At the start of the COVID‐19 pandemic, individuals with cystic fibrosis (CF) and their families were encouraged to shield as it was feared SARS‐CoV‐2 infection would have devastating consequences. Over the subsequent 2 years, the low number of symptomatic infections and the small number of CF deaths have reassured patients, clinicians and authorities to relax restrictions. Children in the United Kingdom, including those with CF, are now back in school with few restrictions. We report the distressing case of the death of an adolescent with CF who died following COVID‐19 and Nocardia farcinia infection. We hope this case will highlight the continued risk to individuals with CF posed by COVID‐19.
A 16‐year‐old boy with CF was hospitalized after a short history of pyrexia, breathlessness, cough, and lethargy. He tested positive for SARS‐CoV‐2 on a polymerase chain reaction (PCR) test 2 days earlier after being the household contact of a confirmed case. His CF diagnosis (homozygous Phe508del) was made in infancy due to failure to thrive. He had moderate bronchiectasis and chronic Pseudomonas aeruginosa infection. His lung function had deteriorated through adolescence but he had an excellent response to elexacaftor–tezacaftor–ivacaftor with forced expiratory volume in one second increasing from 43% to 106% over 6 months.
On admission, he was tachycardic (149 beats/min), tachypnoeic (52 breaths/min), pyrexial (39.0oC), and hypoxic (requiring 8 L/min oxygen to maintain SpO2 ≥ 92%). He had increased work of breathing including tracheal tug and was struggling to speak in full sentences. Auscultation revealed coarse bilateral crackles and decreased air entry on the left posteriorly. Abdominal examination was normal. Investigations revealed neutrophilia (9.60 × 109/L), lymphopenia (0.40 × 109/L), elevated C‐reactive protein (370 mg/L), and hyponatraemia (127 mEq/L). A repeat SARS‐CoV‐2 PCR was positive and chest radiography showed dense left lower lobe (LLL) opacification (Figure 1A). He was commenced on IV tobramycin, IV ceftazidime, and IV dexamethasone. Tocilizumab was discussed but not started due to concerns regarding worsening pneumonia or sepsis.
Figure 1.
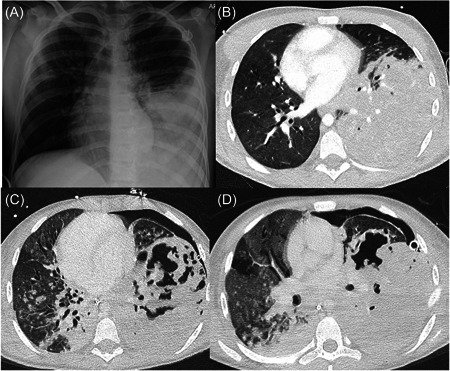
(A) Chest x‐ray on Day 1 of admission showing left lower lobe consolidation. A totally implantable venous access device is also visible. (B) High‐resolution computed tomography (CT) chest on Day 4 of admission showing dense consolidation of the left lower lobe with minimal aerated parenchyma. Right‐sided bronchiectasis also present. (C and D) Repeat high‐resolution CT chest scans undertaken on days 13 and 19 showing left lower lobe necrosis and cavitation. A left‐sided pneumothorax is also seen (different size drains are seen on the two scans).
Over the next 24 h his work of breathing increased and he developed acute confusion which persisted despite management of his hypoxia with noninvasive ventilation. He was therefore intubated and underwent brain magnetic resonance imaging (MRI) and computerized tomography (CT) pulmonary angiogram. The MRI did not show encephalitis, ischemia, thrombus, or hemorrhage. The CT revealed dense LLL consolidation but no necrosis or pulmonary embolus (Figure 1B). Over the next 6 days, he remained intubated requiring increasing ventilatory support as well as nitric oxide, proning and insertion of a chest drain for pneumothorax. Serial lower airway samples isolated N. farcinia prompting his antibiotic regimen to be changed to meropenem and cotrimoxazole. He had not previously isolated this organism. He intermittently required fluid boluses and inotropes for hypotension. Although they did not all occur simultaneously, he developed features of the pediatric multisystem inflammatory syndrome (PIMS) including elevated ferritin (4977 ng/ml), fibrinogen (>7 g/L), lactate dehydrogenase (467 U/L), N‐terminal pro‐B‐type natriuretic peptide (562 ng/L), d‐dimer (4977 ng/ml), and Troponin I (185.5 ng/L). His case was discussed with the regional PIMS multidisciplinary team (MDT), who did not think any additional treatment should be started. On Day 12, due to continued instability and high ventilation requirements, he was transferred to another pediatric ICU for consideration of extracorporeal membranous oxygenation (ECMO) as an escalation of treatment and a possible bridge to transplant.
A further chest CT showed the development of necrosis and cavitation in the LLL (Figure 1C) with the persistence of the left pneumothorax. The ECMO MDT continued conventional ventilation and performed video‐assisted thoracoscopic surgery with debridement and drainage of the left hemithorax. Linezolid was commenced. During the following 5 days, he remained on high pressures and nitric oxide. He did not tolerate prone positioning due to cardiovascular instability. Despite sedation, he was able to open his eyes on command. His clinical condition then deteriorated over the following 3 days. He became unresponsive and his oxygen saturation and blood gases worsened. His CT head remained normal but CT chest (Figure 1D) again showed LLL necrosis. The ECMO MDT agreed additional treatment would be futile and care should be withdrawn. The family were in agreement and he died peacefully 20 days after admission to the hospital, and 22 days after testing positive for COVID‐19.
Individuals with CF have not been as badly affected by COVID‐19 as was first feared. As of January 2023, there had been 1394 cases of COVID‐19 reported to the UK CF Registry (approximately 13% of the UK CF population). 1 Seven percent were hospitalized and there had been 10 deaths. 1 The mild illness experienced by most children with COVID‐19, including those with the respiratory disease, has resulted in those with CF no longer being defined as clinically extremely vulnerable. As such, they do not have to shield and are attending school as normal. In contrast, adults with CF in the United Kingdom are still classed as extremely clinically vulnerable.
Necrotizing pneumonia (NP) is a rare complication of community‐acquired pneumonia. Parenchymal cell death caused by ischemia and microbial toxins results in abscess formation, cavitation and in severe cases, pulmonary gangrene. The high mortality relates to sepsis and respiratory failure. Although not common, there are several case series making the association between severe COVID‐19 lung disease and the development of lung abscesses, 2 NP, 3 and cavitation. 4 These cases are most commonly caused by Staphylococcus aureus, Klebsiella pneumonia, or P. aeruginosa. 2 , 3 , 4 It is notable that the underlying pathology of COVID‐19 pneumonitis and the parenchymal ischemia of NP both include vasculitis and microthrombi. Nocardiosis is the term used to describe NP caused the Nocardia species. 5 These are a group of opportunistic, gram‐positive, branching filamentous bacilli known cause a range of local and disseminated infections usually in immunocompromised hosts. 5 N. farcinia is one of this group which has also been reported in individuals with CF.
To our knowledge, this is the first published case of NP associated with COVID‐19 in an individual with CF and the first associated with Nocardia infection. We suspect the combination of cystic fibrosis, COVID‐19 pneumonitis and co‐infection with N. farcinia caused this young man's NP and ultimately his untimely death. We hope this case will highlight individuals with CF of all ages are at risk of severe COVID‐19 infection.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
We would like to thank the family of this young man for agreeing to the publication of this case report.
Driscoll S, Carroll WD, Nichani S, Fishwick R, Bakewell K, Gilchrist F. COVID‐19 infection and nocardiosis causing the death of an adolescent with cystic fibrosis. Pediatric Pulmonology. 2022;57:1823‐1825. 10.1002/ppul.25954
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Cystic Fibrosis Trust . COVID‐19 in people with cystic fibrosis; 2021. https://www.cysticfibrosis.org.uk/the-work-we-do/uk-cf-registry/reporting-and-resources/covid-19-in-people-with-cf
- 2. Beaucoté V, Plantefève G, Tirolien J‐A, Desaint P, Fraissé M, Contou D. Lung abscess in critically ill coronavirus disease 2019 patients with ventilator‐associated pneumonia: a french monocenter retrospective study. Critical Care Explorations. 2021;3:e0482. 10.1097/CCE.0000000000000482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hidron A, Quiceno W, Cardeño JJ, Roncancio G, García C. Post‐COVID‐19 necrotizing pneumonia in patients on invasive mechanical ventilation. Infect Dis Rep. 2021;13:835‐842. 10.3390/idr13030075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zoumot Z, Bonilla M‐F, Wahla AS, et al. Pulmonary cavitation: an under‐recognized late complication of severe COVID‐19 lung disease. BMC Pulm Med. 2021;21:24. 10.1186/s12890-020-01379-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87:403‐407. 10.1016/j.mayocp.2011.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


