Dear Editor,
One of the hallmarks of COVID‐19, is an abnormal coagulation response, characterized by marked coagulopathy and the presence of systemic microvascular injury and thrombosis. 1 There is a high incidence (31%) of thrombotic complications despite thromboprophylaxis. 2 Recent studies suggest that fibrinolysis may be an important contributor to the hypercoagulable state in COVID‐19. 3 , 4 A study by Zuo et al. found markedly elevated tissue‐type plasminogen activator (tPA) and plasminogen activator inhibitor‐1 (PAI‐1) in COVID‐19 with extremely high levels associated with mortality. 3 We aimed to investigate how fibrinolysis may be affected in COVID‐19 patients by using the overall hemostatic potential (OHP) assay.
The first available citrated samples requested for clinical indications, of consecutive patients with confirmed COVID‐19 admitted to Northern Hospital, a tertiary hospital, between March and September 2020, were collected. The samples were centrifuged at 2000G for 10 min to obtain platelet‐poor plasma (PPP), stored at −80°C and batch‐tested for OHP, a spectrophotometric assessment of fibrin‐aggregation (see below), providing whole of fibrinolytic system analysis. 5 The results were correlated to the clinical evolution of the patients, obtained from medical records, and compared to previously collected healthy controls 6 as well as random samples obtained from non‐COVID‐19 patients with sepsis with C‐reactive protein (CRP) greater than 100 mg/L. Samples of patients on therapeutic anticoagulation were excluded from the OHP analysis. The project has ethics approval from the Alfred Hospital Ethics Committee (Project ID 67614). Given surplus status of PPP, a waiver of consent was allowed.
OHP assay: Seventy‐five microlitre of thawed PPP was added to wells with 75 μl of buffer containing either (i) Tris, NaCl, CaCl2 (final concentration 66 nM Tris, 130 mM NaCl, 35 ml CaCl2; pH 7.0) and thrombin (0.006 IU/ml) to generate the overall coagulation potential (OCP) or (ii) Tris, NaCl, CaCl2, thrombin and tPA (600 ng/ml) to generate the OHP. The two fibrin‐aggregation curves (OCP and OHP) are calculated from the FLUOstar Optima (BMG Labtech) plate reader at 405 nM. The difference between the area underneath the two curves gives the OFP.
Statistical analysis: Analysis of data was performed using Stata/IC 17.0. Normally distributed continuous variables were reported as mean and standard deviation (SD), and non‐normally distributed variables were reported as median and interquartile range (IQR). For univariate analysis, the t‐test was used for normally distributed variables and Mann–Whitney U‐test for skewed continuous variables. For multivariate analysis, where possible, skewed variables were transformed into a normal distribution before linear regression was performed. For variables that were right skewed, Poisson regression was conducted for variables with count data. Both these models included age and gender, and the significance was set at p < 0.05.
One‐hundred sixteen patients' plasma were available for testing (median age 67 years, 49.1% male, 35 [30.2%] from residential care facilities) (Table 1). The majority of the patients (n = 107, 92.2%) scored 0 to 1 point on the Quick Sequential Organ Failure Assessment (qSOFA), which consists of three components (respiratory rate ≥ 22/min, change in mental status, systolic blood pressure ≤ 100 mmHg). 7 Chest imaging was performed on 101 patients (n = 87.1%) – 89 patients (88.1%) had chest X‐ray while 12 (11.9%) had computed tomography performed. The most common changes seen on imaging were consolidation (n = 50) followed by ground‐glass opacity (n = 14) and pulmonary infiltrates (n = 6). The majority of patients were commenced on prophylactic anticoagulation (Table 1). In terms of laboratory investigations, COVID‐19 patients had reduced median lymphocyte count (0.9 × 109/L), increased LDH (median 2820 U/L), CRP (median 47 mg/L) and ferritin (median 400 μg/L), as well as elevated fibrinogen and D‐dimer. Overall, there were 20 deaths of (including 19 patients assigned goals of care C – forward management only and not for intubation or cardiopulmonary resuscitation [CPR]). Of those with goals of care A and B (patients suitable for intubation +/− CPR, n = 73), 20 patients required ventilatory support (5 high‐flow oxygen via nasal prongs, 2 non‐invasive ventilation, 13 mechanical ventilation).
TABLE 1.
Basic demographics, coagulation studies and OHP of patients with COVID‐19 compared to healthy controls, and the subanalysis comparison of COVID‐19 patients with CRP > 100 mg/L and non‐COVID‐19 patients with CRP > 100 mg/L (median, interquartile range reported unless otherwise stated)
| Healthy controls | COVID‐19 patients | Adjusted p value b | COVID‐19 patients with CRP > 100 mg/L | Non‐COVID‐19 septic patients with CRP > 100 mg/L | Adjusted p value b | |
|---|---|---|---|---|---|---|
| N | 153 | 116 | 22 | 24 | ||
| Age (years) | 39.0 (24.0, 57.0) | 67.0 (50.0, 84.0) | 61.5 (18.3) | 71.0 (15.3) | 0.061 a | |
| Male gender | 55 (35.9%) | 57 (49.1%) | 13 (59.1%) | 12 (50.0%) | 0.54 | |
| Co‐morbidities | ||||||
| Hypertension | 0 | 67 (57.8%) | 14 (63.6%) | 19 (79.2%) | 0.24 | |
| Diabetes mellitus | 0 | 39 (33.6%) | 6 (27.3%) | 9 (37.5%) | 0.46 | |
| Ischemic heart disease | 0 | 21 (18.1%) | 3 (13.6%) | 10 (41.7%) | 0.021 | |
| Anticoagulation | 0.35 | |||||
| None | 153 | 8 (6.9%) | 0 | 5 (20.8%) | ||
| Prophylactic | 0 | 89 (76.7%) c | 19 (86.7%) | 15 (62.5%) | ||
| Intermediate | 0 | 3 (2.6%) c | 0 | 0 | ||
| Therapeutic | 0 | 16 (13.8%) c | 3 (13.6%) | 4 (16.7%) | ||
| Prothrombin time (s) | 11.0 (10.3, 12.4) | 13.0 (12.3, 14.6) | 0.006 | 13.1 (12.0, 14.2) | 15.4 (13.9, 16.1) | 0.020 |
| Activated partial thromboplastin time (s) | 28.2 (26.0, 31.0) | 33.4 (30.9, 37.3) | <0.001 | 34.0 (29.3, 37.3) | 30.6 (27.9, 32.7) | 0.058 |
| Fibrinogen (g/L) | 2.9 (2.5, 3.5) | 5.2 (4.2, 6.2) | <0.001 | 6.3 (5.4, 7.0) | 7.1 (5.8, 7.7) | 0.71 |
| D‐dimer (μg/ml FEU) | 0.2 (0.1, 0.3) | 0.7 (0.4, 1.4) | <0.001 | 1.1 (0.5, 1.8) | 2.7 (1.7, 3.7) | 0.020 |
| Overall hemostatic potential assay (OHP) d | ||||||
| N | 142 | 97 | 18 | 20 | ||
| Overall coagulation potential, OCP (unit), mean (SD) | 35.5 (9.7) | 61.8 (21.7) | <0.001 | 76.4 (21.5) | 81.2 (23.8) | 0.70 |
| Overall hemostatic potential, OHP (unit) | 6.4 (4.8, 9.5) | 16.4 (12.1, 24.3) | <0.001 | 24.5 (19.8, 34.0) | 23.2 (15.4, 28.1) | 0.015 |
| Overall fibrinolytic potential (%) | 81.1 (77.5, 84.1) | 70.2 (63.5, 75.7) | <0.001 | 64.7 (62.1, 70.2) | 73.2 (70.3, 78.9) | <0.001 |
| Maximum optical density | 0.6 (0.5, 0.8) | 1.0 (0.7, 1.3) | <0.001 | 1.3 (1.1, 1.5) | 1.5 (1.1, 1.7) | 0.95 |
| Time to 50% lysis (min) | 7.0 (6.0. 8.0) | 12.0 (10.0, 15.0) | <0.001 | 14.5 (12.0, 19.0) | 8.5 (7.0, 12.0) | <0.001 |
| Lysis ratio | 1.0 (0.9, 1.0) | 0.9 (0.7, 1.0) | <0.001 | 0.7 (0.6, 0.9) | 1.0 (0.9, 1.0) | <0.001 |
Univariate p value.
Adjusted for age and gender.
Types of anticoagulant (i) prophylaxis: 88/89 patients on prophylactic enoxaparin, 1/89 on prophylactic unfractionated heparin; (ii) all three intermediate dose patients were on enoxaparin; (iii) therapeutic: 7/16 patients on warfarin, 6/16 on apixaban, 2/16 on rivaroxaban, 1 on enoxaparin.
OHP results of patients on therapeutic anticoagulation were excluded from this analysis. Not all patients had sufficient plasma for OHP. FEU, fibrinogen equivalent unit; SD standard deviation.
OHP in COVID‐19: Patients with COVID‐19 showed evidence of markedly elevated fibrin generation (OCP and OHP, p < 0.001) with reduced OFP compared to healthy controls (Table 1, Figure 1). Furthermore, on analysis of fibrin generation rate changes, COVID‐19 patients had higher maximum OD and demonstrated a longer time to achieve 50% lysis, maximum lysis and end of lysis when compared to healthy controls. Patients on prophylactic anticoagulation had higher OCP compared to those on no anticoagulation (62.8 vs. 39.3, p = 0.003) and a trend towards higher OHP (19.9 vs. 11.9, p = 0.074) with similar OFP (68.7 vs. 69.0%, p = 0.95). However, it is noted that patients on prophylactic anticoagulation tended to be older and more unwell (lower lymphocyte count, higher CRP, more likely to require ICU admission and have imaging changes).
FIGURE 1.
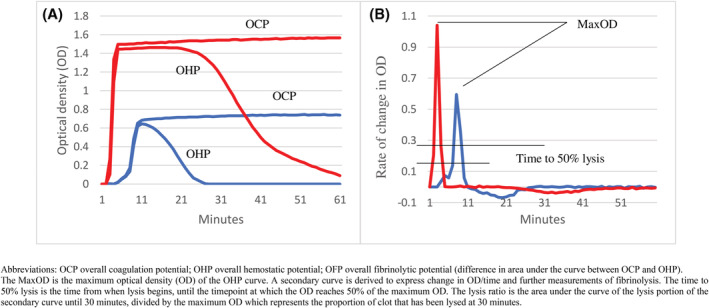
shows an example of OHP (A) trace with secondary curves (B) of a healthy control (solid line) and COVID‐19 patient (dotted line). COVID‐19 patients had higher OCP, OHP with reduced OFP (difference in area under the curve between OCP and OHP). On further analysis, the COVID‐19 patient also demonstrated significantly higher maximum OD with longer time to achieve 50% lysis.
A subanalysis was performed comparing COVID‐19 patients with CRP > 100 mg/L (n = 22) to non‐COVID‐19 septic patients with CRP > 100 mg/L (n = 24) (median CRP 170 vs. 218 mg/L, p = 0.075) (Table 1). There was no difference in the median qSOFA score (p = 0.38). COVID‐19 patients demonstrated significantly increased OHP, lower overall fibrinolytic potential with longer time to achieve 50% lysis and reduced lysis ratio.
OHP and clinical/laboratory outcomes (Table 2): When comparing patients who had the highest OHP (tertile 3) to those with the lowest levels (tertile 1), higher OHP was associated higher neutrophil count (5.8 vs. 3.8 × 109/L, p = 0.020), fibrinogen (6.7 vs. 3.9 g/L, p < 0.001) and biochemistry parameters including lower albumin (30.0 vs. 35.0 g/L, p < 0.001), higher CRP (91.0 vs. 23.0 mg/L, p < 0.001), LDH (345.0 vs. 197.0 U/L, p < 0.001) and ferritin (719.5 vs. 225.5 μg/L, p < 0.001). Higher OHP was also demonstrated in more unwell patients including requirement for ventilatory support (RR 6.2, 95% CI 1.5–25.5) and ICU support (RR 3.8, 95% CI 1.2–12.3).
TABLE 2.
Compares patient outcomes according to tertile levels of OHP (median, interquartile range reported unless otherwise stated)
| OHP tertile 1 (n = 33) | OHP tertile 3 (n = 32) | p Value | |
|---|---|---|---|
| Age, years (mean, SD) | 78.0 (53.0, 87.0) | 62.5 (48.0, 77.5) | 0.20 |
| Male gender | 12 (36.4%) | 18 (56.3%) | 0.27 |
| D‐dimer (μg/ml FEU) | 0.6 (0.3, 1.6) | 1.0 (0.5, 1.5) | 0.020 |
| Tachypnoea (Respiratory rate > 22) (n, %) | 8 (25.0%) | 20 (64.5%) |
0.004 (RR 2.6, 95% CI 1.3–5.0) |
| Consolidation (n, %) | 11 (33.3%) | 21 (67.7%) |
0.011 (RR 2.0, 95% CI 1.1–3.4) |
| Antibiotic use (n, %) | 18 (54.6%) | 27 (84.4%) |
0.013 (RR 1.5, 95% CI 1.1–2.2) |
| Glucocorticoid use (n, %) | 11 (33.3%) | 23 (71.9%) |
0.007 (RR 2.2, 95% CI 1.3–3.7) |
| Thrombosis (n, %) | 0 | 1 (3.2%) | 0.35 |
| Intensive care unit admission (n, %) | 3 (9.1%) | 11 (34.4%) |
0.018 (RR 3.8, 95% CI 1.2–12.3) |
| Ventilatory support (n, %) | 2 (6.1%) | 12 (37.5%) |
0.005 (RR 6.2, 95% CI 1.5–25.5) |
| Death (n, %) | 7 (21.2%) | 6 (19.4%) | 0.20 |
Abbreviations: FEU, fibrinogen equivalent unit; OHP, overall hemostatic potential; SD, standard deviation; RR, relative risk.
This is the first study to utilize OHP to investigate the complex coagulopathy in COVID‐19 patients. This study demonstrated impaired fibrinolysis in COVID‐19 patients as evidenced by the higher OHP (indicating reduced response to tPA) and markedly reduced OFP. While D‐dimer was increased in COVID‐19 patients, it did not appear to be a good discriminator for mortality (p = 0.11) or clinical deterioration (p = 0.090) similar to the findings of Juneja et al. 8 in contrast to other studies. 9 On the other hand, patients with markedly elevated OHP (tertile 3) (Table 2) were more likely to show biochemical and clinical signs of disease severity, suggesting OHP may have the potential to be a predictor of disease severity.
In support of our findings, Juneja et al. found that higher clot lysis time, lower plasminogen levels and higher PAI‐1 may predict mortality in COVID‐19 ICU patients, 8 similar to Zuo et al. who found extremely high levels of tPA predicted mortality. 3 Similarly, Nougier et al. reported higher t‐PA, PAI‐1 and thrombin activatable fibrinolysis inhibitor (TAFI) in ICU patients compared to non‐ICU patients with COVID‐19. 10 One possible explanation is that inflammation and activated platelets can promote local release PAI‐1 from endothelial cells resulting in hypofibrinolysis and fibrin persistence. 11 Despite elevated t‐PA, the patients were hypofibrinolytic suggesting that higher levels of PAI‐1 and TAFI is likely to overwhelm the t‐PA capacity leading to microvascular deposition. 4 These laboratory findings are consistent with postmortem findings of platelet‐fibrin thrombi in small arterial vessels in patients who succumbed to COVID‐19. 1 Medcalf et al. suggested that raised D‐dimer may signify a ‘failing’ fibrinolytic system to remove fibrin and necrotic tissue from the lung parenchyma, being consumed or overwhelmed, and that the fibrinolytic system may be harnessed by SAR‐CoV‐2 to promote infectivity. 12
Fibrinolysis appeared more impaired in COVID‐19 patients compared to the non‐COVID‐19 patients with CRP > 100 mg/L, again indicating that impaired fibrinolysis is an important pathophysiological mechanism in COVID‐19. Bouck et al. reported that while patients with sepsis have delayed plasmin generation, patients with COVID‐19 still had significantly increased plasmin peak and endogenous plasmin potential with hypofibrinolysis in plasma clots. 11 Medcalf et al. hypothesised a theory of plasmin paradox in which plasmin formation can be either advantageous or deleterious depending on timing. 12 Loss of coagulation factors and coagulopathy is typically seen in later stages of severe COVID‐19 compared to sepsis, in which DIC often occurs earlier. Further work is warranted to define how fibrinolysis may differ in COVID‐19.
This study has a number of limitations including that of a retrospective study and the lack of standardization of blood sampling to DVT prophylaxis. While the blood samples for COVID patients were the first available samples on admission, the blood samples for non‐COVID sepsis patients were random samples at points when the CRP was documented to be >100 mg/L although of note, no clinical outcomes were compared between these two cohorts. In addition, we acknowledge the lack of thromboembolic complications and mortality observed in this study, and the relatively small sample size. The relative success of Australia in containing COVID‐19 compared to other countries during this wave may mean patients who were admitted to hospital may not be as critically ill as those in other countries.
In conclusion, COVID‐19 patients demonstrated markedly impaired fibrinolysis, even when compared to unwell non‐COVID‐19 septic patients. Higher OHP appeared to be predictive of worse biochemical parameters, clinical signs, ICU admission and ventilatory support. These findings are suggestive of dysregulation between the coagulation and fibrinolytic pathway which may contribute to the overall hypercoagulable state in COVID‐19. This may provide a framework for future therapeutic interventions.
CONFLICT OF INTEREST
The authors do not have any conflict of interest to disclose.
ACKNOWLEDGEMENTS
We would like to acknowledge all the patients who have contributed samples for this study.
Funding information National Health and Medical Research Council, Grant/Award Number: APP1151535; Heart Foundation Health Professional Scholarship; Dr Lim is a recipient of the co‐funded National Health and Medical Research Council (NHMRC) postgraduate scholarship and Heart Foundation Health Professional Scholarship. This funding, however, is independent of this research work.
DATA AVAILABILITY STATEMENT
Authors elect to not share data.
REFERENCES
- 1. Falasca L, Nardacci R, Colombo D, et al. Postmortem findings in Italian patients with COVID‐19: a descriptive full autopsy study of cases with and without comorbidities. J Infect Dis. 2020;222:1807‐1815. doi: 10.1093/infdis/jiaa578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klok FA, Kruip M, van der Meer N, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. doi: 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zuo Y, Warnock M, Harbaugh A, et al. Plasma tissue plasminogen activator and plasminogen activator inhibitor‐1 in hospitalized COVID‐19 patients. Sci Rep. 2021;11:1580. doi: 10.1038/s41598-020-80010-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meizoso JP, Moore H, Moore EE. Fibrinolysis shutdown in COVID‐19: clinical manifestations, molecular mechanisms, and therapeutic implications. J Am Coll Surg. 2021;232:995‐1003. doi: 10.1016/j.jamcollsurg.2021.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. He S, Antovic A, Blombäck M. A simple and rapid laboratory method for determination of haemostasis potential in plasma: II. Modifications for use in routine laboratories and research work. Thromb Res. 2001;103:355‐361. doi: 10.1016/s0049-3848(01)00332-2 [DOI] [PubMed] [Google Scholar]
- 6. Lim HY, Lui B, Tacey M, et al. Global coagulation assays in healthy controls: are there compensatory mechanisms within the coagulation system? J Thromb Thrombolysis. 2021;52:610‐619. doi: 10.1007/s11239-021-02400-y [DOI] [PubMed] [Google Scholar]
- 7. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis‐3). JAMA. 2016;315:762‐774. doi: 10.1001/jama.2016.0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Juneja GK, Castelo M, Yeh CH, et al. Biomarkers of coagulation, endothelial function, and fibrinolysis in critically ill patients with COVID‐19: a single‐center prospective longitudinal study. J Thromb Haemost. 2021;19:1546‐1557. doi: 10.1111/jth.15327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z. D‐dimer levels on admission to predict in‐hospital mortality in patients with Covid‐19. J Thromb Haemost. 2020;18:1324‐1329. doi: 10.1111/jth.14859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nougier C, Benoit R, Simon M, et al. Hypofibrinolytic state and high thrombin generation may play a major role in SARS‐COV2 associated thrombosis. J Thromb Haemost. 2020;18:2215‐2219. doi: 10.1111/jth.15016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bouck EG, Denorme F, Holle LA, et al. COVID‐19 and sepsis are associated with different abnormalities in plasma procoagulant and fibrinolytic activity. Arterioscler Thromb Vasc Biol. 2021;41:401‐414. doi: 10.1161/ATVBAHA.120.315338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Medcalf RL, Keragala C, Myles PS. Fibrinolysis and COVID‐19: a plasmin paradox. J Thromb Haemost. 2020;18:2118‐2122. doi: 10.1111/jth.14960 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Authors elect to not share data.


