control group, TIR was significantly higher in the remote management group (baseline: 43% ± 24.56% vs. 3 months: 63% ± 48.74%; P < .001). 36
3.3. Average glucose
3.3.1. Before versus during LD
Data from 27 studies, comprising 4546 type 1 diabetes participants, showed that, compared with before LD, average glucose values remained unchanged during LD (mean difference 0.09 mg/dl, 95% CI −3.42 to 3.61; P = .96), with moderate heterogeneity observed between studies (I 2 = 59%) (Figure S4). The test for subgroup differences indicated no statistically significant subgroup difference for geographical location (P = .17, I 2 = 46.3%), and subgroup by diabetes type was not applicable, as all studies were conducted in type 1 diabetes patients. Removing studies at a high risk of bias (n = 3) did not change the statistical interpretation of the test (mean difference −0.94%, 95% CI −3.94% to 2.05%; P = .54).
3.3.2. Before versus after LD
Data from 12 studies, comprising 4810 type 1 diabetes and type 2 diabetes participants, resulted in average glucose statistically significantly decreasing after LD compared with before LD (mean difference −6.86 mg/dl, 95% CI −8.54 to −5.18; P < .001), with no heterogeneity observed between studies (I 2 = 0%) (Figure 4). The test for subgroup differences indicated no statistically significant subgroup difference for diabetes type (P = .28, I 2 = 12.9%) or geographical location (P = .18, I 2 = 45.3%). No studies were judged to be at a high risk of bias (all had Newcastle‐Ottawa Scale >3), therefore removing studies at a high risk of bias was not applicable.
FIGURE 4.

Forest plot of effect sizes (means ± 95% confidence intervals) for studies evaluating average glucose before and after lockdown (LD)
3.3.3. Before versus after telemedicine/other
Only two studies, including 266 patients, were meta‐analysed. Average glucose was lower following telemedicine, but the difference was not statistically significant (mean difference −4.29 mg/dl, 95% CI −8.67 to 0.10; P = .06), with no heterogeneity observed between studies (I 2 = 0%).
Four studies not included in the quantitative synthesis reported a subjective comparison with prepandemic for glucose control among 4538 patients (Table S4). The percentage of patients reporting a worsening in glycaemic control varied considerably between studies, ranging from 13.5% to 78.4%. Two of the four studies reported improvements in glycaemic control; again, the percentage of patients reporting improvements varied considerably, from 7.0% to 42.7%. Of note, risk of bias was considered high in three of the four studies.
3.4. Outcomes synthesized without meta‐analysis
Because of the limited availability of data, fasting glucose, severe hypoglycaemia and DKA were synthesized without meta‐analysis (Table 1). Please see the supporting information for within‐study differences.
4. DISCUSSION
This comprehensive systematic review and meta‐analysis includes 59 studies, of which 44 and a total of 15 464 people with diabetes were quantitatively synthesized. Overall, glycaemic control tended to improve or remain unchanged during and following LD compared with before LD. Quantitively, no pooled results indicated a worsening of glycaemic control, although unexplained statistical heterogeneity limits certainty in many of the outcomes.
Our observed improvements in glycaemic control during LD are mostly consistent with previous research. 6 , 8 , 37 One meta‐analysis reported a statistically significant decrease in HbA1c during LD; this was not observed in our review. 6 In addition to a greater number of studies and patients included in the current review, this discrepancy could be attributed to the previous review only including estimated HbA1c and glucose management indicator, which may not as accurately reflect laboratory‐derived HbA1c values. 38 , 39 Statistical heterogeneity probably in part reflects the range of different contexts in which these studies were conducted. Most notably, the removal of Verma et al. 16 moderated the statistical interpretation of our pooled analysis of HbA1c before compared with during LD and decreased the observed heterogeneity. This was the only study in which HbA1c statistically significantly increased during LD, and the only study that described disruptions to healthcare access. It was also one of the few studies from a low‐income setting. The authors report that 27% and 39% of the population assessed missed insulin administration and blood glucose monitoring, respectively. This was attributed to either non‐availability of insulin/glucostrips during LD, financial issues, or other unspecified reasons. 16 Additionally, one patient who experienced DKA did so because of managing their diabetes with homeopathic medications only, and two of five coeliac patients did not have access to gluten‐free food.
In contrast to Verma et al., studies in other settings largely showed no difference or an improvement in glycaemic control. The latter is particularly interesting to note as it may point to targets for future interventions. A multitude of reasons could contribute to the improvements observed in glycaemic control among people with diabetes. First, it was widely publicized that having either type 1 diabetes or type 2 diabetes was a major risk factor for becoming severely ill from COVID‐19, 40 which in turn could have increased management efforts among individuals in people with diabetes. Another possible reason for improved glycaemic control could be attributed to more predictable daily schedules, including mealtimes and sleeping habits. 6 This is supported by a study we narratively synthesized that found TIR and average glucose improved in those who stopped working during LD, but not in those who continued to work. 41 However, another study reported that TIR statistically significantly decreased in individuals with type 1 diabetes who stopped working during LD. 17 Further research is needed to ascertain the reasons for improved glycaemic control so people with diabetes can, in turn, continue to benefit from lessons learnt during LD and apply them during any potential future LDs. Currently, insufficient data exist to make meaningful conclusions regarding the effects of LD on fasting glucose, severe hypoglycaemia and DKA.
Although pooled data were grouped by diabetes type, there were notably more data available for patients with type 1 diabetes compared with those with type 2 diabetes. For example, average glucose before and after LD had 11 and one studies available for type 1 diabetes and type 2 diabetes, respectively. Additional type 2 diabetes data are needed before meaningful comparisons can be made across types of diabetes. Interpretations of between‐group differences reported within studies were limited because of few studies reporting subgroup differences, and future researchers should consider this when reporting outcomes. From the available data there was very little evidence to support a mediating effect of gender, treatment therapy (continuous subcutaneous insulin infusion vs. multiple daily injections) or glucose monitoring systems on glycaemic control during LD. Only one study subgrouped by socioeconomic status. Studies subgrouping by age, baseline HbA1c and work status during LD are conflicting, making it difficult to draw any meaningful conclusions based on the available data. The only statistically significant subgroup effect observed in this meta‐analysis was for TIR based on geographical location; however, only one study contributed to the Asia subgroup. With the exception of Verma et al., 16 it is unlikely we have captured data from individuals living in low‐ and middle‐income countries with already limited access to healthcare, and as such, those whose healthcare was most affected by the pandemic are probably not reflected in the data we synthesized.
5. LIMITATIONS
With the available data, it is probable we have only been able to capture data from people with diabetes who have advanced health systems, regular access to healthcare and who have the means to fund technological devices. Subsequently we are unable to generalize our findings to people with diabetes from low‐ and middle‐income countries, whom, when it comes to healthcare, have limited quality, geographical accessibility, availability, financial accessibility and acceptability of services. 42 We strongly recommend that future research assesses the impact of COVID‐19 on diabetes management in low‐ and middle‐income countries, and in groups with less healthcare coverage and/or access to diabetes technologies in high‐income countries, and that it reports subgroup differences according to patient demographics. The reliance on observational evidence within this review is also a confounding variable that may have increased heterogeneity and the risk of within‐study and across‐study biases. 43 The authors acknowledge that additional studies have been published since their comprehensive search in February 2021, although further works correspond with the summarized findings. 44 , 45 , 46
The LD measures and restrictions imposed by multiple governments in the context of the COVID‐19 pandemic differ from country to country and thus could also be responsible for some of the observed heterogeneity, as could issues with study reporting and risk of bias. To limit the potential for bias, this review adhered to the PRISMA guidelines, including prespecifying the protocol online. For the first time we also narratively synthesized between‐group differences within studies considering demographics, baseline glycaemic control and treatment technologies.
In summary, the COVID‐19 pandemic improved multiple outcomes of glycaemic control in people with diabetes and, in pooled analyses, showed no detrimental effects on any of the glycaemic outcomes assessed. Data predominantly come from a selected population, with access to healthcare, and research is needed to assess the impact of COVID‐19 on people with diabetes living in low‐ and middle‐income countries and in people with diabetes with lower levels of healthcare access and engagement.
AUTHOR CONTRIBUTIONS
Conceptualization: KK and JHB. Data curation: LLO, PH, LK, JM, RL, ES, KR, AK, NS, AO, SK and OJ. Formal analysis and original draft: LLO and JHB. Funding acquisition: JHB. All authors were involved in reviewing and editing the final manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors have no conflict of interests relevant to this article to declare.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14771.
Supporting information
Appendix S1. Supporting Information
ACKNOWLEDGEMENTS
KK is supported by the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands (ARC EM) and the NIHR Leicester Biomedical Research Centre (BRC). This work was supported in part by the National Institute for Health Research (NIHR) Applied Research Collaboration Oxford, NIHR Applied Research Collaboration East Midlands and Thames Valley at Oxford Health NHS Foundation Trust.
O'Mahoney LL, Highton PJ, Kudlek L, et al. The impact of the COVID‐19 pandemic on glycaemic control in people with diabetes: A systematic review and meta‐analysis. Diabetes Obes Metab. 2022;24(9):1850‐1860. doi: 10.1111/dom.14771
Funding information This work was supported in part by the National Institute for Health Research (NIHR) Applied Research Collaboration Oxford, NIHR Applied Research Collaboration East Midlands and Thames Valley at Oxford Health NHS Foundation Trust
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. CDC COVID‐19 Response Team , Bialek S, Boundy E, Bowen V, Chow N. Severe outcomes among patients with coronavirus disease 2019 (COVID‐19)—United States, February 12–March 16, 2020. Morb Mortal Wkly Rep. 2020;69(12):343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID‐19‐related mortality in England: a whole‐population study. Lancet Diabetes Endo. 2020;8(10):813‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holman N, Knighton P, Kar P, et al. Risk factors for COVID‐19‐related mortality in people with type 1 and type 2 diabetes in England: a population‐based cohort study. Lancet Diabetes Endo. 2020;8(10):823‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGurnaghan SJ, Weir A, Bishop J, et al. Risks of and risk factors for COVID‐19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021;9(2):82‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scott ES, Jenkins AJ, Fulcher GR. Challenges of diabetes management during the COVID‐19 pandemic. Med J Aust. 2020;213(2):56‐57. [DOI] [PubMed] [Google Scholar]
- 6. Garofolo M, Aragona M, Rodia C, et al. Glycaemic control during the lockdown for COVID‐19 in adults with type 1 diabetes: a meta‐analysis of observational studies. Diabetes Res Clin Pract. 2021;180:109066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dover AR, Ritchie SA, McKnight JA, et al. Assessment of the effect of the COVID‐19 lockdown on glycaemic control in people with type 1 diabetes using flash glucose monitoring. Diabet Med. 2021;38(1):e14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eberle C, Stichling S. Impact of COVID‐19 lockdown on glycemic control in patients with type 1 and type 2 diabetes mellitus: a systematic review. Diabetol Metab Syndr. 2021;13(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Higgins JPT, Thomas J, Chandler J, et al. (eds). Cochrane Handbook for Systematic Reviews of Interventions , version 6.2 (updated February 2021). Cochrane. www.training.cochrane.org/handbook. Accessed October 2021.
- 10. Wells GA, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐Analyses. Oxford, UK; 2000. [Google Scholar]
- 11. Hartmann‐Boyce J, Gunnell J, Drake J, et al. Asthma and COVID‐19: review of evidence on risks and management considerations. BMJ Evid Based Med. 2021;26(4):195. [DOI] [PubMed] [Google Scholar]
- 12. Al Agha AE, Alharbi RS, Almohammadi OA, Yousef SY, Sulimani AE, Alaama RA. Impact of COVID‐19 lockdown on glycemic control in children and adolescents. Saudi Med J. 2021;42(1):44‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anjana RM, Pradeepa R, Deepa M, et al. Acceptability and utilization of newer technologies and effects on glycemic control in type 2 diabetes: lessons learned from lockdown. Diabetes Technol Ther. 2020;22(7):527‐534. [DOI] [PubMed] [Google Scholar]
- 14. Assaloni R, Pellino VC, Puci MV, et al. Coronavirus disease (Covid‐19): how does the exercise practice in active people with type 1 diabetes change? A preliminary survey. Diabetes Res Clin Pract. 2020;166:108297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mesa A, Viñals C, Is P, et al. The impact of strict COVID‐19 lockdown in Spain on glycemic profiles in patients with type 1 diabetes prone to hypoglycemia using standalone continuous glucose monitoring. Diabetes Res Clin Pract. 2020;167:108354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Verma A, Rajput R, Verma S, Balania VKB, Jangra B. Impact of lockdown in COVID 19 on glycemic control in patients with type 1 diabetes mellitus. Diabetes Metab Syndr. 2020;14(5):1213‐1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barchetta I, Cimini FA, Bertoccini L, et al. Effects of work status changes and perceived stress onglycaemiccontrol in individuals with type 1 diabetes during COVID‐19 lockdown in Italy. Diabetes Res Clin Pract. 2020;170:108513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Biancalana E, Parolini F, Mengozzi A, Solini A. Short‐term impact of COVID‐19 lockdown on metabolic control of patients with well‐controlled type 2 diabetes: a single‐centre observational study. Acta Diabetol. 2021;58(4):431‐436. doi: 10.1007/s00592-020-01637-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dovc K, Osredkar S, Smigoc D, Battelino T, Bratina N. Nationwide digital/virtual diabetes care of children, adolescents and young adults with type 1 diabetes during a COVID‐19 pandemic in Slovenia. Slov M J. 2020;89:626‐633. doi: 10.6016/ZdravVestn.3104 [DOI] [Google Scholar]
- 20. Khare J, Jindal S. Observational study on Effect of Lock Down due to COVID 19 on glycemic control in patients with Diabetes: Experience from Central India. Diabetes Metab Syndr. 2020;14(6):1571‐1574. doi: 10.1016/j.dsx.2020.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pla B, Arranz A, Knott C, et al. Impact of COVID‐19 lockdown on glycemic control in adults with type 1 diabetes mellitus. J Endocr Soc. 2020;4(12):bvaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khader MA, Jabeen T, Namoju R. A cross sectional study reveals severe disruption in glycemic control in people with diabetes during and after lockdown in India. Diabetes Metab Syndr. 2020;14(6):1579‐1584. doi: 10.1016/j.dsx.2020.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kamrath C, Mönkemöller K, Biester T, et al. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID‐19 pandemic in germany. JAMA. 2020;324(8):801‐804. doi: 10.1001/jama.2020.13445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lawrence N, Natarajan A, Petkar R, et al. Impact of COVID‐19 lockdown on glycaemic control in young people with type 1 diabetes: a retrospective review at a large hospital. Diabetes Care for Children & Young People. 2020:10(2). ISSN 2050‐1528. [Google Scholar]
- 25. Ludvigsson J. Effect of COVID‐19 pandemic on treatment of Type 1 diabetes in children. Acta Paediatr. 2021;110(3):933‐934. doi: 10.1111/apa.15627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lui DTW, Lee CH, Chow WS, et al. A territory‐wide study on the impact of COVID‐19 on diabetes‐related acute care. J Diabetes Investig. 2020;11(5):1303‐1306. doi: 10.1111/jdi.13368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Misra S, Khozoee B, Huang J, et al. Comparison of Diabetic Ketoacidosis in Adults During the SARS‐CoV‐2 Outbreak and Over the Same Time Period for the Preceding 3 Years. Diabetes Care. 2021;44(2):e29‐e31. doi: 10.2337/dc2 [DOI] [PubMed] [Google Scholar]
- 28. Odeh R, Gharaibeh L, Daher A, Kussad S, Alassaf A. Caring for a child with type 1 diabetes during COVID‐19 lockdown in a developing country: Challenges and parents' perspectives on the use of telemedicine. Diabetes Res Clin Pract. 2020;168:108393. doi: 10.1016/j.diabres.2020.108393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Önmez A, Gamsızkan Z, Özdemir Ş, et al. The effect of COVID‐19 lockdown on glycemic control in patients with type 2 diabetes mellitus in Turkey. Diabetes Metab Syndr. 2020;14(6):1963‐1966. doi: 10.1016/j.dsx.2020.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kishimoto M, Ishikawa T, Odawara M. Behavioral changes in patients with diabetes during the COVID‐19 pandemic. Diabetol Int. 2020;12(2):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weingarten SJ, Clare CA. 150 management of diabetes in pregnancy during the COVID‐19 pandemic at a New York City hospital. Am J Obstet Gynecol. 2021;224(2):S103‐S103. [Google Scholar]
- 32. Rachmiel M, Lebenthal Y, Mazor‐Aronovitch K, et al. Glycaemic control in the paediatric and young adult population with type 1 diabetes following a single telehealth visit ‐ what have we learned from the COVID‐19 lockdown? Acta Diabetol. 2021;58(6):697‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tourkmani A, ALHarbi T, Rsheed AMB, et al. The impact of telemedicine on patients with uncontrolled type 2 diabetes mellitus during the COVID‐19 pandemic in Saudi Arabia: findings and implications. J Telemed Telecare. 2021;1357633x20985763. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khare J, Jindal S. Observational study on effect of lock down due to COVID 19 on HBA1c levels in patients with diabetes: experience from central India. Prim Care Diabetes. 2021;14(6):1571‐1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Batten L, Chandrajay D, Burkinshaw C, Gill J, Jayagopal V. Service restriction during the COVID‐19 pandemic and its impact on HbA(1c): a surprising outcome. Diabet Med. 2021;38(1):e14414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kang J, Chen Y, Zhao Y, Zhang C. Effect of remote management on comprehensive management of diabetes mellitus during the COVID‐19 epidemic. Prim Care Diabetes. 2021;15(3):417‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Silverii GA, Delli Poggi C, Dicembrini I, Monami M, Mannucci E. Glucose control in diabetes during home confinement for the first pandemic wave of COVID‐19: a meta‐analysis of observational studies. Acta Diabetol. 2021;58(12):1603‐1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hu Y, Shen Y, Yan R, et al. Relationship between estimated glycosylated hemoglobin using flash glucose monitoring and actual measured glycosylated hemoglobin in a Chinese population. Diabetes Ther. 2020;11(9):2019‐2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perlman JE, Gooley TA, McNulty B, Meyers J, Hirsch IB. HbA1c and glucose management indicator discordance: a real‐world analysis. Diabetes Technol Ther. 2021;23(4):253‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. NHS . Who is at high risk from coronavirus (COVID‐19)? https://www.nhs.uk/conditions/coronavirus-covid-19/people-at-higher-risk/who-is-at-high-risk-from-coronavirus/. Accessed December 2021.
- 41. Bonora BM, Boscari F, Avogaro A, Bruttomesso D, Fadini GP. Glycaemic control among people with type 1 diabetes during lockdown for the SARS‐CoV‐2 outbreak in Italy. Diab Ther Res Treat Educ Diab Related Disord. 2020;11(6):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peters DH, Garg A, Bloom G, Walker DG, Brieger WR, Rahman MH. Poverty and access to health care in developing countries. Ann N Y Acad Sci. 2008;1136:161‐171. [DOI] [PubMed] [Google Scholar]
- 43. Metelli S, Chaimani A. Challenges in meta‐analyses with observational studies. Evid Based Mental Health. 2020;23(2):83‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. D'Onofrio L, Pieralice S, Maddaloni E, et al. Effects of the COVID‐19 lockdown on glycaemic control in subjects with type 2 diabetes: the glycalock study. Diabetes Obes Metab. 2021;23(7):1624‐1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hartmann B, Tittel SR, Femerling M, et al. COVID‐19 lockdown periods in 2020: good maintenance of metabolic control in adults with type 1 and type 2 diabetes. Exp Clin Endocrinol Diabetes. 2022. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 46. Hansel B, Potier L, Chalopin S, et al. The COVID‐19 lockdown as an opportunity to change lifestyle and body weight in people with overweight/obesity and diabetes: results from the national French COVIDIAB cohort. Nutr Metab Cardiovasc Dis. 2021;31(9):2605‐2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


