Abstract
Historically, passive immunotherapy is an approved approach for protecting and treating humans against various diseases when other alternative therapeutic options are unavailable. Human polyclonal antibodies (hpAbs) can be made from convalescent human donor serum, although it is considered limited due to pandemics and the urgent requirement. Additionally, polyclonal antibodies (pAbs) could be generated from animals, but they may cause severe immunoreactivity and, once "humanized," may have lower neutralization efficiency. Transchromosomic bovines (TcBs) have been developed to address these concerns by creating robust neutralizing hpAbs, which are useful in preventing and/or curing human infections in response to hyperimmunization with vaccines holding adjuvants and/or immune stimulators over an extensive period. Unlike other animal‐derived pAbs, potent hpAbs could be promptly produced from TcB in large amounts to assist against an outbreak scenario. Some of these highly efficacious TcB‐derived antibodies have already neutralized and blocked diseases in clinical studies. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has numerous variants classified into variants of concern (VOCs), variants of interest (VOIs), and variants under monitoring. Although these variants possess different mutations, such as N501Y, E484K, K417N, K417T, L452R, T478K, and P681R, SAB‐185 has shown broad neutralizing activity against VOCs, such as Alpha, Beta, Gamma, Delta, and Omicron variants, and VOIs, such as Epsilon, Iota, Kappa, and Lambda variants. This article highlights recent developments in the field of bovine‐derived biotherapeutics, which are seen as a practical platform for developing safe and effective antivirals with broad activity, particularly considering emerging viral infections such as SARS‐CoV‐2, Ebola, Middle East respiratory syndrome coronavirus, Zika, human immunodeficiency virus type 1, and influenza A virus. Antibodies in the bovine serum or colostrum, which have been proved to be more protective than their human counterparts, are also reviewed.
Keywords: transchromosomic bovines, antibody‐based therapies, bovine‐derived biotherapeutics, Ebola, emerging viruses, HIV‐1, influenza A virus, MERS‐CoV, SARS‐CoV‐2, Zika
1. INTRODUCTION
Antibodies are important antiviral defenses as they have broad therapeutic potential against many infectious agents, such as Zika, Ebola, human immunodeficiency virus type 1 (HIV‐1), influenza A virus, and Middle East respiratory syndrome coronavirus (MERS‐CoV), and notably, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and its emerging variants. 1 , 2 Antibody‐based therapy is now considered a viable therapeutic modality for infectious disease targets. 3 Polyclonal antibodies (pAb) isolated from hyperimmunized host serum are critical antibody pools from different B cells that detect different epitopes on the target protein or antigen. “Poly” clonality of pAbs permits many antigenic determinants of the target to be bound. This allows pAbs to be more sensitive in certain assays against a variety of target proteins, cells, or organisms and are more likely to result in high‐avidity binding, with a low risk of antigen “escape variants” emerging. 4 Currently, there are seven human polyclonal immunoglobulins (Igs) products. 5 Human polyclonal antibodies (hpAbs) or human immunoglobulins (hIgs) derived from the plasma of healthy and convalescing human donors, or hyperimmunized animals have been approved against various viral/bacterial infections, such as a respiratory syncytial virus (RSV). 5 hIgG is the most effective and is a life‐saving tool in medical emergency crises, such as severe acute respiratory syndrome (SARS) or the MERS‐CoV outbreaks, for which no appropriate treatment is available 6 , 7 , 8 and, recently, also for COVID‐19 caused by SARS‐CoV‐2. 9 , 10 , 11 , 12 , 13 , 14 The administration of human intravenous immunoglobulin (IVIg), monoclonal antibodies (mAbs), and animal‐derived pAbs are examples of current immunotherapy technology.
Using current hpAbs products has several limitations, including the need for large amounts of plasma from convalescent human donors with high titers to make the commercial product 15 , 16 and the scarcity of serum from convalescent human donors containing hpAbs. mAbs have the disadvantage of being directed against a single epitope, making them vulnerable to the pathogen's mutational escape. 17 , 18 , 19 The modification of epitopes such that they are not recognized by most N‐terminal domain (NTD)‐ and receptor‐binding domain (RBD)‐antibodies underpin viral immune evasion by altering local conformation, charge, and hydrophobic microenvironments. 20 Furthermore, the cost of producing mAb products is exceedingly expensive. 21 So, hpAbs derived from transgenic animals may be a feasible alternative to human plasma‐derived IVIg therapy. 22 , 23 The large‐scale production of hpAbs in the most commonly transgenic animal species, involving mice 24 and rabbits, is inappropriate because they have small body sizes. Since heterologous animal‐derived antibody products are foreign proteins in humans their reactogenicity is often high. That can cause severe allergic reactions (anaphylaxis), 25 , 26 serum sickness disease, 27 and may provide "xenosialitis." 28 , 29 Serum sickness, 27 , 30 , 31 and type III hypersensitivity are mediated by immunoglobulin M (IgM) and IgG in immune complexes with the therapeutic Igs of animal origin. 31 These immune complexes can be deposited in small arteries, renal glomeruli, and synovium of the joints, causing vasculitis, nephritis, and arthritis. 28 , 30 To prevent such side effects, animal‐derived mAbs may be humanized or chimerized to human Fc fragments. However, they are directed against a single epitope, susceptible to rapid mutational escape, and also exhibit reduced neutralization efficiency. Oligoclonal cocktails were developed, but their enough production to assist in an outbreak scenario is challenging. Therefore, technical, logistical, and financial constraints will make it challenging to generate enough mAbs, convalescent plasma, and/or hyperimmune human‐derived Igs on time. Additionally, combinatorial transchromosomic (Tc)‐pAb preparations can be used to combat coinfections with divergent pathogens, demonstrating that the transchromosomic bovine (TcB) platform could be beneficial in geographical areas where multiple infectious diseases coincide or in the case of circulating of multiple pathogens.
Most mammals rely on Ig transfer, and bovine IgG plays a role in human therapy. IgG is one of the most important components with immunological action in cow colostrum. Researchers have investigated the immunological role of bovine immune milk (BIM) consumed by humans for decades. 32 The significance of cattle in supplying humans with protective antibodies in serum and milk, particularly specific antibodies against human or similar bovine viruses, cannot be denied. 33 , 34 Passive immunotherapy has been recommended for multiple deadly and emerging infectious diseases, such as severe seasonal influenza, 35 SARS, 8 MERS, 36 Ebola, 37 , 38 and SARS‐CoV‐2. 14 , 39 However, collecting enough human plasma for production is often limited. 35 , 40 Here, we review the potential promising role of therapeutic antibodies derived in normal or Tc cattle to be effective against the most common human viruses (Figure 1), focusing on a few examples of the recent viruses.
Figure 1.
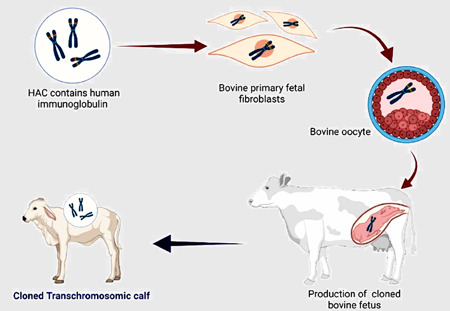
Procedure for production of cloned transchromosomic bovine (TcB). Cloned TcB is accomplished by employing microcell‐mediated chromosomal transfer to introduce a human artificial chromosome (HAC) vector containing the entire unrearranged sequences of the human immunoglobulin heavy‐chain (H) and lambda (λ) light‐chain loci into bovine primary fetal fibroblasts through microcell‐mediated chromosome transfer. Tc fibroblasts and enucleated oocyte couplets are fused, resulting in the transfer of the fibroblast nucleus and the formation of an embryo. The reconstituted Tc embryos were cultured in vitro to the blastocyst stage and then implanted into recipient cows.
2. TRANSCHROMOSOMIC BOVINES
TcBs produce potent human antibody neutralizers. TcB vaccination activates the bovine adaptive immune response, allowing TcB B cells to secrete human polyclonal Ig. pAbs act against a wide variety of epitopes, limiting the potential of viral pathogens to develop mutational resistance. These antibodies play a role in public health infection control and neutralizing viruses. They increase virus clearance in natural killer (NK) cells and cytotoxic T lymphocytes by triggering an antibody‐dependent cellular cytotoxicity (ADCC) mechanism. This immunological response eliminates viral reservoirs by killing virus‐infected cells. 41
SAB Biotherapeutics has developed the TcB (Figure 2) as a viable source of human antibodies for passive immunotherapy 42 to circumvent the constraints found in using mAbs and convalescent plasma (Table 1). Mature and functional hIgs were isolated from the blood of a Tc calf in 2002. 42 That is achieved by introducing a human artificial chromosome (HAC) vector containing the complete unrearranged sequences of the hIg heavy‐chain (H) and lambda (λ) light‐chain loci into bovine primary fetal fibroblasts using microcell‐mediated chromosome transfer. 42 TcB develops entirely potent neutralizing hpAbs endogenously and mounts a strong antibody immune response after hyperimmunization. Tc calves that produce human Igs can effectively protect their human IgGs, which have implications for the successful large‐scale production of therapeutic antibodies. 43 Bovine neonatal Fc‐receptor is involved in IgG homeostasis. Human IgG binds to the bFcRn more strongly than bovine IgG and has a serum half‐life of 33 days in Tc calves, which is more than twice as long as its bovine counterpart. TcBs have a triple deletion in the heavy chain genes, and lambda cluster light chain genes (IGHM/IGHML1/IGL), and HAC containing the information for the human antibody heavy chain and kappa chain has been inserted, enabling TcBs to develop fully human antibodies. 21 , 22 , 44 , 45 Depending on TcB, hIgG, chimeric IgG (human gamma heavy chain and bovine kappa chain), and trans‐class‐switched bovine IgG are the three types of IgG antibodies generated by TcBs. Significantly, 70%–80% of the generated antibodies are completely hIgG. 44 Since the bovine Ig light chain gene has not been deleted from Tcb, chimeric IgG is also produced. 44 TcBs can be hyperimmunized for a long time with vaccines containing potent adjuvants and/or immune stimulators. After hyperimmunization, each cow can produce 150–600 g of purified TcB fully human IgG (Tc‐hIgG) every month, depending on its age and size. Tc‐hIgG is purified from pooled convalescent plasma collected from vaccinated TcBs 46 , 47 , 48 , 49 and is separated from chimeric and bovine IgGs. The purified hpAbs are quickly produced with a highly concentrated form and do not require further treatments. Compared to human‐derived IVIg, TcB antibodies have identical amounts of galactose‐α‐1,3‐galactose carbohydrates (α‐gal). After equine antivenom or cetuximab administration or red meat intake, the α‐gal induces a human‐immunologic barrier to xenotransplantation or anaphylaxis/hypersensitivity reactions in certain humans. 50 , 51 , 52 pAbs are effective against a broad spectrum of epitopes, reducing the viral infectious agent's chances of mutational escape. For treating a wide variety of acute and chronic conditions, transgenic cows may be immunized with antigens from several cancers, infectious agents (including antibiotic‐resistant), or cytokines involved in inflammatory processes to develop high titers of pAbs. Furthermore, through hyperimmunization, the titer of pAbs raised against specific antigens may be closely monitored and improved. 42 , 53 Antigen‐specific human antibodies to diverse viral pathogens have been developed using the TcB platform (Table 2).
Figure 2.
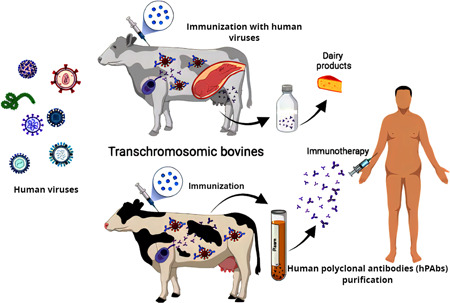
Schematic diagram shows the contribution of bovine‐derived biotherapeutics to human health. Humans can be supplied with hyperimmunized milk from transchromosomic (Tc) bovines, which can be utilized to make dairy products with protective antibodies. Also, Tc bovine vaccination triggers the adaptive immune response in cattle, allowing Tc bovine B cells to release human polyclonal antibodies that target a wide range of epitopes, reducing the risk of viral infections gaining mutational resistance.
Table 1.
Advantages of Tc bovine‐based system for producing therapeutic hPABs 41
| 1. | Production of large amounts of humanized antibodies. |
| 2. | Possibility of hyperimmunization against almost any human pathogen or other peptide antigens. |
| 3. | Easily testing a large number of antigens. |
| 4. | No need for isolation of a target virus for vaccine development. |
| 5. | At any stage of antibody development, no patient intervention is required. |
| 6. | A short time from immunization to antibodies purification (3–5 months). |
| 7. | Low cost (compared to mAb development). |
| 8. | Binding to multiple targets. |
| 9. | Theoretical resistance to escape mutation/reduction of the potential for escape mutants. |
| 10. | Potential intervention to solve infections epidemic/pandemic outbreaks. |
Abbreviations: hpAbs, human polyclonal antibodies; mAbs, monoclonal antibody; Tc, transchromosomic.
Table 2.
Examples of human monoclonal/polyclonal neutralizing antibody products (hpAbs) produced in TcB against human viruses
| hpAbs | Virus | Animal model |
|---|---|---|
| SAB‐159 | HTNV | Syrian hamsters– marmoset |
| (SAb Biotherapeutics) | ||
| SAB‐159P | PUUV | Syrian hamsters– marmoset |
| (SAb Biotherapeutics) | ||
| SAB‐155 | Zika virus | STAT2 knockout golden Syrian hamsters |
| (SAb Biotherapeutics) | ||
| SAB‐139 | EBOV | Mice–Rhesus macaques |
| (SAb Biotherapeutics) | ||
| Tc bovine‐derived VEEV‐specific TcPAbs | VEEV | Mice |
| (SAb Biotherapeutics) | ||
| SAB‐300 | MERS‐CoV | In vitro–mice |
| SAB‐301 | Phase I clinical trial | |
| (SAb Biotherapeutics) | ||
| SAB‐100 | Influenza A virus | In vitro |
| 53C10 | ||
| SAB‐176 | Phase I clinical trial | |
| (SAb Biotherapeutics) | ||
| SAB‐185 | SARS‐CoV‐2 | In vitro |
| (SAb Biotherapeutics) | Phase II clinical trial |
Abbreviations: EBOV, Ebola virus; HTNV, hantavirus; MERS‐CoV, Middle East respiratory syndrome coronavirus; PUUV, Puumala virus; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; VEEV, Venezuelan equine encephalitis virus.
3. SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS 2
The current new coronavirus, officially named SARS‐CoV‐2, was first detected in Wuhan, China, in late December 2019. 54 It was thought to have originated from wild animals at the Huanan market and then rapidly spread by person‐to‐person transmission, causing the pandemic disease named COVID‐19, with various degrees of severity, from mild flu‐like symptoms to pneumonia and death. 54 SARS‐CoV‐2 is a member of the Coronaviridae family, which comprises many virulent strains that infect animals and humans, including SARS‐CoV and MERS‐CoV. 55 As of April 25, 2022, 510 million laboratory‐confirmed human COVID‐19/SARS‐CoV‐2 infection cases, including 6.2 million (1.22%) deaths, had been reported (https://coronavirus.jhu.edu/map.html accessed on April 25, 2022). SARS‐CoV‐2 is a highly transmissible virus with low mortality, and COVID‐19 is considered the third most severe epidemic caused by coronaviruses in the past two decades. Despite more than two years of intensive study since the virus was first isolated, developing an effective and specific SARS‐CoV‐2 treatment remains a daunting challenge though few vaccine candidates have been developed successfully, and vaccination is in progress globally. 56
Recent papers reviewed the therapeutic potential of bovine Ig‐rich colostrum against SARS‐CoV‐2. 32 , 57 , 58 The impact of BIM on human health has been studied for decades (Table 3). Furthermore, colostrum or antibodies‐rich milk from bovines can be used against human diseases caused by viruses and bacteria, 33 , 59 where cow's milk is available to the general public. Currently, different colostrum‐based products are commercially used. 57 Humans can get short‐term protection against COVID‐19 by drinking microfiltered immune milk from SARS‐CoV‐2‐immunized cows. 32 Recent reports showed using heterologous passive immunity of coronavirus BIM as an immunostimulant therapy to control SARS‐CoV‐2 infection, activate the intestinal immune system, and combat the viral infection. 60 Moreover, bovine colostrum‐derived proteins such as lactoferrin may be used to treat COVID‐19 due to their potent antiviral and anti‐inflammatory properties (reviewed by da Silva Galdino et al. 57 ). Lactoferrin may help to reduce the cytokine storm associated with severe COVID‐19 infection, 61 inhibiting the SARS‐CoV‐2 binding to the host cells. 61 Additionally, bovine IgG enriched fraction can neutralize SARS‐CoV‐2 through specific binding to the RBD of SARS‐CoV‐2 S protein, but it has less potency activity against NTD of the spike protein of SARS‐CoV‐2. 62 FM‐CBAL74 (cow's milk fermented with the probiotic Lactobacillus paracasei CBAL74) also showed antiviral activity against SARS‐CoV‐2. 63 SARS‐CoV‐2 infection is significantly inhibited in vitro by bovine lactoferrin (bLF) due to direct entry inhibition and immunomodulatory mechanisms. That backs up the great specificity of bLF's anti‐SARS‐CoV‐2 activity, which is not seen in other bioactive milk proteins. 64 The in vitro antiviral efficacy of bLF has been shown against SARS‐CoV‐2 variants of concerns (VOCs), such as Alpha, Beta, Gamma, Delta, and Omicron variants. 64
Table 3.
Bovine based‐products
| Bovine based‐product | Activity | References |
|---|---|---|
| BMAP‐27 (27‐residue bovine cathelicidin peptide) | Anti‐HIV activity | [65] |
| Lactoperoxidases (bLPO) | Anti‐HSV‐1 activity | [66] |
| Anti‐influenza activity | [67] | |
| Lactoferrin (bLf) | Anti‐HCV activity | [68] |
| Anti‐SARS‐ COV‐2 | [69] | |
| Block HCMV infection | [70] | |
| Anti‐HIV‐l activity | [71] | |
| Anti‐influenza activity | [72] | |
| Anti‐HBV activity | [73] | |
| Bovine lactoferrin has been granted generally recognized safe status by FDA | ||
| Lactoferricin (β‐turn structure peptide) | Anti‐HCMV activity | [74] |
| Anti‐HSV activity | [75] | |
| Indolicidin (extended‐structure peptide) | Anti‐HIV‐1 activity | [76] |
| Anti‐HSV activity | [77] | |
| Indolicidin are cationic antimicrobial peptide isolated from bovine neutrophils | ||
| Bovine milk/colostrum | Anti‐influenza activity | [78, 79] |
| β‐lactoglobulin "modified by 3‐hydroxyphthalic anhydride” | Anti‐HIV activity | [80, 81] |
Abbreviations: bLf, bovine lactoferrin; FDA, Food and Drug Administration; HBV, hepatitis B virus; HCMV, human cytomegalovirus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HSV, human simplex virus; SARS‐ COV‐2, severe acute respiratory syndrome coronavirus 2.
Anti‐SARS‐CoV‐2 (Tc‐hIgG‐SARS‐CoV‐2) Ig was produced using TcB. 82 TcBs were hyperimmunized twice with plasmid DNA encoding the SARS‐CoV‐2 Wuhan‐Hu‐1 strain Spike (S) gene 41 , 83 , 84 then repeatedly immunized with S protein purified from insect cells. The Tc‐hIgG‐SARS‐CoV‐2, termed SAB‐185, efficiently neutralizes SARS‐CoV‐2 and vesicular stomatitis virus SARS‐CoV‐2 chimeras in vitro. 82 SAB‐185 was investigated in vitro for its neutralizing capacity against five SARS‐CoV‐2 variant strains: Munich (Spike D614G), UK (B.1.1.7), Brazil (P.1), and South Africa (SA) (B.1.3.5) variants, as well as a variant, derived from a chronically infected immunocompromised patient (Spike Δ144–146). SAB‐185 neutralized all the SARS‐CoV‐2 variants similarly in Vero E6 cells; however, a control convalescent human blood sample was less efficient in neutralizing the SA variant. 41 , 85 A novel human angiotensin‐converting enzyme 2 (hACE2) transgenic Syrian hamster was employed as the animal model, which protected from a fatal disease and had fewer clinical signs of infection after receiving prophylactic SAB‐185, implying that SAB‐185 may be a successful treatment for patients infected with SARS‐CoV‐2 variants. 85 In the most recent study for assessing the efficacy of SAB‐185 against the most current emerging variants, Luke et al., used recombinant lentivirus pseudoviruses as an alternative pseudovirus platform to express the multiple mutations in VOC/variants of interest (VOI) S proteins using a stably transduced 293T‐ACE2 cell line expressing both ACE2 and TMPRSS2 (293TACE2.TMPRSS2S). 84 For screening tests, this pseudovirus system may offer the properties of safety, genetic stability, and scalability. 41 SAB‐185 (V4) and SAB‐185 (V3–V5) isolated from hyperimmunized TcB plasma showed strong antibody binding avidity to the SARS‐CoV‐2 spike of the vaccine‐homologous WA‐1 strain, as well as the stabilized prefusion spike of the Alpha and Beta VOCs. In investigations from different laboratories, SARS‐CoV‐2 neutralizing activity evaluated by pseudovirus neutralization assay corresponds well with plaque reduction neutralization tests with actual SARS‐CoV‐2 virus. 86 , 87 , 88
TcB sera and purified SAB‐185 displayed high antibody avidity and neutralizing capacity against VOCs/VOIs; Alpha, Epsilon, Iota, Gamma, Beta, Kappa, and Delta strains. As a result, anti‐SARS‐CoV‐2 purified SAB‐185 may likely result in effective virus neutralization and protection against new SARS‐CoV‐2 strains and might potentially serve as an effective therapy for COVID‐19 patients, including those infected with circulating SARS‐CoV‐2 VOCs/VOIs. 1 Recently, SAB‐185 showed efficacy against Delta (VOC) and Lambda (variants being monitored) variants. 84 In an in vitro pseudovirus model, SAB‐185 showed extensive neutralization of Omicron and other VOCs, and it is currently being studied in the NIH1‐sponsored phase 3 COVID trial, which has begun enrolling patients in October 2021. Scientists at the US Food and Drug Administration's (FDA) Center for Biologics Evaluation and Research compiled these findings using a lentiviral‐based pseudovirus assay conducted in a BSL2 environment that included a stable 293T cell line expressing hACE2 and transmembrane serine protease 2 (TMPRSS2). SAB‐185 can still neutralize a recombinant S protein lentiviral pseudovirus that mimics the SARS‐CoV‐2 Omicron variant. SAB‐185 was still able to neutralize the Omicron variant as compared to the wild‐type SARS‐CoV‐2; however, it exhibited a mild‐moderate drop in potency. SAB Biotherapeutics is attempting to improve SAB‐185 by including Omicron‐specific activity. Suddenly, in March 2022, SAB Biotherapeutics reported NIH discontinuing the Phase 3 ACTIV‐2 trial evaluating SAB‐185 for COVID‐19 treatment due to a decrease in COVID hospitalizations. Importantly, by binding to several epitopes on the RBD, pAbs can effectively block viral entry receptors while also activating immune effector cells, increasing the individual's immune response. According to a preclinical study, SAB‐185 is also significantly more potent than human‐derived convalescent IgG. 41
4. MIDDLE EAST RESPIRATORY SYNDROME CORONAVIRUS
The MERS‐CoV, which belongs to betacoronavirus lineage C, causes severe acute respiratory disease in humans. 6 MERS‐CoV was first identified in 2012 in Saudi Arabia. 89 Two groups of TcBs were vaccinated with two experimental MERS‐CoV vaccines. SAB‐300 and SAB‐301 are two purified TcB human IgG produced after vaccination with the Jordan strain and Al‐Hasa strain, respectively. They had high enzyme‐linked immunosorbent assay (ELISA) and neutralized antibody titers in vitro without antibody‐dependent enhancement (ADE) and reducing lung virus titers in infected mice. 47 Consequently, SAB‐301 was selected for in vivo and preclinical studies. The US FDA recently approved SAB‐301 applications for MERS CoV (ClinicalTrials.gov numbers NCT02788188). 36 In a phase I clinical trial, SAB‐301 was found to be safe and well‐tolerated (up to 50 mg/kg in healthy participants, 90 , 91 with an average terminal IgG removal half‐life t1/2) of 28 days, which is similar to human‐derived IVIg. 91
5. HUMAN IMMUNODEFICIENCY VIRUS TYPE 1
HIV‐1 was first characterized in 1981 as the causative agent of acquired immunodeficiency syndrome. 92 HIV‐1 belongs to the primate lentiviruses (RNA viruses), genus retroviruses, the Orthoretroviridae subfamily of Retroviridae family. 93 In HIV‐1, the envelope (Env) glycoprotein (GP) spike is the primary target for neutralizing antibodies (nAbs). High concentrations of nAbs act as promising microbicide formulations but producing them in large quantities is currently prohibitively costly. Moreover, in human or animal models, no immunogen has reliably elicited broadly nAbs to HIV. 94 Despite extensive efforts, no HIV‐1 vaccine has been able to elicit bNAbs reliably. Bovine colostrum can be used to obtain low‐cost HIV‐1‐specific NAbs in large amounts quickly and cheaply. Bovine colostrum contains approximately 50 mg/ml of IgG (primarily IgG1) and 4 mg/ml of IgA and IgM. 95 Colostrum‐purified polyclonal IgG showed specificity for the CD4 binding site. Bovine IgG can bind to human B cells and monocytes, 96 mediate effective HIV‐1 neutralization, and stimulate a functional response in human cells. Bovine anti‐HIV colostrum IgG has strong HIV‐1‐specific ADCC activity in vitro, 97 indicating that it may be a good source of antibodies for a fast and effective response to HIV‐1 infection and developing novel Aby‐mediated approaches as HIV‐1 transmission prevention strategy. 97 This approach provides a low‐cost mucosal HIV preventive agent potentially suitable for a topical microbicide. 96
Innate immune cells, including NK cells, are Fc receptors‐bearing effector cells via which ADCC is initiated as soon as the immune cells recognize and bind IgG‐bound infected cells. 98 FcγRIIa (CD32a) was the major receptor responsible for monocyte mediated (CD14+ monocytes) ADCC in response to bovine IgG. Given the high concentration of serum IgG, FcRI (CD64), expressed on monocytes, has a high affinity for monomeric IgG and is thus believed to be saturated under physiological conditions. 97 Contrarily, under physiological conditions, the efficient binding of low‐affinity receptors FcRIIa/b (CD32a/b) and FcRIIIa/b (CD16a/b) to monomeric IgG involves the formation of immune complexes. 99 In the absence of a neutralization function, only a few anti‐HIV‐1 mAbs have been identified to have ADCC activity. 100
Previously, pregnant cows were immunized with HIV‐1 Env gp140 oligomers and elicited high titers of anti‐gp140‐binding IgG in serum and colostrum. 95 In rabbits 100 and macaques, 101 BG505 SOSIP1 (immunogens that antigenically resemble the HIV Env GP) enhanced the elicitation of potent isolate‐specific antibody responses. Still, it has not yet induced widely nAbs. This failure may be because the relevant antibody repertoires are poorly suited to attack the conserved epitope regions on Env, which are occluded relative to the exposed variable epitopes. 93 BG505 SOSIP 102 was given to four cows. Surprisingly, BG505 SOSIP immunization elicited broad and potent serum antibody responses in all four cows quite quickly. Cows have long third heavy chain complementary determining regions in their antibody repertoire, with an ultralong subset that can be over 70 amino acids long. 103 Compared to previous studies in other species, immunizing cows with a well‐ordered Env trimer reliably and quickly elicits broad and potent neutralizing serum responses. Vaccination of cows with uncleaved HIV AD8 strain gp140 Env (HIVAD8 gp140, AD8 clone of ADA) resulted in a high titer of broadly neutralizing antibodies (BrNAbs) in serum, which was obtained in large amounts in the immunized cows' colostrum samples. 95 , 104 Colostrum IgG had a broad neutralizing activity and was able to inhibit anti‐CD4bs mAbs such as b12 and VRC01 95 and have antibody‐dependent cell‐mediated cytotoxicity activity. 96 Generally, the peculiar characteristics of the bovine Ig diversity system indicate that bovine mAbs may be formed in response to antigenic epitopes that are difficult for other species to engage. The humanization of bovine BrNAbs as long‐acting antiviral therapy in HIV‐infected individuals may be an adjunct to established oral antiviral regimens. 105
6. INFLUENZA A VIRUS
Influenza is one of the most common human respiratory illnesses, causing 250 000–500 000 deaths/year worldwide despite vaccines and antiviral drug development efforts. Influenza virus type A, the most virulent of the three influenza viruses, is linked to seasonal (winter) outbreaks in temperate countries. 107 Because nAbs play a crucial role in diminishing the severity of influenza virus infection, 108 passive immunotherapy could be a potential strategy to treat influenza virus infection, modify severe disease consequences, and provide additional benefits to the standard of care. Antibodies that fight the influenza A virus's surface GPs such as haemagglutinin and neuraminidase are essential components of antiviral drugs and may provide an alternative to current countermeasures. The TcB platform was used to characterize pAbs and mAbs against the influenza A virus. 109 After being immunized with H1N1, H3N2, and influenza B virus, TcB developed SAB‐100, a pAb. 110 SAB‐100 antibody recognized three distinct epitopes, one of which is found in HA2 and highly conserved among different subtypes of HAs according to the peptide‐based ELISA. The 53C10 human nonimmunogenic mAb, which was also generated on the Tc cattle platform, was then characterized; this mAb could neutralize various H1 subtype clades. 53C10 recognizes a novel noncontinuous epitope that overlaps with the receptor‐binding site. Further analysis revealed that two substitutions in the escape mutant do not affect antibody binding but may serve as a competitive advantage. Despite the broad binding of 38C2, mAb generated by reactive immunization with a 1,3‐diketone hapten to H1 HAs, 38C2 mAb showed no detectable neutralizing activity against the H1N1 virus. In vitro study showed that this mAb is a potent ADCC. Despite the presence of a neutralizing escape mutant, 53C10 is effective in treating H1 influenza virus infection in humans. 109
More interestingly, SAB‐176 will be moving into phase 2 trials later this year. SAB‐176 is a polyclonal human antibody produced in transgenic cows after TcB were hyperimmunized with quadrivalent influenza strains to help combat seasonal flu. SAb biotherapeutics announced that SAB‐176 appeared to be safe and well‐tolerated in the randomized, double‐blind, placebo‐controlled challenge study of SAB‐176 (25 mg/kg dose) was conducted in 60 healthy adult participants inoculated with a pandemic influenza virus strain (pH1N1) (Identifier NCT04850898). As said, the results showed that SAB‐176 is effective against both known and unknown viral variants, making it a very valuable feature when addressing rapidly mutating pathogens. 110
7. EBOLA (ZAIRE AND SUDAN) VIRUSES
Ebola viruses (EBOVs) are the etiologic agents of Ebola hemorrhagic fever (EHF), a severe form of viral hemorrhagic fever in humans prevalent in central Africa, 111 and have international public health concerns. EBOV belongs to the family Filoviridae in the order of Mononegavirales. 112 The Filoviridae family comprises six species: EBOV, Sudan virus (SUDV), Reston virus (RESTV), Bundibugyo virus (BDBV), Taï forest virus (TAFV), and Bombali virus. 113 Zaire virus (EBOV), BDBV, SUDV, TAFV, and RESTV are the five species that have been identified so far. 114 Protective and potent fully hpAbs with robust neutralizing activity against Zaire EBOV, more commonly known as EBOV, and SUDV were developed after hyperimmunization of TcB with DNA vaccines expressing the codon‐optimized GP genes of both viruses, 49 using the eukaryotic expression plasmid. These pAbs were first tested in the BALB/c and IFNAR−/− mouse models of EHF, where they showed a significant increase in survival in both models with treatments. 49 Dye et al. 46 used a recombinant GP vaccination containing the Makona EBOV isolate from 2014 to hyperimmunize two TcBs resulting in high levels of fully human IgG. Purified fully hpAbs against EBOV were attested in a mouse challenge model using mouse‐adapted Ebola virus (maEBOV). One day after the lethal challenge with maEBOV, BALB/c mice were given an intraperitoneal dose of pure anti‐EBOV IgG (100 mg/kg), which resulted in 90% protection. These antibodies were rapidly elicited in commercially viable quantities. 46 After sequential hyperimmunization with an EBOV, Makona isolate GP nanoparticle vaccine, anti‐EBOV IgG Igs (collectively referred to as SAB‐139) were purified from TcB plasma. 115 It was noticed that NK cells, monocytes, and peripheral blood mononuclear cells are all potently activated by SAB‐139. The obtained results from in vitro and in vivo studies about SAB‐139 motivated the scientists to go to clinical trials in humans. 115 Another study utilized the TcB to produce hpAbs directed against EBOV GP after TcB vaccination with a DNA plasmid encoding EBOV GP. 116 Following a fatal challenge with the EBOV Makona in Rhesus macaques, these TcB pAbs conferred partial protection and resulted in a 50% survival rate. 116
8. ZIKA VIRUS
Zika virus is a flavivirus belonging to the Flaviviridae family. In 1947, in Brazil, the Zika virus was isolated from Aedesafricanus mosquitos on many occasions. 117 Because of the severe outcomes of ZIKV infection during pregnancy, passive immunotherapy to prevent transmission to the fetus could provide the most clinical benefit. 118 Transferring IgG through the placenta during pregnancy provides passive immunity to the fetus and is critical to protecting newborns against infections and immunological diseases. The brain, testis, spleen, and liver of mice exposed to the lethal challenge of the Zika virus were protected from significant tissue damage after treatment with TcB antibodies. These fully hPAbs generated in TcB were produced against the Zika virus GP after TcB vaccination with a DNA vaccine expressing the preM/E protein of Zika virus. 119 ZIKV infects humans by inactivating human type I interferon responses by targeting human STAT2 protein. 120 So, STAT2 knockout (KO) hamsters were used because their innate immune responses would be similar to those seen in humans after ZIKV infection. STAT2 KO golden Syrian hamsters were prophylactically and therapeutically protected from infection by ZIKV after treatment with ZIKV‐specific hpAbs (SAB‐155), developed in TcB. 121 Testicular lesions are also prevented in this hamster model by these antibodies. 121 SAB‐155 antibodies protected wild‐type mice from ZIKV infection and ZIKV‐induced tissue damage in the brain and testis. 119
9. CONCLUSION
Passive immunization remains an important therapeutic modality to prevent and treat human infectious and noninfectious diseases. One of the most well‐established and proven platforms for passive immunization is hpAbs. More than 20 FDA‐approved products address a broad range of targets or pathogens. 5 The source of pAb therapies can be human or animal plasma. Obtaining commercially microfiltered raw milk from supermarkets as a prophylactic and therapeutic regimen is highly recommended. BrNAbs with long CDR H3 are promising candidates for prophylaxis and antibody‐based immunotherapy. 122 , 123 Other viruses, such as influenza or SARS‐CoV‐2, 124 may benefit from a similar approach, eliminating the need for expensive annual vaccinations or offering a therapeutic response to prevent pandemic outbreaks. 125 The TcB platform for producing hIgs is still relatively new, and products are only now making their way into clinical trials. The ability of TcBs to produce multivalent pAbs in their plasma and rapidly generate antibodies to combat disease agents that have evolved to resist human antibody responses, such as HIV, is an interesting area of research. Besides their inherent prophylactic and therapeutic value, antibodies generated against pathogens that have evolved to avoid human immunological responses may aid in defining targets for vaccine and drug design. Antibodies produced in TcB could solve the respiratory viruses‐associated ADE phenomena. The type III hypersensitivity (based on immune‐complex) could also be avoided because the bovine‐derived antibodies are human‐like and not heterologous. Recent studies showed that the anti‐SARS‐CoV‐2 antibodies (antibody‐based vaccines) could increase the severity of COVID‐19 and multiple viral infections such as RSV 126 , 127 and measles 128 , 129 through ADE, which results in failed vaccine trials. Additionally, variants of the SARS‐CoV‐2 with amino acid substitutions and deletions in the spike protein (S) can minimize the efficacy of mAbs and jeopardize vaccine‐induced immunity. 82 DNA vaccines, such as SARS and SARS‐CoV‐2 vaccines, 130 , 131 , 132 combined with a TcB‐based manufacturing platform, can be used to rapidly manufacture potent antiviral NAbs that are protective in animal models. 133 It is possible to target antibody responses against the most antigenic portions of an infectious agent by combining the TcB mechanism with gene‐based vaccine technology. Some TcB antibodies have shown safety and efficacy in human clinical trials, such as anti‐MERS‐CoV (SAB‐301) and anti‐mycoplasma (SAB‐136) antibodies. 91 , 134 Recently, the human zoonotic diseases caused by emerging viruses have rapidly increased and constitute a major public health problem around the world. Some viruses are intrinsically resistant to existing medicines, while others gain resistance‐caused mutations. 135 Strikingly, antibodies derived from Tc cattle could play a crucial role in other human health issues than viral infections, such as chronic multidrug‐resistant infections. In addition, SAB‐176, SAB‐185, and SAB‐142 are under study for their therapeutic activity against seasonal flu, COVID‐19, type 1 diabetes, and organ transplantation, respectively.
AUTHOR CONTRIBUTIONS
AbdulRahman A. Saied: Conceptualization, data curation, visualization, investigation, and writing–review and editing. Manuela Sales Lima Nascimento, Adriano Henrique do Nascimento Rangel, Krzysztof Skowron, Katarzyna Grudlewska‐Buda, Kuldeep Dhama, Jaffer Shah, Ahmed Abdeen, Fouad S. El‐Mayet, Hassan Ahmed, and Asmaa A. Metwally: Writing–original draft and writing–review and editing. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Saied AA, Nascimento MSL, do Nascimento Rangel AH, et al. Transchromosomic bovines‐derived broadly neutralizing antibodies as potent biotherapeutics to counter important emerging viral pathogens with a special focus on SARS‐CoV‐2, MERS‐CoV, Ebola, Zika, HIV‐1, and influenza A virus. J Med Virol. 2022;94:4599‐4610. 10.1002/jmv.27907
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study. All relevant data are presented in the article.
REFERENCES
- 1. Tang J, Grubbs G, Lee Y, et al. Increased antibody avidity and cross‐neutralization of severe acute respiratory syndrome coronavirus 2 variants by hyperimmunized transchromosomic bovine‐derived human immunoglobulins for treatment of coronavirus disease 2019. J Infect Dis. 2022. [DOI] [PMC free article] [PubMed]
- 2. Ali MG, Zhang Z, Gao Q, Pan M, Rowan EG, Zhang J. Recent advances in therapeutic applications of neutralizing antibodies for virus infections: an overview. Immunol Res. 2020;68(6):325‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salazar G, Zhang N, Fu T‐M, An Z. Antibody therapies for the prevention and treatment of viral infections. NPJ Vaccines. 2017;2(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ascoli CA, Aggeler B. Overlooked benefits of using polyclonal antibodies. Biotechniques. 2018;65(3):127‐136. [DOI] [PubMed] [Google Scholar]
- 5. Tharmalingam T, Han X, Wozniak A, Saward L. Polyclonal hyper immunoglobulin: a proven treatment and prophylaxis platform for passive immunization to address existing and emerging diseases. Hum Vaccin Immunother. 2022;18:1886560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rabaan AA, Al‐Ahmed SH, Sah R, et al. MERS‐CoV: epidemiology, molecular dynamics, therapeutics, and future challenges. Ann Clin Microbiol Antimicrob. 2021;20(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hilgenfeld R, Peiris M. From SARS to MERS: 10 years of research on highly pathogenic human coronaviruses. Antiviral Res. 2013;100(1):286‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yeh K‐M, Chiueh T‐S, Siu LK, et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56(5):919‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID‐19. J Clin Invest. 2020;130(6):2757‐2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci USA. 2020;117(17):9490‐9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ye M, Fu D, Ren Y, et al. Treatment with convalescent plasma for COVID‐19 patients in Wuhan, China. J Med Virol. 2020;92(10):1890‐1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID‐19. Lancet Infect Dis. 2020;20(4):398‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dhama K, Khan S, Tiwari R, et al. Coronavirus disease 2019—COVID‐19. Clin Microbiol Rev. 2020;33(4):e00028‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharun K, Tiwari R, Iqbal Yatoo M, et al. Antibody‐based immunotherapeutics and use of convalescent plasma to counter COVID‐19: advances and prospects. Expert Opin Biol Ther. 2020;20(9):1033‐1046. [DOI] [PubMed] [Google Scholar]
- 15. Hemming VG. Use of intravenous immunoglobulins for prophylaxis or treatment of infectious diseases. Clin Diagn Lab Immunol. 2001;8(5):859‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luke TC, Casadevall A, Watowich SJ, Hoffman SL, Beigel JH, Burgess TH. Hark back: passive immunotherapy for influenza and other serious infections. Crit Care Med. 2010;38:e66‐e73. [DOI] [PubMed] [Google Scholar]
- 17. Toth D. Neutralization of SARS‐CoV‐2 variants by A human polyclonal antibody therapeutic (COVID‐HIG, NP‐028) with high neutralizing titers to SARS‐CoV‐2. bioRxiv. 2022.
- 18. Ning L, Abagna HB, Jiang Q, Liu S, Huang J. Development and application of therapeutic antibodies against COVID‐19. Int J Biol Sci. 2021;17(6):1486‐1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berry CM. Antibody immunoprophylaxis and immunotherapy for influenza virus infection: utilization of monoclonal or polyclonal antibodies? Hum Vaccin Immunother. 2018;14(3):796‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cui Z, Liu P, Wang N, et al. Structural and functional characterizations of infectivity and immune evasion of SARS‐CoV‐2 Omicron. Cell. 2022;185(5):860‐871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsushita H, Sano A, Wu H, et al. Species‐specific chromosome engineering greatly improves fully human polyclonal antibody production profile in cattle. PLoS One. 2015;10(6):e0130699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matsushita H, Sano A, Wu H, et al. Triple immunoglobulin gene knockout transchromosomic cattle: bovine lambda cluster deletion and its effect on fully human polyclonal antibody production. PLoS One. 2014;9(3):e90383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jolles S, Sewell WAC, Misbah SA. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol. 2005;142(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lonberg N. Human antibodies from transgenic animals. Nat Biotechnol. 2005;23(9):1117‐1125. [DOI] [PubMed] [Google Scholar]
- 25. Osther K, Wiik A, Black F, et al. PASSHIV‐1 treatment of patients with HIV‐1 infection. A preliminary report of a phase I trial of hyperimmune porcine immunoglobulin to HIV‐1. AIDS. 1992;6(12):1457‐1464. [DOI] [PubMed] [Google Scholar]
- 26. León G, Herrera M, Segura Á, Villalta M, Vargas M, Gutiérrez JM. Pathogenic mechanisms underlying adverse reactions induced by intravenous administration of snake antivenoms. Toxicon. 2013;76:63‐76. [DOI] [PubMed] [Google Scholar]
- 27. Gitelman SE, Gottlieb PA, Rigby MR, et al. Antithymocyte globulin treatment for patients with recent‐onset type 1 diabetes: 12‐month results of a randomised, placebo‐controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2013;1(4):306‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kawanishi K, Dhar C, Do R, Varki N, Varki A, Gordts P. L. S. M. Human species‐specific loss of CMP‐N‐acetylneuraminic acid hydroxylase enhances atherosclerosis via intrinsic and extrinsic mechanisms. Proc Natl Acad Sci U S A. 2019;116(32):16036‐16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dhar C, Sasmal A, Varki A. From “serum sickness” to “xenosialitis”: past, present, and future significance of the non‐human sialic acid Neu5Gc. Front Immunol. 2019;10:807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Couvrat‐Desvergnes G, Salama A, Le Berre L, et al. Rabbit antithymocyte globulin‐induced serum sickness disease and human kidney graft survival. J Clin Invest. 2015;125(12):4655‐4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Higashi H, Naiki M, Matuo S, Ōkouchi K. Antigen of “serum sickness” type of heterophile antibodies in human sera: identification as gangliosides with N‐glycolylneuraminic acid. Biochem Biophys Res Commun. 1977;79(2):388‐395. [DOI] [PubMed] [Google Scholar]
- 32. Jawhara S. Can drinking microfiltered raw immune milk from cows immunized against SARS‐CoV‐2 provide short‐term protection against COVID‐19? Front Immunol. 2020;11:1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saied AA, Metwally AA. Bovine‐origin human therapy; need more attention. Int J Curr Microbiol App Sci. 2019;8(9):2766‐2770. [Google Scholar]
- 34. Saied AA, Metwally AA, Mohamed HMA, Haridy MAM. The contribution of bovines to human health against viral infections. Environ Sci Pollut Res Int. 2021;28:46999‐47023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beigel JH, Tebas P, Elie‐Turenne M‐C, et al. Immune plasma for the treatment of severe influenza: an open‐label, multicentre, phase 2 randomised study. Lancet Respir Med. 2017;5(6):500‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arabi YM, Balkhy HH, Hayden FG, et al. Middle East respiratory syndrome. N Engl J Med. 2017;376(6):584‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Winkler AM, Koepsell SA. The use of convalescent plasma to treat emerging infectious diseases: focus on Ebola virus disease. Curr Opin Hematol. 2015;22(6):521‐526. [DOI] [PubMed] [Google Scholar]
- 38. Dhama K, Karthik K, Khandia R, et al. Advances in designing and developing vaccines, drugs, and therapies to counter Ebola virus. Front Immunol. 2018;9:1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gupta A, Karki R, Dandu HR, Dhama K, Bhatt MLB, Saxena SK. COVID‐19: benefits and risks of passive immunotherapeutics. Hum Vaccin Immunother. 2020;16:2963‐2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arabi YM, Hajeer AH, Luke T, et al. Feasibility of using convalescent plasma immunotherapy for MERS‐CoV infection, Saudi Arabia. Emerg Infect Dis. 2016;22(9):1554‐1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saied AA, Metwally AA, Alobo M, Shah J, Sharun K, Dhama K. Bovine‐derived antibodies and camelid‐derived nanobodies as biotherapeutic weapons against SARS‐CoV‐2 and its variants: a review article. Int J Surg. 2022;98:106233. 10.1016/j.ijsu.2022.106233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kuroiwa Y, Kasinathan P, Choi YJ, et al. Cloned transchromosomic calves producing human immunoglobulin. Nat Biotechnol. 2002;20(9):889‐894. [DOI] [PubMed] [Google Scholar]
- 43. Kacskovics I, Kis Z, Mayer B, et al. FcRn mediates elongated serum half‐life of human IgG in cattle. Int Immunol. 2006;18(4):525‐536. [DOI] [PubMed] [Google Scholar]
- 44. Sano A, Matsushita H, Wu H, et al. Physiological level production of antigen‐specific human immunoglobulin in cloned transchromosomic cattle. PLoS One. 2013;8(10):e78119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuroiwa Y, Kasinathan P, Sathiyaseelan T, et al. Antigen‐specific human polyclonal antibodies from hyperimmunized cattle. Nat Biotechnol. 2009;27(2):173‐181. [DOI] [PubMed] [Google Scholar]
- 46. Dye JM, Wu H, Hooper JW, et al. Production of potent fully human polyclonal antibodies against Ebola Zaire virus in transchromosomal cattle. Sci Rep. 2016;6:24897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Luke T, Wu H, Zhao J, et al. Human polyclonal immunoglobulin G from transchromosomic bovines inhibits MERS‐CoV in vivo. Sci Transl Med. 2016;8(326):326ra21. 10.1126/scitranslmed.aaf1061 [DOI] [PubMed] [Google Scholar]
- 48. Hooper JW, Brocato RL, Kwilas SA, et al. DNA vaccine–derived human IgG produced in transchromosomal bovines protect in lethal models of hantavirus pulmonary syndrome. Sci Transl Med. 2014;6(264):264ra162. [DOI] [PubMed] [Google Scholar]
- 49. Bounds CE, Kwilas SA, Kuehne AI, et al. Human polyclonal antibodies produced through DNA vaccination of transchromosomal cattle provide mice with post‐exposure protection against lethal Zaire and Sudan Ebolaviruses. PLoS One. 2015;10(9):e0137786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fischer J, Eberlein B, Hilger C, et al. Alpha‐gal is a possible target of IgE‐mediated reactivity to antivenom. Allergy. 2017;72(5):764‐771. [DOI] [PubMed] [Google Scholar]
- 51. Platts‐Mills TAE, Schuyler AJ, Tripathi A, Commins SP. Anaphylaxis to the carbohydrate side chain alpha‐gal. Immunol Allergy Clin. 2015;35(2):247‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sim DW, Lee JS, Park KH, et al. Accurate assessment of alpha‐gal syndrome using cetuximab and bovine thyroglobulin‐specific IgE. Mol Nutr Food Res. 2017;61(10):1601046. [DOI] [PubMed] [Google Scholar]
- 53. Echelard Y, Meade H. Toward a new cash cow. Nat Biotechnol. 2002;20(9):881‐882. [DOI] [PubMed] [Google Scholar]
- 54. Yang Y, Peng F, Wang R, et al. The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun. 2020;109:102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dai L, Gao GF. Viral targets for vaccines against COVID‐19. Nat Rev Immunol. 2021;21(2):73‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. WHO . DRAFT landscape of COVID‐19 candidate vaccines. Accessed September 23, 2021. https//www.who.int/publications/m/item/draft-l
- 57. da Silva Galdino AB, do Nascimento Rangel AH, Buttar HS, et al. Bovine colostrum: benefits for the human respiratory system and potential contributions for clinical management of COVID‐19. Food Agric Immunol. 2021;32(1):143‐162. [Google Scholar]
- 58. Kangro K, Kurashin M, Gildemann K, et al. Bovine colostrum derived antibodies against SARS‐CoV‐2 show great potential to serve as a prophylactic agent. medRxiv. 2021. [DOI] [PMC free article] [PubMed]
- 59. Hurley WL, Theil PK. Perspectives on immunoglobulins in colostrum and milk. Nutrients. 2011;3(4):442‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Arenas A, Borge C, Carbonero A, et al. Bovine coronavirus immune milk against COVID‐19. Front Immunol. 2021;12:843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kell DB, Heyden EL, Pretorius E. The biology of lactoferrin, an iron‐binding protein that can help defend against viruses and bacteria. Front Immunol. 2020;11:1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Oshiro S, Tada T, Mizutani N, et al. Presence of antibodies against SARS‐CoV‐2 spike protein in bovine whey IgG enriched fraction. Int Dairy J. 2021;117:105002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Paparo L, Bruno C. Ferrucci V, et al. protective effects elicited by cow milk fermented with L. paracasei CBAL74 against SARS‐CoV‐2 infection in human enterocytes. J Funct Foods. 2021;87:104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wotring JW, Fursmidt R, Ward L, Sexton JZ. Evaluating the in vitro efficacy of bovine lactoferrin products against SARS‐CoV‐2 variants of concern. J Dairy Sci. 2022;105(4):2791‐2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang G, Watson KM, Buckheit Jr., RW . Anti‐human immunodeficiency virus type 1 activities of antimicrobial peptides derived from human and bovine cathelicidins. Antimicrob Agents Chemother. 2008;52(9):3438‐3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. El‐Fakharany EM, Uversky VN, Redwan EM. Comparative analysis of the antiviral activity of camel, bovine, and human lactoperoxidases against herpes simplex virus type 1. Appl Biochem Biotechnol. 2017;182(1):294‐310. [DOI] [PubMed] [Google Scholar]
- 67. Shin K, Wakabayashi H, Yamauchi K, et al. Effects of orally administered bovine lactoferrin and lactoperoxidase on influenza virus infection in mice. J Med Microbiol. 2005;54(8):717‐723. [DOI] [PubMed] [Google Scholar]
- 68. El‐Ansary MRM, Asaad A, Khalifa R, Rahman ATA, Abd Elsalam AE. Antiviral and immunomodulatory effects of oral bovine lactoferrin therapy among patients with chronic hepatitis C. Egypt Liver J. 2016;6(4):81‐88. [Google Scholar]
- 69. Hu Y, Meng X, Zhang F, Xiang Y, Wang J. The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS‐CoV‐2 is mediated by targeting the heparan sulfate co‐receptor. Emerg Microbes Infect. 2021;10(1):317‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Harmsen MC, Swart PJ, de Béthune MP, et al. Antiviral effects of plasma and milk proteins: lactoferrin shows potent activity against both human immunodeficiency virus and human cytomegalovirus replication in vitro. J Infect Dis. 1995;172(2):380‐388. [DOI] [PubMed] [Google Scholar]
- 71. Puddu P, Borghi P, Gessani S, Valenti P, Belardelli F, Seganti L. Antiviral effect of bovine lactoferrin saturated with metal ions on early steps of human immunodeficiency virus type 1 infection. Int J Biochem Cell Biol. 1998;30(9):1055‐1063. [DOI] [PubMed] [Google Scholar]
- 72. Superti F, Agamennone M, Pietrantoni A, Ammendolia MG. Bovine lactoferrin prevents influenza A virus infection by interfering with the fusogenic function of viral hemagglutinin. Viruses. 2019;11(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li S, Zhou H, Huang G, Liu N. Inhibition of HBV infection by bovine lactoferrin and iron‐, zinc‐saturated lactoferrin. Med Microbiol Immunol. 2009;198(1):19‐25. [DOI] [PubMed] [Google Scholar]
- 74. Andersen JH, Osbakk SA, Vorland LH, Traavik T, Gutteberg TJ. Lactoferrin and cyclic lactoferricin inhibit the entry of human cytomegalovirus into human fibroblasts. Antiviral Res. 2001;51(2):141‐149. [DOI] [PubMed] [Google Scholar]
- 75. Marr AK, Jenssen H, Moniri MR, Hancock REW, Panté N. Bovine lactoferrin and lactoferricin interfere with intracellular trafficking of Herpes simplex virus‐1. Biochimie. 2009;91(1):160‐164. [DOI] [PubMed] [Google Scholar]
- 76. Robinson Jr., WE , McDougall B, Tran D, Selsted ME. Anti‐HIV‐1 activity of indolicidin, an antimicrobial peptide from neutrophils. J Leukoc Biol. 1998;63(1):94‐100. [DOI] [PubMed] [Google Scholar]
- 77. Yasin B, Pang M, Turner JS, et al. Evaluation of the inactivation of infectious Herpes simplex virus by host‐defense peptides. Eur J Clin Microbiol Infect Dis. 2000;19(3):187‐194. [DOI] [PubMed] [Google Scholar]
- 78. Ng WC, Wong V, Muller B, Rawlin G, Brown LE. Prevention and treatment of influenza with hyperimmune bovine colostrum antibody. PLoS One. 2010;5(10):e13622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yu H, Zhong Y, Zhang Z, et al. Characterization of proteins with Siaα2‐3/6Gal‐linked glycans from bovine milk and role of their glycans against influenza A virus. Food Funct. 2018;9(10):5198‐5208. [DOI] [PubMed] [Google Scholar]
- 80. Neurath AR, Jiang S, Strick N, Lin K, Li Y‐Y, Debnath AK. Bovine β‐lactoglobulin modified by 3‐hydroxyphthalic anhydride blocks the CD4 cell receptor for HIV. Nat Med. 1996;2(2):230‐234. [DOI] [PubMed] [Google Scholar]
- 81. Li L, He L, Tan S, et al. 3‐Hydroxyphthalic anhydride‐modified chicken ovalbumin exhibits potent and broad anti‐HIV‐1 activity: a potential microbicide for preventing sexual transmission of HIV‐1. Antimicrob Agents Chemother. 2010;54(5):1700‐1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Liu Z, Wu H, Egland KA, et al. Human immunoglobulin from transchromosomic bovines hyperimmunized with SARS‐CoV‐2 spike antigen efficiently neutralizes viral variants. Hum Vaccin Immunother. 2021;18:1940652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Luke T, Wu H, Egland KA, Sullivan EJ, Bausch CL. Fully human antibody immunoglobulin from transchromosomic bovines is potent against SARS‐CoV‐2 variant pseudoviruses. bioRxiv. 2021.
- 85. Gilliland T, Liu Y, Li R, et al. Protection of human ACE2 transgenic Syrian hamsters from SARS CoV‐2 variants by human polyclonal IgG from hyper‐immunized transchromosomic bovines. bioRxiv. 2021.
- 86. Neerukonda SN, Vassell R, Herrup R, et al. Establishment of a wellcharacterized SARS‐CoV‐2 lentiviral pseudovirus neutralization assay using 293T cells with stable expression of ACE2 and TMPRSS2. PLoS One. 2021;16(3):e0248348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tang J, Ravichandran S, Lee Y, et al. Antibody affinity maturation and plasma IgA associate with clinical outcome in hospitalized COVID‐19 patients. Nat Commun. 2021;12:1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ravichandran S, Lee Y, Grubbs G, et al. Longitudinal antibody repertoire in “mild” versus “severe” COVID‐19 patients reveals immune markers associated with disease severity and resolution. Sci Adv. 2021;7(10):eabf2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zaki AM, Van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814‐1820. [DOI] [PubMed] [Google Scholar]
- 90. Beigel JH, Voell J, Kumar P, et al. A randomized placebo‐controlled phase 1 safety and tolerability study of a novel human anti‐MERS coronavirus polyclonal intravenous immunoglobulin produced from transchromosomic cattle. Lancet Infect Dis. 2018;18(4):410‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Beigel JH, Voell J, Kumar P, et al. Safety and tolerability of a novel, polyclonal human anti‐MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double‐blind, single‐dose‐escalation study. Lancet Infect Dis. 2018;18(4):410‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Barré‐Sinoussi F, Chermann J‐C, Rey F, et al. Isolation of a T‐lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983;220(4599):868‐871. [DOI] [PubMed] [Google Scholar]
- 93. Munis AM. Gene therapy applications of non‐human lentiviral vectors. Viruses. 2020;12(10):1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sok D, Le KM, Vadnais M, et al. Rapid elicitation of broadly neutralizing antibodies to HIV by immunization in cows. Nature. 2017;548(7665):108‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Stelwagen K, Carpenter E, Haigh B, Hodgkinson A, Wheeler TT. Immune components of bovine colostrum and milk. J Anim Sci. 2009;87(suppl_13):3‐9. [DOI] [PubMed] [Google Scholar]
- 96. Kramski M, Center RJ, Wheatley AK, et al. Hyperimmune bovine colostrum as a low‐cost, large‐scale source of antibodies with broad neutralizing activity for HIV‐1 envelope with potential use in microbicides. Antimicrob Agents Chemother. 2012;56(8):4310‐4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kramski M, Lichtfuss GF, Navis M, et al. Anti‐HIV‐1 antibody‐dependent cellular cytotoxicity mediated by hyperimmune bovine colostrum IgG. Eur J Immunol. 2012;42(10):2771‐2781. [DOI] [PubMed] [Google Scholar]
- 98. Russell MW, Mestecky J. Humoral immune responses to microbial infections in the genital tract. Microbes Infect. 2002;4(6):667‐677. [DOI] [PubMed] [Google Scholar]
- 99. Moldt B, Schultz N, Dunlop DC, et al. A panel of IgG1 b12 variants with selectively diminished or enhanced affinity for Fcγ receptors to define the role of effector functions in protection against HIV. J Virol. 2011;85(20):10572‐10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yamada T, Watanabe N, Nakamura T, Iwamoto A. Antibody‐dependent cellular cytotoxicity via humoral immune epitope of Nef protein expressed on cell surface. J Immunol. 2004;172(4):2401‐2406. [DOI] [PubMed] [Google Scholar]
- 101. McCoy LE, van Gils MJ, Ozorowski G, et al. Holes in the glycan shield of the native HIV envelope are a target of trimer‐elicited neutralizing antibodies. Cell Rep. 2016;16(9):2327‐2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sanders RW, van Gils MJ, Derking R, et al. HIV‐1 neutralizing antibodies induced by native‐like envelope trimers. Science. 2015;349(6244):aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sanders RW, Derking R, Cupo A, et al. A next‐generation cleaved, soluble HIV‐1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non‐neutralizing antibodies. PLoS Pathog. 2013;9(9):e1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wang F, Ekiert DC, Ahmad I, et al. Reshaping antibody diversity. Cell. 2013;153(6):1379‐1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Heydarchi B, Center RJ, Gonelli C, et al. Repeated vaccination of cows with HIV Env gp140 during subsequent pregnancies elicits and sustains an enduring strong Env‐binding and neutralising antibody response. PLoS One. 2016;11(6):e0157353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Heydarchi B, Center RJ, Bebbington J, et al. Trimeric gp120‐specific bovine monoclonal antibodies require cysteine and aromatic residues in CDRH3 for high affinity binding to HIV Env. MAbs. 2017;9:550‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rambaut A, Pybus OG, Nelson MI, Viboud C, Taubenberger JK, Holmes EC. The genomic and epidemiological dynamics of human influenza A virus. Nature. 2008;453(7195):615‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Padilla‐Quirarte HO, Lopez‐Guerrero DV, Gutierrez‐Xicotencatl L, Esquivel‐Guadarrama F. Protective antibodies against influenza proteins. Front Immunol. 2019;10:1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gao R, Sreenivasan CC, Sheng Z, et al. Human monoclonal antibody derived from transchromosomic cattle neutralizes multiple H1 clades of influenza A virus by recognizing a novel conformational epitope in the hemagglutinin head domain. J Virol. 2020;94(22):e00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gao R. Epitope mapping and mechanisms of action of human influenza antibodies derived from transchromosomic cattle (Electronic Theses and Dissertations). South Dakota State University; 2020.
- 111. Singh RK, Dhama K, Malik YS, et al. Ebola virus–epidemiology, diagnosis, and control: threat to humans, lessons learnt, and preparedness plans—an update on its 40 year's journey. Vet Q. 2017;37(1):98‐135. [DOI] [PubMed] [Google Scholar]
- 112. Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011;377(9768):849‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kuhn JH, Amarasinghe GK, Basler CF, et al. ICTV virus taxonomy profile: Filoviridae. J Gen Virol. 2019;100(6):911‐912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Burk R, Bollinger L, Johnson JC, et al. Neglected filoviruses. FEMS Microbiol Rev. 2016;40(4):494‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Luke T, Bennett RS, Gerhardt DM, et al. Fully human immunoglobulin G from transchromosomic bovines treats nonhuman primates infected with Ebola virus Makona isolate. J Infect Dis. 2018;218(suppl_5):S636‐S648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rosenke K, Bounds CE, Hanley PW, et al. Human polyclonal antibodies produced by transchromosomal cattle provide partial protection against lethal Zaire Ebolavirus challenge in Rhesus macaques . J Infect Dis. 2018;218(suppl_5):S658‐S661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Dick GWA, Kitchen SF, Haddow AJ. Zika virus (II). Pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46(5):00‐534. [Google Scholar]
- 118. Khandia R, Munjal A, Dhama K. Consequences of Zika virus infection during fetal stage and pregnancy safe drugs: an update. Int J Pharmacol. 2017;13(4):370‐377. [Google Scholar]
- 119. Stein DR, Golden JW, Griffin BD, et al. Human polyclonal antibodies produced in transchromosomal cattle prevent lethal Zika virus infection and testicular atrophy in mice. Antiviral Res. 2017;146:164‐173. [DOI] [PubMed] [Google Scholar]
- 120. Kumar A, Hou S, Airo AM, et al. Zika virus inhibits type‐I interferon production and downstream signaling. EMBO Rep. 2016;17(12):1766‐1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Siddharthan V, Miao J, Van Wettere A, et al. Human polyclonal antibodies produced from transchromosomal bovine provides prophylactic and therapeutic protections against Zika virus infection in STAT2 KO Syrian hamsters. Viruses. 2019;11(2):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Walker LM, Burton DR. Passive immunotherapy of viral infections: “super‐antibodies” enter the fray. Nat Rev Immunol. 2018;18(5):297‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sok D, Burton DR. Recent progress in broadly neutralizing antibodies to HIV. Nat Immunol. 2018;19(11):1179‐1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Saied AA, Dhawan M, Priyanka, Choudhary OP. SARS‐CoV‐2 and influenza A virus: dual diagnostics and vaccines. Int J Surg. 2022;102:106653. 10.1016/j.ijsu.2022.106653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Burke MJ, Stockley PG, Boyes J. Broadly neutralizing bovine antibodies: highly effective new tools against evasive pathogens? Viruses. 2020;12(4):473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(4):422‐434. 10.1093/oxfordjournals.aje.a120955 [DOI] [PubMed] [Google Scholar]
- 127. Graham BS. Vaccines against respiratory syncytial virus: the time has finally come. Vaccine. 2016;34(30):3535‐3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Nader PR, Horwitz MS, Rousseau J. Atypical exanthem following exposure to natural measles: eleven cases in children previously inoculated with killed vaccine. J Pediatr. 1968;72(1):22‐28. [DOI] [PubMed] [Google Scholar]
- 129. Polack FP. Atypical measles and enhanced respiratory syncytial virus disease (ERD) made simple. Pediatr Res. 2007;62(1):111‐115. [DOI] [PubMed] [Google Scholar]
- 130. Yu J, Tostanoski LH, Peter L, et al. DNA vaccine protection against SARS‐CoV‐2 in Rhesus macaques . Science. 2020;369(6505):806‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Yang Z, Kong W, Huang Y, et al. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428(6982):561‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Smith TRF, Patel A, Ramos S, et al. Immunogenicity of a DNA vaccine candidate for COVID‐19. Nat Commun. 2020;11:2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Perley CC, Brocato RL, Wu H, et al. Anti‐HFRS human IgG produced in transchromosomic bovines has potent hantavirus neutralizing activity and is protective in animal models. Front Microbiol. 2020;11:832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Silver JN, Ashbaugh CD, Miles JJ, et al. Deployment of transchromosomal bovine for personalized antimicrobial therapy. Clin Infect Dis. 2018;66(7):1116‐1119. [DOI] [PubMed] [Google Scholar]
- 135. Maulud SQ, Hasan DA, Ali RK, et al. Deltacron: Apprehending a new phase of the COVID‐19 pandemic. Int J Surg. 2022;102:106654. 10.1016/j.ijsu.2022.106654 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study. All relevant data are presented in the article.


