Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) variants could induce immune escape by mutations of the spike protein which are threatening to weaken vaccine efficacy. A booster vaccination is expected to increase the humoral immune response against SARS‐CoV‐2 variants in the population. We showed that immunization with two doses of wild type receptor‐binding domain (RBD) protein, and booster vaccination with wild type or variant RBD protein all significantly increased binding and neutralizing antibody titers against wild type SARS‐CoV‐2 and its variants in mice. Only the booster immunization by Omicron (BA.1)RBD induced a strong antibody titer against the omicron virus strain and comparable antibody titers against all the other virus strains. These findings might shed the light on coronavirus disease 2019 booster immunogens.
Keywords: booster vaccination, humoral immune response, SARS‐CoV‐2
1. INTRODUCTION
The pandemic of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has rapidly spread worldwide, as of 10th May 2022, there have been more than 515 million confirmed cases of coronavirus disease 2019 (COVID‐19), including more than 6.25 million deaths, reported to WHO. 1 The development of vaccines is urgently needed for the prevention and control of COVID‐19, and different candidates have been developed since 2020. 2 , 3 , 4 , 5 As of 7th May 2022, more than 11 billion vaccine doses had been administered globally, which reduced the morbidity and mortality of COVID‐19 efficiently. All of these first‐generation vaccines were developed based on the full‐length spike glycoprotein or the SARS‐CoV‐2 receptor‐binding domain (RBD) derived from the wild‐type SARS‐COV‐2 strain. 6
As SARS‐CoV‐2 faces multiple evolutionary forces, several new SARS‐CoV‐2 variants emerged worldwide and are increasingly dominating the pandemic recently, raising concerns that they may partially evade neutralizing antibodies and pose a threat to the efficacy of current COVID‐19 vaccines. 7 Since the genome of SARS‐CoV‐2 is under the mutational U‐pressure, radical amino acid substitutions caused by missense C to U transitions are expected. 8 Actually, it has been observed that therapeutic monoclonal antibodies and neutralizing antibodies induced by natural infection by the wild type SARS‐CoV‐2 or its variants or immunization became less effective against several SARS‐CoV‐2 variants, 9 , 10 , 11 which resulting in vast vaccine breakthrough infections. 12 The rapid spread of the SARS‐CoV‐2 Omicron variants suggests that the virus might become globally dominant. Notably, Omicron variants with a large number of spike mutations have occurred globally and become dominant, these present a serious threat to many existing COVID‐19 vaccines and therapies. 13 , 14 Given the current challenges presented by SARS‐CoV‐2 variants, the development of next‐generation SARS‐CoV‐2 vaccines against wild type and mutant variants is in high demand. Since a great part of the global population has already been infected or vaccinated with wild‐type SARS‐CoV‐2, an important question is what antigen should be selected as the potential immunogen of booster vaccination to induce robust and broad neutralizing antibody responses against the evolving SARS‐CoV‐2 virus. In this study, we explored the humoral immune response to authentic circulating SARS‐CoV‐2 variants elicited by booster vaccination with distinct RBD subunits in mice, hoping to provide important information for the selection of a new generation of immunogens of SARS‐CoV‐2.
2. METHODS
2.1. Animals
Six‐week‐old female BALB/c mice were purchased from Shanghai Silaike Laboratory Animal Co. Ltd. and were used for all experiments. Those animals were maintained in individually ventilated cages and immediately euthanized by CO2 asphyxiation after all the experiments had been completed.
2.2. Protein expression and purification
The COVID‐19 virus RBD recombinant protein was expressed in human HEK293F cells as a soluble protein. The soluble COVID‐19 virus RBD were purified by HisTrap HP column (GE Healthcare) and were further purified by size‐exclusion chromatography with a Superdex 200 column (GE Healthcare) in 20 mM Tris, pH 8.0, 150 mM NaCl and then analyzed by reducing sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE).
2.3. Immunizations
The immunization regimen is shown in supporting information. Six‐week‐old female BALB/c mice were immunized intramuscularly twice on Day 0 and Day 28, with two doses of 10 μg RBD protein of wild‐type SARS‐CoV‐2 with aluminum salts adjuvant (ThermoFisher). Sera were collected at Day 14 after immunization. Eight weeks after the second immunization, mice were immunized by a booster with 10 μg of each RBD in aluminum salts adjuvant, respectively. Sera were collected at Day 28 after the final immunization and used for the ELISA and neutralization assays.
2.4. Serum‐neutralization assay
104 Vero cells were seeded 24 h before the infection in a 24‐well plate (Costar). On the day of infection, the cells were washed twice with a cell culture medium. Sera from mice were incubated at 56°C for 30 min, and then diluted first 10‐fold by the cell culture medium (Dulbecco's modified eagle medium), and then twofold (several times). Aliquots (40 μl) of diluted sera (from 20‐ to 5120‐fold dilutions) were added to 50 μl of cell culture medium containing 100 plaque‐forming units (PFU) of wild type or each variant SARS‐CoV‐2 virus strain (isolated from Guangdong Provincial Center for Disease Control and Prevention) on a 96‐well plate and incubated at 37°C for 2 h in CO2 5% v ⁄ v. The mixture was added to a monolayer of Vero cells in a 24‐well plate and incubated for 1 h at 37°C. The mixture was removed, 0.5 ml of 1.0% (w/v) LMP agarose (Promega) in 2 × DMEM supplied with 4% (v/v) FBS was added onto the infected cells. After further incubation at 37°C supplied with 5% CO2 for 2 days, the wells were stained with 1% (w/v) crystal violet dissolved in 4% (v/v) formaldehyde to visualize the plaques. The highest dilution of serum that showed complete inhibition activity of SARS‐CoV‐2 was estimated as the NT titer. NT assays were performed in triplicate with negative control sera. All experiments were performed in a Biosafety Level 3 facility. This experiment was repeated three times.
3. RESULTS
The wild type SARS‐CoV‐2, two currently circulating variants of concern (Delta and Omicron BA.1), three previously circulating variants of concern (Alpha, Beta, Gamma) and one previously circulating variant of interest (Kappa) virus strains are included in this study (Supporting Information: Figure S1). The wild type, Alpha, Beta, Gamma, Delta, Kappa, and Omicron BA.1 RBD monomers were expressed, purified, and analyzed by SDS‐PAGE (Supporting Information: Figure S2). To address the question of what antigen should be selected as the potential immunogen of booster vaccination to induce better neutralizing antibody responses, we immunized 35 BALB/c mice with two doses of soluble wild type (Wu‐Hu‐1) RBD of the spike protein (10 μg) with aluminum salts adjuvant (ThermoFisher), with a 4‐week interval between doses, mimicking an immunization schedule for approved SARS‐CoV‐2 vaccines (Supporting Information: Figure S3). Fourteen days after the second immunization with the wild type RBD, binding antibodies in all mice were detectable against all the tested virus strains including the wild type, Alpha, Beta, Gamma, Delta, and Omicron BA.1, although the binding antibody titer against Omicron BA.1 was significantly reduced compared to the titer against wild type RBD (Supporting Information: Figure S4A and Table S1). All the serum samples efficiently neutralized wild type, Alpha, Beta, Gamma, and Delta SARS‐CoV‐2 virus strains with differentiable neutralization titers. However, 19 of 35 serum samples failed to neutralize Omicron BA.1 (Supporting Information: Figure S4B and Table S2). The ratio of antibody titer against Alpha, Beta, Gamma, Delta, and Omicron BA.1 to antibody titer against wild‐type strains was 0.97, 0.48, 0.64, 0.74, and 0.10, respectively. Of note, antibody titers against Omicron BA.1 virus strain induced by two doses of immunization were extremely reduced.
Eight weeks after the second immunization with the wild type RBD, mice were divided into seven groups and boosted with 10 μg of wild type, Alpha, Beta, Gamma, Delta, Kappa, and Omicron BA.1 RBD in aluminum salts adjuvant, respectively (Supporting Information: Figure S3). Both binding and neutralizing antibody titers against all tested virus strains were substantially boosted by the third immunization as compared to the antibody titers after the second immunization (Supporting Information: Figures S4C,D). Notably, the third‐dose boosting vaccination increased the binding antibody titer 3.62‐fold and the neutralizing antibody titer 6.04‐fold compared to the corresponding antibody titers after the second immunization against the Omicron BA.1 virus strain. Interestingly, all the sera after the third vaccination showed positive neutralization activity against Omicron BA.1 virus strains. The third‐dose booster vaccination by each of the variants (wild type, Alpha, Beta, Gamma, Delta, Kappa, or Omicron BA.1) induced broad binding and neutralizing antibodies against the corresponding virus strains (Figures 1 and 2, Supporting Information: Tables S3 and S5). Of note, a third‐dose booster vaccination with the RBD of Omicron BA.1 increased the neutralizing antibody titer against Omicron BA.1 virus strains significantly and showed comparable neutralization antibody titer against wild type and other variants. This result suggests that the RBD protein of Omicron BA.1 has good immunogenicity and can induce a broad‐spectrum humoral immune response while being used as a booster immunogen. We finally compared the overall binding and neutralizing antibody titers induced by booster vaccination with each RBD variant. Booster immunization with each antigen‐induced comparable overall binding antibody titers with a small difference between each other. The ratio of mean binding antibody titer induced by the immunization of Alpha, Beta, Gamma, Delta, Kappa or Omicron BA.1 to antibody titer against wild type strains was 0.94, 1.06, 1.08, 1.14, 1.02, and 0.97, respectively (Supporting Information: Figure 2G and Table S4). The third‐dose booster immunizations with each RBD showed similar overall neutralizing antibody titers against all the tested virus strains. The ratio of mean neutralizing antibody titer induced by the immunization of Alpha, Beta, Gamma, Delta, Kappa, or Omicron BA.1 to antibody titer against wild type strains was 1.05, 1.10, 1.27, 1.27, 1.05, and 1.00, respectively (Supporting Information: Figure 2H and Table S6). Amino acid sequences of the spike proteins of wild type and variants of SARS‐CoV‐2 virus strains in the neutralization assays of this study were further confirmed finally, the virus strains were not mutated again (Supporting Information: Table S7).
Figure 1.
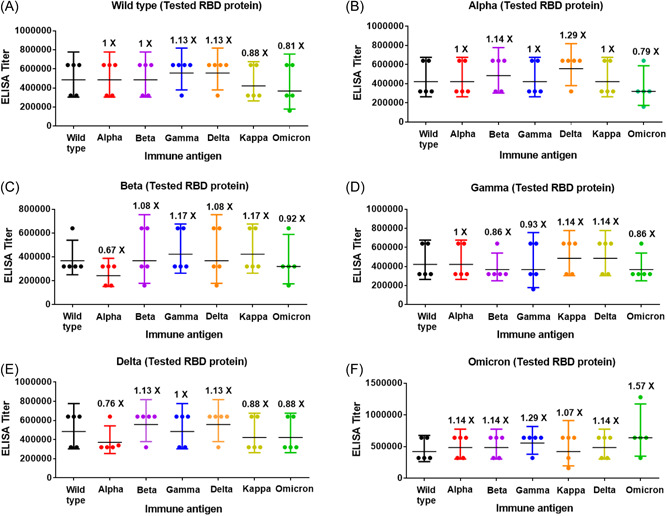
Binding antibody titer induced by the third‐dose booster vaccination with each receptor‐binding domain (RBD) variant against the RBD proteins of wild‐type severe acute respiratory syndrome coronavirus 2 and its variants. Binding antibody titer of the sera after the third‐dose booster vaccination with wild type, Alpha, Beta, Gamma, Delta, Kappa, or Omicron against the RBD of (A) wild type, (B) Alpha, (C) Beta, (D) Gamma, (E) Delta, or (F) Omicron. The ratio of antibody titer against each variant to antibody titer against wild‐type strains is shown, black bars indicate mean values. Statistical analysis was performed using the paired t‐test.
Figure 2.
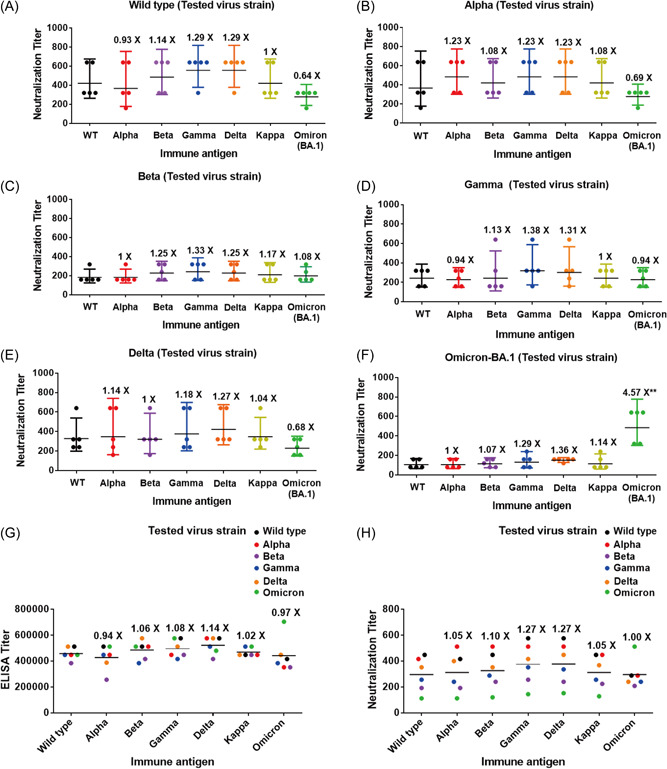
Neutralization antibody titer and overall mean binding and neutralizing antibody titer induced by the third‐dose booster vaccination with each receptor‐binding domain (RBD) variant against the authentic wild‐type Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and its variants. Neutralization antibody titer of the sera after the third‐dose booster vaccination with wild type, Alpha, Beta, Gamma, Delta, Kappa, or Omicron against (A) the wild type, (B) Alpha, (C) Beta, (D) Gamma, (E) Delta, and (F) Omicron SARS‐CoV‐2 virus strain. (G) Overall mean binding and (H) neutralizing antibodies titer induced by booster vaccination with each RBD variant against various SARS‐CoV‐2 virus strains. The ratio of antibody titer against each variant to antibody titer against wild‐type strains was shown, the black bars indicate mean values. Statistical analysis was performed using the paired t‐test.
4. DISCUSSION
Although substantial numbers of people in the world have been vaccinated or infected with SARS‐CoV‐2, reduced efficacy of sera from convalescent and the double vaccinated people against some of the SARS‐CoV‐2 variants has been observed. 15 The choice of an efficient booster is a remarkable scientific issue. This study indicates that the third‐dose booster immunization can significantly increase the binding and the neutralizing antibody titers specific to SARS‐CoV‐2, indicating the importance and necessity of the booster (actually, third) vaccination. In addition, this study used different RBD variants as the third‐dose booster immunogen and compared the humoral immune response induced by each antigen, and surprisingly found that the binding and neutralizing antibody titers in each group were similar. This result indicated that the wild type and variants all revealed good cross‐immunogenicity and might be used as booster immunogens to induce broadly neutralizing antibodies. Encouragingly, mice booster immunized with the RBD of Omicron induced a strong level of neutralizing antibody against authentic Omicron virus strain and a moderate level of neutralizing antibody against other tested SARS‐CoV‐2 viruses, indicating that the antigens from Omicron can be used as a potential booster immunogen to prevent the global pandemic caused by this virus strain. There are some limitations in this study. We did not compare the differences in T cell responses induced by each booster immunogen. The results of this study were carried out in a limited number of mice. To be transformed into new practical approaches, these results should be further confirmed in clinical trials in human participants. Meanwhile, this study did not elucidate the mechanism of the differences in the humoral immune response induced by various immunogens. Nonetheless, our results can provide valuable information for the selection of COVID‐19 booster immunogens.
AUTHOR CONTRIBUTIONS
Chenguang Shen, Baisheng Li, and Xingfen Yang initiated and coordinated the project. Chenguang Shen, Yushan Jiang, and Baisheng Li designed the experiments. Yushan Jiang, Jianhai Yu, Dong Huang, Linlin Zhai, Mengjun Li, and Huanyu Hu conducted the animal experiments. Zhonghua Zheng, Lirong Zou, Zhonghua Zheng, and Baisheng Li evaluated the neutralizing potency of the sera. Yuelin Wang and Zuning Ren evaluated the binding potency of the sera. Junfang Zhang, expressed and purified the RBD proteins. Chenguang Shen, Bao Zhang, and Wei Zhao analyzed the data and wrote the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
This study complies with all relevant ethical regulations. All in vivo studies were performed in accordance with Institutional Animal Care and Use Committee Guidelines and were approved by the Ethics Committee of Southern Medical University Laboratory Animal Center.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
We are grateful to Khrustalev Vladislav Victorovich from Belarusian State Medical University and Khrustaleva Tatyana Aleksandrovna from the Institute of Physiology of the National Academy of Sciences of Belarus for revising the manuscript. This study was supported by grants from the National Natural Science Foundation of China (Grant number 81902058, 32170939, and 82111530302). Guangdong Basic and Applied Basic Research Foundation (Grant number 2020A1515010368 and 2022B1515020075). Shenzhen Science and Technology Innovation Commission for Research and Development Project (Grant number JCYJ20190809183205622). Guangdong Science and Technology Program key projects (No. 2021B1212030014). The Basic Research Project of Key Laboratory of Guangzhou (No. 202102100001).
Jiang Y, Zhang H, Yu J, et al. Humoral immune response to authentic circulating severe acute respiratory syndrome coronavirus 2 variants elicited by booster vaccination with distinct receptor‐binding domain subunits in mice. J Med Virol. 2022;94:4533‐4538. 10.1002/jmv.27882
Yushan Jiang, Huan Zhang, and Jianhai Yu contributed equally to this study.
Contributor Information
Xingfen Yang, Email: yangalice79@smu.edu.cn.
Baisheng Li, Email: libsn@126.com.
Chenguang Shen, Email: a124965468@smu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. https://covid19.who.int/
- 2. Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA‐1273 SARS‐CoV‐2 Vaccine. N Engl J Med. 2021;384:403‐416. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thomas SJ, Moreira ED Jr, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine through 6 Months. N Engl J Med. 2021;385:1761‐1773. 10.1056/NEJMoa2110345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet (London, England). 2021;397:99‐111. 10.1016/s0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector‐based heterologous prime‐boost COVID‐19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet (London, England). 2021;397:671‐681. 10.1016/s0140-6736(21)00234-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265‐269. 10.1038/s41586-020-2008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Copin R, Baum A, Wloga E, et al. The monoclonal antibody combination REGEN‐COV protects against SARS‐CoV‐2 mutational escape in preclinical and human studies. Cell. 2021;184:3949‐3961. 10.1016/j.cell.2021.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khrustalev VV, Giri R, Khrustaleva TA, Kapuganti SK, Stojarov AN, Poboinev VV. Translation‐associated mutational U‐pressure in the first ORF of SARS‐CoV‐2 and other coronaviruses. Front Microbiol. 2020;11:559165. 10.3389/fmicb.2020.559165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Collier DA, De Marco A, Ferreira IATM, et al. Sensitivity of SARS‐CoV‐2 B.1.1.7 to mRNA vaccine‐elicited antibodies. Nature. 2021;593:136‐141. 10.1038/s41586-021-03412-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cao Y, Yisimayi A, Bai Y, et al. Humoral immune response to circulating SARS‐CoV‐2 variants elicited by inactivated and RBD‐subunit vaccines. Cell Res. 2021;31:732‐741. 10.1038/s41422-021-00514-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of SARS‐CoV‐2 variant delta to antibody neutralization. Nature. 2021;596:276‐280. 10.1038/s41586-021-03777-9 [DOI] [PubMed] [Google Scholar]
- 12. Elliott P, Haw D, Wang H, et al. Exponential growth, high prevalence of SARS‐CoV‐2, and vaccine effectiveness associated with the Delta variant. Science (New York, N.Y.). 2021;374:eabl9551. 10.1126/science.abl9551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu L, Iketani S, Guo Y, et al. Striking antibody evasion manifested by the omicron variant of SARS‐CoV‐2. Nature. 2021;602:676‐681. 10.1038/s41586-021-04388-0 [DOI] [PubMed] [Google Scholar]
- 14. Hoffmann M, Krüger N, Schulz S, et al. The omicron variant is highly resistant against antibody‐mediated neutralization: implications for control of the COVID‐19 pandemic. Cell. 2021;185:447‐456. 10.1016/j.cell.2021.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carreño JM, Alshammary H, Tcheou J, et al. Activity of convalescent and vaccine serum against SARS‐CoV‐2 Omicron. Nature. 2021;602:682‐688. 10.1038/s41586-022-04399-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


