Dear Editor,
Reports of diverse clinical adverse events following COVID‐19 vaccination are increasing.
The autoimmune disorder urticarial vasculitis (UV) may be caused by drugs, malignancy, other autoimmune diseases, and infections. 1 And it has recently been associated with COVID‐19 vaccination. 2 , 3 , 4 , 5
A 68‐year‐old man presented to a local doctor with multiple, reddish, elevated, itchy‐lesions that appeared 4 days after his third dose of Pfizer‐BioNTech COVID‐19 vaccine. He reported no reactions to the first two doses. Since treatment with 15 mg prednisolone (PSL) yielded no improvement, he was referred to our clinic. His medical history included angina pectoris, diabetes mellitus, dyslipidemia, and no recent drug changes. His body temperature was 37.8°C. Physical examination revealed edematous erythema with pigmentation and purpura scattered on his trunk, extremities, and thighs (Figure 1A, B). Oral mucosa was normal. Although he reported discomfort in the throat, no laryngeal edema or upper respiratory tract symptoms were evident. Laboratory results included elevated C‐reactive protein (10.04 mg/dl), leukocytes (9400 cells/mm3), neutrophil fraction (89.2%), and platelet, eosinophil granulocyte, C3, C4, and ANA all within normal range. Quantitative antigen test for COVID‐19 was negative. Histopathological examination showed perivascular and interstitial cell infiltration. (Figure 1C). High‐power view showed infiltration of neutrophils throughout the dermis. Leukocytoclastic vasculitis with nuclear dust and erythrocyte leakage was observed (Figure 1D). Direct immunofluorescence was not performed. The patient was diagnosed with UV. Since infection induced hemorrhagic urticaria was initially suspected, PSL was tapered and rupatadine fumarate and intravenous cefazolin were added. Chest and abdominal CT scans revealed no focus of infection. Two days later, the fever resolved and by 4 days later, which was 8 days post‐vaccination, his skin rash had almost disappeared leaving pigmentation on the inner thighs. He was discharged from hospital and has had no recurrence.
FIGURE 1.
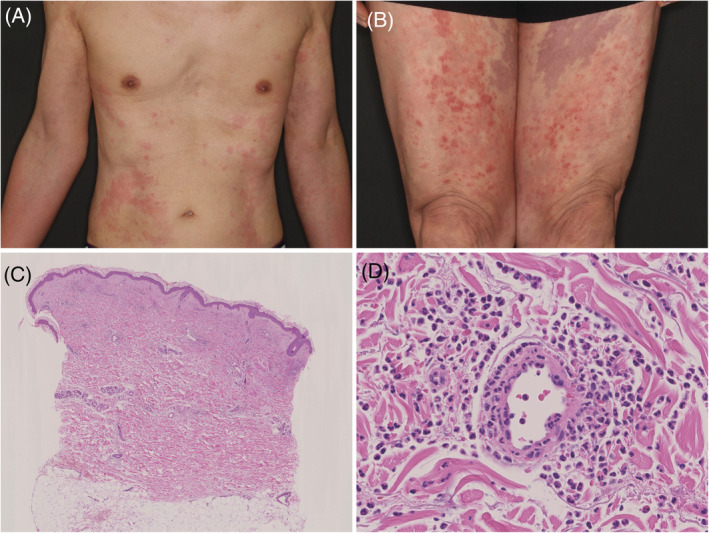
(A) Multiple urticaria‐like skin‐rash lesions on the trunk. (B) Lower extremities covered with sporadic wheals, purpura, and pigmentation lesions. (C) Cellular infiltration was observed perivascular and throughout the dermis (hematoxylin and eosin stain ×100). (D) High‐power view showing perivascular neutrophil infiltration and nuclear debris. Destruction of the vessel wall and erythrocyte leakage are evident (hematoxylin and eosin stain ×400)
There have been at least three case reports 2 , 3 , 4 and 1 review article 5 including incidences of UV following COVID‐19 vaccination. Regarding the case reports; Dash reported a 27‐year‐old man who developed skin rash 1 day after his second dose of inactivated whole virion coronavirus whom was treated with indomethacin, topical calamine lotion and levocetirizine; Nazzaro reported a 27‐year‐old woman who developed skin rash 10 days after her first dose of Moderna COVID‐19 vaccine (mRNA) whom was improved by initial administration of PSL 32 mg/day tapered off over 2 months; and Baraldi reported a 78‐year‐old woman who developed UV 7 days after her first dose of Oxford‐AstraZeneca COVID‐19 vaccine (viral vector) whom was improved by starting with PSL16 mg/day which was tapered off over 16 days, respectively. The latter case and our patient showed typical manifestation of UV with leukocytoclastic vasculitis, while the other cases showed mild clinical phenotypes.
UV is a long‐lasting urticarial‐like skin rash which pathologically shows leukocytoclastic vasculitis. Pathophysiologically, UV is considered to be a type III hypersensitivity reaction with deposition of antigen–antibody immune complexes in the vascular lumen followed by complement activation. 1 Despite the variety of vaccine type including mRNA vaccines, viral vector vaccines, inactivated virion, in previous reports, UV developed. 2 , 3 , 4 , 5 In two of the three cases described above, UV developed 7–10 days following their first vaccination whereas our case showed skin rash 4 days after his third. Interestingly, the other case developed UV 1 day after his second vaccination of inactivated virion vaccine. Taken together it is suggested that the translated or injected SARS COV‐2 NS protein might be involved in the pathogenesis shown in these cases. In addition, there is also a possibility that if patients have a high‐titer of anti‐SARS‐CoV‐2 nucleocapsid protein antibody, the eruption may occur rapidly. Accumulation of similar cases is necessary to clarify the pathogenesis of such vaccine induced UV.
AUTHOR CONTRIBUTIONS
Hiroto Ono, Reimon Yamaguchi, and Akira Shimizu contributed to the preparation of data and finalization of this article.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
ETHICAL STATEMENT
Written informed consent was obtained from the patient for publication of this report and accompanying images.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Kolkhir P, Grakhova M, Bonnekoh H, Krause K, Maurer M. Treatment of urticarial vasculitis: a systematic review. J Allergy Clin Immunol. 2019;143:458‐466. [DOI] [PubMed] [Google Scholar]
- 2. Dash S, Behera B, Sethy M, Mishra J, Garg S. COVID‐19 vaccine‐induced urticarial vasculitis. Dermatol Ther. 2021;34:e15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nazzaro G, Maronese C. Urticarial vasculitis following mRNA anti‐COVID‐19 vaccine. Dermatol Ther. 2022;35:e15282. [DOI] [PubMed] [Google Scholar]
- 4. Baraldi C, Boling L, Patrizi A, et al. Unique case of urticarial skin eruptions after COVID‐19 vaccination. Am J Dermatopathol. 2022;44:198‐200. [DOI] [PubMed] [Google Scholar]
- 5. McMahon D, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol. 2021;85:46‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


