Abstract
Introduction
This study compared the outcomes of dialysis patients who received SARS‐CoV‐2 vaccine with those who did not use data from the Japanese COVID‐19 registry.
Methods
A total of 1260 dialysis patients with confirmed positive SARS‐CoV‐2 infection was included in this study. Patients were divided into two groups: patients who experienced breakthrough infection and those who were unvaccinated. The need of oxygen supplementation and mortality risks were compared using multivariate logistic regression analysis.
Results
The mortality rate was 24.2% in unvaccinated patients and 8.6% in breakthrough patients. The odds ratio of need of oxygen supplementation in the breakthrough patients relative to unvaccinated patients was 0.197. The hazard ratio of mortality in the breakthrough patients relative to unvaccinated patients was 0.464.
Conclusion
Our prospective observational study showed that SRAS‐CoV‐2 vaccination in hemodialysis patients is vital for reducing need of oxygen supplementation and mortality risk.
Keywords: breakthrough infection, COVID‐19, dialysis, SARS‐CoV‐2, SARS‐CoV‐2 vaccine
1. INTRODUCTION
The new coronavirus disease (COVID‐19) has rapidly spread around the world; a total of 318 648 834 COVID‐19 cases and 5 518 343 deaths were reported worldwide as of January 15, 2022 [1]. In Japan, a total of 1 852 958 COVID‐19 cases and 18 431 deaths were reported in the general population as of January 16, 2022 [2]. In dialysis patients, on the other hand, a total of 2693 COVID‐19 cases and 426 deaths were reported [3]. After the first case of COVID‐19 was reported in a dialysis patient, the Japanese Association of Dialysis Physicians, the Japanese Society for Dialysis Therapy, and the Japanese Society of Nephrology jointly established COVID‐19 Task Force Committee [4, 5].
SARS‐CoV‐2 vaccination is thought to be vital to reduce SARS‐CoV‐2 infection, severe disease, and mortality risks. The effectiveness of the SARS‐CoV‐2 vaccination in the general population has been reported [6, 7]; in dialysis patients, antibody titers against SARS‐CoV‐2 receptor binding domain spike protein after vaccination are reported to be positive at a rate similar to that of the general population, but the titers are low [8, 9]. At this point, it is still unknown how the low antibody titer in dialysis patients is associated with increased severity or mortality in dialysis patients in clinical practice. Thus, we investigated the outcome of dialysis patients who received the SARS‐CoV‐2 vaccine and those who did not using the COVID‐19 registry of the COVID‐19 Task Force Committee, which has been collecting nationwide data in Japan.
2. MATERIALS AND METHODS
2.1. Patients
Surveillance of new cases of COVID‐19 in dialysis facilities in Japan was initiated by the COVID‐19 Task Force Committee of the Japanese Association of Dialysis Physicians, the Japanese Society for Dialysis Therapy, and the Japanese Society of Nephrology on April 8, 2020 [4]. Data of a total of 2621 dialysis patients with COVID‐19 who were registered before October 11, 2021 (the end of 5th wave in Japan) were extracted from this registry. Among those, data of 1361 patients (1164 patients whose outcome was unknown, 87 patients whose age, dialysis history, and gender were unknown, and 110 patients with missing data for factors required for analysis) were excluded; a total of 1260 patients were included in this analysis.
It should be noted that the treatment policy defined by the Ministry of Health, Labour, and Welfare in Japan for dialysis patients diagnosed with COVID‐19 requires hospitalization [4].
2.2. Outcomes and statistical methods
SARS‐CoV‐2 infection positive was diagnosed by antigen test or PCR test. Patients who reported SARS‐CoV‐2 infection positive 2 weeks or more after the second SARS‐CoV‐2 vaccination (BNT162b2 vaccine) were considered as breakthrough infections. Need of oxygen supplementation and mortality risks were compared between patients who received the vaccine and patients who did not. Patients who needed oxygen supplementation were defined as those who received oxygen supplementation, ventilator, or extracorporeal membrane oxygenation (ECMO) after diagnosis.
Multivariate logistic regression analysis was performed for SARS‐CoV‐2 vaccination and need of oxygen supplementation, with age, gender, dialysis history, remdesivir administration, and presence of diabetes as adjustment factors. In addition, conditional logistic regression analysis was performed to compare the patients with breakthrough infection and patients who were unvaccinated using propensity score for age, gender, and dialysis history, at the ratio of 1:3. Multivariate logistic regression analysis was also performed for SARS‐CoV‐2 vaccination and mortality, with age, gender, dialysis history, remdesivir administration, and presence of diabetes as adjustment factors. In addition, stratified log‐rank test and stratified Cox regression analysis were performed to compare the patients with breakthrough infection and patients who were unvaccinated using propensity score for age, gender, and dialysis history, at the ratio of 1:3.
All analyses were performed using SPSS Statistics Ver.21 (IBM SPSS Statistics for Windows, IBM, Armonk, NY), and p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Age and mortality
The number of unvaccinated and breakthrough patients and their mortality rates are shown in Table 1; the patients' backgrounds are shown in Table 2. A total of 1167 infection and 282 death cases were reported in unvaccinated patients; the mortality rate was 24.2%. On the other hand, in breakthrough patients, 93 infection and 8 death cases were reported; the mortality rate was 8.6%, which was significantly less than that in unvaccinated patients (p < 0.05). In terms of mortality by age group, breakthrough patients had a 0% mortality rate under the age of 70, and those over 70 had a lower mortality rate compared to unvaccinated patients.
TABLE 1.
The number of unvaccinated patients and breakthrough patients and their mortality rates
| Age group | <50 | 50s | 60s | 70s | ≥80 | Total | |
|---|---|---|---|---|---|---|---|
| Unvaccinated | n | 154 | 194 | 249 | 300 | 270 | 1167 |
| Deaths | 8 | 21 | 43 | 92 | 118 | 282 | |
| % | (5.2) | (10.8) | (17.3) | (30.7) | (43.7) | (24.2) | |
| Breakthrough | n | 3 | 15 | 15 | 37 | 23 | 93 |
| Deaths | 0 | 0 | 0 | 4 | 4 | 8 | |
| % | (0.0) | (0.0) | (0.0) | (10.8) | (17.4) | (8.6) |
TABLE 2.
Patients' background
| Unmatched | Matched | ||||||
|---|---|---|---|---|---|---|---|
| Unvaccinated | Breakthrough | p‐Value | Unvaccinated | Breakthrough | p‐Value | ||
| Gender | M/F | 819/348 | 61/32 | 0.350 | 168/84 | 56/28 | 1.000 |
| Age | <60 | 348 | 18 | 0.019 | 51 | 17 | 1.000 |
| 60 | 249 | 15 | 45 | 15 | |||
| ≥70 | 570 | 60 | 156 | 52 | |||
| Dialysis history | <1 year | 144 | 4 | 0.220 | 12 | 4 |
1.000 |
| 1–5 years | 438 | 42 | 117 | 39 | |||
| 5–10 years | 283 | 23 | 63 | 21 | |||
| 10–15 years | 149 | 11 | 30 | 10 | |||
| ≥15 years | 153 | 13 | 30 | 10 | |||
| Primary disease | Chronic glomerulonephritis | 197 | 14 | 0.941 | 35 | 11 | 0.970 |
| Diabetes mellitus | 553 | 48 | 135 | 46 | |||
| Nephrosclerosis | 159 | 14 | 31 | 12 | |||
| Others | 169 | 14 | 36 | 13 | |||
| Hypertension | No/yes | 572/558 | 16/75 | <0.001 | 129/117 | 15/68 | <0.001 |
| Diabetes mellitus | No/yes | 550/617 | 41/52 | 0.591 | 101/151 | 34/50 | 1.000 |
| Ischemic heart disease | No/yes | 772/334 | 62/27 | 1 | 168/73 | 57/25 | 1.000 |
| Cerebrovascular disease | No/yes | 895/202 | 73/18 | 0.779 | 194/44 | 69/14 | 0.869 |
| Chronic respiratory disease | No/yes | 1013/101 | 77/14 | 0.061 | 222/21 | 71/13 | 0.096 |
| Peripheral arterial disease | No/yes | 917/176 | 69/20 | 0.137 | 195/42 | 63/19 | 0.328 |
| Malignant tumor | No/yes | 940/168 | 73/18 | 0.231 | 195/46 | 68/16 | 1.000 |
| Number of complications | 0 | 167 | 4 | 0.006 | 29 | 2 | 0.060 |
| 1 | 339 | 26 | 74 | 25 | |||
| >2 | 661 | 63 | 149 | 57 | |||
| Clinical laboratory values | Alb (g/dl) | 3.2 ± 0.6 | 3.3 ± 0.6 | 0.065 | 3.1 ± 0.5 | 3.3 ± 0.6 | 0.001 |
| UN (mg/dl) | 56.7 ± 22.2 | 50.8 ± 19.8 | 0.078 | 55.0 ± 21.0 | 50.7 ± 20.2 | 0.425 | |
| Cr (mg/dl) | 10.4 ± 8.3 | 9.2 ± 3.3 | 0.381 | 8.9 ± 3.5 | 9.3 ± 3.4 | 0.332 | |
| CRP (mg/dl) | 5.56 ± 6.81 | 5.46 ± 6.77 | 0.587 | 5.55 ± 6.93 | 5.59 ± 6.90 | 0.582 | |
| WBC (/μl) | 5732 ± 3693 | 5992 ± 3167 | 0.266 | 5611 ± 2907 | 5998 ± 3242 | 0.458 | |
| Hb (g/dl) | 11.1 ± 1.8 | 11.6 ± 1.6 | 0.031 | 11.4 ± 1.6 | 11.7 ± 1.6 | 0.575 | |
| Height and weight | Height (cm) | 162 ± 10 | 161 ± 10 | 0.149 | 161 ± 11 | 161 ± 10 | 0.737 |
| Dry weight (kg) | 61 ± 18 | 60 ± 15 | 0.717 | 59 ± 17 | 61 ± 15 | 0.311 | |
Abbreviations: Alb, albumin; Cr, creatinine; CRP, C‐reactive protein; Hb, hemoglobin; UN, urea nitrogen; WBC, white blood cell count.
3.2. SARS‐CoV‐2 vaccination and oxygen supplementation
The odds ratio of need of oxygen supplementation in the breakthrough patients relative to unvaccinated patients was 0.197 (95% CI: 0.120–0.322, p < 0.001). Among matched patients (84 breakthrough patients and 252 unvaccinated patients), the odds ratio of need of oxygen supplementation in the breakthrough patients relative to unvaccinated patients was 0.243 (95% CI: 0.142–0.416, p < 0.001). In both matched and unmatched cohorts, breakthrough patients had significantly less need of oxygen supplementation.
3.3. SARS‐CoV‐2 vaccination and mortality
The hazard ratio of mortality in the breakthrough patients relative to unvaccinated patients was 0.464 (95% CI: 0.228–0.945, p < 0.05) (Figure 1). Among matched patients (84 breakthrough patients and 252 unvaccinated patients), the hazard ratio of mortality in the breakthrough patients relative to unvaccinated patients was 0.357 (95% CI: 0.139–0.920, p < 0.05). In both matched and unmatched cohorts, breakthrough patients had significantly less mortality risk.
FIGURE 1.
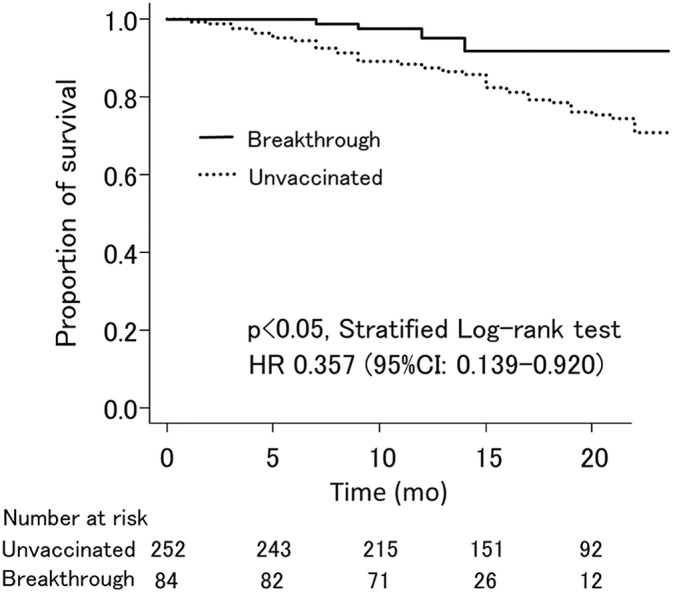
Survival of breakthrough patients and unvaccinated patients
4. DISCUSSION
Since the low antibody titer in dialysis patients after SARS‐CoV‐2 vaccination has not known to affect the outcome, we conducted a cohort study using real‐world data. In our analysis, patients with breakthrough infection had significantly less need of oxygen supplementation and mortality risk compared to patients who were unvaccinated.
Breakthrough infection in dialysis patients at U.S. Renal Care has been reported [10]. Antibody titers in dialysis patients were found to drop to almost the same levels in about 6 months, albeit with variations depending on age, whether the patient has a previous infection with SARS‐CoV‐2, and the type of vaccine they received. In addition, the peak IgG index in the 60 days after vaccination and the IgG index value in the period immediately preceding infection are important factors for the occurrence of breakthrough infections. Thus, to prevent SRAS‐CoV‐2 infection, high antibody titer is important.
A large‐scale, retrospective, observational study examining the efficacy of two mRNA SARS‐CoV‐2 vaccines, BNT162b2 and mRNA‐1273, in hemodialysis patients in the United States has been reported [11]. The results showed that vaccination with BNT162b2 or mRNA‐1273 was associated with a lower risk of COVID‐19 diagnosis and lower risk of hospitalization or death in hemodialysis patients. In this prospective observational study using the Japanese registry, multivariate analysis showed a decreased need of oxygen supplementation by 80.3% (95% CI: 67.8–88.0) and improved survival outcomes by 53.6% (95% CI: 5.5–77.2). Furthermore, propensity score matching analysis also showed similarly a decrease in the need of oxygen supplementation by 75.7% (95% CI: 58.4–85.8) and improved survival outcomes by 64.3% (95% CI: 8.0–86.1). Taken together, SRAS‐CoV‐2 vaccination in hemodialysis patients is vital for reducing disease severity and improving survival outcome. There are some limitations: not all facilities measure antibody titers since this is a nationwide survey, and we selected only five adjustment factors (i.e., age, gender, dialysis history, remdesivir administration, and presence of diabetes) for the logistic regression model.
5. CONCLUSION
Our prospective observational study showed that SRAS‐CoV‐2 vaccination in hemodialysis patients is vital for reducing the need of oxygen supplementation and mortality risk.
FUNDING INFORMATION
No funding source to declare.
CONFLICT OF INTEREST
The authors have no conflicts of interest directly relevant to the content of this article.
ACKNOWLEDGMENTS
We thank the staff members in the dialysis facilities who participated in this study.
Kikuchi K, Nangaku M, Ryuzaki M, Yamakawa T, Yoshihiro O, Hanafusa N, et al. Effectiveness of SARS‐CoV‐2 vaccines on hemodialysis patients in Japan: A nationwide cohort study. Ther Apher Dial. 2022. 10.1111/1744-9987.13887
REFERENCES
- 1. World Health Organization(WHO) . Coronavirus disease (COVID‐19) Pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed January 15, 2022.
- 2. The Japanese Ministry of Health Labour and Welfare . Current status of COVID‐19 in the general population in Japan (as of January 16, 2022). [in Japanese]. https://www.mhlw.go.jp/stf/covid-19/kokunainohasseijoukyou.html. Accessed, January 17, 2022.
- 3. COVID‐19 Task Force Committee of the Japanese Association of Dialysis Physicians, the Japanese Society for Dialysis Therapy, and the Japanese Society of Nephrology . Current status of COVID‐19 in the dialysis patients in Japan (as of January 13, 2022). [in Japanese]. https://jsn.or.jp/medic/data/COVID-19number-of-infected_20220114.pdf. Accessed, January 17, 2022.
- 4. Kikuchi K, Nangaku M, Ryuzaki M, et al. COVID‐19 task force Committee of the Japanese Association of dialysis physicians; the Japanese Society for Dialysis Therapy; the Japanese Society of Nephrology, COVID‐19 of dialysis patients in Japan: current status and guidance on preventive measures. Ther Apher Dial. 2020;24:361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kikuchi K, Nangaku M, Ryuzaki M, Yamakawa T, Yoshihiro O, Hanafusa N, et al. Survival and predictive factors in dialysis patients with COVID‐19 in Japan: a Nationwide cohort study. Ren Replace Ther. 2021;7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid‐19 vaccine in a Nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, Kohane IS, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID‐19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398(10316):2093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rincon‐Arevalo H, Choi M, Stefanski AL, Halleck F, Weber U, Szelinski F, et al. Impaired humoral immunity to SARS‐CoV‐2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6:eabj1031. [DOI] [PubMed] [Google Scholar]
- 9. Grupper A, Sharon N, Finn T, Cohen R, Israel M, Agbaria A, et al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2021;16:1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anand S, Montez‐Rath ME, Han J, et al. SARS‐CoV‐2 vaccine antibody response and breakthrough infection in patients receiving dialysis. Ann Intern Med. 2021. Dec;14:M21–4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sibbel S, McKeon K, Luo J, Wendt K, Walker AG, Kelley T, et al. Real‐world effectiveness and immunogenicity of BNT162b2 and mRNA‐1273 SARS‐CoV‐2 vaccines in patients on hemodialysis. J Am Soc Nephrol. 2022;33:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]


