Abstract
Cytokines play pivotal functions in coronavirus disease 2019 (COVID‐19) pathogenesis. However, little is known about the rationale and importance of genetic variations associated with immune system responses, so‐called “immunogenetic profiling.” We studied whether polymorphisms of IL6, IL6R, TNFA, and IL1RN affect the disorder severity and outcome in patients infected with COVID19. We recruited 317 hospitalized patients with laboratory‐confirmed COVID‐19 from Bu‐Ali hospital and 317 high‐risk participants who had high exposure to COVID‐19 patients but with a negative real‐time‐polymerase chain reaction (PCR) test. Multiple regression analyses were applied. We indicated that participants carrying the A allele in TNFA‐rs361525, G>A (p < .004), the C allele in IL1RN‐rs419598 T>C (p < .004), the A allele in IL6R‐rs2228145, A>C (p = .047) are more susceptible to develop COVID‐19. In contrast, those who carry the G allele of IL6‐rs2069827, G>T (p = .01), are more protected from COVID‐19. Also, we compared the various genotypes regarding the disorder severity and poor prognosis; we found that the AA genotype in TNFA is related to more aggressive illness and bad prognostic in contrast to the other inflammatory cytokines' genotypes. In addition, a high level of inflammatory indications, such as neutrophil‐to‐lymphocyte ratio and systemic immune‐inflammation index, was observed in deceased patients compared with the survived subjects (p < .0001). We advised considering inflammatory cytokines polymorphisms as the main item to realize the therapeutic response against the acute respiratory distress syndrome induced by the SARS‐CoV‐2 virus.
Keywords: Clinical features, COVID‐19, Pathogenesis, polymorphism, proinflammatory cytokine, SARS‐CoV‐2
1. INTRODUCTION
The current increase in the rate of global morbidity and mortality has lately been attributed to coronavirus disease 2019 (COVID‐19), instigated by a novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) (Guo et al., 2020; Rokni, Ghasemi et al., 2020; Sivasankarapillai et al., 2020). The clinical course of COVID‐19 is of paramount importance mostly due to its diversity; that is, although the symptoms remain completely invisible in some individuals, a vast majority of infected patients present a wide array of complications such as increased cytokine release syndrome (CRS) and even death (Sheervalilou, Shirvaliloo, Sargazi, Shirvalilou et al., 2021; Yuki et al., 2020).
At the moment, there is no definite treatment to stop SARS‐CoV‐2 replication (Sheervalilou et al., 2021). Interestingly, molecular docking and structural dynamics experiments have shown that a variety of bioactive compounds (Bhardwaj et al., 2021; Bhardwaj et al., 2021a, 2021b; Sharma et al., 2021) or chemically synthesized (Bhardwaj et al., 2021) or turmeric‐derived (Singh, Bhardwaj & Purohit, 2021) compounds with good docking scores could bind to different proteins of the virus, and therefore, phonetically inhibit its replication (Singh, Bhardwaj, Das et al., 2021).
Cytokines or interleukins (with a molecular weight between 8 and 40 kDa) are the critical mediator regulators of the host's response to diseases such as infection and hyper inflammation. They trigger and balance the immune response and also systemic and local intracellular regulatory mediators. They can be divided into anti‐inflammatory (IL‐1RA [Encoded by the IL1RN gene], IL‐10 and TGF‐β [transforming growth factor beta]) and proinflammatory (IL‐1, IL‐6, IL‐6R [IL‐6 receptor], and tumor necrosis factor α [TNF‐α]) proteins, also anti‐inflammatory proteins can repress the upregulation of proinflammatory proteins (Homeostasis)(Dinarello, 2000). The permeation of SARS‐CoV‐2 into respiratory epithelial cells induces pathogenic T helper 1 (Th1) cells to secrete proinflammatory cytokines such as interleukin‐1 (IL‐1) and IL‐6. These cytokines, in turn, trigger CD14+CD16+ inflammatory monocytes to generate vast amounts of IL‐6, TNF‐α, and other cytokines (Qin et al., 2020; Rokni Hamblin et al., 2020; Sheervalilou, Shirvaliloo, Sargazi, Shirvalilou et al., 2021).
Epidemiological investigations have shown an increase in acute phase proteins [such as ferritin, serum amyloid, and, more importantly, C‐reactive protein (CRP)] in patients infected with the SARS‐CoV‐2 virus, representing the prompt initiation of the innate immune response (Ghaznavi et al., 2022; Jamilloux et al., 2020; Sheervalilou, Shirvaliloo, Sargazi, Bahari, et al., 2021). Meanwhile, the effectiveness of the innate immune response against the SARS‐CoV‐2 virus depends on the production of intrinsic type 1 interferon (IFN‐I) and its downstream signaling factors, which control the replication of the virus and induce an adequate adaptive immune response (Infantino et al., 2020; Prompetchara et al., 2020). Yet, because of the complex immune dysregulation caused by the virus, the virus can avoid this attack (Khosroshahi & Rezaei). Cytokine storm (CS) is mainly caused by chronic stimulation of T cells, weakening the body's defenses and making the infected patients more susceptible (Yazdanpanah et al., 2020). It has been established that these patients have higher levels of circulating MCP‐1, MCP‐3, MIP‐1α, G‐CSF, IP‐10, TNF‐α, IFN‐ γ, IL‐1β, IL‐1RA, IL‐6, sIL‐2Rα, IL‐10, IL‐17, and IL‐18 (Huang et al., 2020; Yang et al., 2020). A substantial secretion of IL‐6, TNF‐α, and IL‐1 signifies the CS in COVID‐19 (Copaescu et al., 2020). A relevant study in a murine model demonstrated that IL‐6 plays a pivotal role in acute lung injury (ALI). In this scenario, loss of IL‐6 alleviated ALI severity (Imai et al., 2008).
Apart from IL‐6, tissue damage can also arise from a soar in several cytokines in CRS. In roughly all acute inflammatory reactions, inflammation gets aggravated by TNF‐α. This cytokine is always manifested in the immune response and, consequently, in the excessively damaging inflammatory phase of COVID‐19, which is conventionally termed as hyperinflammation or CS; thus, counteracting the effect of TNF‐α is the logic underlying its use in anti‐TNF‐α therapies in COVID‐19 (Feldmann et al., 2020). With the IL‐1 family as the central mediator, a response is produced by the host immune system once viral infection initiates. As a result of the function of this family, potent proinflammatory cytokines, including IL‐1α and IL‐1β, are merged with IL‐1 receptor antagonist (IL‐1RA), and their negative regulators possess anti‐inflammatory effects (Rider et al., 2011; Werman et al., 2004). The transmission of proinflammatory signals is hindered when the IL‐1 cell receptor is targeted by IL‐1RA competing with IL‐1α and IL‐1β (Kobayashi et al., 1990; Werman et al., 2004). Moreover, in the case of an acute and lengthy inflammatory response, IL‐1RA guards cells against damage and acts in harmony with the immune response by elevating its levels during the ending stages of an inflammatory response (called homeostasis) (Kavita & Mizel, 1995).
The variations in the unique level of cytokines are primarily determined by the exclusive contributions of their genetic components sincedate, it is not clear whether it has been proved that polymorphisms embodied in the genes coding cytokines can affect tassociated with the risk ofheir transcriptional function. This is why myriad investigations have concentrated on the genetic variants of inflammatory cytokine genes in patients with SARS‐CoVs (Wang et al., 2008) and SARS‐CoV‐2 infection (Saleh et al., 2020). Genetic variations within some inflammatory cytokines, including TNF‐α (rs1800629), IFNAR2 (rs2236757), IFNB (rs2071430), IFNG (rs2430561), IL4 (rs2070874), and IL1RN (rs315952), have been already associated with the risk of COVID‐19 (Paim et al., 2021; Saleh et al., 2020). However, to date, it is not clear whether polymorphisms in IL12, IL10, and CCL7, could affect COVID‐19 risk and severity. Therefore, a vast area of research should be dedicated to attaining a profound perception of the susceptibility factors that affect the disease outcome. Under the given role of IL‐6 known as +2018 CD4+ and associates suggested cells, Kirtipal and Bharadwaj (2020) suggested that IL6 polymorphisms can serve as indicator of the severity of COVID‐19 in patients or subjects with asymptomatic symptoms. In other words, heightened levels of IL‐6 are directly tied with the gravity of COVID‐19 (Sun et al., 2020), and thus, the host response against SARS‐CoVs, and also SARS‐CoV‐2 cknown as +2018an be highly linked to IL6 genetic variants (Kirtipal & Bharadwaj, 2020). Moreover, the progression of several disorders can be expedited by the impact of functional polymorphisms in the IL1RN gene, confirmed by multiple genetic association studies (Dinarello, 2018; Simón et al., 1998).
The IL6 rs2069827 is located in a putative transcription factor binding site (Soerensen et al., 2013). Previous studies have shown that genotypes of this variation do not affect plasma levels of IL‐6 (Singh et al., 2020; Soerensen et al., 2013). The rs2228145 is a missense variation that has been described as an important determinant of circulating IL‐6R levels in the blood, serum, and cerebrospinal fluid (Garbers et al., 2018; Strafella et al., 2020). This variation resides within the extracellular domain of the IL‐6R, which is necessary for the receptor's interaction with extracellular ligands. It has been hypothesized that it may influence protein function due to the amino acid exchange (p.Asp358Ala) (Strafella et al., 2020). As a functional polymorphism, the IL1RN rs419598 (also known as +2018) polymorphism is located in chromosome 2 (position: 113129630) (Mesa et al., 2017), with a minor allele frequency (MAF) of 0.192, based on data provided by 1000 genome project. The majority of large population studies on TNFA variations have not included the −238G/A (rs361525) polymorphism. The MAF of this single‐nucleotide polymorphism (SNP) was reported to be between 3% and 6% in Caucasians, and even though previous data showed no elevation in plasma expression of TNF‐α in the presence of this variation, it is likely to serve a fundamental role in the clinical phenotype of inflammatory diseases and their progression (Sapey et al., 2010).
The current study hypothesizes a direct linkage between the prognostic/outcome of COVID‐19 patients and polymorphisms of the cytokine genes, including the promoteric variations of TNFA (rs361525, G>A), IL6 (rs2069827, G>T), and exonic variations of IL6R (rs2228145, A>C) and IL1RN (rs419598, T>C). The rationale behind selecting these genes was their fundamental roles in immune responses and inflammation regulation, and more importantly, polymorphisms within these inflammatory cytokines can affect gene expression and, therefore, contribute to the pathophysiology of the disease (Ferreira et al., 2013; Zhu et al., 2014).
2. MATERIALS and METHODS
2.1. Study population
This study was conducted in a central hospital for COVID‐19 patients in Zahedan, Iran, between July 2020 and February 2021. The unaffected controls (317 participants) were carefully selected among subjects with a high probability of exposure to the SARS‐CoV‐2 virus, which had a family history of COVID‐19 and/or health care workers in high exposure with COVID‐19 patients (Asymptomatic group), but tested several times in a given 8 months and showed negative real‐time reverse‐transcriptase‐polymerase chain reaction (RT‐PCR) results for the SARS‐CoV‐2 RNA. The case group consisted of 317 hospitalized patients with laboratory‐confirmed SARS‐CoV‐2. Laboratory confirmation was defined as a positive result for SARS‐CoV‐2 RNA on RT‐PCR assay of oro‐ and nasopharyngeal swab specimens. Patients were diagnosed according to the guidelines for the treatment and diagnosis of COVID‐19 (Xu et al., 2020). Patients with mild/moderate (nonsevere) COVID‐19 had less than 39.1°C fevered respiratory symptoms and blood oxygen saturation levels (SpO2) ≤ 93%. Accordingly, patients with severe or critical COVID‐19 had SpO2 ≤ 90%, severe respiratory distress (respiratory rate [RR] > 30/min), acute respiratory failure needing mechanical ventilation (intubation), and combined organ failure requiring mandatory admission to intensive care unit (Zhang et al., 2013). Clinical and demographic data of all participants were recorded (Table 2). Unaffected controls were selected from healthy individuals (or health care workers) who came to the hospital for a checkup with a high probability of exposure to the SARS‐CoV‐2 virus but negative RT‐qPCR test and patients group were selected from hospitalized patients with laboratory‐confirmed SARS‐CoV‐2 RNA. Controls with a history of COVID‐19 diseases (severe form) and COVID‐19 vaccination and lesion in chest computed tomography (CT)‐scan were excluded from the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written consent was obtained from all participants.
Table 2.
Clinical and demographic characteristics of COVID‐19 patients and controls, parameters described as mean ± SD or number (percentage%)
| Parameter evaluated | COVID‐19, N (%) or (mean ± SD) | Controls, N (%) or (mean ± SD) | p value |
|---|---|---|---|
| Age (year) | 55.24 ± 14.03 | 53.85 ± 15.38 | .124 |
| Gender (female/male) | 123/194 | 152/165 | .239 |
| WBC count (×109/L) | 9.31 ± 4.62 | 8.16 ± 5.83 | <.001 * |
| Plt count (×109/L) | 246.93 ± 97.42 | 273.13 ± 73.67 | <.001 * |
| Lymph count (×109/L) | 0.99 ± 0.54 | 2.90 ± 2.54 | <.001 * |
| Neut count (×109/L) | 7.73 ± 4.41 | 4.51 ± 2.89 | <.001 * |
| CRP (mg/L) | 15.20 ± 4.53 | 4.28 ± 0.66 | <.001 * |
| ESR (mm/h) | 49.68 ± 23.25 | 13.23 ± 7.10 | <.001 * |
| NLR (index) | 10.00 ± 7.84 | 1.92 ± 1.93 | <.001 * |
| PLR (index) | 313.14 ± 218.21 | 114.63 ± 51.64 | <.001 * |
| SII (index) | 2517.20 ± 2226.21 | 523.78 ± 476.76 | <.001 * |
| SpO2 (%) | 84.97 ± 8.28 | 98.60 ± 96.40 | <.001 * |
| LDH (IU/L) | 709.91 ± 309.36 | 229.11 ± 50.82 | <.001 * |
| Density pattern | |||
| No lesion | 8 (2.5) | 317 (100.0) | ‐ |
| GGO | 163 (51.4) | 0 | |
| Consolidation | 39 (12.3) | 0 | |
| Mixed | 107 (33.8) | 0 | |
| Lesion location | |||
| No lesion | 8 (2.5) | 317 (100.0) | ‐ |
| Right lateral | 44 (13.9) | 0 | |
| Left lateral | 31 (9.8) | 0 | |
| Bilateral | 234 (73.8) | 0 | |
| Disease form | |||
| Asymptomatica | 0 | 317 (100.0) | ‐ |
| Nonsevere | 114 (36.0) | 0 | |
| Severe/critical | 203 (64.0) | 0 | |
| Status | |||
| Deceased | 26 (8.2) | 0 | ‐ |
| Survived | 291 (91.8) | 317 (100.0) | |
Abbreviations: COVID‐19, coronavirus 2019; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; GGO, grand glass opacity; LDH, lactate dehydrogenase; Lymph, lymphocyte; Neut; neutrophil; NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio; Plt, platelet; SII, systemic immune‐inflammation index; SpO2, blood oxygen saturation levels measured by pulse oximetry; WBC, white blood cell.
p < .05 (bolded p values) was considered statistically significant.
Asymptomatic with negative RT‐PCR test.
2.2. DNA extraction, SNP selection, and genotyping
Five milliliters of peripheral blood specimen were collected from each participant, and extraction of genomic DNA (gDNA) was done using a simple salting‐out procedure (MWer et al., 1988; Rokni et al., 2019; Sandoughi et al., 2020) and the quality of the extracted gDNA was verified by nano‐drop (OD260/OD280 ratio [≥1.8]). Both PCR‐amplification refractory mutation system (ARMS) for genotyping of IL6 (rs2069827, G>T) and restriction fragment length polymorphism (RFLP)‐PCR techniques were used for genotyping of TNFA (rs361525, G>A), IL6R (rs2228145, A>C) and IL1RN (rs419598, T>C) polymorphisms. Information regarding the studies SNPs (With a MAF > 0.2 based on the data from the 1000 genomes project) were retrieved from the national center for biotechnology information (NCBI) database.
Allele‐specific primers (Table 1) were designed using Gene Runner 3.05 (http://www.generunner.com) and synthesized by Sinaclon Co., Ltd. The reactions were set by the following protocol: a total volume of 20 µl containing 1 µl of gDNA (60 ng/ml), 1 µl of each primer (6 pmol), 12 μl of Taq 2x Master Mix Red‐Mgcl2 1.5 mM (Ampliqon Inc.) and 5 μl of distilled water. Each reaction mixture was heated to 95°C for 5 min for initial denaturation and underwent 30 cycles at 95°C for 45 s, annealing at different temperatures (according to Table 1 for each SNP) for 45 s with an extension at 72°C for 45 s, followed by a final extension at 72°C for 5 min. Following HinfI (for IL6R [rs2228145, A>C]) and MspI (for TNFA [rs361525, G>A] and IL1RN [rs419598, T>C]) restriction enzymes digestion in incubating at 37°C for 16 h, the digested products were subjected to electrophoresis on 1.5% agarose gel. The gel was then stained with safe stain load dye (Cinna clon) and visualized under ultraviolet light (Figure 1). For genotyping of IL6 (rs2069827, G>T) polymorphism, an ARMS‐PCR method was established (see in Table 1). At least 20% of the samples were randomly re‐genotyped, and genotyping accuracy was 100%.
Table 1.
Designed primers for genotyping of the studied SNPs
| Genes/SNPs | Function of SNPs | Genotyping methods | Primer sequences | Annealing temp (°C) | RE | Product size (bp) |
|---|---|---|---|---|---|---|
| TNFA | ||||||
| rs361525 G>A | Promoteric | RFLP‐PCR | F: ATCTGGAGGAAGCGGTAGTG | 55 | MspI | G: 132 + 20 |
| R: AGAAGACCCCCCTCGGAACC | A: 152 | |||||
| IL1RN | ||||||
| rs419598 T>C | Synonymous | RFLP‐PCR | F: TTCCGTCTCTTGAAACTTCTACCT | 56 | MspI | T: 314 |
| R: AAAGACCCAACAAGGATTAGGACAT | C: 157 | |||||
| IL6R | ||||||
| rs2228145 A>C | Missense | RFLP‐PCR | F: GTTAAGCTTGTCAAATGGCCTGTT | 55 | HinfI | A: 258 |
| R: CAGAGGAGCGTTCCGAAGG | C: 188 + 170 | |||||
| IL6 | ||||||
| rs2069827 G>T | Promoteric | ARMS‐PCR | F (G‐allele): CAACTGAGGTCACTGTTTTAGCG | 62 | ‐ | G or T: 150 |
| F (T‐allele): CAACTGAGGTCACTGTTTTAGCT | ||||||
| R (Common): GACAGCTCTGAGATGGCTTCA |
Abbreviations: ARMS‐PCR, amplification refractory mutation system polymerase chain reaction; bp, base pair; F, forward; R, reverse; RE, restriction enzyme; RFLP‐PCR, restriction fragment length polymorphism polymerase chain reaction; SNP, single‐nucleotide polymorphism.
Figure 1.
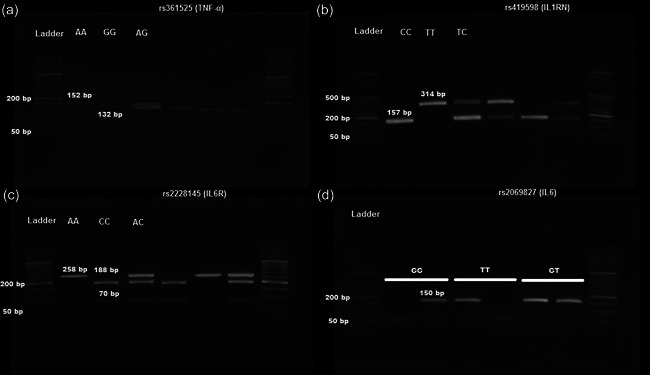
Electrophoresis images of polymerase chain reaction restriction fragments length polymorphism (RFLP‐PCR) for TNFA, IL‐1RN, IL‐6R polymorphism ([a] rs361525 G>A, [b] rs419598 T>C, and [c] rs2228145 A>C) and ARMS‐PCR for IL‐6 polymorphism ([d] rs2069827 G>T). 50 bp DNA ladder
2.3. Laboratory, radiology assessment, and inflammatory indications
For all of the participants, venous blood was collected for para‐clinical evaluation, complete cell blood count or full blood count, erythrocyte sedimentation rate (ESR), CRP, lactate dehydrogenase (LDH), and also chest radiological/CT‐scan were done. Also, inflammation indications were calculated using specific parameters of blood analysis such as neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR). Systemic immune‐inflammation indication (SII) was performed based on platelet count multiplied by the NLR (Supporting Information file).
3. STATISTICS ANALYSIS
In this study, data were presented by mean ± standard deviation. Statistics analysis was used with the IBM SPSS version 23.0 software. The sample size calculator server was used to calculate the sample size with allelic frequencies obtained by both groups at the power of 80% [https://clincalc.com/stats/SampleSize.aspx] (Noordzij et al., 2010). Deviation from the Hardy‐Weinberg equilibrium was checked using the χ 2 test. Student sample t‐test and one‐way analysis of variance with post hoc Bonferroni correction (p < .0125) test were performed to detect multiple comparisons. Descriptive statistics were done to determine the paraclinical and clinical features. Qualitative data were performed using the χ 2 spearman's test. Additionally, the independent effect of each polymorphism was done by logistic regression test. The odds ratios (OR) with 95% confidence intervals (CI) were performed to determine the association between the polymorphisms case groups. p Values less than .05 were shown statistically significant.
4. RESULTS
4.1. Clinical and paraclinical characteristics of the participants
The mean age was 55.24 in COVID‐19 patients and 53.85 in unaffected controls. No significant difference was noticed between cases and unaffected control groups concerning age (p = .124) and gender (p = .239). The clinical features of the studied subjects are shown in Table 2. Compared to healthy individuals, COVID‐19 patients had markedly higher leukocytes/white blood cells (WBCs), neutrophil counts, CRP, ESR, LDH (p < .001) values, and inflammatory indices such as NLR, PLR, and SII index (p < .001). In contrast, platelet, lymphocyte counts, and SpO2 (%) were significantly lower in cases than in unaffected controls (p < .001).
On admission, in the case group, ground‐glass opacity was the most typical radiographic finding on chest CT patterns (51.4%). No radiologic or chest CT pattern abnormality was found in 8 of 317 COVID‐19 patients (2.5%). In the case groups, 203 (64%) of the patients were presented with severe/critical and 114 (36%) nonsevere form of the disease, and 8.2% (26/317) of them were deceased (Table 2).
Student t‐test indicated that the inflammatory index levels (NLR and SII), SpO2, and laboratory characters were statistically different in the case groups with nonsevere (mild/moderate) or severe (critical) COVID‐19 when compared with the decease groups (p < .001) except for serum CRP, ESR levels, and PLR index. In the case groups, most of the underlying diseases in the infected patients with COVID‐19 had diabetes mellitus (86 [27.1%]) and hypertension (81 [21.6%]). Also, data analysis demonstrated that patients with underlying diseases such as coronary heart disease were more than suffer from COVID‐19 (p < .012, see in Table 3).
Table 3.
Risk factors of death and underly diseases among studied cases, parameters described as mean ± SD
| Blood routine (unit, normal range) | Stat | Total (N = 317) | Mean ± SD | Sig. (two‐tailed) | |
|---|---|---|---|---|---|
| Leukocyte count (×109/L, range 3.5–9.5) | Deceased | 26 | 12.49 ± 6.24 | <.0001** | |
| Survived | 291 | 09.02 ±04.35 | |||
| Platelet count (×109/L, range 125–450) | Deceased | 26 | 198.77 ± 90.13 | <.008* | |
| Survived | 291 | 251.23 ± 97.03 | |||
| Neutrophil count (×109/L, range 1.8–6.3) | Deceased | 26 | 11.04 ± 06.22 | <.007* | |
| Survived | 291 | 07.43 ± 04.11 | |||
| Lymphocyte count (×109/L, range 1.1–3.2) | Deceased | 26 | 0.64 ± 0.36 | <.0001** | |
| Survived | 291 | 1.03 ± 0.54 | |||
| Neutrophil/lymphocyte ratio (index, <5) | Deceased | 26 | 19.04 ± 12.88 | <.0001** | |
| Survived | 291 | 09.14 ± 06.64 | |||
| Platelet/lymphocyte ratio (index, <200) | Deceased | 26 | 386.12 ± 290.48 | .078 | |
| Survived | 291 | 307.30 ± 210.33 | |||
| Systematic inflammatory index (index, <500) | Deceased | 26 | 3890.55 ± 3420.04 | <.001* | |
| Survived | 291 | 2402.99 ± 2078.99 | |||
| C‐reactive protein (mg/L, 0.0–6.0) | Deceased | 26 | 15.85 ± 04.01 | .401 | |
| Survived | 291 | 15.14 ± 04.58 | |||
| Blood oxygen saturation levels (%, range 93–98) | Deceased | 26 | 74.19 ± 10.86 | <.0001** | |
| Survived | 291 | 86.34 ± 06.75 | |||
| Erythrocyte sedimentation rate (mm/h, 2‐22) | Deceased | 26 | 54.15 ± 21.77 | .285 | |
| Survival | 291 | 49.27 ± 23.37 | |||
| Lactate dehydrogenase (IU/L, range 140–280) | Deceased | 26 | 1031.04 ± 456.29 | <.0001** | |
| Survival | 291 | 681.22 ± 276.15 | |||
| Underlying diseases, total N = 317 (%) | |||||
| Hypertension diseases, N = 81 (21.6) | Deceased | 6 (1.9%) | ‐ | ‐ | .486 |
| Survival | 75 (23.7%) | ‐ | ‐ | ||
| Autoimmune diseases, N = 21 (06.6) | Deceased | 1 (0.3%) | ‐ | ‐ | .470 |
| Survival | 20 (6.3%) | ‐ | ‐ | ||
| Chronic diseases, N = 44 (13.9) | Deceased | 3 (0.9%) | ‐ | ‐ | .499 |
| Survival | 41 (12.9%) | ‐ | ‐ | ||
| Coronary heart diseases, N = 42 (13.2) | Deceased | 8 (2.5%) | ‐ | ‐ | <.012* |
| Survival | 34 (10.7%) | ‐ | ‐ | ||
| Diabetes mellitus diseases, N = 86 (27.1) | Deceased | 5 (1.6%) | ‐ | ‐ | .242 |
| Survival | 81 (25.6%) | ‐ | ‐ | ||
Abbreviation: SD, standard deviation.
*p < .05 (bolded p values) was considered statistically significant, **p < .001 (bolded p values) was considered statistically significant.
4.2. Genetic association analysis, distribution, disease severity, and prognosis
4.2.1. Polymorphism in TNFA gene (rs361525, G>A)
We found a significant association between rs361525 G>A (TNFA) polymorphism and risk of COVID‐19 under codominant AA versus GG (OR = 2.10; 95% CI: 1.33−3.31, p < .001), and recessive AA versus GG + GA (OR = 2.19; 95% CI: 1.47‐3.27, p < .0001) contrasted genetic models (Table 4). There was no significant difference between the different genotypes from rs361525 G>A (TNFA) polymorphism regarding the para‐clinical, inflammatory indications characters and underlying diseases (Table 5). Moreover, the A allele of rs361525 G>A (TNFA) was associated with a 1.38‐fold increase in COVID‐19 risk. Also, our study highlighted the disease severity and outcome in different genotypes from cytokines polymorphism of the studied cases. There was no statistical difference between the various genotypes of cytokines for the prognosis of the disorder, except for TNFA (rs361525, G>A) that the AA genotype, the disorder was severe (or critical) in comparison to cases in the GA/GG genotype with P1 = 0.033/P 3 = 0.038 (Table 6).
Table 4.
Allelic and genotypic distribution of the studied polymorphisms, number (percentage%)
| SNP | COVID‐19 N (%) | Control N (%) | Genetic model | OR (95% CI) | p value |
|---|---|---|---|---|---|
| rs361525 G>A ( TNFA ) | |||||
| GG | 90 (28.4) | 101 (31.9) | 1 [Reference] | ||
| GA | 141 (44.5) | 170 (53.6) | GA versus GG | 0.93 (0.65−1.34) | .697 |
| AA | 86 (27.1) | 46 (14.5) | AA versus GG | 2.10 (1.33−3.31) | <.001 * |
| HWE | ‐ | 0.059 | Dominant | 1.18 (0.84−1.66) | .341 |
| Recessive | 2.19 (1.47−3.27) | <.0001* | |||
| Over dominant | 0.69 (0.51−0.95) | <.021 * | |||
| G | 321 (50.6) | 372 (58.7) | Allelic | 1 [Reference] | |
| A | 313 (49.4) | 262 (41.3) | Allelic | 1.38 (1.11−1.73) | <.004 * |
| rs419598 T>C (IL1RN) | |||||
| TT | 145 (45.7) | 179 (56.5) | 1 [Reference] | ||
| TC | 134 (42.3) | 113 (35.6) | TC versus TT | 1.46 (1.05−2.04) | .024 * |
| CC | 38 (12.0) | 25 (7.9) | CC versus TT | 1.88 (1.08−3.25) | .024 * |
| HWE | ‐ | 0.234 | Dominant | 1.54 (1.12−2.10) | <.007 * |
| Recessive | 1.59 (0.94−2.70) | .084 | |||
| Over dominant | 1.32 (0.96−1.82) | .087 | |||
| T | 424 (66.9) | 471 (74.3) | Allelic | 1 [Reference]− | |
| C | 210 (33.1) | 163 (25.7) | Allelic | 1.43 (1.12−1.82) | <.004 * |
| rs2228145 A>C (IL6R) | |||||
| CC | 96 (30.3) | 107 (33.7) | 1 [Reference]‐ | ||
| AC | 155 (48.9) | 168 (53.0) | AC versus CC | 1.03 (0.72−1.46) | .876 |
| AA | 66 (20.8) | 42 (13.2) | AA versus CC | 1.75 (1.09−2.82) | .020 * |
| HWE | ‐ | 0.058 | Dominant | 1.17 (0.84−1.64) | .349 |
| Recessive | 1.72 (1.13−2.63) | .011 * | |||
| Over dominant | 0.85 (0.62−1.16) | .302 | |||
| C | 347 (54.7) | 382 (60.3) | Allelic | 1 [Reference] | |
| A | 287 (45.3) | 252 (39.7) | Allelic | 1.25 (1.00−1.57) | .047 * |
| rs2069827 G>T (IL6) | |||||
| GG | 143 (45.1) | 116 (36.6) | 1 [Reference]‐ | ||
| GT | 136 (42.9) | 146 (46.0) | GT versus GG | 0.76 (0.54−1.06) | .104 |
| TT | 38 (12.0) | 55 (17.4) | TT versus GG | 0.56 (0.35−0.91) | .018 * |
| HWE | ‐ | 0.439 | Dominant | 0.70 (0.51−0.97) | .029 * |
| Recessive | 0.65 (0.41−1.01) | .056 | |||
| Over dominant | 0.88 (0.64−1.20) | .424 | |||
| G | 422 (66.6) | 378 (59.6) | Allelic | 1 [Reference] | |
| T | 212 (33.4) | 256 (40.4) | Allelic | 0.74 (0.59−0.93) | .010 * |
Abbreviations: CI, confidence interval; COVID‐19, coronavirus 2019; HWE, Hardy−Weinberg equilibrium; OR, odds ratio; SNP, single‐nucleotide polymorphism.
p < .05 (bolded p values) was considered statistically significant.
Table 5.
Demographic, radiologic (CT‐scan) and laboratory characters of the studied cases according to genotype distribution, parameters described as mean ± SD, number (percentage%)
| Parameter evaluated | Case genotypes (TNFA) rs361525 G>A | Test of Sig | Within‐group significant | Case genotypes (IL1RN) rs419598 T>C | Test of Sig | Within‐group significant | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GG | GA | AA | TT | TC | CC | |||||||
| N = 90 | N = 141 | N = 86 | N = 145 | N = 134 | N = 38 | |||||||
| Age (year) | 55.81 ± 13.67 | 53.26 ± 14.13 | 57.87 ± 13.91 | F: 3.03 | P 1: 0.529 | 54.64 ± 14.49 | 56.84 ± 12.51 | 51.87 ± 16.7 | F: 2.11 | P 1: 0.574 | ||
| p = .05 | P 2: 0.049 | p: .123 | P 2: 0.162 | |||||||||
| P 3: 0.983 | P 3: 0.832 | |||||||||||
| Gender (female/male) | 32/58 | 56/85 | 35/51 | χ 2: 0.579 | P 1: 0.676 | 55/90 | 50/84 | 18/20 | χ 2: 1.35 | P 1: 0.809 | ||
| p: .749 | P 2: 0.576 | p: .510 | P 2: 0.833 | |||||||||
| P 3: 0.314 | P 3: 0.945 | |||||||||||
| WBC count (×109/L) | 9.46 ± 5.2 | 9.27 ± 4.16 | 9.21 ± 4.7 | F: 0.07 | P 1: 1.000 | 9.42 ± 5.05 | 9.15 ± .4.2 | 9.43 ± 4.28 | F: 0.136 | P 1: 1.000 | ||
| p: .931 | P 2: 1.000 | p: .873 | P 2: 1.000 | |||||||||
| P 3: 1.000 | P 3: 1.000 | |||||||||||
| Plt count (× 109/L) | 244.32 ± 102.09 | 248.01 ± 98.72 | 247.88 ± 91.14 | F: 0.045 | P 1: 1.000 | 246.55 ± 100.4 | 245.69 ± 95.17 | 252.76 ± 96.03 | F: 0.080 | P 1: 1.000 | ||
| p: .956 | P 2: 1.000 | p: .923 | P 2: 1.000 | |||||||||
| P 3: 1.000 | P 3: 1.000 | |||||||||||
| Lymph count (×109/L) | 1.06 ± 0.57 | 1.02 ± 0.57 | 0.91 ± 0.45 | F: 1.54 | P 1: 1.000 | 1.02 ± 0.53 | 0.94 ± 0.56 | 1.09 ± 0.53 | F: 1.49 | P 1: 0.650 | ||
| p: .214 | P 2: 0.700 | p: .227 | P 2: 0.365 | |||||||||
| P 3: 0.248 | P 3: 1.000 | |||||||||||
| Neut count (×109/L) | 7.86 ± 4.83 | 7.65 ± 4.11 | 7.69 ± 4.49 | F: 0.08 | P 1: 1.000 | 7.74 ± 4.74 | 7.66 ± 4.16 | 7.87 ± 4.03 | F: 0.035 | P 1: 1.000 | ||
| p: .935 | P 2: 1.000 | p: .965 | P 2: 1.000 | |||||||||
| P 3: 1.000 | P 3: 1.000 | |||||||||||
| CRP (mg/L) | 14.62 ± 4.97 | 15.49 ± 4.43 | 15.33 ± 4.02 | F: 0.05 | P 1: 0.471 | 14.99 ± 4.59 | 15.52 ± 4.36 | 14.84 ± 4.91 | F: 0.607 | P 1: 0.993 | ||
| p: .990 | P 2: 1.000 | p: .545 | P 2: 1.000 | |||||||||
| P 3: 0.912 | P 3: 1.000 | |||||||||||
| LDH (IU/L) | 664.37 ± 257.69 | 750.5 ± 366.91 | 691.03 ± 243.37 | F: 2.37 | P 1: 0.117 | 713.52 ± 280.44 | 713.78 ± 343.67 | 682.55 ± 292.95 | F: 0.168 | P 1: 1.000 | ||
| p: .095 | P 2: 0.478 | p: .845 | P 2: 1.000 | |||||||||
| P 3: 1.000 | P 3: 1.000 | |||||||||||
| ESR (mm/h) | 48.38 ± 25.81 | 50.29 ± 21.61 | 50.02 ± 23.26 | F: 0.198 | P 1: 1.000 | 51.54 ± 21.76 | 47.21 ± 24.94 | 51.24 ± 22.32 | F: 1.311 | P 1: 0.361 | ||
| p: .820 | P 2: 1.000 | p: 0.271 | P 2: 1.000 | |||||||||
| P 3: 1.000 | P 3: 1.000 | |||||||||||
| NLR (index) | 09.53 ± 6.77 | 10.15 ± 8.11 | 10.06 ± 8.36 | F: 0.184 | P 1: 1.000 | 09.72 ± 8.23 | 10.62 ± 7.71 | 8.48 ± 6.22 | F: 1.222 | P 1: 1.000 | ||
| p: 0.832 | P 2: 1.000 | p: 0.296 | P 2: 0.414 | |||||||||
| P 3: 1.000 | P 3: 1.000 | |||||||||||
| PLR (index) | 291.64 ± 183.71 | 325.4 ± 242.1 | 317.80 ± 211.64 | F: 0.675 | P 1: 0.760 | 299.84 ± 224.71 | 339.57 ± 225.96 | 275.9 ± 151.82 | F: 1.809 | P 1: 0.388 | ||
| 8 | p: .510 | P 2: 1.000 | p: .166 | P 2: 0.339 | ||||||||
| P 3: 1.000 | P 3: 1.000 | |||||||||||
| SII (index) | 2494.3 ± 2235.6 | 2548.2 ± 2262.1 | 2519.1 ± 2268.2 | F: 0.016 | P 1: 1.000 | 2477.2 ± 2359.26 | 2710.21 ± 2255.2 | 2054.5 ± 1703.72 | F: 1.321 | P 1: 1.000 | ||
| p: .984 | P 2: 1.000 | p: .268 | P 2: 0.340 | |||||||||
| P 3: 1.000 | P 3: 0.908 | |||||||||||
| SpO2 (%) | 85.35 ± 8.32 | 84.78 ± 8.73 | 86.25 ± 5.66 | F: 0.933 | P 1: 1.000 | 86.16 ± 7.34 | 84.24 ± 8.93 | 86.10 ± 5.29 | F: 2.294 | P 1: 0.388 | ||
| p: .394 | P 2: 0.519 | p: .103 | P 2: 0.339 | |||||||||
| P 3: 1.000 | P 3: 1.000 | |||||||||||
| No lesion in CT | 2 (2.2) | 2 (1.4) | 4 (4.7) | χ 2: 0.738 | P 1: 0.527 | 4 (2.8) | 4 (3.0) | 0 (0) | χ 2: 1.63 | P 1: 0.441 | ||
| P 2: 0.702 | p: .442 | P 2: ‐‐ | ||||||||||
| Lesion in CT | 88 (97.8) | 139 (98.6) | 82 (95.3) | p: .691 | P 3: 0.383 | 141 (97.2) | 130 (97.0) | 38 (100.0) | P 3: ‐‐ | |||
| Parameter evaluated | Case genotypes ( IL6R ) rs2228145 A > C | Test of Sig | Within‐group significant | Case genotypes ( IL6 ) rs2069827 G > T | Test of Sig | Within‐group significant | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | AC | CC | GG | GT | TT | |||||||
| N = 66 | N = 155 | N = 96 | N = 143 | N = 136 | N = 38 | |||||||
| Age (year) | 54.33 ± 11.93 | 56.63 ± 14.39 | 53.61 ± 14.68 | F: 1.54 | P 1: 0.799 | 55.44 ± 14.94 | 54.92 ± 13.06 | 55.61 ± 14.20 | F: 0.063 | P 1: 1.000 | ||
| p: .215 | P 2: 0.297 | p: .939 | P 2: 1.000 | |||||||||
| P 3: 1.000 | P 3: 1.000 | |||||||||||
| Gender (female/male) | 22/44 | 63/92 | 38/58 | χ 2: 1.08 | P 1: 0.462 | 55/88 | 54/82 | 14/24 | χ 2: 0.12 | P 1: 0.928 | ||
| p: .583 | P 2: 0.209 | p: .944 | P 2: 0.675 | |||||||||
| P 3: 0.616 | P 3: 0.715 | |||||||||||
| WBC count (×109/L) | 9.92 ± 5.73 | 8.99 ± 3.87 | 9.38 ± 4.87 | F: 0.962 | P 1: 0.512 | 9.58 ± 4.99 | 8.94 ± 3.84 | 9.57 ± 5.64 | F: 0.744 | P 1: 0.741 | ||
| p: .383 | P 2: 1.000 | p: .476 | P 2: 1.000 | |||||||||
| P 3: 1.000 | P 3: 1.000 | |||||||||||
| Plt count (×109/L) | 249.65 ± 113.1 | 241.25 ± 87.71 | 254.24 ± 101.3 | F: 0.558 | P 1: 1.000 | 244.17 ± 97.49 | 250.60 ± 99.06 | 244.21 ± 93.19 | F: 0.168 | P 1: 1.000 | ||
| p: .573 | P 2: 0.918 | p: .884 | P 2: 1.000 | |||||||||
| P 3: 1.000 | P 3: 1.000 | |||||||||||
| Lymph count (×109/L) | 0.99 ± 0.49 | 0.91 ± 0.48 | 1.13 ± 0.64 | F: 4.854 | P 1: 1.000 | 1.02 ± 0.53 | 0.97 ± 0.56 | 0.97 ± 0.55 | F: 0.40 | P 1: 1.000 | ||
| p : <.008 * | P 2 : < 0.006 * | p: .846 | P 2: 1.000 | |||||||||
| P 3: 0.278 | P 3: 1.000 | |||||||||||
| Neut count | 8.22 ± 5.1 | 7.53 ± 3.92 | 7.7 ± 4.67 | F: 0.585 | P 1: 0.842 | 7.99 ± 4.64 | 7.41 ± 3.8 | 7.87 ± 5.48 | F: 0.64 | P 1: 0.801 | ||
| (×109/L) | p: .558 | P 2: 1.000 | p: .528 | P 2: 1.000 | ||||||||
| P 3: 1.000 | P 3: 1.000 | |||||||||||
| CRP (mg/L) | 15.18 ± 4.45 | 15.52 ± 4.31 | 14.69 ± 4.91 | F: 1.007 | P 1: 1.000 | 14.73 ± 4.71 | 15.81 ± 4.14 | 14.79 ± 5.02 | F: 2.17 | P 1: 0.139 | ||
| p: .366 | P 2: .471 | p: .115 | P 2: 1.000 | |||||||||
| P 3: 1.000 | P 3: 1.000 | |||||||||||
| LDH (IU/L) | 717.17 ± 251.58 | 706.58 ± 323. | 710.31 ± 324.14 | F: 0.027 | P 1: 1.000 | 713.31 ± 318.86 | 703.16 ± 296.42 | 721.29 ± 325.89 | F: 0.066 | P 1: 1.000 | ||
| 8 | p: .973 | P 2: 1.000 | p: .936 | P 2: 1.000 | ||||||||
| P 3: 1.000 | P 3: 1.000 | |||||||||||
| ESR (mm/h) | 51.26 ± 22.74 | 48.01 ± 23.96 | 51.28 ± 22.45 | F: 0.780 | P 1: 1.000 | 50.10 ± 23.60 | 48.89 ± 23.32 | 50.89 ± 22.13 | F: 0.153 | P 1: 1.000 | ||
| p: .459 | P 2: 0.838 | p: .858 | P 2: 1.000 | |||||||||
| P 3: 1.000 | P 3: 1.000 | |||||||||||
| NLR (index) | 10.22 ± 8.8 | 10.22 ± 7.85 | 9.32 ± 7.00 | F: 0.444 | P 1: 1.000 | 10.41 ± 9.04 | 9.53 ± 6.74 | 09.72 ± 06.26 | F: 0.461 | P 1: 1.000 | ||
| p: .642 | P 2: 1.000 | p: .631 | P 2: 1.000 | |||||||||
| P 3: 1.000 | P 3: 1.000 | |||||||||||
| PLR (index) | 292.97 ± 178.52 | 328.69 ± 235. | 303.97 ± 215.79 | F: 0.456 | P 1: 0.802 | 299.27 ± 217.68 | 330.98 ± 233.94 | 306.69 ± 155.69 | F: 0.755 | P 1: 0.681 | ||
| 2 | p: .471 | P 2: 1.000 | p: .471 | P 2: 1.000 | ||||||||
| P 3: 1.000 | P 3: 1.000 | |||||||||||
| SII (index) | 2622.3 ± 2581.2 | 2507.9 ± 2198.2 | 2485.7 ± 2104.2 | F: 0.080 | P 1: 1.000 | 2553.8 ± 2372.2 | 2464.7 ± 2104.3 | 2632.6 ± 2329.3 | F: 0.104 | P 1: 1.000 | ||
| p: .923 | P 2: 1.000 | p: .902 | P 2: 1.000 | |||||||||
| P 3: 1.000 | P 3: 1.000 | |||||||||||
| SpO2 (%) | 84.61 ± 9.15 | 85.53 ± 7.56 | 85.56 ± 7.53 | F:0.363 | P 1: 1.000 | 84.71 ± 8.57 | 86.22 ± 7.04 | 84.57 ± 8.1 | F: 1.49 | P 1: 0.334 | ||
| p: .696 | P 2: 1.000 | p: .230 | P 2: 0.772 | |||||||||
| P 3: 1.000 | P 3: 1.000 | |||||||||||
| No lesion in CT | 3 (4.5) | 4 (2.6) | 1 (1.0) | χ 2: 6.59 | P 1: 0.875 | 5 (3.5) | 2 (1.5) | 1 (2.6) | χ 2: 3.40 | P 1: 0.877 | ||
| p: .360 | P 2: 0.456 | p: .757 | P 2: 0.568 | |||||||||
| Lesion in CT | 63 (95.5) | 151 (97.4) | 95 (99.0) | P 3: 0.324 | 138 (96.5) | 134 (98.5) | 37 (97.4) | P 3: 0.996 | ||||
Abbreviations: CRP, C‐reactive protein; CT, computed tomography; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; Lymph, lymphocyte; Neut, neutrophil; NLR: neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; Plt: platelet; SII, systemic immune‐inflammation index; SpO2, blood oxygen saturation levels, WBC, white blood cell.
p < .0125 (bolded p values) was considered statistically significant. In TNFA, P 1: difference between GG & GA, P 2: difference between AA & GA, P 3: difference between GG & AA. In IL1RN, P 1: difference between TT & TC, P 2: difference between CC & TC, P 3: difference between CC & TT. In IL6R, P 1: difference between AA & AC, P 2: difference between CC & AC, P 3: difference between AA & CC. In IL6, P 1: difference between GG & GT, P 2: difference between TT & GT, P 3: difference between GG & TT.
Table 6.
Disease severity and prognosis in different genotypes of the studied cases, parameters described as mean ± SD, number (percentage%)
| Parameter evaluated | Case genotypes (TNFA) rs361525 G>A | Test of significance | Within‐group significant | Case genotypes (IL1RN) rs419598 T>C | Test of significance | Within‐group significant | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| GG | GA | AA | TT | TC | CC | |||||
| N = 90 | N = 141 | N = 86 | N = 145 | N = 134 | N = 38 | |||||
| Severity of disease | ||||||||||
| Severe | 49 (54.4) | 95 (67.4) | 59 (68.6) | χ 2: 5.05 | P 1: 0.033 * | 86 (59.3) | 92 (68.7) | 25 (65.8) | χ 2: 2.69 | P 1: 0.067 |
| Nonsevere | 41 (45.6) | 46 (32.6) | 27 (31.4) | p: .08 | P 2: 0.483 | 59 (40.7) | 42 (31.3) | 13 (34.2) | p: .259 | P 2: 0.440 |
| P 3: 0.038 * | P 3: 0.296 | |||||||||
| Survival | ||||||||||
| Survived | 82 (91.1) | 131 (92.9) | 78 (90.7) | χ 2: 0.42 | P 1: 0.398 | 132 (91.0) | 123 (91.8) | 36 (94.7) | χ 2: 0.548 | P 1: 0.496 |
| Deceased | 8 (8.9) | 10 (7.1) | 8 (9.3) | p: .808 | P 2: 0.360 | 13 (9.0) | 11 (8.2) | 2 (5.3) | p: .760 | P 2: 0.420 |
| P 3: 0.565 | P 3: 0.361 | |||||||||
| Parameter evaluated | Case genotypes ( IL6R ) rs2228145 A>C | Test of significance | Within‐group significant | Case genotypes ( IL6 ) rs2069827 G>T | Test of significance | Within‐group significant | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| AA | AC | CC | GG | GT | TT | |||||
| N = 66 | N = 155 | N = 96 | N = 143 | N = 136 | N = 38 | |||||
| Severity of disease | ||||||||||
| Severe | 40 (60.6) | 102 (65.8) | 61 (63.5) | χ 2: 0.558 | P 1: .0278 | 95 (66.4) | 83 (61.0) | 25 (65.8) | χ 2: 0.94 | p 1: 0.208 |
| P 2: 0.408 | p 2: 0.368 | |||||||||
| Nonsevere | 26 (39.4) | 53 (34.2) | 35 (36.5) | p: .756 | P 3: 0.414 | 48 (33.6) | 53 (39.0) | 13 (34.2) | p: .625 | p 3: 0.542 |
| Survival | ||||||||||
| Survived | 63 (95.5) | 139 (89.7) | 89 (92.7) | χ 2: 2.20 | P 1: 0.125 | 128 (89.5) | 128 (94.1) | 35 (92.1) | χ 2: 1.97 | P 1: 0.118 |
| P 2: 0.283 | P 2: 0.445 | |||||||||
| Deceased | 3 (4.5) | 16 (10.3) | 7 (7.3) | p: .332 | P 3: 0.359 | 15 (10.5) | 8 (5.9) | 3 (7.9) | p: .373 | P 3: 0.452 |
p < .05 (bolded p values) was considered statistically significant. In TNFA, P 1: difference between GG & GA, P 2: difference between AA & GA, P 3: difference between GG & AA. In IL1RN, P 1: difference between TT & TC, P 2: difference between CC & TC, P 3: difference between CC & TT. In IL6R, P 1: difference between AA & AC, P 2: difference between CC & AC, P 3: difference between AA & CC. In IL6, P 1: difference between GG & GT, P 2: difference between TT & GT, P 3: difference between GG & TT.
4.2.2. Polymorphism in IL1RN gene (rs419598 T>C)
In this study, enhanced risk of COVID‐19 infection was observed under codominant TC versus TT [OR = 1.46; 95% CI: 1.05−2.04, p = .024], CC versus TT (OR = 1.88; 95% CI: 1.08−3.25, p = .024) and dominant TC + TT versus CC (OR = 1.54; 95% CI: 1.12−2.10, p < .007) genetic models of rs419598 T>C (IL1RN) polymorphism (Table 4). There was no significant difference between the different genotypes from rs419598 T>C (IL1RN) polymorphism regarding the para‐clinical, inflammatory indications characters, disorder severity, outcome, and underlying diseases.
4.2.3. Polymorphism in IL6R (rs2228145 A>C) and IL6 (rs2069827 G>T) genes
The result of this study depicted that increased risk of COVID‐19 infection was observed under codominant AA versus CC [OR = 1.75; 95% CI: 1.09−2.82, p = .020], recessive AA versus AC + CC [OR = 1.72; 95% CI: 1.13−2.63, p = .011] models of rs2228145 A>C (IL6R) polymorphisms. As regards the rs2069827 G>T (IL6) polymorphism, decreased risk of COVID‐19 infection was found under codominant TT versus GG [OR = 0.56; 95% CI: 0.35−0.91, p = .018], dominant GG + GT versus TT [OR = 0.70; 95% CI: 0.51−0.97, p = .029] modes of inheritance (Table 4). There was no significant difference between the different genotypes from cytokines polymorphism regarding the para‐clinical and inflammatory indications characters, except for IL6R (rs2228145, A>C) that the AC genotype increased the degree the lymphocytopenia in comparison to cases in the CC genotype with P 2 = .006 (Table 5). In the studied cases of COVID‐19, the distribution of the CC genotype compared to the AA genotype of the IL6R gene indicated a significant difference regarding suffering from hypertension (p < .01).
Also, there was no significant difference between the different genotypes of IL6 as regards the comorbidities except for diabetes mellitus (GT vs. TT) and hypertension (GT vs. GG) with p = .039 and 0.029, respectively.
So, the A allele of both rs361525 G>A and rs2228145 A>C and the C allele of rs419598 T>C enhanced COVID‐19 susceptibility, while the G allele of rs2069827 G>T (IL6) indicated powerful protection against this pandemic disease.
Table 7 indicates the interaction analysis of the tested SNPs on COVID‐19 risk. The results of relationship between the studied variations demonstrated that the AA/CT/CA/GT (OR = 6.67; 95% CI: 1.14−39.10, p = .021), AA/CT/CC/GT (OR = 8.00; 95% CI: 1.41−45.23, p < .009), AG/CT/CC/GG (OR = 3.67; 95% CI: 1.19−11.31, p = .021), AG/TT/CA/GG (OR = 4.33; 95% CI: 1.74−10.76, p < .001), AG/TT/CC/GG (OR = 6.09; 95% CI: 2.01−18.47, p < .001), GG/CT/AA/GG (OR = 5.33; 95% CI: 1.15−24.79, p = .023), GG/CT/CC/GG (OR = 6.67; 95% CI: 1.14−39.10, p = .021), GG/TT/CA/GT (OR = 5.33; 95% CI: 1.95−14.62, p < .001), GG/TT/CC/GT (OR = 12.00; 95% CI: 2.26−63.72, p < .001) and GG/TT/CC/TT (OR = 4.00; 95% CI: 0.96−16.69, p = .048) combinations statistically increased COVID‐19 susceptibility.
Table 7.
Interaction analysis of the studied SNPs on COVID‐19 risk, number (percentage%)
| rs361525 G>A | rs419598 T>C | rs2228145 A>C | rs2069827 G>T | COVID‐19 | Control | ||
|---|---|---|---|---|---|---|---|
| (TNFA) | (IL1RN) | (IL6R) | (IL6) | N (%) | N (%) | OR (95% CI) | p value |
| AA | CT | CC | GG | 12 (3.8) | 32 (10.1) | 1 [Reference] | |
| GG | TT | CC | GG | 5 (1.6) | 11 (3.5) | 1.21 (0.35−4.22) | .762 |
| AA | CT | CA | GT | 5 (1.7) | 2 (0.7) | 6.67 (1.14−39.10) | .021 * |
| AA | CT | CC | GT | 6 (2.1) | 2 (0.7) | 8.00 (1.41−45.23) | <.009 * |
| AA | TT | CA | GG | 2 (0.6) | 7 (2.2) | 0.76 (0.14−4.19) | .754 |
| AA | TT | CA | GT | 3 (1.0) | 4 (14) | 2.00 (0.39−10.28) | .401 |
| AA | TT | CC | GG | 3 (0.9) | 5 (1.6) | 1.60 (0.33−7.75) | .557 |
| AA | TT | CC | GT | 3 (0.9) | 4 (1.3) | 2.00 (0.39−10.28) | .401 |
| AG | CT | AA | GG | 3 (0.9) | 7 (2.2) | 1.14 (0.25−5.15) | .862 |
| AG | CT | CC | GG | 11 (3.5) | 8 (2.5) | 3.67 (1.19−11.31) | .021 * |
| AG | TT | AA | GG | 7 (2.2) | 10 (3.2) | 1.87 (0.58‐6.02) | .293 |
| AG | TT | CA | GG | 26 (8.2) | 16 (5.0) | 4.33 (1.74−10.76) | <.001 * |
| AG | TT | CC | GG | 16 (5.0) | 7 (2.2) | 6.09 (2.01−18.47) | <.001 * |
| AG | TT | CC | GT | 8 (2.50 | 12 (3.8) | 1.78 (0.58−5.41) | .309 |
| GG | CT | AA | GG | 6 (1.9) | 3 (0.9) | 5.33 (1.15−24.79) | .023 * |
| GG | CT | AA | GT | 2 (0.6) | 4 (1.3) | 1.33 (0.22−8.25) | .756 |
| GG | CT | CA | GG | 4 (1.3) | 3 (0.9) | 3.56 (0.69−18.28) | .114 |
| GG | CT | CA | GT | 3 (0.9) | 6 (1.9) | 1.33 (0.29−6.20) | .713 |
| GG | CT | CC | GG | 5 (1.6) | 2 (0.6) | 6.67 (1.14−39.10) | .021 * |
| GG | CT | CC | GT | 5 (1.6) | 7 (2.2) | 1.90 (0.51−7.17) | .336 |
| GG | TT | AA | GG | 3 (0.9) | 7 (2.2) | 1.14 (0.25−5.15) | .862 |
| GG | TT | CA | GG | 8 (2.5) | 11 (3.5) | 1.94 (0.63−5.98) | .246 |
| GG | TT | CA | GT | 20 (6.3) | 10 (3.2) | 5.33 (1.95−14.62) | <.001 * |
| GG | TT | CC | GT | 9 (2.8) | 2 (0.6) | 12.00 (2.26−63.72) | <.001 * |
| GG | TT | CC | TT | 6 (1.9) | 4 (1.3) | 4.00 (0.96−16.69) | .048 * |
Note: Genotype frequencies less than 0.01 were excluded.
Abbreviations: CI, confidence interval; COVID‐19, coronavirus 2019; OR, odds ratio.
p < .05 (bolded p values) was considered statistically significant.
5. DISCUSSION
A sizeable body of evidence backs the genetically determined cytokine response in humans as hosts. There are consistent and reproducible disparities in cytokine production in healthy individuals due to their close link with genetic variations in the encoding genes. Although SNPs are abundant in genes encoding cytokines as well as in patients with COVID‐19, the cost and time of scrutinizing the potential connection between SNPs and diseases in laboratories are prohibitive. Therefore, a thorough investigation of the precise function of human immunogenetic factors in inducing various vulnerabilities to viral infection and various clinical manifestations caused by SARS‐CoV‐2, in particular, is an ambitious project the human genetics community should undertake (Casanova et al., 2020; Mehrian‐Shai et al., 2020). So, most cytokines genes, such as IL6, IL6R, IL1RN, and TNFA in humans, are polymorphic, potentially affecting cytokine expression. SNPs were likely associated with the genetic susceptibility of COVID‐19, which needs a human immunogenetics initiative for fighting the SARS‐CoV‐2 pandemic (Rokni & Ahmadikia et al., 2020).
The present study is a case‐control report on the inflammatory cytokines' polymorphism and clinical/para‐clinical features of 317 COVID‐19 patients and 317 unaffected controls referred to our central lab/hospital over 8 months. We observed no difference between the cases and unaffected control groups regarding the demographic characteristics (age and gender), demonstrating that the two groups are cross‐matched. Moreover, there was a significant difference between the cases and unaffected control groups in terms of the clinical, paraclinical, and inflammatory indications characteristics (Table 2). Noticeable inflammatory responses co‐occur with a drop in the absolute count of lymphocytes in the peripheral blood circulation. Conversely, a host of studies documented a surge in the number of neutrophils, a phenomenon known as a distinct characteristic among COVID‐19 cases (Liu et al., 2020). Recently, Rokni et al. demonstrated that NLR and PLR could be considered valuable prognostic factors in multiple disorders such as sepsis, pneumonia, acute respiratory distress syndrome (ARDS), and severe COVID‐19; yet, the SII indicator is a prognostic index in the follow‐up of COVID‐19 patients (Rokni & Ghasemi et al., 2020). Our study indicated similar findings. Between studied variations, some studies have been previously associated with COVID‐19 risk. Table 8 summarizes the previous studies on the association between the IL6R and TNF polymorphism with COVID‐19.
Table 8.
Previous studies for the association between the IL6R and TNF polymorphism with COVID‐19
| Gene | SNP | Results | References |
|---|---|---|---|
| IL6R | rs2228145 | The prevalence of the A and C alleles were 0.673 and 0.327, respectively. | Strafella et al. (2020) |
| IL6R | rs2228145 | The frequency of AC genotype was higher in populations of Japan, Spain, Mexico, Brazil, Russia, Poland, Italy, and the Netherland, while the AA genotype was more frequent in from Indian, Swedish and South Africa populations. The CC genotype was only observed in the UK population. | Karcioglu Batur and Hekim (2021) |
| IL6R | rs2228145 | The rs2228145 polymorphism elevated serum sIL‐6R concentrations in subjects with heterozygous or homozygous genotypes. | Garbers et al. (2018) |
| IL6R | rs2228145 | Frequency of the CC genotype was higher compared with the AC and AA genotypes in a population. | Smieszek et al. (2021) |
| TNFA | rs1800629 | The AA genotype of TNFA is related with a high aggressive pattern of the disease. | Saleh et al. (2020) |
| TNFA/TNFB | rs1800629 rs909253 | The association between TNFA/TNFB polymorphisms and COVID‐19 disease. | Heidari Nia et al. (2021) |
Abbreviation: SNP, single‐nucleotide polymorphism.
After genotyping in the current research, a statistically significant difference between the case and unaffected control groups was indicated concerning the genotype distribution in anti‐inflammatory (IL‐1RA) and proinflammatory (IL‐6R, IL‐6, and TNF‐α) cytokines. This shows that participants carrying the A allele (AA and GA) in TNFA‐rs361525 G>A (p < .004), the C allele (CC and TC) in IL1RN‐rs419598 T>C (p < .004), the A allele (AA and AC) in IL6R‐rs2228145 A>C (p = .047) are more susceptible to develop COVID‐19 and the G allele (GG and GT) in IL6‐rs2069827 G>T (p = .01) are powerful protection against the patients with COVID‐19.
Ahmed Saleh et al. indicated that the A allele is high expressed in case versus unaffected control groups in the TNF‐Α; G‐308‐polymorphism with p < .005 (Saleh et al., 2020). Heidari Nia et al. (2021) demonstrated the association between TNFA/TNFB polymorphisms and COVID‐19 disease. These findings are confirmed in patients with various genotype expressions in the inflammatory disease such as inflammatory bowel disease (IBD) and suffering from COVID‐19 who benefited from anti‐TNF‐α therapies that ultimately showed impressive recovery as against those on alternative therapies (Brenner et al., 2020). Quite identically, the application of anti‐TNF‐α therapies to patients with a rheumatic disease (such as RA [rheumatoid arthritis] and SSc [systemic sclerosis]) resulted in diminished hospital admission rates for COVID‐19 (Gianfrancesco et al., 2020).
There exists a strong likelihood that organ damage and ARDS in patients with COVID‐19 can be mitigated by TNF‐α blockade, including adalimumab since it is employed as a therapeutic approach to alleviate over 10 different proinflammatory diseases (Feldmann et al., 2020). The host defense can be fortified against a broad spectrum of pathogenic microbes by the instrumental role of TNF‐α as a critical mediator of the inflammatory response. However, the speed of disease recovery can diminish as a result of the overexpression of this cytokine. The gene regulation, mainly occurring in the promoter or other region of this gene, can be complicated due to the dual role TNF‐α plays as an agent of innate immunity and inflammatory pathology (Wang et al., 2008). Different individuals depict substantially varying capacities for producing cytokines, which is determined by genetics and various genotype expression (Juszczynski et al., 2002).
Our study indicated that the AC genotype of IL6R (rs2228145, A>C) correlated with lymphocytopenia level. Interestingly, Zulvikar et al. observed that IL6‐174‐G/C polymorphism was significantly correlated to the severity of pneumonia (C vs. G), particularly in the Caucasian society (CC + GC vs. GG and CC vs. GG) (Ulhaq & Soraya, 2020a, 2020b). Feng et al. (2015) demonstrated that carriers of the IL6‐174G/C had a 2.5‐fold higher risk of developing severe pneumonia with a degree of lymphopenia. In fact, the CC genotype has been associated with significantly enhanced IL‐6 levels. On the other hand, many studies concerning the IL6R gene results show that carriers of rs12083537AA genotype and CC genotype for rs11265618 have a better prognostic to immunotherapy (Perricone et al., 2020).
Having been adapted to the demographic features and comorbidities, IL‐6 was shown to be the most potent prognostic marker of survival, overshadowing or outperforming CRP, d‐dimer, ferritin, and NLR (Khosroshahi et al., 2021). Although elevated rates of IL‐6 and the critical state of the disease necessitate urgent mechanical ventilation, the administration of tocilizumab (Or siltuximab and/or sarilumab are humanized monoclonal antibodies) to COVID‐19‐associated ARDS (CARDS) patients yielded promising outcomes as the preliminary data reported (Rojas‐Marte et al., 2020). Thus, the prospective responders to tocilizumab (TCZ or Actemra) during the COVID‐19 pandemic can be detected based on specific genetic markers such as several SNPs to prognosticate the response. Regarding the IL6R gene, TCZ is deemed an efficacious treatment considering the responses yielded by several genotypes and SNP (Tong et al., 2010; Ulhaq & Soraya, 2020a, 2020b). Interestingly, the remarkable recovery of acute COVID‐19‐infected patients was substantiated by the results of multiple studies when treatments targeted anti‐IL‐6R antibodies and were characterized by the suppression of CRP and alleviation of clinical symptoms and lymphopenia degree. This can be achieved by inhibiting TCZ and suppressing inflammatory responses due to transcriptional induction of the CRP gene during SARS‐CoV‐2 infection (Luo et al., 2020; Sargazi et al., 2021). Owing to the invaluable data obtained from lung and viral diseases regarding the immunogenetic impact of IL6 polymorphisms, IL6 and/or IL6R polymorphism can be the focal points of investigating the potential therapeutic responses against COVID‐19 in infected human populations to launch a population‐based therapeutic approach similar to personal medicine discovery (Del Valle et al., 2020).
Mamoor et al. indicated that more than 1.5‐fold enhanced IL‐6 upregulation and moreover, less than 1.5‐fold increase in IL1RN expression in the lungs of mice infected with SARS coronavirus family to the lungs of control‐infected mice. As a result, they stated that modulation of IL6 and/or IL1RN upregulation might display a therapeutic strategy in SARS coronavirus disease mainly and specifically in COVID‐19 infection (Mamoor, 2020). On the other hand, some studies show that the IL1RN SNP (like rs4251961) plays a prominent role in the pathophysiology of human infectious diseases (Carrol et al., 2011). Our results also demonstrated similar results findings. As a recombinant form of human IL‐1RA, anakinra impedes the function of the proinflammatory cytokine IL‐1 and is administered as a treatment for proinflammatory disorders mainly owing not only to its satisfactory safety records in patients suffering from hyper inflammation and pneumonia (such as COVID‐19) but also its short half‐life which leads to prompt discontinuation (Nemchand et al., 2020; Shakoory et al., 2013). A consistent plunge in the severity of inflammatory diseases, spanning from RA and COVID‐19 to inherited autoinflammatory syndromes such as cryopyrin‐associated syndromes (CAPS), can be achieved by obstructing IL‐1 activity, which is a highly active proinflammatory cytokine, via mono‐therapy (Tarp et al., 2016).
To further know the diverse presentations and progression of COVID‐19 disease, we compared the various genotypes as regards the disorder severity and poor prognosis; we found that the AA genotype in TNF‐Α (rs361525, G>A) is related to a more aggressive illness and poor prognostic in contrast to the other inflammatory cytokines genotypes. Similarly, one study demonstrated that the AA genotype of TNF‐Α is the more invasive disease pattern (Saleh et al., 2020).
We also noticed that the levels of neutrophil, leucocyte/WBC, platelet, lymphocyte count, SpO2, and inflammatory indexes such as NLR and SII (increased, p < .001) were significantly different in deceased patients when compared to survived patients (Table 3). This suggests multiple organ dysfunction syndromes (MODS) due to hyperinflammation in our deceased patients, as observed in previous studies (Rokni, Ghasemi, et al., 2020). In addition, the upregulation of neutrophil‐endothelial cell adhesion molecules and chemokines is modulated by proinflammatory cytokines and their receptors, for example, TNF‐α and IL‐6R, which triggers the accumulation of leukocytes at the site of infection (Zahr et al., 2016).
So, diminishing the COVID‐19 mortality rate has been possible via deploying some promising approaches such as immune‐modulatory therapies, targeting CS, and identifying polymorphisms in genes encoding cytokines in elderly patients (Panigrahy et al., 2020; Khosroshahi & Rezaei, 2019).
Our study had limitations. First of all, the frequency of the studied SNPs was not entirely consistent throughout the different populations in the world. This might be due to ethnic variations as well as limited sample size. Second, we did not assess the cytokine levels in the patients. Replicated studies on other ethnicities with larger sample sizes are needed to confirm these findings. Moreover, performing SNP‐SNP interaction analysis to determine the combined effect of SNPs on cytokine levels and COVID‐19 risk is highly encouraged.
6. CONCLUSION
This study demonstrated that the A allele in TNFA, the C allele in IL1RN, the A allele in IL6R, and the G allele in IL6 are more prone to the disease. The AA genotype in TNF‐α and AC genotype of IL6R are related to a more invasive type of the disease. We advised considering cytokines polymorphism as the main item to realize the therapeutic response against the ARDS induced by SARS‐CoV‐2 infection in human populations to obtain a population‐based therapeutic development as in personalized medicine.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
The study protocol was approved by the ethics committee of Zahedan University of medical sciences (Ethical code: IR. ZAUMS. REC.1399.122) (The web page of ethical approval is available at: https://ethics.research.ac.ir/ProposalCertificateEn.php?id=140933%26Print=true%26NoPrintHeader=true%26NoPrintFooter=true%26NoPrintPageBorder=true%26LetterPrint=true).
Supporting information
Supporting information.
ACKNOWLEDGMENTS
We highly appreciate the cooperation of Bu‐Ali hospital, Zahedan, Iran. This study was financially supported by Zahedan University of Medical Sciences (grant number: 9859). The funders had no role in the study design, data analysis, or manuscript preparation.
Rokni, M. , Sarhadi, M. , Heidari Nia, M. , Mohamed Khosroshahi, L. , Asghari, S. , Sargazi, S. , Mirinejad, S. , & Saravani, R. (2022). Single nucleotide polymorphisms located in TNFA, IL1RN, IL6R, and IL6 genes are associated with COVID‐19 risk and severity in an Iranian population. Cell Biology International, 46, 1109–1127. 10.1002/cbin.11807
DATA AVAILABILITY STATEMENT
The data presented in this manuscript will be available by the corresponding author upon reasonable request
REFERENCES
- Bhardwaj, V. K. , Singh, R. , Das, P. , & Purohit, R. (2021). Evaluation of acridinedione analogs as potential SARS‐CoV‐2 main protease inhibitors and their comparison with repurposed anti‐viral drugs. Computers in Biology and Medicine, 128, 104117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj, V. K. , Singh, R. , Sharma, J. , Rajendran, V. , Purohit, R. , & Kumar, S. (2021a). Bioactive molecules of Tea as potential inhibitors for RNA‐dependent RNA polymerase of SARS‐CoV‐2. Frontiers in Medicine, 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj, V. K. , Singh, R. , Sharma, J. , Rajendran, V. , Purohit, R. , & Kumar, S. (2021b). Identification of bioactive molecules from tea plant as SARS‐CoV‐2 main protease inhibitors. Journal of Biomolecular Structure and Dynamics, 39(10), 3449–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, E. J. , Ungaro, R. C. , Gearry, R. B. , Kaplan, G. G. , Kissous‐Hunt, M. , Lewis, J. D. , & Ruemmele, F. M. (2020). Corticosteroids, but not TNF antagonists, are associated with adverse COVID‐19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology, 159(2), 481–491 .e483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrol, E. D. , Payton, A. , Payne, D. , Miyajima, F. , Chaponda, M. , Mankhambo, L. A. , Banda, D. L. , Molyneux, E. M. , Cox, H. , Jacobson, G. , Carr, D. F. , Molyneux, M. E. , Stewart, J. P. , Quinn, J. P. , Hart, C. A. , & Ollier, W. E. (2011). The IL1RN promoter rs4251961 correlates with IL‐1 receptor antagonist concentrations in human infection and is differentially regulated by GATA‐1. The Journal of Immunology, 186(4), 2329–2335. [DOI] [PubMed] [Google Scholar]
- Casanova, J. L. , Su, H. C. , & COVID Human Genetic Effort . (2020). A global effort to define the human genetics of protective immunity to SARS‐CoV‐2 infection. Cell, 181(6), 1194–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copaescu, A. , Smibert, O. , Gibson, A. , Phillips, E. J. , & Trubiano, J. A. (2020). The role of IL‐6 and other mediators in the cytokine storm associated with SARS‐CoV‐2 infection. Journal of Allergy and Clinical Immunology, 146(3), 518–534. e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle, D. M. , Kim‐Schulze, S. , Huang, H. H. , Beckmann, N. D. , Nirenberg, S. , Wang, B. , Lavin, Y. , Swartz, T. H. , Madduri, D. , Stock, A. , Marron, T. U. , Xie, H. , Patel, M. , Tuballes, K. , Van Oekelen, O. , Rahman, A. , Kovatch, P. , Aberg, J. A. , Schadt, E. , … Gnjatic, S. (2020). An inflammatory cytokine signature predicts COVID‐19 severity and survival. Nature Medicine, 26(10), 1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello, C. A. (2000). Proinflammatory cytokines. Chest, 118(2), 503–508. [DOI] [PubMed] [Google Scholar]
- Dinarello, C. A. (2018). Overview of the IL‐1 family in innate inflammation and acquired immunity. Immunological Reviews, 281(1), 8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann, M. , Maini, R. N. , Woody, J. N. , Holgate, S. T. , Winter, G. , Rowland, M. , Richards, D. , & Hussell, T. (2020). Trials of anti‐tumour necrosis factor therapy for COVID‐19 are urgently needed. The Lancet, 395(10234), 1407–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, B. , Mao, Z. R. , Pang, K. , Zhang, S. L. , & Li, L. (2015). Association of tumor necrosis factor α ‐308G/A and interleukin‐6 ‐174G/C gene polymorphism with pneumonia‐induced sepsis. Journal of Critical Care, 30(5), 920–923. 10.1016/j.jcrc.2015.04.123 [DOI] [PubMed] [Google Scholar]
- Ferreira, R. C. , Freitag, D. F. , Cutler, A. J. , Howson, J. M. , Rainbow, D. B. , Smyth, D. J. , Kaptoge, S. , Clarke, P. , Boreham, C. , Coulson, R. M. , Pekalski, M. L. , Chen, W. M. , Onengut‐Gumuscu, S. , Rich, S. S. , Butterworth, A. S. , Malarstig, A. , Danesh, J. , & Todd, J. A. (2013). Functional IL6R 358Ala allele impairs classical IL‐6 receptor signaling and influences risk of diverse inflammatory diseases. PLoS Genetics, 9(4), e1003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers, C. , Heink, S. , Korn, T. , & Rose‐John, S. (2018). Interleukin‐6: Designing specific therapeutics for a complex cytokine. Nature Reviews Drug Discovery, 17(6), 395–412. [DOI] [PubMed] [Google Scholar]
- Ghaznavi, H. , Shirvaliloo, M. , Sargazi, S. , Mohammadghasemipour, Z. , Shams, Z. , Hesari, Z. , & Shirvalilou, S. (2022). SARS‐CoV‐2 and influenza viruses: Strategies to cope with co‐infection and bioinformatics perspective. Cell Biology International . [DOI] [PMC free article] [PubMed]
- Gianfrancesco, M. , Hyrich, K. L. , Al‐Adely, S. , Carmona, L. , Danila, M. I. , Gossec, L. , Gossec, L. , Izadi, Z. , Jacobsohn, L. , Katz, P. , Lawson‐Tovey, S. , Mateus, E. F. , Rush, S. , Schmajuk, G. , Simard, J. , Strangfeld, A. , Trupin, L. , Wysham, K. D. , Bhana, S. , Costello, W. , … COVID‐19 Global Rheumatology Alliance . (2020). Characteristics associated with hospitalisation for COVID‐19 in people with rheumatic disease: Data from the COVID‐19 global rheumatology alliance physician‐reported registry. Annals of the Rheumatic Diseases, 79(7), 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari Nia, M. , Rokni, M. , Mirinejad, S. , Kargar, M. , Rahdar, S. , Sargazi, S. , Sarhadi, M. , & Saravani, R. (2021). Association of polymorphisms in tumor necrosis factors with SARS‐CoV‐2 infection and mortality rate: A case‐control study and in silico analyses. Journal of Medical Virology, 94, 1502–1512. 10.1002/jmv.27477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , Zhang, L. , Fan, G. , Xu, J. , Gu, X. , Cheng, Z. , Yu, T. , Xia, J. , Wei, Y. , Wu, W. , Xie, X. , Yin, W. , Li, H. , Liu, M. , … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet, 395(10223), 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, Y. , Kuba, K. , Neely, G. G. , Yaghubian‐Malhami, R. , Perkmann, T. , van Loo, G. , Ermolaeva, M. , Veldhuizen, R. , Leung, Y. H. , Wang, H. , Liu, H. , Sun, Y. , Pasparakis, M. , Kopf, M. , Mech, C. , Bavari, S. , Peiris, J. S. , Slutsky, A. S. , Akira, S. , … Penninger, J. M. (2008). Identification of oxidative stress and Toll‐like receptor 4 signaling as a key pathway of acute lung injury. Cell, 133(2), 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infantino, M. , Damiani, A. , Gobbi, F. L. , Grossi, V. , Lari, B. , Macchia, D. , Casprini, P. , Veneziani, F. , Villalta, D. , Bizzaro, N. , Cappelletti, P. , Fabris, M. , Quartuccio, L. , Benucci, M. , & Manfredi, M. (2020). Serological assays for SARS‐CoV‐2 infectious disease: Benefits, limitations and perspectives. Israel Medical Association Journal, 22(4), 203–210. [PubMed] [Google Scholar]
- Jamilloux, Y. , Henry, T. , Belot, A. , Viel, S. , Fauter, M. , El Jammal, T. , Walzer, T. , François, B. , & Sève, P. (2020). Should we stimulate or suppress immune responses in COVID‐19? Cytokine and anti‐cytokine interventions. Autoimmunity Reviews, 19(7), 102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juszczynski, P. , Kalinka, E. , Bienvenu, J. , Woszczek, G. , Borowiec, M. , Robak, T. , Kowalski, M. , Lech‐Maranda, E. , Baseggio, L. , Coiffier, B. , Salles, G. , & Warzocha, K. (2002). Human leukocyte antigens class II and tumor necrosis factor genetic polymorphisms are independent predictors of non‐Hodgkin lymphoma outcome. Blood, 100(8), 3037–3040. [DOI] [PubMed] [Google Scholar]
- Karcioglu Batur, L. , & Hekim, N . (2021). Correlation between interleukin gene polymorphisms and current prevalence and mortality rates due to novel coronavirus disease 2019 (COVID‐2019) in 23 countries. Journal of Medical Virology, 93(10), 5853–5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavita, U. , & Mizel, S. B. (1995). Differential sensitivity of interleukin‐1α and‐β precursor proteins to cleavage by calpain, a calcium‐dependent protease. Journal of Biological Chemistry, 270(46), 27758–27765. [DOI] [PubMed] [Google Scholar]
- Khosroshahi, L. M. , & Rezaei, N. (2019). Dysregulation of the immune response in coronavirus disease. Cell Biology International, 45(4), 702–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosroshahi, L. M. , Rokni, M. , Mokhtari, T. , & Noorbakhsh, F. (2021). Immunology, immunopathogenesis and immunotherapeutics of COVID‐19; an overview. International Immunopharmacology, 93, 107364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtipal, N. , & Bharadwaj, S. (2020). Interleukin 6 polymorphisms as an indicator of COVID‐19 severity in humans. Journal of Biomolecular Structure and Dynamics, 39(12), 4563–4565. [DOI] [PubMed] [Google Scholar]
- Kobayashi, Y. , Yamamoto, K. , Saido, T. , Kawasaki, H. , Oppenheim, J. J. , & Matsushima, K. (1990). Identification of calcium‐activated neutral protease as a processing enzyme of human interleukin 1 alpha. Proceedings of the National Academy of Sciences, 87(14), 5548–5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Liu, Y. , Xiang, P. , Pu, L. , Xiong, H. , Li, C. , Zhang, M. , Tan, J. , Xu, Y. , Song, R. , Song, M. , Wang, L. , Zhang, W. , Han, B. , Yang, L. , Wang, X. , Zhou, G. , Zhang, T. , Li, B. , … Wang, X. (2020). Neutrophil‐to‐lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. Journal of Translational Medicine, 18, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, P. , Liu, Y. , Qiu, L. , Liu, X. , Liu, D. , & Li, J. (2020). Tocilizumab treatment in COVID‐19: A single center experience. Journal of Medical Virology, 92(7), 814–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamoor, S. (2020). IL6 and IL1RN is differentially expressed and transcriptionally induced in models of SARS coronavirus infection.
- Mehrian‐Shai, R. , Novelli, G. , Vasiliou, V. , Watt, J. , & Reichardt, J. K. V. (2020). Genomics of COVID‐19: Molecular mechanisms going from susceptibility to severity of the disease. BioMed Central, 14(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa, F. , Lanza, E. , García, L. , Marfil‐Alvarez, R. , & Magan‐Fernandez, A. (2017). Polymorphism IL‐1RN rs419598 reduces the susceptibility to generalized periodontitis in a population of European descent. PLoS One, 12(10), e0186366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MWer, S. , Dykes, D. , & Polesky, H. (1988). A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Research, 16(3), 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemchand, P. , Tahir, H. , Mediwake, R. , & Lee, J. (2020). Cytokine storm and use of anakinra in a patient with COVID‐19. BMJ Case Reports, 13(9), e237525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordzij, M. , Tripepi, G. , Dekker, F. W. , Zoccali, C. , Tanck, M. W. , & Jager, K. J. (2010). Sample size calculations: Basic principles and common pitfalls. Nephrology, Dialysis, Transplantation, 25(5), 1388–1393. 10.1093/ndt/gfp732 [DOI] [PubMed] [Google Scholar]
- Paim, A. A. O. , Lopes‐Ribeiro, Á. , e Silva, D. S. O. D. , Andrade, L. A. F. , Moraes, T. F. S. , Barbosa‐Stancioli, E. F. , & Coelho‐dos‐Reis, J. G. (2021). Will a little change do you good? A putative role of polymorphisms in COVID‐19. Immunology Letters, 235, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahy, D. , Gilligan, M. M. , Huang, S. , Gartung, A. , Cortés‐Puch, I. , Sime, P. J. , Phipps, R. P. , Serhan, C. N. , & Hammock, B. D. (2020). Inflammation resolution: A dual‐pronged approach to averting cytokine storms in COVID‐19? Cancer and Metastasis Reviews, 39(2), 337–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricone, C. , Conigliaro, P. , Ciccacci, C. , Marcucci, E. , Cafaro, G. , Bartoloni, E. , Perricone, R. , Novelli, G. , Borgiani, P. , & Gerli, R. (2020). The differential response to anti IL‐6 treatment in COVID‐19: the genetic counterpart. Clinical and Experimental Rheumatology, 38(3), 580. [PubMed] [Google Scholar]
- Prompetchara, E. , Ketloy, C. , & Palaga, T. (2020). Immune responses in COVID‐19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pacific Journal of Allergy and Immunology, 38(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Qin, C. , Zhou, L. , Hu, Z. , Zhang, S. , Yang, S. , Tao, Y. , Xie, C. , Ma, K. , Shang, K. , Wang, W. , & Tian, D. S. (2020). Dysregulation of immune response in patients with coronavirus 2019 (COVID‐19) in Wuhan, China. Clinical Infectious Diseases, 71(15), 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider, P. , Carmi, Y. , Guttman, O. , Braiman, A. , Cohen, I. , Voronov, E. , White, M. R. , Dinarello, C. A. , & Apte, R. N. (2011). IL‐1α and IL‐1β recruit different myeloid cells and promote different stages of sterile inflammation. The Journal of Immunology, 187(9), 4835–4843. [DOI] [PubMed] [Google Scholar]
- Rojas‐Marte, G. , Khalid, M. , Mukhtar, O. , Hashmi, A. T. , Waheed, M. A. , Ehrlich, S. , Aslam, A. , Siddiqui, S. , Agarwal, C. , Malyshev, Y. , Henriquez‐Felipe, C. , Sharma, D. , Sharma, S. , Chukwuka, N. , Rodriguez, D. C. , Alliu, S. , Le, J. , & Shani, J. (2020). Outcomes in patients with severe COVID‐19 disease treated with tocilizumab: A case–controlled study. QJM: An International Journal of Medicine, 113(8), 546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokni, M. , Ahmadikia, K. , Asghari, S. , Mashaei, S. , & Hassanali, F. (2020). Comparison of clinical, para‐clinical and laboratory findings in survived and deceased patients with COVID‐19: Diagnostic role of inflammatory indications in determining the severity of illness. BMC Infectious Diseases, 20(1), 869. 10.1186/s12879-020-05540-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokni, M. , Ghasemi, V. , & Tavakoli, Z. (2020). Immune responses and pathogenesis of SARS‐CoV‐2 during an outbreak in Iran: Comparison with SARS and MERS. Reviews in Medical Virology, 30(3), e2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokni, M. , Hamblin, M. R. , & Rezaei, N. (2020). Cytokines and COVID‐19: Friends or foes? Human Vaccines & Immunotherapeutics, 16(10), 2363–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokni, M. , Salimi, S. , Sohrabi, T. , Asghari, S. , Teimoori, B. , & Saravani, M. (2019). Association between miRNA‐152 polymorphism and risk of preeclampsia susceptibility. Archives of Gynecology and Obstetrics, 299(2), 475–480. [DOI] [PubMed] [Google Scholar]
- Saleh, A. , Sultan, A. , Elashry, M. A. , Farag, A. , Mortada, M. I. , Ghannam, M. A. , Saed, A. M. , & Ghoneem, E. (2020). Association of TNF‐α G‐308 a Promoter Polymorphism with the Course and Outcome of COVID‐19 Patients. Immunological Investigations, 51, 1–12. 10.1080/08820139.2020.1851709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoughi, M. , Saravani, M. , Rokni, M. , Nora, M. , Mehrabani, M. , & Dehghan, A . (2020). Association between COX‐2 and 15‐PGDH polymorphisms and SLE susceptibility. International Journal of Rheumatic Diseases, 23(5), 627‐632. [DOI] [PubMed] [Google Scholar]
- Sapey, E. , Wood, A. M. , Ahmad, A. , & Stockley, R. A. (2010). Tumor necrosis factor–α rs361525 polymorphism is associated with increased local production and downstream inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 182(2), 192–199. [DOI] [PubMed] [Google Scholar]
- Sargazi, S. , Sheervalilou, R. , Rokni, M. , Shirvaliloo, M. , Shahraki, O. , & Rezaei, N. (2021). The role of autophagy in controlling SARS‐CoV‐2 infection: An overview on virophagy‐mediated molecular drug targets. Cell Biology International, 45, 1599–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoory, B. , Carcillo, J. , Zhao, H. , Cron, R. , Dinarello, C. , & Opal, S. (2013). 1105: IL‐1 Receptor antagonist improves mortality in severe sepsis subset with hemophagocytic syndrome. Critical Care Medicine, 41(12), A279–A281. [Google Scholar]
- Sharma, J. , Bhardwaj, V. K. , Singh, R. , Rajendran, V. , Purohit, R. , & Kumar, S. (2021). An in‐silico evaluation of different bioactive molecules of tea for their inhibition potency against non structural protein‐15 of SARS‐CoV‐2. Food Chemistry, 346, 128933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheervalilou, R. , Ahmadzadeh, J. , Alavi, S. , Mobaraki, K. , Sargazi, S. , Shirvaliloo, M. , Golchin, A. , Yekanlou, A. , & Mehranfar, S. (2021). Evaluation of diagnostic modalities for SARS‐Cov‐2: A review study. International Journal of Epidemiologic Research, 8, 129–137. [Google Scholar]
- Sheervalilou, R. , Shirvaliloo, M. , Sargazi, S. , Shirvalilou, S. , Shahraki, O. , Pilehvar‐Soltanahmadi, Y. , Sarhadi, A. , Nazarlou, Z. , Ghaznavi, H. , & Khoei, S. (2021). Application of nanobiotechnology for early diagnosis of SARS‐CoV‐2 infection in the COVID‐19 pandemic. Applied Microbiology and Biotechnology, 105(7), 2615–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheervalilou, R. , Shirvaliloo, M. , Sargazi, S. , Bahari, S. , Saravani, R. , Shahraki, J. , & Shams, Z. (2021). Convalescent blood: Current perspective on the efficacy of a legacy approach in COVID‐19 treatment. Blood Purification, 51,1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón, C. , Valbuena, D. , Krüssel, J. , Bernal, A. , Murphy, C. R. , Shaw, T. , & Polan, M. L. (1998). Interleukin‐1 receptor antagonist prevents embryonic implantation by a direct effect on the endometrial epithelium. Fertility and sterility, 70(5), 896–906. [DOI] [PubMed] [Google Scholar]
- Singh, M. , Mastana, S. , Singh, S. , Juneja, P. K. , Kaur, T. , & Singh, P. (2020). Promoter polymorphisms in IL‐6 gene influence pro‐inflammatory cytokines for the risk of osteoarthritis. Cytokine, 127, 154985. [DOI] [PubMed] [Google Scholar]
- Singh, R. , Bhardwaj, V. K. , Das, P. , & Purohit, R. (2021). A computational approach for rational discovery of inhibitors for non‐structural protein 1 of SARS‐CoV‐2. Computers in Biology and Medicine, 135, 104555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, R. , Bhardwaj, V. K. , & Purohit, R. (2021). Potential of turmeric‐derived compounds against RNA‐dependent RNA polymerase of SARS‐CoV‐2: An in‐silico approach. Computers in Biology and Medicine, 139, 104965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, R. , Bhardwaj, V. K. , Sharma, J. , Purohit, R. , & Kumar, S. (2021). In‐silico evaluation of bioactive compounds from tea as potential SARS‐CoV‐2 nonstructural protein 16 inhibitors. Journal of Traditional and Complementary Medicine, 9, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasankarapillai, V. S. , Pillai, A. M. , Rahdar, A. , Sobha, A. P. , Das, S. S. , Mitropoulos, A. C. , Mokarrar, M. H. , & Kyzas, G. Z. (2020). On facing the SARS‐CoV‐2 (COVID‐19) with combination of nanomaterials and medicine: possible strategies and first challenges. Nanomaterials, 10(5), 852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smieszek, S. P. , Przychodzen, B. P. , Polymeropoulos, V. M. , Polymeropoulos, C. M. , & Polymeropoulos, M. H . (2021). Assessing the potential correlation of polymorphisms in the IL6R with relative IL6 elevation in severely ill COVID‐19 patients. Cytokine, 148, 155662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soerensen, M. , Dato, S. , Tan, Q. , Thinggaard, M. , Kleindorp, R. , Beekman, M. , Suchiman, H. E. , Jacobsen, R. , McGue, M. , Stevnsner, T. , Bohr, V. A. , de Craen, A. J. , Westendorp, R. G. , Schreiber, S. , Slagboom, P. E. , Nebel, A. , Vaupel, J. W. , Christensen, K. , & Christiansen, L. (2013). Evidence from case‐control and longitudinal studies supports associations of genetic variation in APOE, CETP, and IL6 with human longevity. Age, 35(2), 487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella, C. , Caputo, V. , Termine, A. , Barati, S. , Caltagirone, C. , Giardina, E. , & Cascella, R. (2020). Investigation of genetic variations of IL6 and IL6r as potential prognostic and pharmacogenetics biomarkers: Implications for covid‐19 and neuroinflammatory disorders. Life, 10(12), 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, D. , Li, H. , Lu, X. ‐X. , Xiao, H. , Ren, J. , Zhang, F. ‐R. , & Liu, Z. ‐S. (2020). Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: A single center's observational study. World Journal of Pediatrics, 16, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarp, S. , Amarilyo, G. , Foeldvari, I. , Christensen, R. , Woo, J. M. , Cohen, N. , Pope, T. D. , & Furst, D. E. (2016). Efficacy and safety of biological agents for systemic juvenile idiopathic arthritis: A systematic review and meta‐analysis of randomized trials. Rheumatology, 55(4), 669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, Y. , Wang, Z. , Geng, Y. , Liu, J. , Zhang, R. , Lin, Q. , Li, X. , Huang, D. , Gao, S. , Hu, D. , Li, Y. , Cheng, J. , & Lu, Z. (2010). The association of functional polymorphisms of IL‐6 gene promoter with ischemic stroke: Analysis in two Chinese populations. Biochemical and Biophysical Research Communications, 391(1), 481–485. [DOI] [PubMed] [Google Scholar]
- Ulhaq, Z. S. , & Soraya, G. V. (2020a). Anti‐IL‐6 receptor antibody treatment for severe COVID‐19 and the potential implication of IL‐6 gene polymorphisms in novel coronavirus pneumonia. Medicina Clinica (English Ed), 155(12), 548–556. 10.1016/j.medcle.2020.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulhaq, Z. S. , & Soraya, G. V. (2020b). Interleukin‐6 as a potential biomarker of COVID‐19 progression. Medecine et Maladies Infectieuses, 50, 382–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. , Wei, M. , Han, Y. , Zhang, K. , He, L. , Yang, Z. , Su, B. , Zhang, Z. , Hu, Y. , & Hui, W. (2008). Roles of TNF‐α gene polymorphisms in the occurrence and progress of SARS‐Cov infection: A case‐control study. BMC Infectious Diseases, 8(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werman, A. , Werman‐Venkert, R. , White, R. , Lee, J. K. , Werman, B. , Krelin, Y. , Voronov, E. , Dinarello, C. A. , & Apte, R. N. (2004). The precursor form of IL‐1α is an intracrine proinflammatory activator of transcription. Proceedings of the National Academy of Sciences, 101(8), 2434–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y. , Chen, Y. , & Tang, X. (2020). Guidelines for the diagnosis and treatment of coronavirus disease 2019 (COVID‐19) in China. Global Health & Medicine, 2(2), 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Shen, C. , Li, J. , Yuan, J. , Yang, M. , Wang, F. , & Peng, L. (2020). Exuberant elevation of IP‐10, MCP‐3 and IL‐1ra during SARS‐CoV‐2 infection is associated with disease severity and fatal outcome. MedRxiv.
- Yazdanpanah, F. , Hamblin, M. R. , & Rezaei, N. (2020). The immune system and COVID‐19: Friend or foe? Life Sciences, 256, 117900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki, K. , Fujiogi, M. , & Koutsogiannaki, S. (2020). COVID‐19 pathophysiology: A review. Clinical Immunology, 215, 108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr, A. , Alcaide, P. , Yang, J. , Jones, A. , Gregory, M. , Dela Paz, N. G. , Patel‐Hett, S. , Nevers, T. , Koirala, A. , Luscinskas, F. W. , Saint‐Geniez, M. , Ksander, B. , D'Amore, P. A. , & Argüeso, P. (2016). Endomucin prevents leukocyte−endothelial cell adhesion and has a critical role under resting and inflammatory conditions. Nature Communications, 7(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. H. , Zhao, Y. , Li, N. , Peng, Y. C. , Giannoulatou, E. , Jin, R. H. , Yan, H. P. , Wu, H. , Liu, J. H. , Liu, N. , Wang, D. Y. , Shu, Y. L. , Ho, L. P. , Kellam, P. , McMichael, A. , & Dong, T. (2013). Interferon‐induced transmembrane protein‐3 genetic variant rs12252‐C is associated with severe influenza in Chinese individuals. Nature Communications, 4(1), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Q. , Sun, J. , & Chen, Y. (2014). Preterm birth and single nucleotide polymorphisms in cytokine genes. Translational Pediatrics, 3(2), 120–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data presented in this manuscript will be available by the corresponding author upon reasonable request


