Abstract
Background
The COVID‐19 pandemic has caused significant morbidity and mortality in solid organ transplant (SOT) recipients. However, it remains unclear whether the risk factor for SOT patients is the immunosuppression inherent to transplantation versus patient comorbidities.
Methods
We reviewed outcomes in a cohort of SOT (n = 129) and non‐SOT (NSOT) patients (n = 708) admitted to the University of California, Los Angeles for COVID‐19 infection. Data analyses utilized multivariate logistic regression to evaluate the impact of patient demographics, comorbidities, and transplant status on outcomes. SOT patients were analyzed by kidney SOT (KSOT) versus nonkidney SOT (NKSOT) groups.
Results
SOT and NSOT patients with COVID‐19 infection differed in terms of patient age, ethnicity, and comorbidities. NKSOT patients were the most likely to experience death, with a mortality rate of 16.2% compared with 1.8% for KSOT and 8.3% for NSOT patients (p = .013). Multivariable analysis of hospitalized patients revealed that patient age (odds ratio [OR] 2.79, p = .001) and neurologic condition (OR 2.66, p < .001) were significantly associated with mortality. Analysis of ICU patients revealed a 2.98‐fold increased odds of death in NKSOT compared with NSOT patients (p = .013).
Conclusions
This study demonstrates the importance of transplant status in predicting adverse clinical outcomes in patients hospitalized or admitted to the ICU with COVID‐19, especially for NKSOT patients. Transplant status and comorbidities, including age, could be used to risk stratify patients with COVID‐19. This data suggests that immunosuppression contributes to COVID‐19 disease severity and mortality and may have implications for managing immunosuppression, especially for critically ill patients admitted to the ICU.
Keywords: comorbidities, COVID‐19, solid organ transplant
1. INTRODUCTION
The COVID‐19 pandemic caused by infection with SARS‐CoV‐2 continues to demonstrate worldwide impact, with over 400 million cases and over 5 million deaths. 1 Given the demonstrated importance of the T‐cell immune response for control of COVID‐19, 2 , 3 it seems likely that immunosuppression will impact vulnerability to infection and severe infection, as seen in other community‐acquired respiratory viral infections such as influenza. 4 Conversely, high levels of pro‐inflammatory cytokines are associated with severe COVID‐19 disease, 5 and anti‐inflammatory therapies have been shown to improve clinical outcomes, 6 suggesting that the possibility that immunosuppression might temporize disease manifestations. However, there remains a lack of clarity on the impact of immunosuppression on clinical outcomes, which we sought to address by utilizing a large patient cohort of solid organ transplant (SOT) and non‐SOT patients hospitalized with COVID‐19.
The US Centers for Disease Control and Prevention (CDC) recognizes immunosuppression as associated with an increased risk of severe COVID‐19 illness. 7 Previous case series demonstrated increased rates of hospitalization, as well as significant morbidity and mortality in both SOT and stem cell transplant recipients with rates ranging from 13% to 30%. 8 , 9 , 10 , 11 Initial case series demonstrated the highest mortality rates at 30%, significantly higher than that reported for nontransplant recipients during similar time periods. 12 , 13 A report from the United Kingdom demonstrated increased mortality of kidney transplant recipients compared with those remaining on dialysis (25.8% compared with 10.2%). 14 In contrast, an analysis of mortality due to COVID‐19 in a US cohort demonstrated a similar incidence of mortality before and after kidney transplantation. 15 These ranges in mortality could be attributable to the various geographical areas and times during which the COVID‐19 pandemic was peaking in the United States, as well as the introduction of remdesivir and dexamethasone treatment. 16 Recent reports suggest that mortality rates for SOT recipients may be improving with the advent of new treatment approaches 17 ; however, this retrospective cohort did not include a comparison with non‐SOT (NSOT) patients.
Previous studies comparing mortality in SOT versus NSOT patients are generally limited by small cohort sizes, data reported prior to the widespread use of remdesivir and dexamethasone, and the inclusion of a significant majority of kidney transplant recipients, limiting extrapolation to nonkidney transplant patients. 18 , 19 , 20 , 21 , 22 , 23 Some reports demonstrated an association between SOT status and risk of clinical outcomes: As of now, the largest published report on 128 SOT recipients demonstrated increased rates of mortality and increased rates of mechanical intubation compared with nontransplant patients. 24 Likewise, a large study from Italy demonstrated an association between mortality and transplant status in patients hospitalized with COVID‐19, with a 3.83 adjusted odds ratio (OR) for SOT patients with COVID‐19 infection compared with NSOT patients adjusted by age and sex. 25 Furthermore, meta‐analyses have demonstrated an increased risk of severe disease with immunosuppression. 26 , 27
In contrast, other retrospective reviews have demonstrated that comorbidities, but not transplant status, are associated with mortality, 21 , 28 , 29 Other reviews of clinical outcomes in cohorts of SOT patients are limited by the absence of a parallel non‐SOT group for comparison. 30 , 31 Therefore, there is a lack of published data as to whether SOT status contributes to mortality in hospitalized patients adjusted for comorbid conditions.
Analyses of specific organ types demonstrate a similar incidence of mortality in kidney transplant patients compared with nontransplant patients. 32 , 33 For liver transplantation, one publication demonstrated increased mortality compared with nontransplant patients, 34 whereas another using a registry analysis approach did not show differences in outcome compared with other hospitalized patients. 35 Studies of heart transplant patients have either demonstrated a similar incidence of mortality compared with nontransplant recipients, 36 , 37 or increased mortality rates. 38 In lung transplantation, one study reported a mortality of 25% in a small cohort, 39 whereas other small case series have shown a lower mortality (11%), 40 however, without comparison to an NSOT cohort.
Therefore, given the limitations of previous studies, the study presented here is uniquely suited to address the question of transplant status versus comorbidities as the driving factor behind observed increased mortality in SOT patients using parallel SOT and NSOT cohorts, including a large number of nonkidney transplant recipients.
2. METHODS
Data regarding the incidence and impact of COVID‐19 infection in SOT recipients was collected as part of an ongoing quality improvement and retrospective research project at the University of California, Los Angeles (UCLA). This information was compared with data on SOT and NSOT patients diagnosed with COVID‐19 at our center under a UCLA IRB‐approved study (PI: S. Saab).
Study data was extracted from the UCLA electronic medical record. The data was managed using REDCap (Research Electronic Data Capture). The database was composed of patients who underwent testing for SARS‐CoV‐2 by our Clinical Microbiology Laboratory. Data on variant testing is not available during the period of study. The test used was the FDA‐approved CDC COVID‐19 PCR test via nasopharyngeal swab.
For all patients testing positive for SARS‐CoV‐2, transplant status and need for hospitalization was determined through database review; symptoms and level of disease severity were not determined for outpatients. We focused solely on patients admitted to the Ronald Reagan UCLA Medical Center (RRMC), the UCLA tertiary care center where the majority of transplant recipients are admitted. Given the different incidences and manifestations of COVID‐19 in the pediatric population, patients under the age of 18 were excluded from the analysis. Stem cell transplant recipients were also not included in the cohort of transplant patients given our primary focus on SOT and to reduce the confounder of immunosuppression use in the NSOT population. Given the small number of intestinal and pancreas transplant recipients, these patients were excluded from the analysis. Patients requiring extracorporeal membrane oxygenation (ECMO) were excluded from our analysis due to RRMC's status as a tertiary referral center outside of the typical catchment area for UCLA hospitals and therefore a common location for referrals for patients requiring ECMO and given the known poor prognosis of patients with COVID‐19 on ECMO (n = 25). Given our center's status as a specific referral center for patient's requiring ECMO, these patients were almost entirely NSOT patients with critical illness transferred from other hospitals. We additionally excluded patients without full follow‐up data available (n = 13). No patients remained in the ICU at the end of the follow‐up period. We subsequently identified patients who had previously received a SOT as identified by ICD‐10 coding (Figure 1). Dual organ transplant recipients were classified based on their primary (nonkidney) organ.
FIGURE 1.
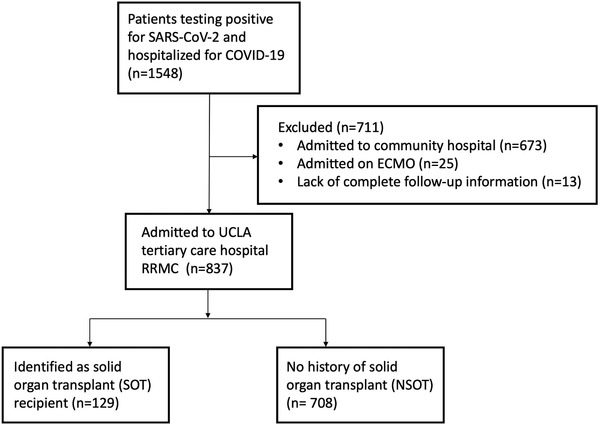
Consort diagram demonstrating identification of patient cohort for analysis. Abbreviations: ECMO, extracorporeal membrane oxygenation; NSOT, nonsolid organ transplant; RRMC, Ronald Reagan UCLA Medical Center; SOT, solid organ transplant; UCLA, University of California, Los Angeles
Comorbidities were defined as follows using review of recorded past medical history and ICD‐10 codes: Cardiovascular conditions included hypertension, hyperlipidemia, arrhythmia, myocardial infarction, congestive heart failure, or peripheral vascular disease; neurologic conditions included cerebrovascular disease, dementia, chronic neurologic disorder, paraplegia; lung disease was defined as asthma, obstructive sleep apnea, chronic pulmonary disease; gastrointestinal disease was defined as gastroesophageal reflux disease, peptic ulcer disease, irritable bowel syndrome, chronic diarrhea, chronic constipation, celiac disease, prior pancreatitis; hepatobiliary disease was defined as prior biliary disease or liver disease; and cancer was defined as any malignancy except for skin neoplasms. The majority of patients classified as having neurologic disease were due to cerebrovascular disease. Based on CDC guidelines, a cutoff of BMI 30 was used to define obesity.
Data was extracted between December 13, 2019 and February 2, 2021. We used a cutoff date of July 1, 2020 as the beginning of a `second wave’ of COVID‐19 infection due to a new peak of SARS‐CoV‐2 infection, as well as marking a time when remdesivir and dexamethasone were used routinely for inpatient clinical care. Numeric values were analyzed by mean and standard deviation (SD). Long length of stay (LOS) was defined as greater than 6 days and the median LOS in our cohort. Death was defined as either death or discharge on hospice care while hospitalized for COVID‐19 infection.
Patient characteristics and study variables were summarized using mean (SD) or frequency (%) and compared between groups using the t‐test or chi‐square test. Prespecified outcomes of interest were defined as LOS, ICU admission, intubation, and death as previously described markers of clinical outcomes in hospitalized patients with COVID‐19. Univariable and multivariable (stepwise reduced) logistic regression models were constructed for our outcomes of interest (long LOS, ICU admission, intubation, mortality) and results presented with ORs (95% confidence interval [CI]). We used forward stepwise selection with entry criteria (0.05) and exit criteria (0.10) based on the Wald p‐value. For exploratory purposes (e.g., Table 2A), we took the stepwise reduced model and forced in transplant status in order to assess the impact on outcome. Statistical analyses were run using IBM SPSS V27 (Armonk, NY) and p‐values < .05 were considered statistically significant.
TABLE 2A.
Risk factors for mortality in non‐kidney transplant (NKSOT) and non‐transplant recipients (NSOT) hospitalized with COVID‐19 (OR [95% CI])
| OR for univariate analysis | p‐Value | OR for multivariate analysis | p‐Value | |
|---|---|---|---|---|
| Older age | 3.81 (2.11–6.86) | <.001 | 2.79 (1.51–5.16) | .001 |
| Female sex | 0.81 (0.49–1.33) | .405 | ||
| White race | 1.19 (0.73–1.94) | .487 | ||
| Hispanic ethnicity | 0.75 (0.45–1.25) | .266 | ||
| Diabetes | 1.23 (0.75–2.03) | .416 | ||
| Cardiovascular condition | 2.61 (1.59–4.28) | <.001 | ||
| Neurologic condition | 3.71 (2.24–6.12) | <.001 | 2.66 (1.57–4.50) | <.001 |
| Obesity | 0.59 (0.34–1.05) | .073 | ||
| Lung disease | 1.81 (1.08–3.04) | .024 | ||
| Gastrointestinal disease | 0.36 (0.05–2.70) | .322 | ||
| Hepatobiliary disease | 0.88 (0.45–1.72) | .700 | ||
| Chronic kidney disease | 2.59 (1.55–4.31) | <.001 | ||
| Rheumatologic disorder | 1.68 (0.68–4.13) | .257 | ||
| Cancer | 1.40 (0.73–2.71) | .221 | ||
| Second wave (admit July 2020 or later) | 0.70 (0.39–1.24) | .221 | ||
| Nonkidney transplant recipient (NKSOT) | 2.13 (1.09–4.17) | .028 | 1.75 (0.87–3.50) | .114 |
Note: Multivariate analysis represents results from a stepwise selected approach, those with p < 0.05 included in multivariate model with the addition of transplant status for exploratory purposes.
P values <0.05 are in bold.
Abbreviations: CI, confidence interval; OR, odds ratio.
3. RESULTS
3.1. Demographic and clinical characteristics of solid organ transplant and non‐SOT patients
We identified 129 SOT patients who were diagnosed with COVID‐19 by SARS‐CoV‐2 PCR testing and hospitalized at RRMC. We compared these patients with 708 NSOT patients, who were hospitalized at RRMC during the same period.
We observed that of the 6874 patients who tested positive for SARS‐CoV‐2 during the period of data analysis, SOT patients were more likely to be hospitalized compared with nontransplant patients regardless of symptom severity. Specifically, 63% of SOT patients testing positive for SARS‐CoV‐2 were hospitalized for COVID‐19 infection compared with 21% NSOT patients (p < .001). The total group of 129 SOT patients hospitalized at RRMC included kidney (n = 55), liver (n = 33), lung (n = 24), and heart (n = 17) transplant recipients.
Given that kidney transplant recipients represented the largest group of SOT patients in our cohort, we repeated analyses to determine the differences among NSOT, kidney SOT (KSOT) recipients, and nonkidney SOT (NKSOT) groups (Table 1A). This division revealed that NKSOT patients were older, with 67.6% above the age of 60 years, whereas 50.6% of the NSOT and 34.5% of the KSOT patients were over 60‐year old (p = .001). Significant differences were also seen in the frequency of comorbidities (Table 1A). Both KSOT and NKSOT patients demonstrated a significantly higher proportion of Hispanic patients and an increased incidence of diabetes compared with NSOT patients. Both KSOT and NSOT patients were more likely to be obese compared with NKSOT patients (Table 1A).
TABLE 1A.
Demographic and clinical characteristics of kidney versus non‐kidney solid organ transplant recipients compared with non‐transplant patients hospitalized with COVID‐19 (n (%))
| Kidney solid organ transplant (KSOT) n = 55 | Non‐kidney solid organ transplant (NKSOT) n = 74 | Non‐Solid Organ Transplant (NSOT) n = 708 | p‐Value | |
|---|---|---|---|---|
| Age older than 60 years | 19 (34.5%) | 50 (67.6%) | 358 (50.6%) | .001 |
| Female sex | 25 (45.5%) | 27 (36.5%) | 318 (44.9%) | .374 |
| White | 18 (32.7%) | 29 (39.2%) | 304 (42.9%) | .296 |
| Black | 4 (7.3%) | 8 (10.8%) | 63 (8.9%) | .776 |
| Asian | 6 (10.9%) | 2 (2.7%) | 63 (8.9%) | .153 |
| Hispanic | 37 (67.3%) | 45 (60.8%) | 279 (39.4%) | <.001 |
| Diabetes mellitus | 34 (61.8%) | 47 (63.5%) | 227 (32.1%) | <.001 |
| Cardiovascular condition | 20 (36.4%) | 47 (63.5%) | 181 (25.6%) | <.001 |
| Neurologic condition | 7 (12.7%) | 23 (31.1%) | 145 (20.5%) | .031 |
| Obesity | 18 (36.0%) | 18 (25.4%) | 266 (41.4%) | .028 |
| Lung disease | 10 (18.2%) | 42 (56.8%) | 147 (20.8%) | <.001 |
| Gastrointestinal disease | 4 (7.3%) | 8 (10.8%) | 20 (2.8%) | .001 |
| Hepatobiliary disease | 11 (20.0%) | 44 (59.5%) | 90 (12.7%) | <.001 |
| Chronic kidney disease | 54 (98.2%) | 53 (71.6%) | 118 (16.7%) | <.001 |
| Rheumatologic disorder | 2 (3.6%) | 8 (10.8%) | 35 (4.9%) | .870 |
| Cancer | 6 (10.9%) | 20 (27.0%) | 82 (11.6%) | .001 |
| Second wave (admit July 2020 or later) | 47 (85.5%) | 63 (85.1%) | 574 (81.1%) | .525 |
P values <0.05 are in bold.
3.2. Clinical outcomes in kidney transplant (KSOT), nonkidney solid organ transplant (NKSOT), and nonsolid organ transplant (NSOT) patients
Analyses of outcomes by a patient group revealed that the NKSOT group had more severe clinical outcomes, whereas the KSOT patients often did as well, or for some outcomes, better, compared with the NSOT patient group (Table 1B). The incidence of intubation was not significantly different between the NSOT and KSOT patients, at 14.3% and 5.5%, respectively. In addition, the incidence of death was similar between NSOT (8.3%) and KSOT (1.8%) patients. In contrast, NKSOT patients had a significantly increased rate of long LOS (54.1%, p = .032), intubation (23.0%, p = .019), and mortality (16.2%, p = .013), compared with both the KSOT and NSOT groups (Table 1B). In terms of comorbidities and outcomes, direct comparisons between the NKSOT and NSOT patient groups demonstrated similar findings to the three‐group analysis presented above (Table S1).
TABLE 1B.
Clinical outcomes of kidney versus nonkidney solid organ transplant recipients compared with nontransplant patients hospitalized with COVID‐19, n
| Kidney solid organ transplant (KSOT) n = 55 | Non‐kidney solid organ transplant (NKSOT) n = 74 | Non‐solid organ transplant (NSOT) n = 708 | p‐Value | |
|---|---|---|---|---|
| Long length of staya | 23 (41.8%) | 40 (54.1%) | 272 (38.4%) | .032 |
| ICU admission | 11 (20.0%) | 28 (37.8%) | 249 (35.2%) | .060 |
| Intubation | 3 (5.5%) | 17 (23.0%) | 101 (14.3%) | .019 |
| Death | 1 (1.8%) | 12 (16.2%) | 59 (8.3%) | .013 |
Long length of stay defined as greater than 6 days.
P values <0.05 are in bold.
3.3. Differences in presentation and outcomes by transplant organ type
Kidney transplant recipients were the most common type of transplant patient diagnosed with COVID‐19. This was an expected finding given that the larger numbers of kidney transplant recipients compared with other transplant types (Table S2A). Sex, race, and ethnicity were similar across transplant types. In addition, there was no difference by organ type in terms of time posttransplantation. However, both heart and lung transplant recipients were more likely to be older, whereas kidney transplant recipients were more likely to be younger (p < .001). Furthermore, there was a nonsignificant higher proportion of kidney and liver transplant patients having higher incidences of obesity compared with heart and lung transplant recipients (p = .095). Incidence of diabetes was similar across organ types (p = .303). Heart transplant recipients were more likely to have cardiac disease (p < .001) and lung transplant recipients lung disease (p < .001) compared with other transplant types. The time posttransplant did not differ significantly by SOT type, with a mean of 4 years or greater in each group (Table S2A).
Incidence of intubation and death was different by organ type. Lung and liver transplant recipients had the highest incidences of intubation and death, whereas both outcomes were significantly lower in kidney transplant recipients (p = .021 and p = .010, respectively) (Table S2B). LOS did not differ significantly by transplant organ type. However, a nonsignificant higher proportion of lung transplant recipients were admitted to the ICU compared with other organ types (p = .067). Overall, kidney transplant recipients demonstrated the best clinical outcomes compared with other organ transplant recipients.
3.4. Evaluation of NKSOT (lung, heart, liver SOT) patients compared with NSOT patients and corrected for comorbidities
Given the differences seen between KSOT and NKSOT patients as described earlier, we set out to evaluate the differences between these patient groups to better understand the risk factors for heart, lung, and liver transplant recipients (NKSOT) compared with nontransplant patients. As shown in Table 1A, NKSOT patients were more likely to be older than NSOT patients, and they were more likely to have multiple comorbid conditions, including diabetes, heart disease, lung disease, and chronic kidney disease.
Analysis of predictors of long LOS identified several comorbidities associated with LOS, including age, Hispanic ethnicity, diabetes, cardiac disease, neurologic disease, lung disease, chronic kidney disease, earlier wave of COVID‐19 hospitalization, and transplant status (all p < .05). In a multivariable analysis, older age (OR 1.95 [95% CI 1.42–2.67] p < .001), Hispanic ethnicity (OR 1.63 [95% CI 1.19–2.23] p = .002), chronic kidney disease (OR 1.80 [95% CI 1.23–2.65] p = .003), and wave of disease (OR 0.57 [95% CI 0.39–0.82] p = .003 for later wave) were all associated with longer LOS.
In terms of other clinical outcomes, we also constructed a multivariable to predict intubation. This analysis demonstrated that only transplant status was found to be statistically significant for both univariate and multivariable analyses, with an OR of 1.79 for intubation (95% CI [1.00–3.20] p = .049).
Given the observed contribution of increased patient age and comorbidities to mortality in NKSOT and NSOT patients, we performed a multivariable analysis in order to understand the contribution of transplantation and immunosuppression to outcomes as separate from differences in patient demographic and comorbidity characteristics. Analysis of the NSOT versus NKSOT groups controlling for comorbid conditions demonstrated a strong association between older age and death in both the univariate and multivariate models (OR 2.79 [95% CI 1.51–5.16], p = .001) (Table 2A). When transplant status was included in the multivariate model, we noted an OR of 1.75 for NKSOT patient status, although this did not reach statistical significance (95% CI 0.87–3.50, p = .114). Repeating this analysis with neurologic condition eliminated, given that it was colinear with age, demonstrated similar results, with increased patient age as the primary variable associated with death (OR 3.25 [95% CI 1.78–5.92], p < .001), and now with the addition of heart disease in the multivariate model (OR 1.94 [95% CI 1.16–3.26], p = .012).
For NSOT and NKSOT patients admitted to the ICU (n = 277), analysis was performed for intubation and death to evaluate the impact of transplant status (Table 2B). Both hepatobiliary disease (p = .001) and transplant status (p = .005) were predictive of intubation in a univariate analysis, with hepatobiliary disease retaining statistical significance in the multivariate model (OR 2.47 [95% CI 1.17–5.22] p = .017). Transplant status demonstrated a nonsignificant increase in odds of intubation (OR 2.03 [95% CI 0.84–4.89] p = .114). An analysis of risk for death in patients admitted to the ICU identified age (p < .001), cardiac condition (p = .002), neurologic condition (p < .001), obesity (p = .006), chronic kidney disease (.010), and transplant status (p = .0115) to be statistically significant in the univariate model (Table 2B). Multivariable analysis revealed that older age (OR 3.11 [95% CI 1.58–6.12] p = .001), neurologic disease (OR 2.19 [95% CI 1.18–4.07] p = .013), and transplant status (OR 2.98 [95% CI 1.25–7.09] p = .013) were all predictive of mortality in patients admitted to the ICU. Repeating this analysis without the inclusion of neurologic disease demonstrated that age, cardiac disease, and transplant status were all associated with death. These analyses demonstrate that both comorbidities and transplant status contribute to adverse clinical outcomes.
TABLE 2B.
Risk factors for mortality in nonkidney transplant and nontransplant recipients admitted to the ICU for COVID‐19 infection (OR [95% CI])
| OR for univariate analysis | p‐Value | OR for multivariate analysis | p‐Value | |
|---|---|---|---|---|
| Older age | 3.53 (1.87–6.67) | <.001 | 3.11 (1.58–6.12) | .001 |
| Female sex | 0.93 (0.53–1.64) | .808 | ||
| White race | 1.22 (0.70–2.12) | .486 | ||
| Hispanic ethnicity | 0.57 (0.32–1.00 | .052 | ||
| Diabetes | 0.72 (0.41–1.27) | .261 | ||
| Cardiovascular condition | 2.40 (1.37–4.22) | .002 | ||
| Neurologic condition | 3.06 (1.70–5.49) | <.001 | 2.19 (1.18–4.07) | .013 |
| Obesity | 0.42 (0.23–0.79) | .006 | ||
| Lung disease | 1.72 (0.95–3.12) | .076 | ||
| Gastrointestinal disease | 0.27 (0.03–2.16) | .220 | ||
| Hepatobiliary disease | 1.23 (0.58–2.61) | .597 | ||
| Chronic kidney disease | 2.14 (1.19–3.85) | .011 | ||
| Rheumatologic disorder | 1.78 (0.63–5.01) | .275 | ||
| Cancer | 1.87 (0.82–4.28) | .138 | ||
| Second wave (admit July 2020 or later) | 0.82 (0.43–1.56) | .556 | ||
| Nonkidney transplant recipient | 2.65 (1.18–5.92) | .018 | 2.98 (1.25–7.09) | .013 |
Note: Multivariate analysis represents results from a stepwise selected approach, those with p < .05 included in multivariate model.
P values <0.05 are in bold.
Abbreviations: CI, confidence interval; OR, odds ratio.
4. DISCUSSION
A review of outcomes in contemporaneous cohorts of SOT and NSOT patients demonstrated increased rates of intubation and death in nonkidney transplant recipients. Most notably, in our multivariate analyses corrected for comorbid conditions and age, transplant status was significantly associated with death in patients admitted to the ICU in liver, heart, and lung transplant recipients. These findings highlight the importance of analyzing kidney transplant patients apart from nonkidney transplant patients given their different clinical trajectories. Differences in KSOT versus NKSOT patients may at least partially explain the varying results reported in the literature in terms of impact of transplant status on clinical outcomes.
As seen in other previous publications, we observed that comorbid conditions, including cardiovascular disease, lung disease, and chronic kidney disease, contributed to ICU admission and death. 41 , 42 , 43 Similar comorbid conditions have been previously identified as associated with mortality in SOT patients as well. 19 Interestingly, we found that neurologic disease remained significant for the prediction of death in a multivariate model, which is not a comorbid condition that has received significant attention in the literature. However, given that cerebrovascular disease was the major component in our cohort, this association is presumably related to the adverse effect of cardiovascular disease as described in other publications. Surprisingly, in contrast to other published reports, diabetes was not associated with death. This could be due to the fact that patients identified with this medical condition included both mild and severe conditions.
Patient age is another key component shown to be associated with adverse outcomes in patients with COVID‐19 across a multitude of studies. 44 The impact of age may be manifold, including association with a greater number of comorbidities, association with frailty, or association with immune senescence and other components of age‐related immune dysfunction. 44 , 45 It is important to note that age has been found to be associated with poor outcomes in other studies of SOT patients with COVID‐19, including relatively small studies on lung transplant recipients. 46 , 47 This observation may partially explain the relatively low incidence of death on our KSOT cohort.
One important strength of our study is the racial and ethnic diversity of our SOT patient cohort. Of note, although previous studies have demonstrated an increased incidence of COVID‐19 in non‐White patients as well as worse clinical outcomes, 48 , 49 , 50 in our cohort of patients hospitalized with COVID‐19, neither race nor ethnicity was associated with intubation or death, although Hispanic ethnicity was associated with longer LOS in SOT patients. This lack of association between race and ethnicity and death was also observed in a multicenter registry of SOT patients with COVID‐19, 16 confirming the importance of studying patient outcomes in diverse patient groups. Future studies should explore the root cause of these differences to identify effective approaches to address this issue.
A review of the literature on SOT versus NSOT patient outcomes revealed a variety of conclusions, with some authors demonstrating that SOT status is associated with mortality independent of age and comorbidities, 24 , 25 whereas others not finding an association with SOT status when models were corrected for comorbidities. 16 , 22 Given the known worse outcomes in lung transplant recipients compared with other transplant organ types, the inclusion of larger percentages of nonkidney transplant patients within the SOT cohort may also be a factor. 8 Although a cohort of kidney transplant recipients in France demonstrated that transplant status was significantly associated with mortality in multivariate analysis, 51 many cohorts containing a majority of KSOT patients have demonstrated outcomes more comparable to the NSOT population. 16 , 22 Our analysis demonstrated that there were significant differences in ICU admission, intubation, and death in the KSOT versus NKSOT patient groups. We hypothesize that although our multivariable analysis corrected for age, the increased median age of NKSOT patients compared with KSOT patients may imply an increased level of patient frailty that was not captured in this analysis, given that frailty has been shown to play a role in worse outcomes in patients hospitalized with COVID‐19. 52 Further studies should explore this potential factor.
These findings underscore the importance of considering KSOT versus NKSOT patients separately in transplant center policies for patient testing and triaging during the COVID‐19 pandemic. This difference appeared to primarily be driven by lung transplant and liver transplant recipients, whose outcomes were significantly worse compared to other patient groups.
Evaluation of our data in the context of other published studies suggests that both transplant status and medical comorbidities play a role in adverse patient outcomes. Given the increased frequency of medical issues, including cardiovascular disease, neurologic conditions, and lung disease in the SOT population, our analyses highlight the importance of risk stratifying transplant recipients by age and comorbid conditions. However, the impact of NKSOT status for patients admitted to the ICU suggests that for patients with severe disease, immunosuppression appears to increase risk for death independent of comorbidities. This hypothesis is supported by observations that SOT patients demonstrate impaired antibody and T‐cell response to both natural and vaccine‐induced antigen exposure compared with NSOT patients. 53 , 54 , 55 This observation supports the recommendation by national transplant organizations, such as the American Society of Transplantation and the International Society for Heart and Lung Transplantation, to reduce immune suppression for transplant patients with severe COVID‐19. 56
The apparent paradox that immune suppressive therapies such as dexamethasone may meliorate disease suggests the importance of differentiating types of immune suppression. Transplant recipients are on chronic T‐ and B‐cell‐directed therapies, including calcineurin inhibitors and mycophenolate mofetil to prevent alloantibody formation and rejection, leading to impaired response to vaccination and infection, especially due to novel antigens. These medications impair T‐cell response and are likely associated with persistent viral replication, a known factor in severe or fatal COVID‐19. 57 In contrast, immunomodulating therapies with benefits in COVID‐19 infection, such as dexamethasone or tocilizumab, act primarily as an anti‐inflammatory intervention, stemming the cytokine storm associated with severe COVID‐19. 58 Whether anti‐inflammatory therapy provides additional benefit to patients on chronic anti‐cellular immune suppression remains unknown but does raise the possibility of additional clinical complications, including secondary bacterial or fungal infections.
Previous studies have not shown an impact of specific immunosuppression regimen, although these studies have generally not included measurement of calcineurin inhibitor levels. 59 Other laboratory‐based analysis has demonstrated improved antiviral T‐cell response with reduction in immunosuppression supporting this approach 60 ; further study is needed to better understand T‐cell dysfunction and antiviral antibody production in the setting of immunosuppression.
From a quality assurance and performance improvement perspective, the observations presented may have important information for triaging KSOT and NKSOT patients in outpatient settings. In addition, the observation regarding the impact of age and comorbidities may have implications in terms of remote monitoring, patient follow‐up and education, hospital admission, and advocacy for booster vaccination and preventative monoclonal antibody therapies. This information can also be used to improve care for transplant patients at risk for COVID‐19, as well as to prepare for future pandemic infections.
A strength of this study is that SOT and NSOT cohorts were examined during the same time period in a single center, so that SARS‐CoV‐2 transmission and treatment patterns were equivalent between groups. As discussed earlier, study limitations include that the threshold for hospitalization may have differed between groups and that severity of illness at the time of testing or admission was not available. We attempted to address this issue by performing a prespecified analysis on patients admitted to the ICU with similar disease severity. Another potential limitation is that as a tertiary care referral center, NSOT patients are often referred from other hospitals with severe infection. We have tried to address this potentially confounding issue by restricting our analysis to patients not requiring ECMO, thereby allowing for a more equitable comparison between patient groups.
In many regards, despite vaccination and other preventative strategies, the COVID‐19 pandemic continues to exercise a significant toll on transplant recipients, with unknown times ahead given the likely advent of new variants with unpredictable patterns of transmissibility and virulence. The data presented in this study identifies that patient age and certain comorbid conditions are associated with adverse clinical outcomes. This provides evidence that for patients requiring ICU admission, NKSOT status provides additional risk for patient death independent from the impact of age and comorbidities.
FUNDING INFORMATION
None.
AUTHOR CONTRIBUTIONS
J.S. designed the analysis, interpreted results, and wrote the paper; H.B. conceived the study, interpreted results, and wrote the paper; T.G. performed data analysis and interpreted results; Y.M. collected data and assisted in database creation; O.B. interpreted results and wrote the paper; M.K., E.L, K.M., D.S., and D.V. interpreted results and participated in study design; S.S. conceived the study and interpreted results. All authors reviewed the results and approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
5.
Supporting information
Supporting information
ACKNOWLEDGMENTS
We greatly appreciate all of the physicians, nurses, and transplant coordinators at UCLA who have participated in patient care during the COVID‐19 pandemic. We also appreciate the support from the UCLA Clinical and Translational Science Institute for database creation and statistical support.
Schaenman J, Byford H, Grogan T, et al. Impact of solid organ transplant status on outcomes of hospitalized patients with COVID‐19 infection. Transpl Infect Dis. 2022;e13853. 10.1111/tid.13853
REFERENCES
- 1. Organization WH . WHO coronavirus (COVID‐19) dashboard. 2022. Accessed on May 8, 2022. https://covid19.who.int
- 2. Bonifacius A, Tischer‐Zimmermann S, Dragon AC, et al. COVID‐19 immune signatures reveal stable antiviral T cell function despite declining humoral responses. Immunity. 2021;54(2):340‐354.e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ni L, Cheng ML, Feng Y, et al. Impaired cellular immunity to SARS‐CoV‐2 in severe COVID‐19 patients. Front Immunol. 2021;12:603563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kumar D, Michaels MG, Morris MI, et al. Outcomes from pandemic influenza A H1N1 infection in recipients of solid‐organ transplants: a multicentre cohort study. Lancet Infect Dis. 2010;10(8):521‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koutsakos M, Rowntree LC, Hensen L, et al. Integrated immune dynamics define correlates of COVID‐19 severity and antibody responses. Cell Rep Med. 2021;2(3):100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mouffak S, Shubbar Q, Saleh E, El‐Awady R. Recent advances in management of COVID‐19: a review. Biomed Pharmacother. 2021;143:112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Control CfD. People with Certain Medical Conditions . COVID‐19 2022. Accessed on May 8, 2022. https://www.cdc.gov/coronavirus/2019‐ncov/need‐extra‐precautions/people‐with‐medical‐conditions.html
- 8. Kates OS, Haydel BM, Florman SS, et al. COVID‐19 in solid organ transplant: a multi‐center cohort study. Clin Infect Dis. 2021;73(11):e4090‐e4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fung M, Babik JM. COVID‐19 in immunocompromised hosts: what we know so far. Clin infect Dis. 2021;72(2):340‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Azzi Y, Bartash R, Scalea J, Loarte‐Campos P, Akalin E. COVID‐19 and solid organ transplantation: a review article. Transplantation. 2021;105(1):37‐55. [DOI] [PubMed] [Google Scholar]
- 11. Quante M, Brake L, Tolios A, et al. SARS‐CoV‐2 in solid organ transplant recipients: a structured review of 2020. Transplant Proc. 2021;53(8):2421‐2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trapani S, Masiero L, Puoti F, et al. Incidence and outcome of SARS‐CoV‐2 infection on solid organ transplantation recipients: a nationwide population‐based study. Am J Transplant. 2020;21(7):2509‐2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ravanan R, Callaghan CJ, Mumford L, et al. SARS‐CoV‐2 infection and early mortality of waitlisted and solid organ transplant recipients in England: a national cohort study. Am J Transplant. 2020;20(11):3008‐3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mohan S, King KL, Husain SA, Schold JD. COVID‐19‐Associated mortality among kidney transplant recipients and candidates in the United States. Clin J Am Soc Nephrol. 2021;16(11):1695‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heldman MR, Kates OS, Safa K, et al. Changing trends in mortality among solid organ transplant recipients hospitalized for COVID‐19 during the course of the pandemic. Am J Transplant. 2022;22:279‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heldman MR, Kates OS, Safa K, et al. Changing trends in mortality among solid organ transplant recipients hospitalized for COVID‐19 during the course of the pandemic. Am J Transplant. 2022;22(1):279‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miarons M, Larrosa‐Garcia M, Garcia‐Garcia S, et al. COVID‐19 in solid organ transplantation: a matched retrospective cohort study and evaluation of immunosuppression management. Transplantation. 2021;105(1):138‐150. [DOI] [PubMed] [Google Scholar]
- 19. Chaudhry ZS, Williams JD, Vahia A, et al. Clinical characteristics and outcomes of COVID‐19 in solid organ transplant recipients: a cohort study. Am J Transplant. 2020;20(11):3051‐3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rinaldi M, Bartoletti M, Bussini L, et al. COVID‐19 in solid organ transplant recipients: no difference in survival compared to general population. Transpl Infect Dis. 2021;23(1):e13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Linares L, Cofan F, Diekmann F, et al. A propensity score‐matched analysis of mortality in solid organ transplant patients with COVID‐19 compared to non‐solid organ transplant patients. PLoS One. 2021;16(3):e0247251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Avery RK, Chiang TP, Marr KA, et al. Inpatient COVID‐19 outcomes in solid organ transplant recipients compared to non‐solid organ transplant patients: a retrospective cohort. Am J Transplant. 2021;27(7):2498‐2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ringer M, Azmy V, Kaman K, et al. A retrospective matched cohort single‐center study evaluating outcomes of COVID‐19 and the impact of immunomodulation on COVID‐19‐related cytokine release syndrome in solid organ transplant recipients. Transpl Infect Dis. 2021;23(2):e13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fisher AM, Schlauch D, Mulloy M, et al. Outcomes of COVID‐19 in hospitalized solid organ transplant recipients compared to a matched cohort of non‐transplant patients at a national healthcare system in the United States. Clin Transplant. 2021;35(4):e14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trapani S, Masiero L, Puoti F, et al. Incidence and outcome of SARS‐CoV‐2 infection on solid organ transplantation recipients: a nationwide population‐based study. Am J Transplant. 2021;21(7):2509‐2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gao Y, Chen Y, Liu M, Shi S, Tian J. Impacts of immunosuppression and immunodeficiency on COVID‐19: a systematic review and meta‐analysis. J Infect. 2020;81(2):e93‐e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Belsky JA, Tullius BP, Lamb MG, Sayegh R, Stanek JR, Auletta JJ. COVID‐19 in immunocompromised patients: a systematic review of cancer, hematopoietic cell and solid organ transplant patients. J Infect. 2021;28(3):329‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pereira MR, Arcasoy S, Farr MA, et al. Outcomes of COVID‐19 in solid organ transplant recipients: a matched cohort study. Transpl Infect Dis. 2021;23(4):e13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hadi YB, Naqvi SFZ, Kupec JT, Sofka S, Sarwari A. Outcomes of COVID‐19 in solid organ transplant recipients: a propensity‐matched analysis of a large research network. Transplantation. 2021;105(6):1365‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vinson AJ, Agarwal G, Dai R, et al. COVID‐19 in solid organ transplantation: results of the National COVID Cohort Collaborative. Transplant Direct. 2021;7(11):e775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heldman MR, Kates OS, Safa K, et al. COVID‐19 in hospitalized lung and non‐lung solid organ transplant recipients: a comparative analysis from a multicenter study. Am J Transplant. 2021;21(8):2774‐2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lum E, Bunnapradist S, Multani A, et al. Spectrum of coronavirus disease 2019 outcomes in kidney transplant recipients: a single‐center experience. Transplant Proc. 2020;52(9):2654‐2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chavarot N, Gueguen J, Bonnet G, et al. COVID‐19 severity in kidney transplant recipients is similar to nontransplant patients with similar comorbidities. Am J Transplant. 2021;21(3):1285‐1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fraser J, Mousley J, Testro A, Smibert OC, Koshy AN. Clinical presentation, treatment, and mortality rate in liver transplant recipients with coronavirus disease 2019: a systematic review and quantitative analysis. Transplant Proc. 2020;52(9):2676‐2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Webb GJ, Marjot T, Cook JA, et al. Outcomes following SARS‐CoV‐2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5(11):1008‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hsu JJ, Al‐Saffar F, Ardehali R, et al. Heart transplantation in the early phase of the COVID‐19 pandemic: a single‐center case series. Clin Transplant. 2020;34(9):e14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Al‐Darzi W, Aurora L, Michaels A, et al. Heart transplant recipients with confirmed 2019 novel coronavirus infection: the Detroit experience. Clin Transplant. 2020;34(12):e14091. [DOI] [PubMed] [Google Scholar]
- 38. Bottio T, Bagozzi L, Fiocco A, et al. COVID‐19 in heart transplant recipients: a multicenter analysis of the Northern Italian outbreak. JACC Heart Fail. 2021;9(1):52‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Myers CN, Scott JH, Criner GJ, et al. COVID‐19 in lung transplant recipients. Transpl Infect Dis. 2020;22(6):e13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Verleden GM, Godinas L, Lorent N, et al. COVID‐19 in lung transplant patients: a case series. Am J Transplant. 2020;20(11):3234‐3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ejaz H, Alsrhani A, Zafar A, et al. COVID‐19 and comorbidities: deleterious impact on infected patients. J Infect Public Health. 2020;13(12):1833‐1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Z, Deng H, Ou C, et al. Clinical symptoms, comorbidities and complications in severe and non‐severe patients with COVID‐19: a systematic review and meta‐analysis without cases duplication. Medicine (Baltimore). 2020;99(48):e23327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Callender LA, Curran M, Bates SM, Mairesse M, Weigandt J, Betts CJ. The impact of pre‐existing comorbidities and therapeutic interventions on COVID‐19. Front Immunol. 2020;11:1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen Y, Klein SL, Garibaldi BT, et al. Aging in COVID‐19: vulnerability, immunity and intervention. Ageing Res Rev. 2021;65:101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mueller AL, McNamara MS, Sinclair DA. Why does COVID‐19 disproportionately affect older people?. Aging (Albany NY). 2020;12(10):9959‐9981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nacif LS, Zanini LY, Waisberg DR, et al. COVID‐19 in solid organ transplantation patients: a systematic review. Clinics (Sao Paulo). 2020;75:e1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ali T, Al‐Ali A, Fajji L, et al. Coronavirus disease‐19: disease severity and outcomes of solid organ transplant recipients: different spectrums of disease in different populations?. Transplantation. 2021;105(1):121‐127. [DOI] [PubMed] [Google Scholar]
- 48. Sharma P, Chen V, Fung CM, et al. COVID‐19 outcomes among solid organ transplant recipients: a case–control study. Transplantation. 2021;105(1):128‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Willicombe M, Gleeson S, Clarke C, et al. Identification of patient characteristics associated With SARS‐CoV‐2 infection and outcome in kidney transplant patients using serological screening. Transplantation. 2021;105(1):151‐157. [DOI] [PubMed] [Google Scholar]
- 50. Acosta AM, Garg S, Pham H, et al. Racial and ethnic disparities in rates of COVID‐19‐associated hospitalization, intensive care unit admission, and in‐hospital death in the United States from March 2020 to February 2021. JAMA Netw Open. 2021;4(10):e2130479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Caillard S, Chavarot N, Francois H, et al. Is COVID‐19 infection more severe in kidney transplant recipients?. Am J Transplant. 2021;21(3):1295‐1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pranata R, Henrina J, Lim MA, et al. Clinical frailty scale and mortality in COVID‐19: a systematic review and dose‐response meta‐analysis. Arch Gerontol Geriatr. 2021;93:104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schmidt T, Klemis V, Schub D, et al. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector‐based COVID‐19 vaccine regimens in solid organ transplant recipients. Am J Transplant. 2021;21(12):3990‐4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bertrand D, Hamzaoui M, Lemee V, et al. Antibody and T cell response to SARS‐CoV‐2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32(9):2147‐2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sattler A, Schrezenmeier E, Weber UA, et al. Impaired humoral and cellular immunity after SARS‐CoV‐2 BNT162b2 (tozinameran) prime‐boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131(14):e150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Raja MA, Mendoza MA, Villavicencio A, et al. COVID‐19 in solid organ transplant recipients: a systematic review and meta‐analysis of current literature. Transplant Rev (Orlando). 2021;35(1):100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. El Zein S, Chehab O, Kanj A, et al. SARS‐CoV‐2 infection: initial viral load (iVL) predicts severity of illness/outcome, and declining trend of iVL in hospitalized patients corresponds with slowing of the pandemic. PLoS One. 2021;16(9):e0255981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. An PJ, Zhu YZ, Yang LP. Biochemical indicators of coronavirus disease 2019 exacerbation and the clinical implications. Pharmacol Res. 2020;159:104946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Merli M, Pasulo L, Perricone G, et al. Impact of immunosuppressive therapy on the severity of COVID‐19 in solid organ transplant recipients. J Infect. 2021;82:414‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Candon S, Guerrot D, Drouot L, et al. T cell and antibody responses to SARS‐CoV‐2: experience from a French transplantation and hemodialysis center during the COVID‐19 pandemic. Am J Transplant. 2021;21(2):854‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


