Summary
Symptoms of insomnia are an important risk factor for the development of mental disorders, especially during stressful life periods such as the coronavirus disease 2019 (COVID‐19) pandemic. However, up to now, most studies have used cross‐sectional data, and the prolonged impact of insomnia symptoms during the pandemic on later mental health remains unclear. Therefore, we investigated insomnia symptoms as a predictor of other aspects of mental health across 6 months, with altogether seven assessments (every 30 days, t0–t6), in a community sample (N = 166–267). Results showed no mean‐level increase of insomnia symptoms and/or deterioration of mental health between baseline assessment (t0) and the 6‐ month follow‐up (t6). As preregistered, higher insomnia symptoms (between persons) across all time points predicted reduced mental health at the 6‐month follow‐up. Interestingly, contrary to our hypothesis, higher insomnia symptoms at 1 month, within each person (i.e., compared to that person's symptoms at other time points), predicted improved rather than reduced aspects of mental health 1 month later. Hence, we replicated the predictive effect of averagely increased insomnia symptoms on impaired later mental health during the COVID‐19 pandemic. However, we were surprised that increased insomnia symptoms at 1 month predicted aspects of improved mental health 1 month later. This unexpected effect might be specific for our study population and a consequence of our study design. Overall, increased insomnia symptoms may have served as a signal to engage in, and successfully implement, targeted countermeasures, which led to better short‐term mental health in this healthy sample.
Keywords: anxiety, depression, mental wellbeing, psychological resilience, sleep, stress‐related symptoms
Sleep is an important component of mental health (e.g., Baglioni et al., 2010; Harvey et al., 2011), and sleep disturbances, such as insomnia symptoms, often accompany other mental disorders like depression and anxiety, as well as more general stress‐related symptoms (Baglioni et al., 2016; Freeman et al., 2020). Furthermore, insomnia symptoms also serve as a risk factor for the development of other mental disorders (e.g., Harvey, 2008). For example, a recent meta‐analysis by Hertenstein et al. (2019) showed that insomnia is a significant predictor of later depression (odds ratio [OR] 2.83; 12–24 months later), anxiety (OR 3.23; 12 months up to 20 years later), and alcohol abuse (OR 1.35; 12 months up to 7 years later). Similar findings had already been found in an earlier meta‐analysis by Baglioni et al. (2011) for the link between insomnia and later depression. Buysse et al. (2008) extended these findings by showing that even short‐term insomnia of 2–4 weeks predicted major depressive episodes at subsequent assessments (five assessments across 18 years: first follow‐up assessment after ~2 years). However, these studies did not take into account whether insomnia remitted or remained on a clinically significant level during the following study period until the next assessment.
Some research additionally investigated the time course of insomnia and its impact on later mental disorders, mainly focusing on symptoms of depression and anxiety. Those studies showed that, in particular, individuals who experience insomnia symptoms across a prolonged period of time are at greater risk of developing new or increased depressive symptoms (e.g., Ellis et al., 2014; Okajima et al., 2011; Suh et al., 2013). For example, Ellis et al. (2014) found that individuals whose acute insomnia symptoms persisted across 3 months had a higher risk of developing new, clinically relevant depressive symptoms compared to good sleepers or individuals whose insomnia symptoms naturally remitted. Other studies, supporting these findings, used even longer periods of 2–3 years between subsequent assessments (Okajima et al., 2011; Suh et al., 2013). Although it seems plausible that increased insomnia symptoms exert a continuous effect, which might lead to reduced mental health within shorter periods, such as 1 month, to our knowledge no study has yet investigated the short‐term effects of insomnia symptoms on mental health.
Acute insomnia symptoms predominantly arise due to stressful life events or longer stressful life periods (e.g., Li et al., 2019; Sinha, 2016), especially in vulnerable individuals (e.g., Riemann et al., 2010). The coronavirus disease 2019 (COVID‐19) pandemic affected the whole world and served as a stressful life period for many people. Cross‐sectional studies have shown elevated levels of distress and increased symptoms of various mental health disorders (including symptoms of insomnia, depression, and anxiety) during the pandemic when compared to before the pandemic (e.g., Deng et al., 2021; Franceschini et al., 2020; Petzold et al., 2020; Scarpelli et al., 2021; Vindegaard & Benros, 2020). Furthermore, longitudinal data, with a follow‐up assessment after 4 weeks, suggests that these increased symptom levels are relatively stable (Brose, 2020; Vindegaard & Benros, 2020) or even slightly increasing further (Salfi et al., 2020). Therefore, the period of the COVID‐19 pandemic may serve as a unique opportunity to conduct a longitudinal study to assess insomnia symptoms as a potential risk factor for other aspects of mental health in the general population.
Some studies specifically investigated the development of insomnia symptoms and mental health during the COVID‐19 pandemic. Salfi et al. (2020) showed that, overall, insomnia symptoms, as well as depression, anxiety, and stress‐related symptoms, increased across 4 weeks during the first lockdown in an Italian population. Another study by Morin et al. (2021) showed declining symptom levels across 5 months during the first wave of the COVID‐19 pandemic, with no assessment point during the second wave. In a second study by Salfi, D'Atri, Tempesta, and Ferrara (2021), they compared insomnia symptoms (and other aspects of sleep, e.g., overall sleep quality) and mental health between the first and second contagion wave of COVID‐19 in a population‐based sample of 2,013 individuals in Italy. They found that overall sleep disturbances and depressive symptoms remained elevated during the second wave compared to the first wave, despite the second wave being characterised by fewer restrictive measures. In contrast, insomnia symptoms slightly decreased across this period. Additionally, Salfi et al. (2020) found diverging trajectories for men and women across 4 weeks, with women showing generally higher mean levels of symptoms. In women, these symptoms diminished while the lockdown continued, whereas men reported increased symptoms 4 weeks later, despite lower mean levels of symptoms compared to women. However, neither of these studies investigated whether insomnia symptoms during the first assessment predicted developing symptoms of other mental disorders. Therefore, the prolonged impact of insomnia symptoms during the COVID‐19 pandemic on mental health remains unclear.
Taken together, the present investigation builds on two lines of research. First, clinical studies support a predictive effect of persisting versus remitting insomnia on the development of other mental disorders, e.g., depression. Second, studies with a focus on the effects of the COVID‐19 pandemic found increased subclinical insomnia symptoms that are cross‐sectionally associated with other increased psychological symptoms. Combining the findings of both lines of research, the first aim of the study was to replicate the overall, prolonged effect of increased insomnia symptoms on later mental health during the COVID‐19 pandemic, in a community sample with subclinical symptom levels. Second, to fill the gap on short‐term consequences of insomnia symptoms (regardless of whether they are remitting later or not), we wanted to investigate the short‐term effect of increased insomnia symptoms on mental health 1 month later. Insomnia symptoms, depression, anxiety, stress‐related symptoms, and overall mental wellbeing across 6 months were assessed with a survey every 30 days on average. On the one hand, to operationalise persisting insomnia symptoms across the study period, we looked at differences in the average experience of symptoms on a between‐person level, namely that experiencing more insomnia symptoms than others across all time points negatively impacts one's long‐term mental health. On the other hand, we investigated the effect of within‐person fluctuations of insomnia symptoms on mental health, that is, whether experiencing more insomnia symptoms than one usually would experience has a direct short‐term effect on one's mental health the next month. Specifically, we expected higher between‐person insomnia symptoms across all time points (i.e., higher than the average of the whole sample during the study period) to predict reduced mental health at the 6‐month follow‐up (H1). 1 In addition, we expected higher within‐person insomnia symptoms at 1 month (i.e., higher than one person has on average during the study period) to predict reduced mental health as soon as 1 month later (H2). These hypotheses were preregistered, along with the analysis strategy, to model the assumed effects separately for men and women, as there is some evidence suggesting different mean symptom levels, as well as different slopes in men and women (e.g., Salfi et al., 2020).
Furthermore, although the majority of studies suggest a stronger influence of insomnia symptoms on the development of other psychological symptoms, especially depression (for review, see Alvaro et al., 2013; Freeman et al., 2020), there are also studies that support bidirectional links between insomnia symptoms and depression and/or anxiety symptoms (e.g., Jansson & Steven, 2006). In exploratory analyses, we therefore also investigated the reverse effects of mental health on later insomnia symptoms for both hypotheses. Based on earlier research comparing persisting versus remitting insomnia symptoms, we additionally wanted to explore whether greatly increased insomnia symptoms that eventually decrease during the study period would indeed have no effect on later mental health (c.f., Ellis et al., 2014; Okajima et al., 2011; Suh et al., 2013). Therefore, we also analysed the effect of one's personal highest peak in insomnia symptoms during one of the first 4 months (within‐person, consequently indicating a lower level of symptoms at the other months) on mental health at the 6‐month follow‐up.
METHODS
This project is part of the Corona couple experience sampling method (ESM) study that contains several separate, preregistered research questions, including codebooks of the assessed variables (https://osf.io/72ejg/); for the preregistration of the present work, please see https://osf.io/6eah2/. Overall, the goal of our study was to investigate the impact of COVID‐19 on several aspects of daily life (during the ESM period with a focus on relationship behaviour and emotion regulation), as well as the prolonged consequences on mental health (through monthly questionnaire assessments). The present research question focuses on the influence of insomnia symptoms on mental health (i.e., depression, anxiety, stress‐related symptoms, and mental wellbeing), and the analyses only focus on the monthly questionnaire assessments (collected between April 2020 and January 2021). Other research questions and related measures (e.g., during the ESM period) will be presented elsewhere.
Participants
As mentioned in the preregistration preamble of the Corona couple ESM study (https://osf.io/72ejg/), sample size was determined by the aim to finish data collection (including the 6‐month follow‐up) by the start of 2021, so that data could be analysed and reported in a timely manner. Therefore, through July 2020, as many individuals as possible were recruited for participation. Participants were included if they were part of a heterosexual relationship, were aged ≥18 years, were German‐speaking, used a personal smartphone with Android or iOS, and gave written informed consent. Overall, 273 individuals from the German general population started the study; 267 individuals completed at least the baseline assessment. Hence, the data set consists of a mixture of individual participants who are in a relationship (n = 47) and couples (n = 110) where both partners participated.
Measures
At baseline, a broad range of sociodemographic variables (e.g., age, gender, level of education, mental health status, healthcare worker status, and children) was collected. In addition, the extent of COVID‐19 restrictions at each of the seven monthly assessments was measured on a 5‐point scale (1 = “no lockdown/home confinement”, 2 = “no lockdown/home confinement, but recommendation for social distancing”, 3 = “lockdown/home confinement, but not being in quarantine”, 4 = “being in quarantine, but partner not”, 5 = “both being in quarantine”). Furthermore, the following questionnaires were used to collect data on symptoms of insomnia, depression, anxiety, stress, and overall mental wellbeing.
Insomnia severity index (ISI)
Insomnia symptoms were measured by the German version of the ISI (possible range 0–28; Gerber et al., 2016). The ISI assesses difficulties initiating or maintaining sleep and early morning awakenings within the past 2 weeks. For example, “Please rate the current (i.e., in the last 2 weeks) severity of your insomnia problem(s)” on a scale from 0 = “none” to 4 = “very severe.” Additionally, it assesses insomnia‐related worries. For presentation of the descriptive statistics, we used a clinical cut‐off of 15, which indicates moderate (i.e., clinically significant) insomnia. The internal consistency in our sample was good (McDonald's ωtotal at t0 = 0.84). 2
Depression‐Anxiety‐Stress scales (DASS)
Depression, anxiety, and stress‐related symptoms were measured using separate subscales of the German version of the DASS (possible range 0–63; Nilges & Essau, 2015). The DASS measures psychological core aspects of depression (within the past week), like the lack of activities and positive emotions (DASS‐D; clinical cut‐off >10), anxiety‐like physiological hyperarousal and increased tension (DASS‐A; clinical cut‐off >6), and further stress‐related symptoms, such as general irritability (DASS‐S; clinical cut‐off >10). The internal consistencies of all subscales were good in the present sample (DASS‐D: McDonald's ωtotal at t0 = 0.89; DASS‐S: McDonald's ωtotal at t0 = 0.82; DASS‐S: McDonald's ωtotal at t0 = 0.88).
Warwick–Edinburgh Mental Wellbeing Scale (WEMWBS)
Mental wellbeing was measured using the German version of the WEMWBS (possible range 14–70; Taggart et al., 2016). It assesses subjective wellbeing and psychological functioning as aspects of positive mental health within the past 2 weeks. Thus, it is based on a model of mental health that represents more than the absence of mental illness and involves both feeling good and functioning well. The internal consistency of the WEMWBS at baseline was excellent (McDonald's ωtotal at t0 = 0.94). Please note that we had to impute item number 14 at baseline (t0; i.e., by using the mean of all other items within each person), due to a programming error in the survey system that was resolved for all subsequent assessments.
Procedure
Individuals were recruited via email distribution lists, online fora, blog posts, and word of mouth. Figure 1 shows an overview of the different parts of the study. It started with a baseline assessment (t0), followed by an ESM period (Csikszentmihalyi & Larson, 1987; Trull & Ebner‐Priemer, 2013) of 28 days, five monthly assessments (t1–t5), and a final follow‐up assessment (t6). In the baseline assessment (t0), participants received several psychological and mental health questionnaires. In the subsequent ESM period, participants reported three times per day on their relationship satisfaction (among other variables) and were encouraged to reflect upon possible new behaviours they showed to promote closeness to their partners. Moreover, we asked about positive and negative emotions, as well as which specific strategies (from a list of around 16 strategies) participants used to deal with these emotions, along with the effectiveness of the applied strategies. No ESM variable is relevant for the present paper. Directly after the ESM period, participants received the first monthly assessment (t1). This assessment and the following four assessments (t2–t5) included a reduced number of questionnaires. The final follow‐up assessment (t6) again included a broader range of psychological and mental health questionnaires. For all monthly assessments (t1–t6), participants received an email every 28 days, including a link to the corresponding questionnaire, with instructions to complete the questionnaire within 1 week; the mean (SD) duration between completion of the questionnaires was 29.61 (5.19) days. Participants were offered individual feedback based on their data and the opportunity to win a shopping voucher (amount between 50 and 250 euros) as incentives for participating. The probability of winning a voucher increased with higher response rates in the ESM period, more completed monthly assessments, and when both partners in a couple participated in the study. The study was approved by the ethics committee of the LMU Munich.
FIGURE 1.
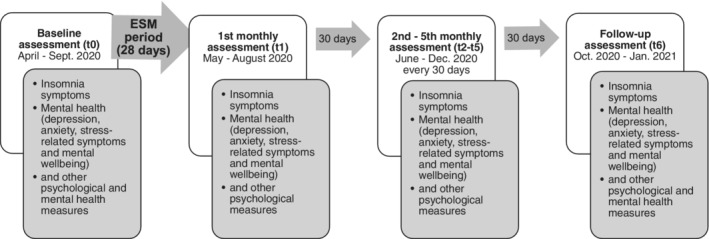
Overall study procedure
Data were manually checked to remove participant errors in questionnaire completion where possible. In addition to aspects specified in the preregistration preamble, the following criteria were applied: If questionnaires were started twice, we only included the one that was completed or that included more responses. Additionally, in two cases, the date of questionnaire completion did not correspond to the respective time point. In one of these cases, questionnaires from t1 and t2 were completed at the same time (when t2 was due), hence we only kept the answers from the t2 questionnaire but excluded answers on the relevant variables from the t1 questionnaire. In the other case, the questionnaire link for t2 was completed when t4 was due; however, as these assessments contain the same questionnaires, we assigned these answers to the questionnaire for t4.
Statistical analysis
All analyses were conducted in R studio (Version 1.3.1093, R Core Team, 2021), and our analysis code is available at https://osf.io/72ejg/. For the main analyses, first, all variables were z‐standardised using the grand‐mean and grand‐SD across both genders and all time points. We used a multilevel‐modelling approach due to the dyadic data structure in cases where both partners participated, and also due to the repeated measurements of the baseline and monthly follow‐up questionnaires. For repeated measurement of the dependent variable (H2), data were modelled as questionnaires (within‐person, level 1), crossed with participants (between‐person, level 2), who are nested in couples if both partners participated in the study (level 3). Additionally, for H2, we group‐mean centred the z‐standardised within‐person‐predictor on the individual mean across all time points and controlled for the mean of the respective variable of a person as between‐person variable (CWCM‐method; Zhang et al., 2009). For the multilevel model analyses, the R package lmerTest (Kuznetsova et al., 2017) was used, which is based on lme4 (Bates et al., 2015). As we expected differences between women and men on the outcome variables, we used fully distinguishable models that include separate random and fixed intercepts for women and men (modelled by two dummy variables: sexf: 0 = male, 1 = female; sexm: 0 = female, 1 = male), as well as separate random and fixed slopes (where possible 3 ).
As preregistered, all analyses included as covariates the lagged dependent variable (i.e., the autocorrelation of the outcome) and age. To control for linear trends over time, the analyses for H2 additionally included a time index as covariate (ranging from 0 = baseline assessment, 1 = first monthly assessment … to 6 = follow‐up assessment), as specified in the preregistration preamble. The analyses were based on all available data, with a minimal number of 166 individuals (72 men, 94 women) at the follow‐up assessment (t6); for the number of participants at each assessment point, please see Table 1. The p values of all focal hypothesis tests were corrected for multiple testing (separately for each gender) by using the Benjamini‐Hochberg correction to control the false discovery rate at 5% (Benjamini & Hochberg, 1995). As we had four mental health outcome variables per hypothesis, we controlled for four tests in each hypothesis. The alpha‐level for statistical significance was set to 0.05. 4
TABLE 1.
Means and standard deviations for all variables
| t0 | t1 | t2 | t3 | t4 | t5 | t6 | |
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Insomnia symptoms (ISI) | 7.00 (5.23) | 7.85 (5.23) | 7.74 (5.08) | 6.86 (4.85) | 6.71 (5.09) | 7.00 (5.20) | 6.91 (4.95) |
| Depressive symptoms (DASS‐D) | 3.55 (4.08) | 3.99 (4.36) | 3.95 (4.41) | 3.12 (4.06) | 2.94 (3.79) | 3.05 (3.93) | 3.12 (3.92) |
| Anxiety symptoms (DASS‐A) | 1.79 (2.82) | 1.88 (2.65) | 2.05 (2.65) | 1.57 (2.42) | 1.50 (2.31) | 1.56 (2.55) | 1.33 (1.96) |
| Stress‐related symptoms (DASS‐S) | 5.29 (4.34) | 5.96 (4.42) | 5.82 (4.67) | 5.02 (4.42) | 4.68 (3.99) | 4.58 (4.18) | 4.53 (3.78) |
| Mental wellbeing (WEMWBS) | 51.47 (9.31) | 52.13 (9.87) | 50.43 (10.05) | 51.51 (9.54) | 51.70 (9.87) | 52.32 (9.63) | 52.64 (9.72) |
Abbreviation: DASS‐A, Depression‐Anxiety‐Stress‐Scales (subscale anxiety); DASS‐S, Depression‐Anxiety‐Stress‐Scales (subscale stress); DASS‐D, Depression‐Anxiety‐Stress‐Scales (subscale depression); ISI, Insomnia Severity Index; WEMWBS, Warwick–Edinburgh Mental Wellbeing Scale.
Note: Absolute values are presented. For analyses, z‐standardised values were used as described in the methods section.
N t0 = 267, N t1 = 180, N t2 = 174, N t3 = 171, N t4 = 168, N t5 = 167, N t6 = 166.
Nearly half of the sample (46.8%) completed the baseline assessment during the first German lockdown phase (baseline assessments completed in April 2020), whereas the rest of the sample completed the baseline assessment during phases of lighter restrictions. Hence, towards the end of the study restrictions increased again for part of the sample (as a second lockdown phase began in November 2020). Therefore, in order to control for changes in COVID‐19‐related restrictions, we calculated a difference score between the self‐reported restrictions at the relevant time points in each analysis (e.g., restrictions at follow‐up (t6) minus restrictions at baseline (t0) for the models of H1). This difference score was recoded into a contrast variable indicating reduced restrictions (−1), no change in restrictions (0), or increased restrictions (1) between the time points; for the models of H2 the difference score was calculated accordingly between subsequent months. All preregistered analyses were repeated including this contrast variable (as main effect and interaction, followed by another Benjamini‐Hochberg correction) and we only describe emerging changes in our findings in the results section for these additional analyses.
Exploratory analyses on the effects of mental health on later insomnia symptoms were calculated similar to H1 and H2 by exchanging the dependent variable with the focal predictor variables and including lagged insomnia symptoms and age as covariates; the analysis that matched H2, additionally included the time index as covariate. Further exploratory analyses on the influence of remitting insomnia symptoms on later mental health were operationalised as follows: we used one's personal highest peak in insomnia symptoms during one of the first 4 months as a predictor of mental health at the 6‐month follow‐up. This peak variable describes the highest deviation of insomnia symptoms between t0 and t4 from that person's mean insomnia symptoms during the other months. Consequently, symptom levels at other months were lower and 95% of the sample indicated an average reduction in symptoms after the peak (i.e., at t5 and/or t6). The model further contained mean insomnia symptoms at all other time points (excluding the peak time point), the time point itself (to control for effects of the specific assessments), and the dependent variable at t0 (i.e., depression, anxiety, stress‐related symptoms, or mental wellbeing). All exploratory analyses were also repeated including the contrast variable of COVID‐19‐related restrictions (as main effect and interaction) and all analyses were corrected for multiple testing by using the Benjamini‐Hochberg correction (treating the set of analyses with and without the variable of COVID‐19 related restrictions separately). Again, we only describe emerging changes in our findings in the results section for these additional analyses.
RESULTS
Characteristics
The final sample to complete the baseline assessment consisted of 267 individuals, 5 with 56.93% of the sample being female. The mean (SD) age was 30.95 (10.11) years. Most of the sample were highly educated with 77.9% having a higher education entrance qualification, reported not to work in healthcare (81.7%), and had no children in their household (81.7%). With regard to mental health, most individuals reported no current (89.5%) or lifetime mental disorder (76.0%) at the beginning of the study (no information was given by 3.4% and 2.6% of the sample, respectively). Accordingly, 89.1% of the sample reported not undergoing psychotherapy at the moment and 70.0% of all individuals indicated that they had never undergone psychotherapeutic treatment (no information was given by 0.8% and 1.1% of the sample). Furthermore, at the baseline assessment, 10.1% of the present sample reported clinically significant insomnia symptoms; additionally, 27.0% reported subclinical insomnia symptoms (ISI values between 8 and 14). Around 5% to 11% of the sample indicated symptoms above the clinical cut‐off for depression (DASS‐D: 7.5%), anxiety (DASS‐A: 5.6%), and stress symptoms (DASS‐S: 11.2%). Table 1 gives an overview of all variables (means, SDs) across time.
Time course of symptoms across study period (preliminary analyses)
Although not being part of the preregistration, we first analysed the time course of insomnia and other aspects of mental health across the study period (mean‐level comparison of baseline [coded as 0] and follow‐up 6 months later [coded as 1]). Separate characteristics for all assessment points (including the specific participants numbers) for women and men can be found in the Supplement (Table S1). 6 There was no significant mean‐level change of insomnia, depression, anxiety, and stress‐related symptoms between baseline (t0) and the 6‐month follow‐up (t6) for men or women (bs = −0.83 to +0.32, 95% confidence intervals [CIs] [−1.55 to −0.66, −0.11 to +1.30], dfs = 166.73–207.31, ts = −2.25 to +0.64, ps > 0.026; none of the effects remained significant after correcting for multiple testing). Additionally, no significant mean‐level change of mental wellbeing was found for men or women (bs = 0.44 − +0.84, 95% CIs [−1.30 to −0.70, 2.17 to 2.38], dfs = 188.97–196.98, ts = 0.50–1.07, ps > 0.287). The overall pattern of results did not change when accounting for the change in restrictions across the study period.
Even though the change itself was not significantly different from zero for either women or men across the study period, we found a significant main effect of gender at t0 (i.e., a significant difference between women [coded as 1] and men [coded as −1]) for most variables (after correcting for multiple testing). Specifically, women reported higher symptoms of depression, anxiety, and stress (bs = 0.37–1.20, 95% CIs [0.11–0.77, 0.64–1.63], dfs = 181.91–194.43, ts = 2.77–5.48, ps ≤ 0.006), as well as lower mental wellbeing (b = −1.29, 95% CI [−2.20, −0.38], df = 183.16, t = −2.79, p = 0.006) than men at baseline. For symptoms of insomnia, no significant difference between women and men was found (b = 0.47, 95% CI [−0.11, 1.06], df = 178.47, t = 1.58, p = 0.116). When including the change in restrictions in the models (and conducting a subsequent Benjamini‐Hochberg correction), the difference in anxiety between women and men disappeared (p = 0.200) and the differences in depression and mental wellbeing were reduced but remained significant (ps ≤ 0.029). For insomnia and stress‐related symptoms, the pattern of results remained (insomnia symptoms: p > 0.05, stress‐related symptoms: p ≤ 0.001).
Effect of insomnia symptoms on later mental health (primary analyses)
In order to examine the effect of persisting insomnia symptoms, we then investigated the between‐person effect of insomnia symptoms on mental health at follow‐up (H1). Overall, we found effects in the expected direction. Higher insomnia symptoms across all time points predicted increased depression and stress‐related symptoms (but not anxiety), as well as reduced mental wellbeing at the 6‐month follow‐up (see Table 2; for the effects of all included covariates see Table S2 in the Supplement). When including the change of restrictions in the analyses, higher insomnia symptoms also predicted increased anxiety at the 6‐month follow‐up in men (p = 0.046), but not in women.
TABLE 2.
Between‐person effect of insomnia symptoms across all time‐points on mental health at the 6‐month follow‐up (t6), accounting for mental health at baseline (t0)
| Variable | β (95% CI) | df | t | p |
|---|---|---|---|---|
| Dependent variable: depressive symptoms t6 | ||||
| Depressive symptoms t0 | 0.44 (0.29, 0.59) | 153.06 | 5.84 | <0.001 |
| Insomnia symptoms (between‐person) – women | 0.30 (0.06, 0.53) | 153.47 | 2.49 | 0.014 |
| Insomnia symptoms (between‐person) – men | 0.32 (0.12, 0.51) | 99.27 | 3.18 | 0.002 |
| Dependent variable: anxiety symptoms t6 | ||||
| Anxiety symptoms t0 | 0.42 (0.32, 0.54) | 160.00 | 7.76 | <0.001 |
| Insomnia symptoms (between‐person) – women | 0.16 (−0.03, 0.34) | 160.00 | 1.67 | 0.097 |
| Insomnia symptoms (between‐person) – men | 0.16 (−0.01, 0.34) | 160.00 | 1.84 | 0.068 |
| Dependent variable: stress symptoms t6 | ||||
| Stress symptoms t0 | 0.34 (0.21, 0.47) | 159.12 | 4.97 | <0.001 |
| Insomnia symptoms (between‐person) – women | 0.43 (0.22, 0.64) | 159.54 | 3.94 | <0.001 |
| Insomnia symptoms (between‐person) – men | 0.29 (0.10, 0.49) | 157.73 | 2.93 | 0.004 |
| Dependent variable: mental wellbeing t6 | ||||
| Mental wellbeing t0 | 0.56 (0.42, 0.71) | 158.48 | 7.47 | <0.001 |
| Insomnia symptoms (between‐person) – women | −0.29 (−0.52, −0.06) | 157.88 | −2.45 | 0.015 |
| Insomnia symptoms (between‐person) – men | −0.29 (−0.50, −0.07) | 139.28 | −2.64 | 0.009 |
Abbreviation: CI, confidence interval.
Note: Uncorrected, two‐sided p values are presented. We used the Benjamini‐Hochberg correction to adjust for testing multiple dependent variables. Significant effects regarding the hypothesis, after the correction, are highlighted in bold.
N = 166 individuals (332 time‐points: 144 by men, 188 by women).
Second, we investigated the within‐person effect of insomnia symptoms at 1 month on next‐month mental health (H2). Unexpectedly, higher insomnia symptoms did not predict reduced mental health. On the contrary, increased insomnia symptoms at one time point predicted improved aspects of mental health 1 month later (see Table 3; for the effects of all included covariates see Table S3 in the Supplement). Specifically, women showed significantly reduced symptoms of depression and anxiety, as well as increased mental wellbeing, and men showed reduced anxiety symptoms 1 month later. These results indicate that if individuals reported higher insomnia severity at one time point than they reported on average across the 6 months, aspects of their mental health were improved 1 month later. When including the change in restrictions, a significant interaction of insomnia symptoms with change in restrictions emerged for women (β = 0.24, 95% CI 0.06–0.43, df = 348.26, t = 2.59, p = 0.010). This indicates that greater insomnia symptoms predicted increased depressive symptoms 1 month later in women, if restrictions increased during this period.
TABLE 3.
Within‐person effect of insomnia symptoms on next‐month mental health, accounting for mental health at the time of the predictor
| Variable | β (95% CI) | df | t | p |
|---|---|---|---|---|
| Dependent variable: depressive symptoms tx + 1 | ||||
| Depressive Symptoms tx | 0.61 (0.56, 0.66) | 128.62 | 23.12 | <0.001 |
| Insomnia symptoms tx (within‐person) – women | −0.22 (−0.32, −0.12) | 61.35 | −4.15 | <0.001 |
| Insomnia symptoms tx (within‐person) – men | −0.13 (−0.25, −0.01) | 76.68 | −2.09 | 0.040 a |
| Dependent variable: anxiety symptoms tx + 1 | ||||
| Anxiety symptoms tx | 0.49 (0.44, 0.54) | 18.21 | 142.44 | <0.001 |
| Insomnia symptoms tx (within‐person) – women | −0.13 (−0.24, −0.02) | 84.85 | −2.41 | 0.018 |
| Insomnia symptoms tx (within‐person) – men | −0.19 (−0.31, −0.07) | 80.62 | −3.06 | 0.003 |
| Dependent variable: stress symptoms tx + 1 | ||||
| Stress symptoms tx | 0.17 (0.11, 0.23) | 5.46 | 864.99 | <0.001 |
| Insomnia symptoms tx (within‐person) – women | −0.07 (−0.16, 0.02) | 64.85 | −1.60 | 0.114 |
| Insomnia symptoms tx (within‐person) – men | −0.06 (−0.17, 0.05) | 312.79 | −1.10 | 0.270 |
| Dependent variable: mental wellbeing tx + 1 | ||||
| Mental wellbeing tx | 0.27 (0.21, 0.33) | 8.52 | 770.54 | <0.001 |
| Insomnia symptoms tx (within‐person) – women | 0.16 (0.06, 0.25) | 53.27 | 3.37 | 0.001 |
| Insomnia symptoms tx (within‐person) – men | −0.01 (−0.12, 0.10) | 83.98 | −0.17 | 0.867 |
Abbreviation: CI, confidence interval.
Note: Uncorrected, two‐sided p values are presented. We used the Benjamini‐Hochberg correction to adjust for multiple testing. Significant effects regarding the hypothesis, after the correction, are highlighted in bold.
N = 186 individuals (997 time points: 426 by men, 571 by women).
Effect was no longer significant after correcting for multiple testing.
Although not being part of the hypothesis (H2), the between‐person effect was also specified in the analyses, to clearly separate within‐person variation from between‐person variation. After correcting for multiple testing, we found significant between‐person effects of insomnia symptoms on mental health, which also remained significant when including the change of restrictions in the analyses. This indicates that higher insomnia severity across all time points was strongly associated with reduced mental health across all time points for depression, anxiety, and stress‐related symptoms (βs = 0.19–0.61, 95% CIs [0.12–0.47, 0.29–0.75], dfs = 20.44–85.74, ts = 4.91–8.65, ps < 0.001), as well as for mental wellbeing (βs = −0.43 to −0.42, 95% CIs [−0.57 to −0.56, −0.31 to −0.29], dfs = 48.93–66.23, ts = −6.05 to −6.80, ps < 0.001; see Table S3 in the Supplement). This effect includes no information on temporal links between these variables but reflects that those individuals who experience high mean levels of insomnia on average also experience low levels of mental health on average.
Reverse effects of mental health on insomnia symptoms (exploratory analyses I)
In addition, we conducted exploratory analyses to investigate the reverse effects of mental health on later insomnia symptoms. First, between‐person effects showed that reduced mental health across all time points predicted higher insomnia symptoms after 6 months in men, but not in women (reversed H1; see Table 4).
TABLE 4.
Between‐person effect of mental health on insomnia symptoms at the 6‐month follow‐up, accounting for insomnia symptoms at baseline
| Variable | β (95% CI) |
|
|
|
|||
|---|---|---|---|---|---|---|---|
| Dependent variable: insomnia at t6 | |||||||
| Insomnia symptoms (t0) | 0.57 (0.44, 0.71) | 159.66 | 8.48 | <0.001 | |||
| Depressive symptoms (between‐person) – women | 0.08 (−0.11, 0.27) | 158.43 | 0.83 | 0.407 | |||
| Depressive symptoms (between‐person) – men | 0.30 (0.07, 0.53) | 159.78 | 2.58 | 0.011 | |||
| Dependent variable: insomnia at t6 | |||||||
| Insomnia symptoms (t0) | 0.56 (0.43, 0.69) | 159.62 | 8.66 | <0.001 | |||
| Anxiety symptoms (between‐person) – women | 0.12 (−0.08, 0.32) | 159.30 | 1.21 | 0.227 | |||
| Anxiety symptoms (between‐person) – men | 0.50 (0.22, 0.79) | 159.49 | 3.45 | 0.001 | |||
| Dependent variable: insomnia at t6 | |||||||
| Insomnia symptoms (t0) | 0.49 (0.35, 0.63) | 156.80 | 7.01 | <0.001 | |||
| Stress symptoms (between‐person) – women | 0.22 (0.01, 0.42) | 157.97 | 2.07 | 0.041 a | |||
| Stress symptoms (between‐person) – men | 0.50 (0.26, 0.74) | 156.15 | 4.12 | <0.001 | |||
| Dependent variable: insomnia at t6 | |||||||
| Insomnia symptoms (t0) | 0.57 (0.44, 0.69) | 159.47 | 8.63 | <0.001 | |||
| Mental wellbeing (between‐person) – women | −0.11 (−0.30, 0.07) | 159.76 | −1.18 | 0.240 | |||
| Mental wellbeing (between‐person) – men | −0.31 (−0.53, −0.09) | 159.19 | −2.74 | 0.007 |
Abbreviation: CI, confidence interval.
Note: Uncorrected, two‐sided p values are presented. We used the Benjamini‐Hochberg correction to adjust for testing multiple dependent variables. Significant effects regarding the exploratory analyses, after the correction, are highlighted in bold. For the effects of all included covariates see Table S4 in the Supplement.
Effect was no longer significant after correcting for multiple testing.
Second, for within‐person effects across 1 month, we found that higher within‐person depressive, anxiety or stress‐related symptoms did not predict insomnia symptoms 1 month later, either in men or women (reversed H2; see Table 5). However, reduced within‐person mental wellbeing at 1 month predicted fewer next‐month insomnia symptoms for men and women.
TABLE 5.
Within‐person effect of mental health at 1 month on insomnia symptoms 1 month later, accounting for insomnia symptoms at the time of the predictor
| Variable | β (95% CI) |
|
|
|
|||
|---|---|---|---|---|---|---|---|
| Dependent variable: insomnia symptoms tx + 1 | |||||||
| Insomnia Symptoms (tx) | 0.30 (0.23, 0.36) | 591.63 | 9.41 | <0.001 | |||
| Depressive symptoms tx (within‐person) – women | −0.02 (−0.14, 0.11) | 53.43 | −0.29 | 0.774 | |||
| Depressive symptoms tx (within‐person) – men | −0.13 (−0.28, 0.01) | 126.52 | −1.82 | 0.071 | |||
| Dependent variable: insomnia symptoms tx + 1 | |||||||
| Insomnia Symptoms (tx) | 0.32 (0.26, 0.38) | 553.06 | 10.19 | <0.001 | |||
| Anxiety symptoms tx (within‐person) – women | −0.06 (−0.17, 0.05) | 23.83 | −1.07 | 0.297 | |||
| Anxiety symptoms tx (within‐person) – men | −0.10 (−0.24, 0.04) | 164.27 | −1.37 | 0.172 | |||
| Dependent variable: insomnia symptoms tx + 1 | |||||||
| Insomnia symptoms (tx) | 0.32 (0.26, 0.38) | 577.30 | 10.21 | <0.001 | |||
| Stress symptoms tx (within‐person) – women | −0.08 (−0.19, 0.03) | 91.94 | −1.37 | 0.175 | |||
| Stress symptoms tx (within‐person) – men | −0.11 (−0.26, 0.04) | 69.99 | −1.47 | 0.147 | |||
| Dependent variable: insomnia symptoms tx + 1 | |||||||
| Insomnia symptoms (tx) | 0.46 (0.41, 0.52) | 144.81 | 15.98 | <0.001 | |||
| Mental wellbeing tx (within‐person) – women | 0.19 (0.07, 0.32) | 59.19 | 3.00 | 0.004 | |||
| Mental wellbeing tx (within‐person) – men | 0.20 (0.06, 0.34) | 125.66 | 2.77 | 0.006 |
Abbreviation: CI, confidence interval.
Note: Time Index = time point (tx–1, tx). Uncorrected, two‐sided p values are presented. We used the Benjamini‐Hochberg correction to adjust for testing multiple dependent variables. Significant effects regarding the exploratory analyses, after the correction, are highlighted in bold. For the effects of all included covariates see Table S5 in the Supplement.
Taken together, overall reduced mental health in men (but not in women; between persons) predicted higher insomnia severity at the 6‐month follow‐up. In contrast, if men or women reported reduced mental wellbeing at one time‐point (within persons, compared to their average wellbeing across all time points), it predicted fewer insomnia symptoms 1 month later.
Effect of one's peak insomnia symptoms on mental health (exploratory analyses II)
To explore the effect of remitting insomnia symptoms on later mental health, we used one's peak in insomnia symptoms during the first 4 months (mean [SD] 10.88 [5.07]) as a predictor of mental health. However, we found no within‐person effect of peak insomnia symptoms on mental health at the 6‐month follow‐up for depression, anxiety, and stress‐related symptoms (βs = −0.20 to +0.08, 95% CIs [−0.33 to −0.44, 0.04 to 0.49], dfs = 108.24–156.00, ts = −1.59 to +0.39, ps ≥0.113), or for mental wellbeing (βs = −0.31 to –0.10, 95% CIs [−0.38 to −0.74, 0.12 to 0.18], dfs = 146.83–155.65, ts = −1.43 to −0.71, ps ≥0.155) for men or women (see Table S6 in the Supplement). This effect indicates that a peak in insomnia symptoms that remits afterwards shows no effect on one's mental health 2–5 months later.
DISCUSSION
This study investigated the predictive power of insomnia symptoms on several aspects of mental health (i.e., depression, anxiety, stress‐related symptoms, and overall mental wellbeing) across 6 months during the COVID‐19 pandemic, with monthly assessments on average every 30 days, in a community sample. The results replicated previously published results on the between‐person level (H1). However, our within‐person hypothesis (H2) was not supported. Specifically, in line with our predictions in H1, higher insomnia symptoms across all time points (i.e., higher than the average of the whole sample) predicted reduced mental health 6 months later. With regard to our within‐person predictions about monthly changes in H2, we were surprised to find effects in the opposite direction: Increased insomnia symptoms at 1 month, within each person, predicted improved gender‐specific aspects of mental health 1 month later. Exploratory analyses on the reverse effect of mental health on later insomnia symptoms revealed that lower mental health (i.e., lower than the average of the whole sample; between persons) predicted increased insomnia symptoms after 6 months in men, but not in women. In addition, lower mental wellbeing at 1 month (but not depression, anxiety, or stress‐related symptoms), within each person, predicted better sleep 1 month later, in men and women. Furthermore, no effect of one's peak insomnia symptoms on later mental health was found. Overall, increased insomnia symptoms across all time points predicted reduced mental health 6 months later; for men, bidirectional effects were found. Across 1 month, higher insomnia symptoms predicted aspects of better mental health, and lower mental wellbeing predicted better sleep.
We used the opportunity to investigate insomnia symptoms and mental health during the COVID‐19 pandemic, because we expected increased variability in various sleep disturbances (including insomnia symptoms) in the general population due to this challenging and stressful experience. In line with Salfi et al. (2021), we found no mean‐level change in insomnia symptoms and mental health across the 6‐month study period. Our study started during the lockdown phase (with the last participants starting the study in September 2020) in Germany, and individuals were followed for 6 months. Therefore, for some individuals, restrictions were reduced during the study period whereas restrictions increased for other participants. However, controlling for individual changes in restrictions did not change our pattern of results (see also Figure S1 for a visualisation of the symptoms for men and women during the specific months we covered in our study). Other recent studies found decreasing symptoms across time (e.g., Alfonsi et al., 2021; Morin et al., 2021). However, these studies investigated only the first lockdown phase and following months (before the second lockdown) and did not include an additional ESM phase, which – in our study – might have influenced the following symptom levels. This study focused on the influence of subclinical insomnia symptoms on later mental health during the COVID‐19 pandemic, thereby extending earlier findings by showing that if insomnia symptoms remained high across all time points (i.e., compared to the whole sample), reduced mental health was reported at the 6‐month follow‐up. Associated exploratory analyses used one's peak insomnia symptoms during the first 4 months, a variable that included (per definition) reduced insomnia symptoms at all other times points (and reduced insomnia symptoms in 95% of the sample after the peak [at t5 and/or t6]). We found no effect of one's peak insomnia symptoms on mental health at the 6‐month follow‐up, indicating no impact of increased insomnia symptoms if these symptoms naturally remit afterwards. These findings are consistent with Ellis et al. (2014), who, before the COVID‐19 pandemic, found that individuals whose acute insomnia symptoms persisted for a longer period showed a higher risk of developing new, clinically relevant depressive symptoms compared to good sleepers or individuals whose insomnia symptoms remitted. With regard to the reverse effect of mental health on sleep, we found that reduced mental health across all time points predicted increased insomnia symptoms in men but not in women, which might point to differential links between sleep and mental health for men and women. In men, our findings rather point to a bidirectional relationship in which insomnia and other mental health symptoms are mutually maintained by each other.
Interestingly, increased insomnia symptoms at a certain month predicted better mental health aspects 1 month later (within persons). Specifically, in women, increased insomnia symptoms predicted reduced next‐month depressive symptoms and anxiety symptoms, and predicted higher mental wellbeing, while in men increased insomnia symptoms predicted reduced next‐month anxiety symptoms. Accordingly, with regard to the effects of mental health on sleep, reduced mental wellbeing (but not depression, anxiety, or stress) predicted better sleep 1 month later. How can we explain these findings, which are contrary to what we expected? Although speculative, the combination of three aspects could play an important role for these associations. First, according to Salfi et al. (2021), our sample seems to be a relatively healthy sample, because predictors of increased insomnia symptoms during the second wave were low (e.g., older age, low education level, working in healthcare, and living with children). Specifically, with a mean age of 31 years, our sample was relatively young, and ~80% reported having graduated from high school, did not work in healthcare, and had no children in their household. Additionally, all individuals in our sample were (by design) in a committed relationship. Furthermore, the prevalence of clinically significant insomnia symptoms in our sample (10.11%) comply with known prevalence rates in the general population before the pandemic (e.g., 6%–10%, see Ohayon, 2002), indicating no overall increased symptom levels in our sample. These sample characteristics, together with no decreases in mean‐level sleep and mental health across the study period, might indicate a high degree of resilience in our sample, which is described as “the ability to withstand setbacks, adapt positively, and bounce back from adversity” (Luthar & Cicchetti, 2001; cited by Killgore et al., 2020). Second, as mentioned earlier, these analyses are part of the Corona couple ESM study, which also included reporting and reflecting upon relationship behaviour and the use and effectiveness of emotion regulation strategies during the COVID‐19 pandemic. Similar to known reactivity effects in ESM studies (e.g., Conner & Reid, 2012; Reynolds et al., 2016), our specific study design might have prompted specific behaviour, thereby additionally increasing psychological resilience in our sample. Indeed, some of our emotion regulation strategies, such as seeking more social support from friends and family and more daily exercise, were shown to predict greater resilience during the pandemic period (Killgore et al., 2020). Killgore et al. (2020) explicitly stated that “those who actively engaged in these vital activities and nurtured their relationships tended to be the most resilient to the challenges to mental health imposed by the COVID‐19 pandemic”. Third, the reflection on symptoms each month and the time interval between the assessments in our study might have been ideal for participants to be able to realise the presence of increased symptoms and consequently to engage in and successfully implement countermeasures, which might have led to reduced symptoms after 2–4 weeks. This effect seems to be specific for insomnia symptoms with depression, anxiety, and stress exerting no effect on sleep 1 month later. In the reverse direction, only reduced mental wellbeing predicted better sleep 1 month later. Therefore, insomnia symptoms and overall mental wellbeing might be aspects that are easier to detect (and may be less stigmatised) and might therefore be more easily influenced by individuals.
Taken together, this unexpected effect of increased insomnia symptoms on better mental health might be specific for our predominantly healthy and presumably resilient study population, as well as a consequence of our study design. However, it also highlights the potential to be able to counteract some of the adverse effects of the COVID‐19 pandemic: By sensitising individuals to detect reductions in sleep or mental wellbeing, as well as by increasing their psychological resilience by supporting healthy behaviours (e.g., increased emotion regulation, going outside, seeking support from others).
Limitations
Some limitations need to be considered when interpreting the present findings. First, our sample consisted of relatively young, highly educated, partnered adults. As described in the discussion, this, as well as our overarching research questions and related design, might have contributed to our results. Being prompted to detect symptoms along with having specific strategies to deal with these symptoms, might have led to fast improvements. Therefore, future studies focusing only on sleep and mental health and including different samples regarding education, age, and relationship status, could help disentangle these effects. Second, other aspects might have contributed to the present results as well. For example, although we measured and controlled for the change in COVID‐19 restrictions, this was based on a rather crude subjective characterisation by the participants, therefore the actual extent to which restrictions may have influenced the present findings is unclear. In addition, other stressful life events could have happened as well, especially during the pandemic, that also influence sleep and mental health. Third, our questionnaires measured items during the past week for depression, anxiety, and stress, in contrast to insomnia symptoms and mental wellbeing, which were measured during the past 2 weeks. This was done because we wanted to use the original, validated measures. However, we cannot preclude that the similarity/difference between some measures contributed to the present results. Fourth, compared to other epidemiological studies, the sample size of the between‐person analyses is rather small for investigating the extent of sleep disturbances and mental health during the pandemic.
CONCLUSION
In line with earlier research, the present findings support subclinical insomnia symptoms as a risk factor for mental health during the COVID‐19 pandemic in a sample from the general population. However, later mental health seems to only suffer when insomnia symptoms are high on average and persist across a prolonged period, whereas one's peak insomnia symptoms (including reduced insomnia symptoms afterwards) did not exert a negative impact on mental health at the 6‐month follow‐up. On the contrary, increased insomnia symptoms at 1 month may even serve as a signal to engage in, and successfully implement, targeted countermeasures, which may lead to better mental health 1 month later. Overall, most studies highlight the negative impact of the COVID‐19 pandemic on the general population, where support and interventions are definitely needed. However, although our findings might be specific for our sample, we think that it is also important to point out subgroups with possible high resilience, as well as identifying which factors might contribute to psychological resilience during this challenging time. Increasing individuals’ ability to engage in targeted countermeasures (adapted to existing restrictions, e.g., seeking support from others, going outside, or exercising in order to deal with difficult emotions) might prevent increasing rates of mental disorders in subgroups of the general population during the next months and years.
AUTHOR CONTRIBUTIONS
Gabriela G. Werner: conceptualisation, funding acquisition, formal analysis, methodology, writing – original draft; Barbara Cludius: conceptualisation, funding acquisition, methodology, writing – review and editing; Philipp Sckopke: conceptualisation, funding acquisition, methodology, project administration, writing – review and editing; Angelika Stefan: conceptualisation, investigation, methodology, writing – review and editing; Felix Schönbrodt: conceptualisation, funding acquisition, methodology, project administration, writing – review and editing; Caroline Zygar‐Hoffmann: conceptualisation, formal analysis, investigation, methodology, project administration, supervision, writing – review and editing.
CONFLICT OF INTEREST
None.
Supporting information
Table S1 Description of insomnia symptoms and mental health for women and men across all time points
Figure S1, (a–e) Time course of all measures across specific months for men and women
Table S2 Between‐person effect of insomnia symptoms (across all time points) on mental health at the 6‐month follow‐up (H1; including all covariates), accounting for mental health at baseline
Table S3 Within‐ (and between‐) person effect of insomnia symptoms at 1‐month on next‐month mental health (H2; including all covariates), accounting for mental health at the time of the predictor
Table S4 Between‐person effect of mental health on insomnia symptoms at the 6‐month follow‐up (including all covariates), accounting for insomnia symptoms at baseline
Table S5 Within‐ (and between‐) person effect of mental health at 1 month on insomnia symptoms 1 month later (including all covariates), accounting for insomnia symptoms at the time of the predictor
Table S6 Effect of one's peak insomnia symptoms (t0–t4) on mental health at the 6‐month follow‐up (including all covariates), accounting for mental health at baseline
Table S7 Within‐ and between‐person effect of insomnia symptoms on mental health at the 6‐month follow‐up (including all covariates), accounting for mental health at baseline
Table S8 Within‐ and between‐person effect of mental health on insomnia symptoms at the 6‐month follow‐up (including all covariates), accounting for insomnia symptoms at baseline
ACKNOWLEDGMENTS
The WEMWBS was funded by the Scottish Government National Programme for Improving Mental Health and Wellbeing, commissioned by NHS Health Scotland, developed by the University of Warwick and the University of Edinburgh, and is jointly owned by NHS Health Scotland, the University of Warwick, and the University of Edinburgh.
This research was funded by the German Research Foundation (DFG SCHO 1334/5‐1, Felix Schönbrodt). Angelika Stefan was supported by the NWO Research Talent Programme (406.18.556).
The authors would like to thank Diana Schnelle‐Perry for her very helpful and fast proofreading.
Werner, G. G. , Cludius, B. , Sckopke, P. , Stefan, A. , Schönbrodt, F. , & Zygar‐Hoffmann, C. (2022). The predictive power of insomnia symptoms on other aspects of mental health during the COVID‐19 pandemic: a longitudinal study. Journal of Sleep Research, e13641. 10.1111/jsr.13641
Funding information Deutsche Forschungsgemeinschaft, Grant/Award Number: DFG SCHO 1334/5‐1; NWO Research Talent Programme, Grant/Award Number: 406.18.556
Endnotes
This hypothesis corresponds to H2 in the preregistration. After a more comprehensive and in‐depth analysis of the related literature in preparation for this paper, we decided to drop the preregistered H1 concerning the effect of within‐person deviations of insomnia symptoms at t0 on mental health at t6. It no longer seemed adequate to predict the presence of such a relationship, especially not in the assumed direction, as it implied an improvement of insomnia symptoms between t0 and t6. However, a more plausible notion related to H1 (i.e., the effect of a peak in insomnia symptoms with a subsequent symptom reduction) was investigated in exploratory analyses, as will be presented later. The results of the preregistered analyses of the former H1 can be found in the Supplement. Thus, the present H2 respectively corresponds to the former H3 in the preregistration.
We report McDonald's ωtotal as a measure of internal consistency instead of Cronbach's α (Dunn et al., 2014). The results differ only for the reliability of the DASS‐S, where Cronbach's α is 0.81, and for the WEMWBS, where Cronbach's α is 0.93.
As H1 did not include repeated assessments of the dependent variable, only gender‐specific fixed intercepts and slopes were possible for this analysis, but not gender‐specific random intercepts and slopes (in contrast to H2, where these were implemented).
We preregistered to perform directional tests, which would allow us to halve the two‐sided p values in the results when the effect is in the predicted direction. However, as some effects went in the opposite direction as our predictions, we decided to overall report two‐sided p values to provide some indication of how surprising the observed or more extreme data were given the null hypothesis of the effect being zero.
As described in the methods section, the analyses were based on all individuals who also completed the follow‐up assessment (N = 166). For the characteristics of these individuals, please see the Supplement.
These analyses were based on all individuals who also completed the follow‐up assessment (N = 166).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from one of the authors upon reasonable request.
REFERENCES
- Alfonsi, V. , Gorgoni, M. , Scarpelli, S. , Zivi, P. , Sdoia, S. , Mari, E. , Quaglieri, A. , Ferlazzo, F. , Giannini, A. M. , & De Gennaro, L. (2021). Changes in sleep pattern and dream activity across and after the COVID‐19 lockdown in Italy: A longitudinal observational study. Journal of Sleep Research, 00(e13500), e13500. 10.1111/jsr.13500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvaro, P. K. , Roberts, R. M. , & Harris, J. K. (2013). A systematic review assessing Bidirectionality between sleep disturbances, anxiety, and depression. Sleep, 36(7), 1059–1068. 10.5665/sleep.2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglioni, C. , Battagliese, G. , Feige, B. , Spiegelhalder, K. , Nissen, C. , Voderholzer, U. , Lombardo, C. , & Riemann, D. (2011). Insomnia as a predictor of depression: A meta‐analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders, 135(1–3), 10–19. 10.1016/j.jad.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Baglioni, C. , Nanovska, S. , Regen, W. , Spiegelhalder, K. , Feige, B. , Nissen, C. , Reynolds, C. F. , & Riemann, D. (2016). Sleep and mental disorders: A meta‐analysis of polysomnographic research. Psychological Bulletin, 142(9), 969–990. 10.1037/bul0000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglioni, C. , Spiegelhalder, K. , Lombardo, C. , & Riemann, D. (2010). Sleep and emotions: A focus on insomnia. Sleep Medicine Reviews, 14(4), 227–238. 10.1016/j.smrv.2009.10.007 [DOI] [PubMed] [Google Scholar]
- Bates, D. , Maechler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67(1), 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Serie B (Methodological), 57(1), 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Brose, A. , Blanke, E. S. , Schmiedek, F. , Kramer, A. C. , Schmidt, A. , & Neubauer, A. B. (2020). Change in mental health symptoms during the COVID‐19 pandemic: The role of appraisals and daily life experiences – Brose – 2021 – journal of personality – Wiley online library. Journal of Personality, 89, 468–482. 10.1111/jopy.12592 [DOI] [PubMed] [Google Scholar]
- Buysse, D. J. , Angst, J. , Gamma, A. , Ajdacic, V. , Eich, D. , & Rossler, W. (2008). Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep, 31(4), 473–480. 10.1093/sleep/31.4.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner, T. S. , & Reid, K. A. (2012). Effects of intensive mobile happiness reporting in daily life. Social Psychological and Personality Science, 3(3), 315–323. 10.1177/1948550611419677 [DOI] [Google Scholar]
- Csikszentmihalyi, M. , & Larson, R. (1987). Validity and reliability of the experience‐sampling method. Journal of Nervous and Mental Disease, 175, 526–536. 10.1097/00005053-198709000-00004 [DOI] [PubMed] [Google Scholar]
- Deng, J. , Zhou, F. , WhHou, W. , Silver, Z. , Wong, C. Y. , Chang, O. , Drakos, A. , Zuo, Q. K. , & Huang, E. (2021). The prevalence of depressive symptoms, anxiety symptoms and sleep disturbance in higher education students during the COVID‐19 pandemic: A systematic review and meta‐analysis. Psychiatry Research, 301, 113863. 10.1016/j.psychres.2021.113863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, T. J. , Baguley, T. , & Brunsden, V. (2014). From alpha to omega: A practical solution to the pervasive problem of internal consistency estimation ‐ Dunn ‐ 2014 ‐ British Journal of psychology ‐ Wiley online library. British Journal of Psychology, 105(3), 399–412. 10.1111/bjop.12046 [DOI] [PubMed] [Google Scholar]
- Ellis, J. G. , Perlis, M. L. , Bastien, C. H. , Gardani, M. , & Espie, C. A. (2014). The natural history of insomnia: Acute insomnia and first‐onset depression. Sleep, 37(1), 97–106. 10.5665/sleep.3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini, C. , Musetti, A. , Zenesini, C. , Palagini, L. , Scarpelli, S. , Quattropani, M. C. , Lenzo, V. , Freda, M. F. , Lemmo, D. , Vegni, E. , Borghi, L. , Saita, E. , Cattivelli, R. , De Gennaro, L. , Plazzi, G. , Riemann, D. , & Castelnuovo, G. (2020). Poor sleep quality and its consequences on mental health during the COVID‐19 lockdown in Italy. Frontiers in Psychology, 11, 1–15. 10.3389/fpsyg.2020.574475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, D. , Sheaves, B. , Waite, F. , Harvey, A. G. , & Harrison, P. J. (2020). Sleep disturbance and psychiatric disorders. Lancet . Psychiatry, 7(7), 628–637. 10.1016/S2215-0366(20)30136-X [DOI] [PubMed] [Google Scholar]
- Gerber, M. , Lang, C. , Lemola, S. , Colledge, F. , Kalak, N. , Holsboer‐Trachsler, E. , Pühse, U. , & Brand, S. (2016). Validation of the German version of the insomnia severity index in adolescents, young adults and adult workers: Results from three cross‐sectional studies. BMC Psychiatry, 16, 174. 10.1186/s12888-016-0876-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey, A. G. (2008). Insomnia, psychiatric disorders, and the Transdiagnostic perspective. Current Directions in Psychological Science, 17(5), 299–303. 10.1111/j.1467-8721.2008.00594.x [DOI] [Google Scholar]
- Harvey, A. G. , Murray, G. , Chandler, R. A. , & Soehner, A. (2011). Sleep disturbance as transdiagnostic: Consideration of neurobiological mechanisms. Clinical Psychology Review, 31(2), 225–235. 10.1016/j.cpr.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertenstein, E. , Feige, B. , Gmeiner, T. , Kienzler, C. , Spiegelhalder, K. , Johann, A. , Jansson‐Fröjmark, M. , Palagini, L. , Rücker, G. , Riemann, D. , & Baglioni, C. (2019). Insomnia as a predictor of mental disorders: A systematic review and meta‐analysis. Sleep Medicine Reviews, 43, 96–105. 10.1016/j.smrv.2018.10.006 [DOI] [PubMed] [Google Scholar]
- Jansson, M. , & Steven, J. L. (2006). The role of anxiety and depression in the development of insomnia: Cross‐sectional and prospective analyses. Psychology & Health, 21(3), 383–397. 10.1080/14768320500129015 [DOI] [Google Scholar]
- Killgore, W. D. S. , Taylor, E. C. , Cloonan, S. A. , & Dailey, N. S. (2020). Psychological resilience during the COVID‐19 lockdown. Psychiatry Research, 291, 113216. 10.1016/j.psychres.2020.113216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova, A. , Brockhoff, P. B. , & Christensen, R. H. B. (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82(13), 1–26. 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- Li, Y. , Gu, S. , Wang, Z. , Li, H. , Xu, X. , Zhu, H. , Deng, S. , Ma, X. , Feng, G. , Wang, F. , & Huang, J. H. (2019). Relationship between stressful life events and sleep quality: Rumination as a mediator and resilience as a moderator. Frontiers in Psychiatry, 10, 1–9. 10.3389/fpsyt.2019.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin, C. M. , Bjorvatn, B. , Chung, F. , Holzinger, B. , Partinen, M. , Penzel, T. , Ivers, H. , Wing, Y. K. , Chan, N. Y. , Merikanto, I. , Mota‐Rolim, S. , Macêdo, T. , De Gennaro, L. , Léger, D. , Dauvilliers, Y. , Plazzi, G. , Nadorff, M. R. , Bolstad, C. J. , Sieminski, M. , … Espie, C. A. (2021). Insomnia, anxiety, and depression during the COVID‐19 pandemic: An international collaborative study. Sleep Medicine, 87, 38–45. 10.1016/j.sleep.2021.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilges, P. , & Essau, C. (2015). Die Depressions‐Angst‐Stress‐Skalen. Der DASS – ein Screeningverfahren nicht nur für Schmerzpatienten. Der . Schmerz, 6, 649–657. 10.1007/s00482-015-0019-z [DOI] [PubMed] [Google Scholar]
- Ohayon, M. M. (2002). Epidemiology of insomnia: What we know and what we still need to learn. Sleep Medicine Reviews, 6(2), 97–111. 10.1053/smrv.2002.0186 [DOI] [PubMed] [Google Scholar]
- Okajima, I. , Komada, Y. , Nomura, T. , Nakashima, K. , & Inoue, Y. (2011). Insomnia as a risk for depression: A longitudinal epidemiologic study on a Japanese rural cohort. Journal of Clinical Psychiatry, 72(3), 24–34. [DOI] [PubMed] [Google Scholar]
- Petzold, M. B. , Bendau, A. , Plag, J. , Pyrkosch, L. , Mascarell Maricic, L. , Betzler, F. , Rogoll, J. , Große, J. , & Ströhle, A. (2020). Risk, resilience, psychological distress, and anxiety at the beginning of the COVID‐19 pandemic in Germany. Brain and Behavior, 10(9), e01745. 10.1002/brb3.1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing; Retrieved from https://www.R-project.org/ [Google Scholar]
- Reynolds, B. M. , Robles, T. F. , & Repetti, R. L. (2016). Measurement reactivity and fatigue effects in daily diary research with families. Developmental Psychology & Health, 52(3), 442–456. 10.1037/dev0000081 [DOI] [PubMed] [Google Scholar]
- Riemann, D. , Spiegelhalder, K. , Feige, B. , Voderholzer, U. , Berger, M. , Perlis, M. , & Nissen, C. (2010). The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Medicine Reviews, 14(1), 19–31. 10.1016/j.smrv.2009.04.002 [DOI] [PubMed] [Google Scholar]
- Salfi, F. , D'Atri, A. , Tempesta, D. , & Ferrara, M. (2021). Sleeping under the waves: A longitudinal study across the contagion peaks of the COVID‐19 pandemic in Italy. Journal of Sleep Research, e13313, e13313. 10.1111/jsr.13313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salfi, F. , Lauriola, M. , Amicucci, G. , Corigliano, D. , Viselli, L. , Tempesta, D. , & Ferrara, M. (2020). Gender‐related time course of sleep disturbances and psychological symptoms during the COVID‐19 lockdown: A longitudinal study on the Italian population. Neurobiology of Stress, 13, 100259. 10.1016/j.ynstr.2020.100259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpelli, S. , Gorgoni, M. , Alfonsi, V. , Annarumma, L. , Di Natale, V. , Pezza, E. , & De Gennaro, L. (2021). The impact of the end of COVID confinement on pandemic dreams, as assessed by a weekly sleep diary: A longitudinal investigation in Italy. Journal of Sleep Research., 31, e13429. 10.1111/jsr.13429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha, S. S. (2016). Trauma‐induced insomnia: A novel model for trauma and sleep research. Sleep Medicine Reviews, 25, 74–83. 10.1016/j.smrv.2015.01.008 [DOI] [PubMed] [Google Scholar]
- Suh, S. , Kim, H. , Yang, H. C. , Cho, E. R. , Lee, S. K. , & Shin, C. (2013). Longitudinal course of depression scores with and without insomnia in non‐depressed individuals: A 6‐year follow‐up longitudinal study in a Korean cohort. Sleep, 36(3), 369–376. 10.5665/sleep.2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart, F. , Stewart‐Brown, S. , & Parkinson, J. (2016). Warwick–Edinburgh Mental Well‐being Scale (WEMWBS). User Guide ‐ Version 2. NHS Health Scotland. 10.1037/t58709-000 [DOI] [Google Scholar]
- Trull, T. J. , & Ebner‐Priemer, U. (2013). Ambulatory assessment. Annual Review of Clinical Psychology, 9, 151–176. 10.1146/annurev-clinpsy-050212-185510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindegaard, N. , & Benros, M. E. (2020). COVID‐19 pandemic and mental health consequences: Systematic review of the current evidence. Brain, Behavior, and Immunity, 89, 531–542. 10.1016/j.bbi.2020.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. , Zyphur, M. J. , & Preacher, K. J. (2009). Testing multilevel mediation using hierarchical linear models: Problems and solutions. Organizational Research Methods, 12(4), 695–719. 10.1177/1094428108327450 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Description of insomnia symptoms and mental health for women and men across all time points
Figure S1, (a–e) Time course of all measures across specific months for men and women
Table S2 Between‐person effect of insomnia symptoms (across all time points) on mental health at the 6‐month follow‐up (H1; including all covariates), accounting for mental health at baseline
Table S3 Within‐ (and between‐) person effect of insomnia symptoms at 1‐month on next‐month mental health (H2; including all covariates), accounting for mental health at the time of the predictor
Table S4 Between‐person effect of mental health on insomnia symptoms at the 6‐month follow‐up (including all covariates), accounting for insomnia symptoms at baseline
Table S5 Within‐ (and between‐) person effect of mental health at 1 month on insomnia symptoms 1 month later (including all covariates), accounting for insomnia symptoms at the time of the predictor
Table S6 Effect of one's peak insomnia symptoms (t0–t4) on mental health at the 6‐month follow‐up (including all covariates), accounting for mental health at baseline
Table S7 Within‐ and between‐person effect of insomnia symptoms on mental health at the 6‐month follow‐up (including all covariates), accounting for mental health at baseline
Table S8 Within‐ and between‐person effect of mental health on insomnia symptoms at the 6‐month follow‐up (including all covariates), accounting for insomnia symptoms at baseline
Data Availability Statement
The data that support the findings of this study are available from one of the authors upon reasonable request.


