Abstract
Aims
The discovery of antiviral substances to respond to COVID‐19 is a global issue, including the field of drug development based on natural materials. Here, we showed that chitosan‐based substances have natural antiviral properties against SARS‐CoV‐2 in vitro.
Methods and Results
The molecular weight of chitosan‐based substances was measured by the gel permeation chromatography analysis. In MTT assay, the chitosan‐based substances have low cytotoxicity to Vero cells. The antiviral effect of these substances was confirmed by quantitative viral RNA targeting the RdRp and E genes and plaque assay. Among the substances tested, low molecular weight chitooligosaccharide decreased the fluorescence intensity of SARS‐CoV‐2 nucleocapsid protein of the virus‐infected cells in a dose‐dependent manner.
Conclusions
In conclusion, the chitooligosaccharide, a candidate for natural treatment, has antiviral effects against the SARS‐CoV‐2 virus in vitro.
Significance and Impact of Study
In this study, it was suggested for the first time that chitosan‐based substances such as chitooligosaccharide can have an antiviral effect on SARS‐CoV‐2 in vitro.
Keywords: antiviral effect, chitooligosaccharide, COVID‐19, natural treatment, SARS‐CoV‐2
INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is a respiratory syndrome caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), an RNA virus belonging to coronaviridae (Zhu et al., 2020). It has been reported that SARS‐CoV‐2 virus infection can occur not only in respiratory epithelial cells but also in the mucous membrane of the eyes (Rothe et al., 2020). Various symptoms, ranging from fever, malaise, cough, shortness of breath, and, in severe cases, severe pneumonia, have been reported (Huang et al., 2020). In several countries, drugs like palliatives for the respiratory syndrome are being used for limited COVID‐19 treatment (Gavriatopoulou et al., 2021; Varrassi & Rekatsina, 2022). Recently, Pfizer's Paxlovid has received emergency use authorization from the FDA. (Izda et al., 2021; Parums, 2022). Even now, in vitro studies and clinical trials trying to discover effective therapies for SARS‐CoV‐2 infection are ongoing (Drożdżal et al., 2021). In addition, as one of the therapeutic strategies targeting the SARS‐CoV‐2 virus directly, a method using a neutralization reaction specific to the virus surface protein has been proposed (Chatterjee et al., 2020). In the COVID‐19 situation, disinfectants that are effective directly against the virus are considered one of the main strategies to inhibit the spread of infection. Still, the human harm of chemical disinfectants cannot be overlooked (Rai et al., 2020).
Chitin, poly(β‐[1,4]‐N‐acetyl‐d‐glucosamine), is a natural high‐molecular polysaccharide that can be obtained in large amounts from the shells of various crustaceans (Suneeta & Rupak, 2020). Among these chitin‐derived biological materials, chitosan (poly‐[D]glucosamine) and chitooligosaccharide, which are most widely used, have been proposed as natural disinfectants with high biocompatibility, low cytotoxicity, antibacterial activity, and antiviral activity (Cheung et al., 2015; Kim et al., 2021; Kong et al., 2010; Liaqat & Eltem, 2018). In several studies, chitosan‐derived substances have been reported as effective for preventing viral infections, such as HIV‐1, by reducing inflammation and enhancing immunity (Fernandes et al., 2010; Jaber et al., 2021; Lodhi et al., 2014; Marmouzi et al., 2019; Sánchez et al., 2018). On the other hand, an infectious disease drug delivery system using chitosan‐derived compounds as nanoparticles is also being developed (Friedman et al., 2013; Garg et al., 2019; Mohammed et al., 2017).
Here, we showed that chitosan‐based substances have natural antiviral properties against SARS‐CoV‐2 in vitro using Vero cells. In particular, among the chitosan‐based substances, we propose that low molecular weight chitooligosaccharide can serve as a natural antiviral treatment for SARS‐CoV‐2.
MATERIALS AND METHODS
Gel permeation chromatography of chitosan‐based substances
The molecular weight of chitosan‐based substances was measured by the gel permeation chromatography analysis using the columns Ultrahydrogel™ 120 and Ultrahydrogel™ 250 (Waters™) of high‐performance liquid chromatography systems (Shimadzu) (Choi et al., 2004). The mobile phase and dilution solvent were prepared in 0.1 M NaCl (with 0.1% trifluoroacetic acid). The flow rate of the mobile phase is 1 ml/min and was detected using the refractive index detector. The concentration of chitosan‐based substances was diluted to 1%. The samples were analysed for 30 min, and the standard curve was prepared by analysing the standard product before exploring substances.
Cell culture and chitosan‐based substances treatment
Vero E6 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco®) containing 10% fetal bovine serum (FBS) (Gibco®), 2 mM L‐glutamine, 1 mM sodium pyruvate, 1500 mg/L sodium bicarbonate, 15 mM HEPES and 1% penicillin/streptomycin in a 5% CO2 incubator at 37°C. Cells were seeded 24 h before being treated with candidate substances (AMI‐1 to −4) at a different dose (25–200 μg/ml) for 24 h and observed through an inverted microscope (LionHeart FX automated Microscope, Biotek).
Cell viability assay
Vero E6 cells were seeded into 96‐well tissue culture plates 24 h before treatment with candidate substances for 24 h and incubated with 0.04 mg/ml MTT (3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5‐diphenyltetrazolium bromide) solution (Sigma‐Aldrich, USA) at 37 °C for 2 h. The cells were then incubated with 100 μl of dimethyl sulfoxide at 37°C for 30 min to solubilize the formazan precipitate's final product of MTT metabolism. The optical density of each well was measured using a microplate reader set to 570 nm (Synergy H1, BioTek). The cytotoxicity concentration of 50% (CC50) was analysed in GraphPad Prism 5.0 (GraphPad Software).
Viral infection and cytopathic effect (CPE) observation
Vero E6 cells were seeded into 6‐well tissue culture plates 24 h before infection with SARS‐CoV‐2 (National Culture Collection for Pathogens [NCCP]) NCCP43326 in media containing 2% FBS for 1 h. CPE of the infected cells was observed for 96 h through an inverted microscope.
Viral RNA extraction and detection of SARS‐CoV‐2 using real‐time RT‐PCR
The in vitro reaction between the virus and the chitosan‐based substances was performed as described (Chung et al., 2021; Kim et al., 2021). SARS‐CoV‐2 virus (NCCP43326) was incubated with candidate substances at room temperature for 1 h and then infected at a multiplicity of infection (MOI) 0.01 (a dose of 1 × 104 PFU/well, 6‐well) for 1 h to the Vero E6 cells seeded into 6‐well plates. The infected cells were washed with PBS 3 times and then replaced and incubated with fresh media for 2 days. Viral RNA from the cultured media was extracted using QIAamp Viral RNA Mini Kit (QIAGEN). Real‐time RT‐PCR assay was performed using STANDARD M nCoV Real‐Time Detection kit (SD Biosensor) or iScript™ cDNA Synthesis Kit (Bio‐Rad) and iQ SYBR Green Supermix (Bio‐Rad) with designed primers on CFX96™ Real‐Time PCR Detection System (Bio‐Rad) with the extracted viral RNA. The Ct values of the Rdrp and E genes of SARS‐CoV‐2 were quantified. The sequence of the primers used in the experiment is as follows; SARS‐CoV‐2 Rdrp gene forward 5’‐TCAAACCCGTCCTTGATTGG‐3′, reverse 5’‐CCACCGACAATTTCACAAGC‐3′; SARS‐CoV‐2 E gene forward 5’‐TTACTGCGCTTCGATTGTGT‐3′, reverse 5’‐GACCAGAAGATCAGGAACTCTA‐3′. The PCR amplifications with primers for Rdrp and E genes were performed with thermal profile as follows; hold at 95°C for 10 min, 45 cycles of 95°C for 10 s and 57°C for 1 min, melt curve at 65°C for 30 s and up to 95°C.
SARS‐CoV‐2 plaque assay
Plaque assay on SARS‐CoV‐2 was performed as described (Mendoza et al., 2020). Vero E6 cells were seeded into 6‐well tissue culture plates 24 h before infection with serial diluted SARS‐CoV‐2 (NCCP43326) from 10−2 to 10−6 in media containing 2% FBS for 1 h. For the double overlay method, the infected cells were washed with PBS 3 times and cultured with the first overlay 1% low‐melting‐point (LMP) UltraPure™ agarose (Invitrogen) and 2% FBS containing cell culture media in a 5% CO2 incubator at 37°C for 5 days. The second overlay was added with 1% LMP agarose and 0.01% neutral red‐containing media and cultured in a 5% CO2 incubator at 37°C for 24 h. For the fixation staining method, the infected cells were washed with PBS 3 times and cultured with 1% LMP agarose and 2% FBS containing cell culture media in a 5% CO2 incubator at 37°C for 3 days. After solid overlay aspirated from each well, the cells were fixed by 4% paraformaldehyde for 1 h at room temperature and then stained with 0.5% crystal violet solution for 15 min. The plaque was observed using a white‐light transilluminator.
Detection of SARS‐CoV‐2 using immunofluorescence staining
Vero E6 cells were infected with SARS‐CoV‐2 at MOI 0.01 and incubated with the candidate compound as an indicated concentration in a 5% CO2 incubator at 37°C for 36 h. The cells were fixed with 4% paraformaldehyde in PBS for 1 h and permeabilized with 0.1% Triton X‐100 in PBS for 15 min at room temperature. The cells were incubated with anti‐nucleocapsid protein (NP) rabbit antibody (200‐401‐A50, Rockland) (1:100 dilute) in 1% BSA in PBS for 24 h at 4°C. After washing with PBS, the cells were incubated with Alexa 488‐conjugated anti‐rabbit goat antibody (ab150077, Abcam) (1:250 dilute) for 1 h at room temperature, and then stained with Hoechst 33342 (H3570, Thermo Fisher) for 1 h at room temperature. Fluorescent images were obtained using LionHeart FX automated Microscope (Biotek). The fluorescent intensity of images was quantified using the Gen5 software (Biotek).
RESULTS
The average molecular weight of chitosan‐based substances
The substances from Amicogen in the table were used in this study to investigate the antiviral effect of chitosan and chitosan‐based substances against SARS‐CoV‐2 (Figure 1a). The average molecular weight of these chitosan‐based substances, including chitosan, chitooligosaccharide, and water‐soluble chitosan, was analysed through gel permeation chromatography, and each substance was named AMI‐1 (221,620 daltons), AMI‐2 (15,604 daltons) and AMI‐3 (84,504 daltons), respectively (Figure 1b). Ivermectin, named AMI‐4 in this study, is a substance with potential as a therapeutic agent for COVID‐19 (Caly et al., 2020; Jans & Wagstaff, 2021) due to being one of the anti‐parasitic agents and was used in this study to confirm the in vitro ability of antiviral effects against SARS‐CoV‐2.
FIGURE 1.
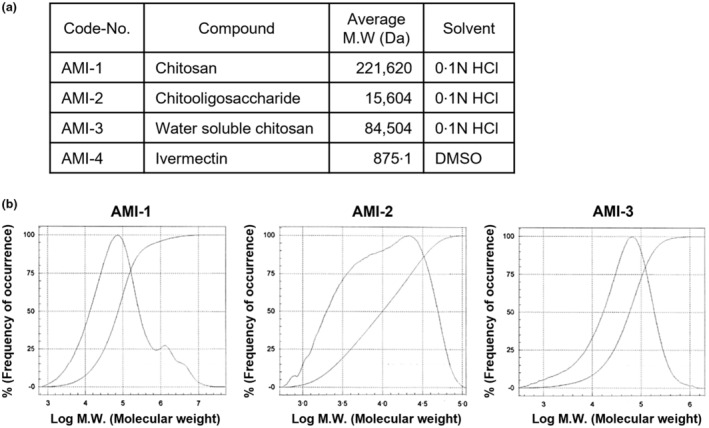
The average molecular weight of chitosan‐based substances. (a) The candidate substances were used for antiviral effects to SARS‐CoV‐2 in the experiment. Synthetic substances such as chitosan‐based substances and ivermectin are named as follows; AMI‐1 (chitosan), AMI‐2 (chitooligosaccharide), AMI‐3 (water soluble chitosan) and AMI‐4 (ivermectin). (b) Measuring the molecular weight of chitosan‐based substances through gel permeation chromatography. The molecular weight of the chitosan‐based substances of 5% concentration was analysed by chromatography and compared with the standard curve of the standard product.
Cytotoxicity of chitosan‐based substances
Before investigating the antiviral effect of chitosan‐based substances, cytotoxicity was measured in Vero cells treated with 25–200 μg/ml concentration of substances through cell viability assay. Before analysing the cell viability assay at the concentration intervals, the cytotoxic effect of the substances was observed by phase‐contrast microscopy (Figure 2a). As shown in Figure 2, the cytotoxic effect of chitosan‐based substances in treated cells was not observed, and AMI‐3 crystallized without being absorbed into cells at two high concentrations (200 and 100 μg/ml). However, there was no cytotoxicity. As a result of quantifying cytotoxicity through the MTT assay, the chitosan‐based substances have very low cytotoxicity to Vero cells in the range of 25–200 μg/ml concentration. There was no significant difference in cytotoxicity compared to the control‐treated with 0.1 N HCl solvent used as a vehicle. On the other hand, AMI‐4 (ivermectin) has a CC50 of 13.98 μM (Figure 2b). As a result of quantifying cytotoxicity in A549 and NCI‐H358 cells, both human non‐small cell lung carcinomas, chitosan‐based substances have low cytotoxicity, but AMI‐4 has CC50 of 10.76 μM and 36.98 μM, respectively (Figure S1).
FIGURE 2.
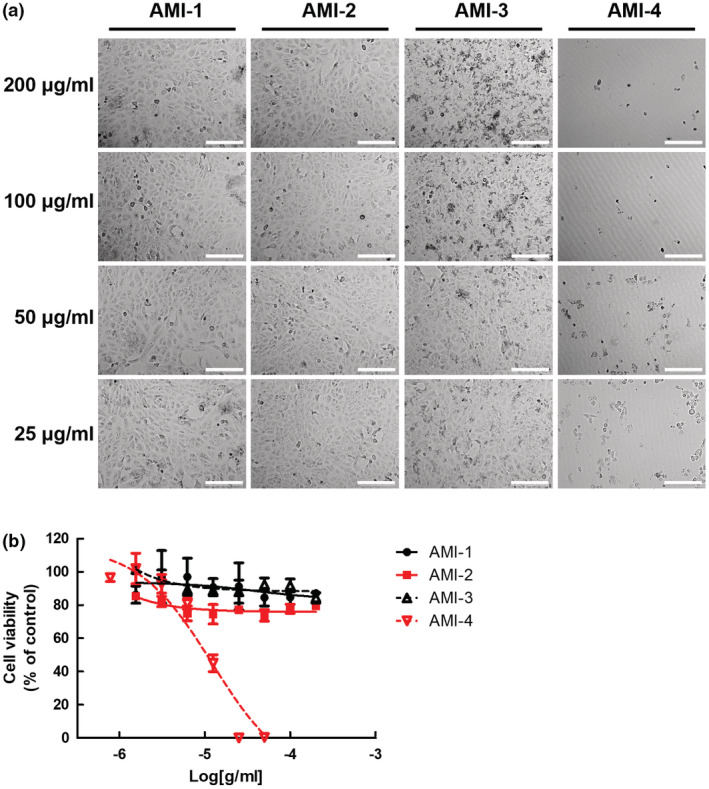
Cytotoxicity of chitosan‐based substances on cultured cells. (a) Morphology of chitosan‐based substances and ivermectin treated cells. Phase‐contrast microscopy images were obtained from Vero E6 cells treated with indicated concentrations (25–200 μg/ml) of AMI‐1 to −4 for 24 h. Representative single optical sections are shown. Scale bars, 200 μm. (b) Measurement of cytotoxicity of cells treated with chitosan‐based substances and ivermectin. MTT assay of Vero E6 cells treated with indicated concentration (25–200 μg/ml) of AMI‐1 to −4 for 24 h. Data are represented as mean ± SD.
Antiviral effects of chitosan‐based substances on SARS‐CoV‐2
The SARS‐CoV‐2 virus was treated with 50 μg/ml of the substances for 1 h and then infected to Vero cells to validate the antiviral effects of chitosan‐based substances. Viral RNA was extracted from the infected cells medium after two days post‐infection and was analysed with quantitative PCR to detect SARS‐CoV‐2 genes (Figure 3a–c). By targeting the RdRp gene (RNA‐dependent RNA polymerase) involved in RNA viral genome synthesis and the E gene (Envelope protein) involved in the component of the virus envelope, the Ct values of these genes were quantified using SARS‐CoV‐2 diagnosis kit (STANDARD M nCoV Real‐Time Detection kit, SD Biosensor). As a result, the RdRp gene and E gene were not detected in Vero cells infected with a mixture of SARS‐CoV‐2 virus and AMI‐2, and the RdRp gene was not detected in the case of AMI‐4. Next, the antiviral effect of AMI‐2 on the ability of the SARS‐CoV‐2 virus to generate a cytopathic effect was verified through plaque assay. As a result of observing plaque formation by treatment with two different concentrations of AMI‐2 (25 and 12.5 μg/ml), it was observed that plaques were not formed by the SARS‐CoV‐2 virus when AMI‐2 was treated at a concentration of 25 μg/ml (Figure 3e). Considering the cytotoxicity test results, we regard that AMI‐2 has an antiviral effect on SARS‐CoV‐2 among the substances used for viral RNA quantitation and plaque formation.
FIGURE 3.
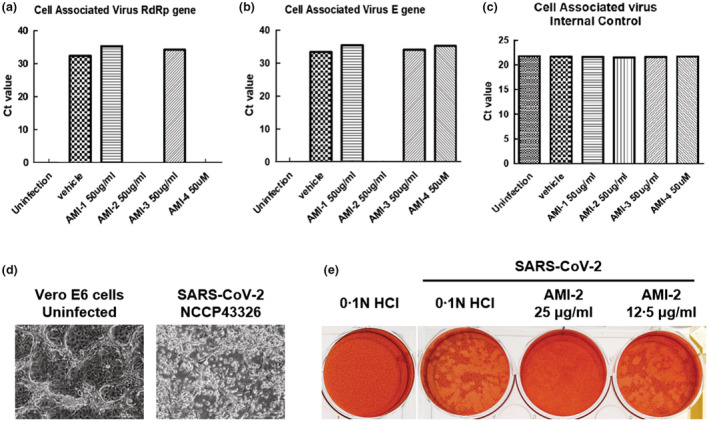
Effects of chitosan‐based substances on SARS‐CoV‐2. (a–c) Antiviral effect of chitosan‐based substances on SARS‐CoV‐2 through qPCR analysis. The amount of SARS‐CoV‐2 virus at MOI 0.01 was incubated with 50 μg/ml of chitosan‐based substances (AMI‐1, −2, and −3) or 50 μM of AMI‐4 for 1 h at room temperature, infected with Vero E6 cells, and incubated with the substances for 48 h. Viral RNA was extracted from the culture medium of the cells, and the SARS‐CoV‐2 gene was detected through qPCR analysis. The graph of Ct value was represented to (a) RNA‐dependent RNA polymerase (RdRP) gene, (b) envelope (E) gene of SARS‐CoV‐2 and (c) internal control of qPCR kits, respectively. (d) The cytopathic effect of SARS‐CoV‐2 on Vero E6 cells. The images of cytopathic effects of Vero E6 cells infected with SARS‐CoV‐2 (NCCP43326) for 4 days were obtained by phase‐contrast microscopy images. Representative single optical sections are shown. Scale bars, 100 μm. (e) Plaque assay of SARS‐CoV‐2 infected Vero E6 cells. After 1 h of the virus within indicated concentration of AMI‐2 adsorption, low‐melting agar containing first overlays were added. Secondary overlays were added after 5 days, and the cells were incubated overnight. Use a white‐light transilluminator (light box) to aid in visualize the plaques.
Dose‐dependent antiviral effect of chitooligosaccharide
Next, dose‐dependent antiviral efficacy of AMI‐2 was confirmed by observation through immunofluorescence staining of the nucleocapsid protein (NP) produced in host cells with the SARS‐CoV‐2 virus. After infecting the SARS‐CoV‐2 virus with three different concentrations in the range of 25–100 μg/ml of AMI‐2 for 36 h, the expression of SARS‐CoV‐2 NP was observed by immunofluorescence microscopy. As shown in Figure 4, the AMI‐2 decreased the expression of SARS‐CoV‐2 NP of the infected cells in a dose‐dependent manner (Figure 4a). As a result of analysing the fluorescence intensity, the expression of NP was reduced by 46.66 ± 2.70% at the 25 μg/ml of AMI‐2, 92.02 ± 7.25% at the 50 μg/ml, and 95.43 ± 1.51% at the 100 μg/ml of AMI‐2, respectively (Figure 4b). Taken together, the chitooligosaccharide, a chitosan‐based substance, exhibited antiviral effects against the SARS‐CoV‐2 virus in vitro, confirming that these effects were correlated with concentration dependence.
FIGURE 4.
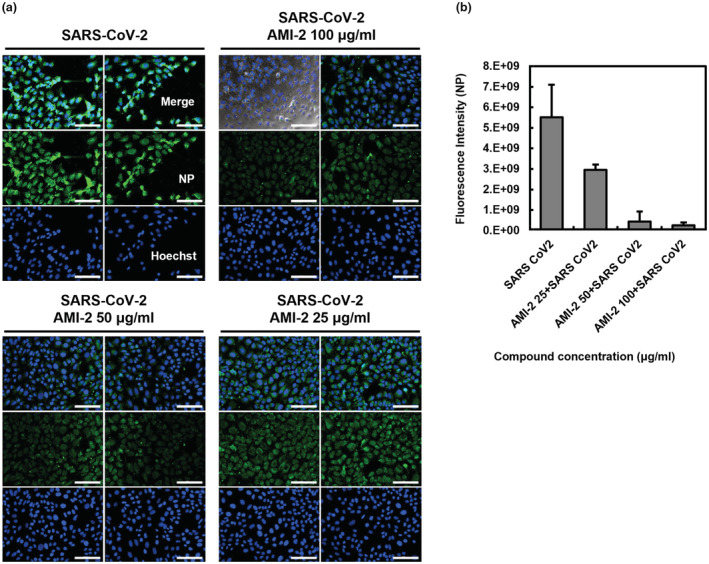
Dose‐dependent antiviral effect of chitooligosaccharide on SARS‐CoV‐2 infected Vero cells. (a, b) Indirect immunofluorescence images of nucleocapsid protein (NP) in SARS‐CoV‐2 infected Vero E6 cells. Vero E6 cells were infected with SARS‐CoV‐2 at MOI 0.01 within indicated concentrations of the AMI‐2 for 36 h. The infected cells were fixed and stained with anti‐nucleocapsid protein antibody followed by Alexa 488‐labelled antibody and analysed by fluorescence microscopy. The nuclei were stained with Hoechst dye. (a) Representative two different single optical sections are shown. Scale bars, 100 μm. (b) The bar graph was converted from the fluorescence intensity of the images. Data are represented as mean ± SD.
Antiviral effect on SARS‐CoV‐2 by the molecular weight of chitooligosaccharide
Finally, the expression of the viral gene and plaque‐forming unit were quantified to investigate the antiviral effect of chitooligosaccharides by molecular weight on SARS‐CoV‐2. The chitooligosaccharides of various molecular weights (2, 10, 30 and 50 kDa) were used in the experiment to confirm the inhibitory effect on SARS‐CoV‐2 replication according to molecular weight (Figure 5a). The virus was reacted with a substance at a concentration of 25 μg/ml for 1 h, then infected with Vero cells to investigate the impact of chitooligosaccharides on the inhibition of SARS‐CoV‐2 replication by molecular weight. The viral RNA was extracted from the infected cells 2 days post‐infection and analysed with quantitative PCR to detect SARS‐CoV‐2 Rdrp and E genes. As a result of analysing the quantitative PCR, the expression of the Rdrp gene was reduced by 43.19% ± 28.81% at the chitooligosaccharide 2 K, 91.04% ± 5.32% at the chitooligosaccharide 30 K, and 60.76% ± 10.53% at the chitooligosaccharide 50 K, respectively (Figure 5b). The E gene expression was reduced by 50.61% ± 27.94% at the chitooligosaccharide 2 K, 83.24% ± 11.87% at the chitooligosaccharide 30 K, and 66.00% ± 3.74% at the chitooligosaccharide 50 K, respectively. In the case of chitooligosaccharide 10 K, it did not significantly reduce the gene expression of SARS‐CoV‐2 (Rdrp gene; 21.86% ± 15.52% reduced, E gene; 25.08% ± 23.80% reduced). Next, plaque formation was quantified to investigate whether chitooligosaccharide 30 K effectively inhibited the viral infectivity of SARS‐CoV‐2. The virus was reacted with 200 μg/ml of chitooligosaccharide 30 K, then infected with Vero cells, and plaque assay was performed. As a result of the plaque‐forming unit measure, the plaque formation was reduced by 30.42% ± 11.88% at chitooligosaccharide 30 K (Figure 5c). Together, these results show that the chitooligosaccharide with a range of specific molecular weight has higher antiviral activity on SARS‐CoV‐2 in vitro than others.
FIGURE 5.
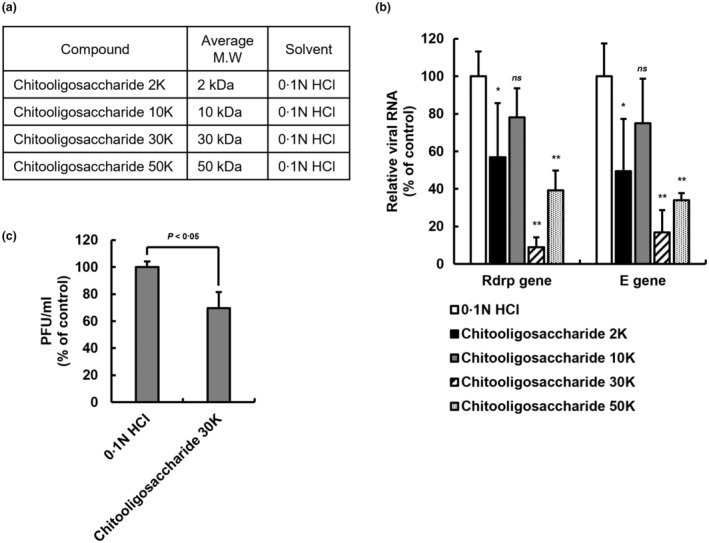
Antiviral effect of chitooligosaccharides of various molecular weights on SARS‐CoV‐2. (a) The chitooligosaccharides of various molecular weights. Separation by molecular weight to confirm the inhibitory effect of chitooligosaccharide on SARS‐CoV‐2 virus replication. (b) Antiviral effect of chitooligosaccharides on SARS‐CoV‐2 through qPCR analysis. The amount of SARS‐CoV‐2 virus at MOI 0.01 was incubated with 25 μg/ml of chitooligosaccharides (2, 10, 30 and 50 kDa) for 1 h at room temperature, infected with Vero E6 cells, and incubated with the chitooligosaccharides for 48 h. Viral RNA was extracted from the culture medium of the cells, and the SARS‐CoV‐2 gene was detected through qPCR analysis. The graph of relative viral RNA was represented to RdRP gene and E gene of SARS‐CoV‐2. Data are represented as mean ± SD (n = 5, ns. not significant, *p < 0.05, **p < 0.001, two‐way ANOVA). (c) Plaque assay of SARS‐CoV‐2 infected Vero E6 cells. After 1 h of the virus within 200 μg/ml of chitooligosaccharide 30 kDa adsorption, low‐melting agar containing overlays were added. After 3 days, the cells were fixed and stained with crystal violet. The plaque forming unit is calculated by plaque number counting. Data are represented as mean ± SD (n = 3, p < 0.05, Student's t test).
DISCUSSION
Antimicrobial effects of chitosan, chitooligosaccharide or modified compounds derived from chitin against bacteria and viruses has already been studied (Liaqat & Eltem, 2018; Lodhi et al., 2014; Tachaboonyakiat, 2017). Among them, it is suggested that chitosan‐derived compounds were proposed as natural antiviral reagents from the results of studies showing that antiviral effects on several types of viruses, such as human immunodeficiency virus (HIV), influenza A and porcine epidemic diarrhoea virus (PEDV) (Artan et al., 2010; Kim et al., 2021; Zheng et al., 2016). It is suggested for the first time in this study that chitosan‐based substances such as chitooligosaccharide can have antiviral efficacy against SARS‐CoV‐2 in vitro. The application of these chitosan‐based substances can be expected in the natural treatment for COVID‐19, which is currently an epidemic worldwide (Jaber et al., 2021; Safarzadeh et al., 2021; Sharma et al., 2021).
Commonly called low molecular weight chitosan, the molecular weight is reduced by shortening the D‐glucosamine bond through an enzymatic reaction or chemical reaction with high molecular weight chitosan (El Knidri et al., 2018). Although the exact mechanism of the antibacterial and antiviral effects of low molecular weight chitosan and chitooligosaccharide is not fully understood, the antibacterial and antiviral action of chitosan on microorganisms suggests a mechanism in which the leakage of intracellular constituents of microorganisms is induced by changing the cell membrane permeability through the interaction of positively charged chitosan molecules with the negatively charged cell membrane of microorganisms (Kong et al., 2010; Kumirska et al., 2011; Nagy et al., 2011). A recent study predicts that the disinfectant feature of chitosan against other types of coronavirus will also be a neutralizing effect (Kim et al., 2021). In this study, the viruses were reacted with chitosan‐based substances in vitro at room temperature for 1 h before infecting the cells. This experimental design includes a part of the host cell's immune response and the virus‐neutralizing effect by the chitosan‐based substances. Recently, a molecular structure study suggested that the receptor‐binding domain of spike protein, which plays a role in the primary infection mechanism of the SARS‐CoV‐2 virus, and chitosan derivatives can interact with a high binding affinity of the ligands (Modak et al., 2021). In particular, their findings are encouraging that chitosan derivatives are considered as anti‐SARS‐CoV‐2 treatments because they have low molecular weight, and it is the advantage of being able to work against the structural features of spike proteins of B.1.7 (UK) and P.1 (Brazil) SARS‐CoV‐2 variants as well as wild type SARS‐CoV‐2. The experimental results we present in this study seem to partially corroborate the previous molecular structural predictions.
The main factors determining the average molecular weight of chitosan and chitooligosaccharides are the number of chitosanases added to the enzymatic reaction and the hydrolysis time during the manufacturing process (Jeon et al., 2001; Qin et al., 2004). Previous studies showed differences in antifungal, antibacterial, and antitumor effects according to the difference in average molecular weight of chitosan and chitooligosaccharides (Jeon et al., 2001; Kendra & Hadwiger, 1984; Omura et al., 2003). In the present study, the chitooligosaccharides with a specific molecular weight seemed suitable for antiviral activity against SARS‐CoV‐2 because chitooligosaccharides with 30 kDa of molecular weight were significantly superior to others. In addition, further study is needed to investigate how chitooligosaccharides of a specific molecular weight can have a higher antiviral effect than substances of other molecular weights.
In the case of ivermectin, one of the substances used in this study, as an FDA‐approved drug, it was proposed as a candidate drug capable of inhibiting the replication of SARS‐CoV‐2 in vitro (Bello, 2021; Caly et al., 2020). However, when the cytotoxicity of ivermectin was measured in vitro using Vero, A549 and NCI‐H358 cells in the present study, it appeared to have relatively high cytotoxicity compared to natural treatment candidates such as chitosan‐based substances (Figure 2b and Figure S1). Previous studies have observed that ivermectin inhibits cell proliferation, arrests the cell cycle through DNA damage, and induces apoptosis via the mitochondrial pathway in vitro (Zhang et al., 2019). Considering the low cytotoxicity and antiviral ability of the chitosan‐based substances identified in this study, we suggest that a natural treatment through the development of chitosan‐derived substances will be suitable for infectious viral pathogens.
Our findings suggest the chitooligosaccharide may be a potent natural treatment for COVID‐19. Additional studies will be needed to verify its effect by molecular weight in more detail. Furthermore, demonstration of its stability and antiviral ability in animal models is needed to develop a harmless treatment using chitooligosaccharide for infectious diseases such as COVID‐19.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest. Through Amicogen (Republic of Korea), the authors have filed patent applications on the chitosan‐derivates substances. The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Supporting information
Figure S1
ACKNOWLEDGEMENTS
Experiments with live SARS‐CoV‐2 virus were conducted at the biosafety level 3 laboratory in Masan Nation Tuberculosis Hospital after obtaining permission from the institutional biosafety committee. The pathogen resource (NCCP43326) for this study was provided from National Culture Collection for Pathogens (NCCP). Gel permeation chromatography of chitosan‐based substances was performed with assistance from Amicogen (Republic of Korea). This work was supported by a Masan National TB Hospital (14‐69‐691). D Jang is a Basic Science Research Program recipient through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (No. 2020R1A6A3A01099841).
Jang, D. , Lee, D. , Shin, Y.C. , Lee, J.S. , Jung, J. & Ryoo, S. (2022) Low molecular weight chitooligosaccharide inhibits infection of SARS‐CoV‐2 in vitro. Journal of Applied Microbiology, 00, 1–10. Available from: 10.1111/jam.15618
Donghwan Jang and Dagyum Lee contributed equally to this work.
REFERENCES
- Artan, M. , Karadeniz, F. , Karagozlu, M.Z. , Kim, M.M. & Kim, S.K. (2010) Anti‐HIV‐1 activity of low molecular weight sulfated chitooligosaccharides. Carbohydrate Research, 345, 656–662. [DOI] [PubMed] [Google Scholar]
- Bello, M. (2021) Elucidation of the inhibitory activity of ivermectin with host nuclear importin α and several SARS‐CoV‐2 targets. Journal of Biomolecular Structure & Dynamics, 10, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly, L. , Druce, J.D. , Catton, M.G. , Jans, D.A. & Wagstaff, K.M. (2020) The FDA‐approved drug ivermectin inhibits the replication of SARS‐CoV‐2 in vitro. Antiviral Research, 178, 104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee, S.K. , Saha, S. & Munoz, M.N.M. (2020) Molecular pathogenesis, immunopathogenesis and novel therapeutic strategy against COVID‐19. Frontiers in Molecular Biosciences, 7, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, R.C. , Ng, T.B. , Wong, J.H. & Chan, W.Y. (2015) Chitosan: an update on potential biomedical and pharmaceutical applications. Marine Drugs, 13, 5156–5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, Y.J. , Kim, E.J. , Piao, Z. , Yun, Y.C. & Shin, Y.C. (2004) Purification and characterization of chitosanase from Bacillus sp. strain KCTC 0377BP and its application for the production of chitosan oligosaccharides. Applied and Environmental Microbiology, 70, 4522–4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, H.C. , Nguyen, V.G. , Kim, C.U. , Do, H.Q. , Park, B.K. , Park, Y.H. et al. (2021) Application of nano‐graphene oxide as nontoxic disinfectant against alpha and betacoronaviruses. Veterinary Medicine and Science, 7, 2434–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drożdżal, S. , Rosik, J. , Lechowicz, K. , Machaj, F. , Szostak, B. , Przybyciński, J. et al. (2021) An update on drugs with therapeutic potential for SARS‐CoV‐2 (COVID‐19) treatment. Drug Resist Update, 59, 100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Knidri, H. , Belaabed, R. , Addaou, A. , Laajeb, A. & Lahsini, A. (2018) Extraction, chemical modification and characterization of chitin and chitosan. International Journal of Biological Macromolecules, 120, 1181–1189. [DOI] [PubMed] [Google Scholar]
- Fernandes, J.C. , Spindola, H. , De Sousa, V. , Santos‐Silva, A. , Pintado, M.E. , Malcata, F.X. et al. (2010) Anti‐inflammatory activity of chitooligosaccharides in vivo. Marine Drugs, 8, 1763–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, A.J. , Phan, J. , Schairer, D.O. , Champer, J. , Qin, M. , Pirouz, A. et al. (2013) Antimicrobial and anti‐inflammatory activity of chitosan‐alginate nanoparticles: a targeted therapy for cutaneous pathogens. The Journal of Investigative Dermatology, 133, 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg, U. , Chauhan, S. , Nagaich, U. & Jain, N. (2019) Current advances in chitosan nanoparticles based drug delivery and targeting. Advanced Pharmaceutical Bulletin, 9, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavriatopoulou, M. , Ntanasis‐Stathopoulos, I. , Korompoki, E. , Fotiou, D. , Migkou, M. , Tzanninis, I.G. et al. (2021) Emerging treatment strategies for COVID‐19 infection. Clinical and Experimental Medicine, 21, 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 395, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izda, V. , Jeffries, M.A. & Sawalha, A.H. (2021) COVID‐19: a review of therapeutic strategies and vaccine candidates. Clinical Immunology, 222, 108634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber, N. , Al‐Remawi, M. , Al‐Akayleh, F. , Al‐Muhtaseb, N. , Al‐Adham, I.S.I. & Collier, P.J. (2021) A review of the antiviral activity of Chitosan, including patented applications and its potential use against COVID‐19. Journal of Applied Microbiology, 132, 41–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans, D.A. & Wagstaff, K.M. (2021) The broad spectrum host‐directed agent ivermectin as an antiviral for SARS‐CoV‐2? Biochemical and Biophysical Research Communications, 538, 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon, Y.J. , Park, P.J. & Kim, S.K. (2001) Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carbohydrate Polymers, 44, 71–76. [Google Scholar]
- Kendra, D.F. & Hadwiger, L.A. (1984) Characterization of the smallest chitosan oligomer that is maximally antifungal to Fusarium solani and elicits pisatin formation in Pisum sativum. Experimental Mycology, 8, 276–281. [Google Scholar]
- Kim, S.J. , Nguyen, V.G. , Kim, C.U. , Park, B.K. , Huynh, T.L. , Shin, S. et al. (2021) Application of chitosan as a natural disinfectant against porcine epidemic diarrhoea virus. Acta Veterinaria Hungarica, 69, 94–99. [DOI] [PubMed] [Google Scholar]
- Kong, M. , Chen, X.G. , Xing, K. & Park, H.J. (2010) Antimicrobial properties of chitosan and mode of action: a state of the art review. International Journal of Food Microbiology, 144, 51–63. [DOI] [PubMed] [Google Scholar]
- Kumirska, J. , Weinhold, M.X. , Thöming, J. & Stepnowski, P. (2011) Biomedical activity of chitin/chitosan based materials—influence of physicochemical properties apart from molecular weight and degree of N‐acetylation. Polymers, 3, 1875–1901. [Google Scholar]
- Liaqat, F. & Eltem, R. (2018) Chitooligosaccharides and their biological activities: a comprehensive review. Carbohydrate Polymers, 184, 243–259. [DOI] [PubMed] [Google Scholar]
- Lodhi, G. , Kim, Y.S. , Hwang, J.W. , Kim, S.K. , Jeon, Y.J. , Je, J.Y. et al. (2014) Chitooligosaccharide and its derivatives: preparation and biological applications. BioMed Research International, 2014, 654913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmouzi, I. , Ezzat, S.M. , Salama, M.M. , Merghany, R.M. , Attar, A.M. , El‐Desoky, A.M. et al. (2019) Recent updates in pharmacological properties of chitooligosaccharides. BioMed Research International, 2019, 4568039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza, E.J. , Manguiat, K. , Wood, H. & Drebot, M. (2020) Two detailed plaque assay protocols for the quantification of infectious SARS‐CoV‐2. Current Protocols in Microbiology, 57, ecpmc105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modak, C. , Jha, A. , Sharma, N. & Kumar, A. (2021) Chitosan derivatives: a suggestive evaluation for novel inhibitor discovery against wild type and variants of SARS‐CoV‐2 virus. International Journal of Biological Macromolecules, 187, 492–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed, M.A. , Syeda, J.T.M. , Wasan, K.M. & Wasan, E.K. (2017) An overview of chitosan nanoparticles and its application in non‐parenteral drug delivery. Pharmaceutics, 20, 9–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy, A. , Harrison, A. , Sabbani, S. , Munson, R.S. , Dutta, P.K. & Waldman, W.J. (2011) Silver nanoparticles embedded in zeolite membranes: release of silver ions and mechanism of antibacterial action. International Journal of Nanomedicine, 6, 1833–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura, Y. , Shigemoto, M. , Akiyama, T. , Saimoto, H. , Shigemasa, Y. , Nakamura, I. et al. (2003) Antimicrobial activity of chitosan with different degrees of acetylation and molecular weights. Biocontrol Science, 8, 25–30. [Google Scholar]
- Parums, D.V. (2022) Editorial: current status of oral antiviral drug treatments for SARS‐CoV‐2 infection in non‐hospitalized patients. Medical Science Monitor, 28, e935952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, C. , Zhou, B. , Zeng, L. , Zhang, Z. , Liu, Y. , Du, Y. et al. (2004) The physicochemical properties and antitumor activity of cellulase‐treated chitosan. Food Chemistry, 84, 107–115. [Google Scholar]
- Rai, N.K. , Ashok, A. & Akondi, B.R. (2020) Consequences of chemical impact of disinfectants: safe preventive measures against COVID‐19. Critical Reviews in Toxicology, 50, 513–520. [DOI] [PubMed] [Google Scholar]
- Rothe, C. , Schunk, M. , Sothmann, P. , Bretzel, G. , Froeschl, G. , Wallrauch, C. et al. (2020) Transmission of 2019‐nCoV infection from an asymptomatic contact in Germany. New England Journal of Medicine, 382, 970–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safarzadeh, M. , Sadeghi, S. , Azizi, M. , Rastegari‐Pouyani, M. , Pouriran, R. & Haji Molla Hoseini, M. (2021) Chitin and chitosan as tools to combat COVID‐19: a triple approach. International Journal of Biological Macromolecules, 183, 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez, Á. , Mengíbar, M. , Fernández, M. , Alemany, S. , Heras, A. & Acosta, N. (2018) Influence of preparation methods of chitooligosaccharides on their physicochemical properties and their anti‐inflammatory effects in mice and in RAW264.7 macrophages. Marine Drugs, 16, 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, N. , Modak, C. , Singh, P.K. , Kumar, R. , Khatri, D. & Singh, S.B. (2021) Underscoring the immense potential of chitosan in fighting a wide spectrum of viruses: a plausible molecule against SARS‐CoV‐2? International Journal of Biological Macromolecules, 179, 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suneeta, K. & Rupak, K. (2020) Chitin and chitosan: origin, properties, and applications. In: Handbook of chitin and chitosan, England, Elsevier. pp. 1‐33. [Google Scholar]
- Tachaboonyakiat, W. (2017) Antimicrobial applications of chitosan, England, Woodhead Publishing, 9, 245–274. [Google Scholar]
- Varrassi, G. & Rekatsina, M. (2022) Updates on palliative medicine in the COVID‐19 era. Journal of Clinical Medicine, 11, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P. , Zhang, Y. , Liu, K. , Liu, B. , Xu, W. , Gao, J. et al. (2019) Ivermectin induces cell cycle arrest and apoptosis of HeLa cells via mitochondrial pathway. Cell Proliferation, 52, e12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, M. , Qu, D. , Wang, H. , Sun, Z. , Liu, X. , Chen, J. et al. (2016) Intranasal administration of chitosan against influenza A (H7N9) virus infection in a mouse model. Scientific Reports, 6, 28729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, N. , Zhang, D. , Wang, W. , Li, X. , Yang, B. , Song, J. et al. (2020) A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine, 382, 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


