Abstract
Aim
Our aim was to investigate the rates of preterm births, live births and stillbirths in Denmark during the first year of the COVID‐19 pandemic.
Methods
This was a national, cross‐sectional registry‐based study that used the Danish Newborn Quality database, which covers all births in Denmark. The proportions of preterm births were compared between the COVID‐19 pandemic period of 1 March 2020 to 28 February 2021 and the preceding 4‐year pre‐pandemic period.
Results
We studied 60 323 and 244 481 newborn infants from the pandemic and pre‐pandemic periods, respectively. The proportion of preterm live births and stillbirths declined slightly, from 6.29% during the pre‐pandemic period to 6.02% during the pandemic period. This corresponded to a relative risk (RR) of 0.96, with a 95% confidence interval (CI) of 0.93–0.99 during the pandemic. The RRs for extremely preterm, very preterm and moderately preterm infants were 0.88 (95% CI 0.76–1.02), 0.91 (95% CI 0.82–1.02) and 0.97 (95% CI 0.93–1.01), respectively.
Conclusion
This comparative study showed a small reduction in just over 4%, from 6.29 to 6.02% in the proportion of all preterm births during the pandemic period, compared with the previous four pandemic‐free years. There were no differences between subcategories of preterm births.
Keywords: COVID‐19, Denmark, pandemic, preterm infants, stillbirths
Abbreviations
- CI
confidence interval
- DNQ
Danish Newborn Quality Database
- EPT
extremely preterm infants
- RR
risk ratio
- SGA
small for gestational age
Key notes.
This national registry‐based study compared 60 323 infants born during the first year of the COVID‐19 pandemic to the 244 481 newborn infants born during the previous 4 years.
Preterm live births and stillbirths were slightly lower in Denmark during the pandemic, than in the pre‐pandemic period, falling from 6.29% to 6.02% of all births.
There were no clear differences in the subcategories of preterm births between the two periods.
1. INTRODUCTION
Healthcare systems all over the world have reported direct and indirect consequences since the World Health Organization declared COVID‐19 a pandemic on 11 March 2020. The Danish Government put varying levels of restrictions in place from 12 March 2020 to 1 September 2021. The most rigorous lockdown periods were from 12 March to 15 April 2020 and 5 January to 8 February 2021.
During the first month of the pandemic, one study reported a remarkable 90% decrease in the proportion of extremely preterm (EPT) infants born alive before 28 weeks of gestation in Denmark. 1 The study compared the proportion of EPT infants with similar periods in the preceding 5 years. These findings, which were published online in August 2020, received substantial scientific, public and political attention. However, the results were regarded as preliminary, due to the study limitations. The analyses included the number of singletons who had survived for 3 days after being admitted to neonatal wards, but other possible perinatal outcomes, such as intrauterine death or death in the labour ward, were not included. It is important to include all stillbirths and live births when evaluating changes in the rates of preterm birth, together with any competing outcomes, if possible. These include the proportion of infants born small for gestational age (SGA) at higher gestational ages and the survival rates of liveborn infants. The study group who produced the first Danish paper 1 subsequently expanded their analyses, by including information on all liveborn infants and the perinatal mortality rates. They still found a significant lower proportion of EPT infants by approximately 70%. 2
A national Swedish study did not replicate this significant decline in EPT births in the first months of the COVID‐19 pandemic. 3 A meta‐analysis comprised 31 studies on maternal and foetal outcomes during the first months of the COVID‐19 pandemic. 4 The rate of preterm births, before 37 weeks of gestation, declined slightly in high‐resource countries. However, a specific decrease in the proportion of EPT infants was not found. Although 12 of the studies that were included in the analysis showed a significant increase in the rate of stillbirths, only a few studies reported stillbirths and preterm births from the same cohorts. An important finding of the meta‐analysis was that some of the obstetric and perinatal outcomes showed disparities between high‐resource and low‐resource settings. The meta‐analysis also underlined the need to treat the results of studies investigating preterm birth during the pandemic with caution. This is because the generalisability of an individual study might be limited.
The aim of this study was to expand on the early findings from Denmark, 1 , 2 by focusing on a longer period. The primary aim was to investigate the rates of preterm birth in Denmark during the first year of the COVID‐19 pandemic. We did this by comparing data from the pandemic period of March 2020 to February 2021 with the same dates in 2016 to 2020. The study also included comparisons of live births and stillbirths, survival rates and other measures, such as rates of SGA and admissions to the neonatal wards.
2. METHODS
2.1. Study design and participants
This national, cross‐sectional study was based on Danish register data. We compared the proportions of preterm births during the two time periods. These were the 1‐year pandemic period from 1 March 2020 to 28 February 2021 and the preceding 4‐year non‐pandemic period from 1 March 2016 to 28 February 2020. Data were obtained from the Danish Newborn Quality Database (DNQ), which was established in 2016. 5 All Danish citizens receive a unique personal identification number shortly after birth, and this can be used to link data from the various national registers. All the data in the DNQ that we used for this study came from the Danish National Patient Register. 6 The DNQ covers all infants born in Denmark and is based on mandatory information reported to the health registers by clinicians. All infants born preterm from 22 completed weeks of gestation to 36 completed weeks and 6 days were included in the analyses. Infants from multiple pregnancies were included, but these could not be differentiated from singletons because the DNQ data did not specify whether the infants were singletons or not. Infants were excluded if they were delivered after a medical termination. These are rare in Denmark after 21 weeks and 6 days, but a few infants might have been delivered after they completed 22 gestational weeks. SGA was defined as a birthweight Z‐score below −2 standard deviations, and these were calculated for infants born at 24 weeks or more, according to Niklasson et al. 7 The authors do not provide data below that age, so we were not able to calculate SGA for more premature infants. Gestational age was primarily estimated by first‐trimester ultrasound scans, which are performed for more than 92% of pregnancies in Denmark. 8
2.2. Data analysis
This study used aggregated, anonymised data from the DNQ to compare the two study periods. First, we compared the rates of all preterm live births and stillbirths during the two periods. Three gestational age categories were also analysed: EPT infants from 22 to 27 weeks and 6 days, very preterm infants from 28 to 31 weeks and 6 days and moderately preterm infants from 32 to 36 weeks and 6 days. We also compared the proportion of preterm infants admitted to neonatal wards, infants who were born SGA and those who survived to a postmenstrual age of 44 weeks.
The aggregated anonymised data were used with the permission of the management secretariat of the Danish Clinical Quality Program—National Clinical Registries. 9
The risks for the outcomes during the pandemic period were compared with the risks during the pre‐pandemic period using risk ratios (RRs) with 95% confidence intervals (CIs). The absolute risk differences were also calculated with 95% CIs. The RRs for each month in the 1‐year pandemic period were calculated for each preterm sub‐category to demonstrate variations from month to month. OpenEpi 10 was used to calculate the RRs, risk differences and CIs.
3. RESULTS
There were 60 332 live births in Denmark during the 1‐year pandemic period and 244 481 live birth during the 4‐year pre‐pandemic period. The final cohort comprised 60 323 and 244 363 infants, respectively, as we excluded 9 and 98 for missing gestational age.
We found that 6.02% were born preterm during the pandemic period, compared to 6.29% during the pre‐pandemic period, which was a reduction of 0.27%. These pandemic and pre‐pandemic preterm births were as follows: EPT (0.36% vs. 0.40%), very preterm (0.62% vs. 0.68%) and moderately preterm (5.05% vs. 5.20%).
The decline in overall preterm births, from 6.29% in the pre‐pandemic period to 6.02% during the pandemic period, equated to a RR of 0.96 (95% CI 0.93–0.99) and a risk difference of −0.26% (95% CI −0.48 to −0.05) (Table 1). Small decreases were also found in the proportion of liveborn preterm SGA infants and in admissions to neonatal wards, but the survival rates were similar in the two periods for all the preterm births (Table 2).
TABLE 1.
Preterm births: risk ratios comparing the pandemic with the pre‐pandemic period
| Pandemic | Pre‐pandemic | Pandemic vs. pre‐pandemic period | ||||
|---|---|---|---|---|---|---|
| Total births, n = 60 323 | Total births, n = 244 363 | Risk ratio (95% CI) | Risk difference, per mille (95% CI) | |||
| Events (n) | Prevalence per 1000 | Events (n) | Prevalence per 1000 | |||
| GA 22–27 weeks + 6 days, all | 214 | 3.55 | 982 | 4.02 | 0.88 (0.76–1.02) | −0.47 (−1.01 to 0.07) |
| Live born | 150 | 2.49 | 711 | 2.91 | 0.85 (0.72–1.02) | −0.42 (−0.87 to 0.03) |
| Stillbirths | 64 | 1.06 | 271 | 1.11 | 0.96 (0.73–1.26) | −0.05 (−0.34 to 0.24) |
| GA 28–31 weeks + 6 days, all | 374 | 6.20 | 1663 | 6.80 | 0.91 (0.82–1.02) | −0.61 (−1.31 to 0.10) |
| Live born | 349 | 5.78 | 1575 | 6.44 | 0.90 (0.80–1.01) | −0.66 (−1.34 to 0.02) |
| Stillbirths | 25 | 0.41 | 88 | 0.36 | 1.15 (0.74–1.79) | 0.05 (−0.12 to 0.23) |
| GA 32–36 weeks + 6 days, all | 3044 | 50.46 | 12 712 | 52.00 | 0.97 (0.93–1.01) | −1.56 (−3.52 to 0.40) |
| Live born | 3003 | 49.77 | 12 564 | 51.39 | 0.97 (0.93–1.01) | −1.63 (−3.58 to 0.31) |
| Stillbirths | 41 | 0.68 | 148 | 0.61 | 1.12 (0.79–1.59) | 0.07 (−0.16 to 0.30) |
| GA 22–36 weeks + 6 days, all | 3632 | 60.21 | 15 357 | 62.85 | 0.96 (0.93–0.99) | −2.64 (−4.76 to −0.51) |
| Live born | 3502 | 58.05 | 14 850 | 60.77 | 0.96 (0.92–0.99) | −2.72 (−4.81 to −0.62) |
| Stillbirths | 130 | 2.16 | 507 | 2.07 | 1.04 (0.86–1.26) | 0.08 (−0.33 to 0.49) |
Note: Pandemic period: the 1‐year period from 1 March 2020 to 28 February 2021. Pre‐pandemic period: the 4‐year period 1 March 2016 to 28 February 2020. Pandemic period: total births: 60 323 (9 with missing gestational age were excluded). Pre‐pandemic period: total births: 244 363 (98 with missing gestational age were excluded).
TABLE 2.
SGA, admission and survival in liveborn preterm infants, risk ratios comparing pandemic with the pre‐pandemic period
| Pandemic | Pre‐pandemic | Pandemic vs. pre‐pandemic period | ||
|---|---|---|---|---|
| Total live born: 60 143 | Total live born: n = 243 592 | Risk ratios (95% CI) | Risk difference % (95% CI) | |
| GA 22–27 weeks + 6 days, live born | n = 150 | n = 711 | ||
| SGA | 10.7% (n = 16) | 13.2% (n = 94) | 0.81 (0.49–1.33) | −2.6 (−8.1 to 3.0) |
| Admitted | 81.3% (n = 122) | 85.1% (n = 605) | 0.96 (0.88–1.04) | −3.8 (−10.5 to 3.0) |
| Survival | 70.7% (n = 106) | 70.2% (n = 499) | 1.01 (0.90–1.13) | 0.5 (−7.5 to 8.5) |
| GA 28–31 weeks + 6 days, live born | n = 349 | n = 1575 | ||
| SGA | 13.2% (n = 46) | 17.0% (n = 268) | 0.77 (0.58–1.04) | −3.8 (−7.8 to 0.2) |
| Admitted | 96.6% (n = 337) | 97.8% (n = 1541) | 0.99 (0.97–1.01) | −1.3 (−3.3 to 0.8) |
| Survival | 97.4% (n = 340) | 96.3% (n = 1517) | 1.01 (0.99–1.03) | 1.1 (−0.8 to 3.0) |
| GA 32–36 weeks + 6 days, live born | n = 3003 | n = 12 564 | ||
| SGA | 8.4% (n = 253) | 9.4% (n = 1175) | 0.90 (0.79–1.03) | −0.9 (−2.0 to 0.2) |
| Admitted | 61.3% (n = 1842) | 63.8% (n = 8015) | 0.96 (0.93–0.99) | −2.5 (−4.4 to −0.5) |
| Survival | 99.6% (n = 2992) | 99.4% (n = 12 492) | 1.00 (1.0–1.01) | 0.2 (0–0.5) |
| GA 22–36 weeks + 6 days, live born | n = 3505 | n = 14 850 | ||
| SGA | 9.0% (n = 315) | 10.4% (n = 1537) | 0.87 (0.77–0.97) | −1.4 (−2.4 to −0.3) |
| Admitted | 65.4% (n = 2301) | 68.4% (n = 10 161) | 0.96 (0.93–0.99) | −2.8 (−4.5 to −1.0) |
| Survival | 98.1% (n = 3438) | 97.7% (n = 14 508) | 1.00 (1.00–1.01) | 0.4 (−0.1 to 0.9) |
Note: Pandemic period: 9 were excluded because of missing GA. Pre‐pandemic: 94 were excluded because of missing GA.
Abbreviation: SGA, small for gestational age.
We also looked at the subcategories of the preterm births and found that the proportions of EPT, very preterm and moderately preterm infants were lower in the pandemic period. However, the RRs were not statistically significant: EPT births (RR 0.88, 95% CI 0.76–1.02), very preterm births (RR 0.91, 95% CI 0.82–1.02) and moderately preterm births (RR 0.97, 95% CI 0.93–1.01) (Table 1). No differences were found in the liveborn infants in these subcategories between the two periods, with regard to the proportion of infants born SGA, admissions to the neonatal wards and survival (Table 2). Figure 1 shows the relative risks for combined liveborn and stillborn EPT infants by month during the pandemic period and pre‐pandemic period. These data suggest that the RRs during the first Danish lockdown period were 0.44 (95% CI 0.23–0.85) in March 2020 and 0.53 (95% CI 0.29–0.98) in April 2020.
FIGURE 1.
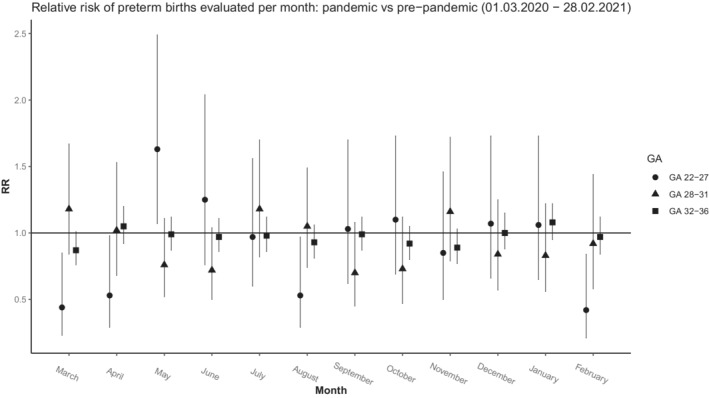
Relative risk of preterm births evaluated per month: pandemic versus pre‐pandemic (1 March 2020–28 February 2021)
4. DISCUSSION
This Danish study was based on the National Danish Newborn Quality Database. It covered all infants born in Denmark during the 1‐year pandemic period from 1 March 2020 to 28 February 2021 and the previous four pre‐pandemic years. We found that there was a small decline in just over 4% in the proportion of preterm births, including all live births and stillbirths, during the pandemic period. Furthermore, the proportion of preterm infants born SGA was lower during the pandemic period, whereas the survival rates among the liveborn infants were similar in the two periods. In the three subcategories of gestational age at birth, namely EPT, very preterm and moderately preterm, the proportions of live births and stillbirths did not differ significantly between the two periods.
Our study was prompted by two previous Danish studies, which suggested that there had been a large decrease in the proportion of liveborn EPT singletons during the first Danish lockdown period, 1 , 2 but no overall changes in preterm births. Our study replicated a decline in the rate of EPT births during this specific 2‐month period, but the aim was to include a higher number of births to provide more robust estimates by investigating information from a longer pandemic period. This meant that our study analysed both restricted and less restricted lockdown periods as one period and a true decline in EPT births due to factors related to the degree of lockdown may have been diluted. Conversely, our risk of type‐2 errors from studying subcategories of preterm births with very few infants, such as infants born EPT, was less pronounced.
Our finding that there was a small overall reduction in the rate of preterm infants born nationally during the pandemic period may not have just been due to chance. Most of the published studies from high‐resource settings appear to point in the same direction, 11 although there has been criticism of possible publication bias. 12 Furthermore, most of the studies that have investigated preterm birth during the COVID‐19 pandemic could be criticised for their methodological limitations. This is because they were mostly based on information from healthcare facilities that did not necessarily represent the general population. 13 There have only been a few population‐based studies, 2 , 3 , 14 , 15 and some of these were Nordic population‐based studies that included data on both live births and stillbirths from well‐defined geographic areas. A study that included population‐based data on live births and stillbirths in Norway, Sweden and Denmark has also been published. 16 This registry‐based study used the difference‐in‐differences method to analyse births from January 2014 to December 2020, before and after the introduction of COVID‐19 restrictions. The data were analysed for the 2 weeks before 12 March 2020, and the day after a global pandemic was declared, and 16 weeks after that date. This showed no reductions in the overall proportion of all preterm live births and stillbirths or of births below 32 weeks of gestation. The study also found a slight decline in all three countries for the rate of preterm births from 2014 to 2020. Thus, our findings of a small overall reduction in the rate of preterm births may be partially explained by a tendency towards a decline that was already present before the pandemic.
The pathways leading to preterm birth are poorly understood, but there are well‐established risk factors, 17 and some of these might be affected during a pandemic. For example, psychosocial, medical, environmental and lifestyle factors may be in play. It has been shown that the risk of preterm birth and perinatal death may be increased in hospitalised pregnant women with COVID‐19. 18 This was also found in a Nordic study that included data from Denmark, 19 but until October 2020, we have data that show that only a few pregnant women were admitted to Danish hospitals due to COVID‐19. 20 Another study found that most of the preterm births in pregnant women with COVID‐19 were iatrogenic. 21 It is possible that a small change in interventions by professionals might have influenced the association between COVID‐19 and preterm births. Change in the pattern of other non‐genital infections was pronounced during the first year of the pandemic and infections such as influenza declined dramatically after the introduction of COVID‐19 restrictions. 22 , 23 This may also have influenced the preterm birth rates. 24 , 25 A change in preterm birth rates during lockdown depends on many factors, and future studies should investigate potential causal factors for such changes.
The strengths of our study were that this was a national cohort using unbiased information on the preterm birth rates for both live births and stillbirths. The gestational ages were based on reliable estimates, and we included additional relevant measures, such as the proportion of infants born SGA and the overall survival by all subcategories of gestational ages.
The limitations were the relatively few births in Denmark and the varying levels of restrictions during the period we analysed. Furthermore, we calculated the risk of preterm births using the total number of births as the denominator instead of the number of pregnant women with risks of specific outcomes. This could be considered a limitation. It is important to point out that we had no information on the putative causes to explain any potential differences in the rates of preterm birth between the two periods.
5. CONCLUSION
This study compared preterm live births and stillbirths during a 1‐year period of the COVID‐19 pandemic and a 4‐year pre‐pandemic period in Denmark. We found that the proportion of preterm births showed a very small reduction of just over 4%, from 6.29% before the pandemic to 6.02% during the first year of the pandemic. There was a small decline in the proportion of infants born SGA and similar survival rates among the liveborn infants.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Mølholm Hansen B, Cueto H, Padkaer Petersen J, Zachariassen G, Sønderby Christensen P, Breindahl M, Preterm birth rates were slightly lower in Denmark during the first year of the COVID‐19 pandemic compared with the previous 4 years. Acta Paediatr. 2022;00:1–6. 10.1111/apa.16401
REFERENCES
- 1. Hedermann G, Hedley PL, Bækvad‐Hansen M, et al. Danish premature birth rates during the COVID‐19 lockdown. Arch Dis Child Fetal Neonatal Ed. 2021;106:93‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hedley PL, Hedermann G, Hagen CM, et al. Preterm birth, stillbirth and early neonatal mortality during the Danish COVID‐19 lockdown. Eur J Pediatr. 2022;181(3):1175‐1184. doi: 10.1007/s00431-021-04297-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pasternak B, Neovius M, Söderling J, et al. Preterm birth and stillbirth during the COVID‐19 pandemic in Sweden: a Nationwide cohort study. Ann Intern Med. 2021;174(6):873‐875. doi: 10.7326/M20-6367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chmielewska B, Barratt I, Townsend R, et al. Effects of the COVID‐19 pandemic on maternal and perinatal outcomes: a systemic review and meta‐analysis. Lancet Glob Health. 2021;9(6):e759‐e772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Accessed May 16, 2022. https://www.rkkp.dk/kvalitetsdatabaser/databaser/dansk‐kvalitetsdatabase‐for‐nyfodte/
- 6. Schmidt M, Schmidt SA, Sandegaard JL, et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Niklasson A, Bertsson‐Wikland K. Continuous growth reference from 24th week of gestation to 24 months by gender. BMC Pediatr. 2008;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Danish Fetal Medicine Database . Annual report 2018 [Internet]. Accessed March 18, 2020. http://www.dfms.dk/images/foetodatabase/Arsrapport_FOTO_2015final_anonymiseret
- 9.Accessed May 16, 2022. https://www.rkkp.dk/
- 10. Dean AG, Sullivan KM, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version. Accessed April 6, 2013. www.OpenEpi.com
- 11. Vaccaro C, Mahmoud F, Aboulatta L, Aloud B, Eltonsy S. The impact of COVID‐19 first wave national lockdowns on perinatal outcomes: a rapid review and meta‐analysis. BMC Pregnancy Childbirth. 2021;21(1):676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Greisen G, Chalak L, Hansen ML, Rasmussen MI. The impact of the COVID pandemic on prematurity rates: conflicting results, publication ethics and academic frustration. Acta Paediatr. 2022;111(2):269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rasmussen MI, Hansen ML, Pichler G, et al. Extremely preterm infant admissions within the SafeBoosC‐III consortium during the COVID‐19 lockdown. Front Pediatr. 2021;9:647880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Been JV, Burgos Ochoa L, Bertens LCM, Schoenmakers S, Steegers EAP, Reiss IKM. Impact of COVID‐19 mitigation measures on the incidence of preterm birth: a national quasi‐experimental study. Lancet Public Health. 2020;5(11):e604‐e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gemmill A, Casey JA, Catalano R, Karasek D, Margerison CE, Bruckner T. Changes in preterm birth and caesarean deliveries in the United States during the SARS‐CoV‐2 pandemic. Paediatr Perinat Epidemiol. 2021. 10.1111/ppe.12811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oakley LL, Örtqvist AK, Kinge J, et al. Preterm birth after the introduction of COVID‐19 mitigation measures in Norway, Sweden and Denmark: a registry‐based difference‐in‐differences study. Am J Obstet Gynecol. 2022;226(4):550.e1‐550.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frey HA, Klebanoff MA. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonatal Med. 2016;21(2):68‐73. [DOI] [PubMed] [Google Scholar]
- 18. Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID‐19) during pregnancy: a systematic review and meta‐analysis. Am J Obstet Gynecol MFM. 2020;2(2):100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Engjom H, Aabakke AJM, Klungsøyr K, et al. COVID‐19 in pregnancy‐characteristics and outcomes of pregnant women admitted to hospital because of SARS‐CoV‐2 infection in the Nordic countries. Acta Obstet Gynecol Scand. 2021;100(9):1611‐1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aabakke AJM, Krebs L, Petersen TG, et al. SARS‐CoV‐2 infection in pregnancy in Denmark‐characteristics and outcomes after confirmed infection in pregnancy: a nationwide, prospective, population‐based cohort study. Acta Obstet Gynecol Scand. 2021;100(11):2097‐2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta‐analysis. BMJ. 2020;370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Emborg HD, Carnahan A, Bragstad K, et al. Abrupt termination of the 2019/2020 influenza season following preventive measures against COVID‐19 in Denmark, Norway and Sweden. Euro Surveill. 2021;26(22):2001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hills T, Kearns N, Kearns C, Beasley R. Influenza control during the COVID‐19 pandemic. Lancet. 2020;396(10263):1633‐1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meijer WJ, van Noorwijk AGA, Bruinse HW, et al. Influenza virus in pregnancy: a review. Acta Obstet Gynecol Scand. 2015;94:797‐819. [DOI] [PubMed] [Google Scholar]
- 25. Fell DB, Savitz DA, Kramer MS, et al. Maternal influenza and birth outcomes: systematic review of comparative studies. BJOG. 2017;124(1):48‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]


