Abstract
To investigate the vaccination status and adverse reactions to the COVID‐19 vaccine among pregnant women in Japan, we conducted an online questionnaire survey from October 5 to November 22, 2021. The number of participants in the online survey was 6576. Of the participants, 4840 (73.6%) were vaccinated twice, and 557 (8.5%) were vaccinated once. A total of 1179 (17.9%) responders had never been vaccinated against COVID‐19. The most frequent adverse reaction was local pain at the injection site. The incidence of local adverse reactions was almost identical after the first and the second vaccinations, while systemic reactions, such as fever and fatigue/malaise, and adverse reactions outside the vaccination site such as headache and arthralgia, were more frequent after the second vaccination than after the first vaccination. Regarding the obstetrical complications, uterine tension and/or contraction was observed in 1.65% of the pregnant women after the first vaccination and in 2.98% after the second vaccination, and uterine pain appeared in 1.06% of the pregnant women after the second vaccination. However, serious symptoms, such as hemorrhage, decreased fetal movement, edema, increased blood pressure, and amniorrhexis, were seen in less than 1% of vaccinated women after both the first and second vaccinations. This study clarified the characteristics of vaccination, adverse reactions, and obstetrical symptoms in pregnant women in Japan who had the COVID‐19 vaccine up to the second dose. As a booster vaccination is currently underway, further study is needed to improve the management of pregnant women during the current pandemic.
Keywords: adverse reaction, COVID‐19, mRNA vaccine, pregnancy, vaccination
Introduction
The coronavirus disease 2019 (COVID‐19) pandemic has been a serious public health concern in the past 2 years. Although vertical transmission of COVID‐19 has been quite rare, pregnant women are reported to be at an increased risk for severe COVID‐19 compared to nonpregnant women. 1 In Japan, the COVID‐19 vaccination program was started in March 2021 using the Pfizer‐Biotech mRNA vaccine (BNT162b2) and the Moderna mRNA vaccine (mRNA‐1273). Reports from countries or areas that were already vaccinating pregnant women showed no increase in the rates of adverse reactions or pregnancy complications and no adverse effect on the fetuses or neonates. 2 The World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC) recommended that pregnant women should be vaccinated against SARS‐CoV‐2 because the high risk of severe disease increases when pregnant women are infected with SARS‐CoV‐2. 3 , 4 , 5 Therefore, a COVID‐19 vaccination program has been proposed for pregnant women in Japan. However, since the mRNA vaccine is a new vaccine, some people were concerned about its safety, especially regarding the adverse reactions and the timing for vaccinating pregnant women. In this study, we conducted a web‐based questionnaire survey to investigate the status and adverse reactions of the COVID‐19 vaccination among pregnant women in Japan.
Methods
Study design and participants
A cross‐sectional study was conducted using an online national survey for pregnant women in Japan between October 5 and November 22, 2021, through the “Baby‐plus” application (HEARZEST Co., Ltd.). Baby‐plus is an application developed for pregnant women under the supervision of the Japan Society of Obstetrics and Gynecology (JSOG). Participants older than 20 years or married minors older than 16 years were recruited for the present study. Informed consent to participate in this study was obtained from all of the potential participants prior to answering the questionnaire. The information that was sent on the internet was encrypted and converted into data through a secure server without individual information. This study was approved by the Ethics Committee of Nihon University School of Medicine (Approval number: 2021‐04‐01). All procedures were performed in accordance with the guidelines of our institutional ethics committee and adhered to the tenets of the Declaration of Helsinki.
Survey questions
The online survey questionnaires included the characteristics and socioeconomic status of the pregnant women, such as age, weeks of gestation, primipara, fetal number, pregnancy method, complications during pregnancy, and employment status. The COVID‐19 vaccine questions that were asked included: “Have you been vaccinated against COVID‐19?” “How many doses of the COVID‐19 vaccine have you had?” and “Which type of the COVID‐19 vaccine did you receive?” The adverse reactions after the first and the second COVID‐19 vaccines included pain and swelling at the inoculation site, fever, fatigue or malaise, headache, being uncomfortable and/or vomiting, diarrhea, abdominal pain, arthralgia, rash, sore throat, and anaphylactic reaction, and the obstetric and gynecological symptoms included uterine tension and/or contraction, genital bleeding, lower abdominal pain, the onset of labor, decreased fetal movement, genital or systemic edema, increased blood pressure, amniorrhexis, and other symptoms.
Statistical analysis
The descriptive statistics were expressed as numbers and percentages for the sociodemographic factors, pregnancy‐related factors, adverse reactions, and obstetric and gynecological symptoms after the first and second vaccinations in the pregnant participants. The differences in the sociodemographic factors and pregnancy‐related factors between the participants with and without the COVID‐19 vaccine were analyzed using the chi‐square test or Fisher's exact test. The univariate and multivariable logistic regression models were analyzed to the related factor with and without the COVID‐19 vaccine. The statistics were expressed as crude odds ratio (cOR) and adjusted odds ratio (aOR) and 95% confidence interval (95% CI). Adverse reaction or obstetric and gynecological symptoms after the first and second COVID‐19 vaccine was evaluated. To avoid the influence of sample size, the effect size of the chi‐square test, Cramer's V ≥ 0.1: small, ≥0.3: medium, ≥0.5: large, was calculated to compare adverse reactions after the first and second vaccination.
All statistical analyses were performed using an assumed type I error rate of 0·05. Statistical analyses were performed using IBM SPSS Statistics 27 for Windows (IBM Japan).
Results
Characteristics of the responders
The flow diagram of the present study is shown in Figure 1. The number of participants in the study was 6576. Of the participants, 4840 (73.6%) were vaccinated twice, and 557 (8.5%) were vaccinated at least once. A total of 1179 (17.9%) responders had never been vaccinated against COVID‐19. The patients who gave insufficient answers to the questionnaire were excluded. The characteristics of the pregnant women with and without vaccinations are shown in Table 1.
FIGURE 1.
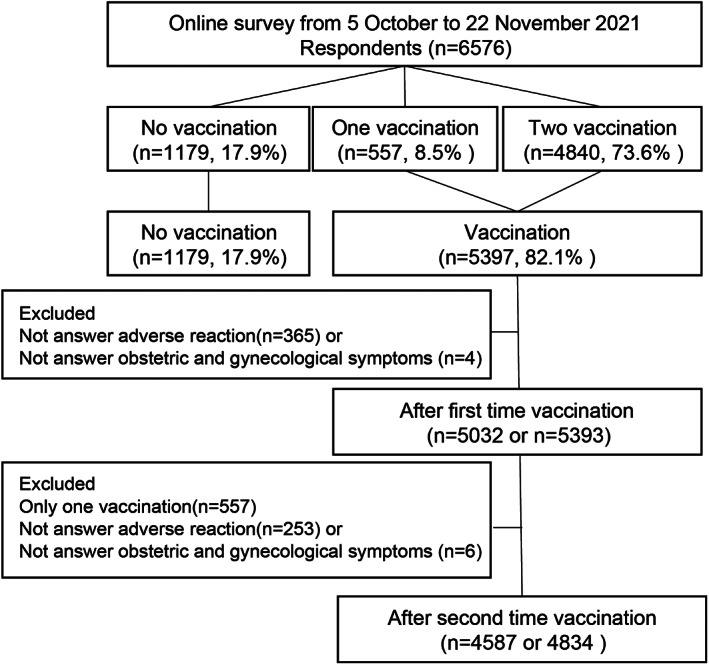
Flow diagram of the present study
Table 1.
Characteristics of pregnant women with and without vaccination
| Total (n = 6576) | Vaccination (−) (n = 1179) | Vaccination (+) (n = 5397) | p‐Value a | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age group | |||||||
| <20 years | 18 | 0.3 | 10 | 0.8 | 8 | 0.1 | <0.001 |
| 20–29 | 1837 | 27.9 | 373 | 31.6 | 1464 | 27.1 | |
| 30–39 | 4202 | 63.9 | 707 | 60.0 | 3495 | 64.8 | |
| ≧40 | 519 | 7.9 | 89 | 7.5 | 430 | 8.0 | |
| Occupation | |||||||
| Housewife | 1514 | 23.0 | 296 | 25.1 | 1218 | 22.6 | <0.001 |
| Employee | 2470 | 37.6 | 423 | 35.9 | 2047 | 37.9 | |
| Public servant | 449 | 6.8 | 68 | 5.8 | 381 | 7.1 | |
| Self employed | 218 | 3.3 | 57 | 4.8 | 161 | 3.0 | |
| Education | 148 | 2.3 | 22 | 1.9 | 126 | 2.3 | |
| Health care workers | 889 | 13.5 | 114 | 9.7 | 775 | 14.4 | |
| Part‐time | 764 | 11.6 | 164 | 13.9 | 600 | 11.1 | |
| Others | 124 | 1.9 | 35 | 3.0 | 89 | 1.6 | |
| Weeks of gestation b | |||||||
| Early pregnancy (≤15 weeks) | 1456 | 22.2 | 358 | 30.4 | 1098 | 20.4 | <0.001 |
| Mid‐pregnancy (16–27 weeks) | 2177 | 33.2 | 359 | 30.5 | 1818 | 33.7 | |
| Late pregnancy (≥28 weeks) | 2933 | 44.7 | 460 | 39.1 | 2473 | 45.9 | |
| Primipara | |||||||
| Yes | 3780 | 57.5 | 655 | 55.6 | 3125 | 57.9 | 0.769 |
| No | 2796 | 42.5 | 524 | 44.4 | 2272 | 42.1 | |
| Fetal number | |||||||
| Single | 6527 | 99.3 | 1171 | 99.3 | 5356 | 99.2 | 0.140 |
| Twins or triplets | 49 | 0.7 | 8 | 0.7 | 41 | 0.8 | |
| Pregnancy | |||||||
| Natural | 5540 | 84.2 | 1014 | 86.0 | 4526 | 83.9 | 0.123 |
| Artificial insemination | 224 | 3.4 | 31 | 2.6 | 193 | 3.6 | |
| In vitro fertilization | 812 | 12.3 | 134 | 11.4 | 678 | 12.6 | |
| Complications during pregnancy b | |||||||
| All | 1655 | 25.8 | 275 | 24.0 | 1380 | 26.2 | 0.116 |
| COVID‐19 | 42 | 0.7 | 18 | 1.6 | 24 | 0.5 | <0.001 |
| Gestational hypertension | 47 | 0.7 | 10 | 0.9 | 37 | 0.7 | 0.544 |
| Gestational diabetes mellitus | 257 | 4.0 | 37 | 3.2 | 220 | 4.2 | 0.136 |
| Anemia | 786 | 12.3 | 128 | 11.1 | 658 | 12.5 | 0.209 |
| Threatened premature delivery | 596 | 9.3 | 109 | 9.5 | 487 | 9.2 | 0.791 |
| Others | 180 | 2.8 | 22 | 1.9 | 158 | 3.0 | 0.044 |
| Diseases under treatment b | |||||||
| All | 1071 | 17.6 | 157 | 14.7 | 914 | 18.2 | 0.007 |
| Asthma | 131 | 2.1 | 20 | 1.9 | 111 | 2.2 | 1.000 |
| Malignancy | 3 | 0.0 | 0 | 0.0 | 3 | 0.1 | 0.425 |
| Thyroid disease | 226 | 3.7 | 31 | 2.9 | 195 | 3.9 | 0.125 |
| Autoimmune disease | 33 | 0.5 | 5 | 0.5 | 28 | 0.6 | 0.719 |
| Allergy | 292 | 4.8 | 34 | 3.2 | 258 | 5.1 | 0.007 |
| Inflammatory bowel disease | 25 | 0.4 | 3 | 0.3 | 22 | 0.4 | 0.467 |
| Hypertension | 24 | 0.4 | 5 | 0.5 | 19 | 0.4 | 0.596 |
| Diabetes mellitus | 30 | 0.5 | 5 | 0.5 | 25 | 0.5 | 0.901 |
| Heart disease | 7 | 0.1 | 2 | 0.2 | 5 | 0.1 | 0.354 |
| Kidney disease | 10 | 0.2 | 2 | 0.2 | 8 | 0.2 | 0.691 |
| Mental disorder | 118 | 1.9 | 16 | 1.5 | 102 | 2.0 | 0.252 |
| Others | 300 | 4.9 | 46 | 4.3 | 254 | 5.1 | 0.306 |
Using the chi‐squared test or Fisher's exact test
Missing values were excluded in the weeks of gestation (n = 10), complications during pregnancy (n = 160), and diseases under treatment (n = 482);
Note: Bold values presented in Tables represent statistically significant.
The vaccination information and the related factors in the pregnant women
The vaccination information and the related factors in the pregnant women are presented in Table 2.
Table 2.
The vaccination information and the related factors in the pregnant women
| Univariate analysis | p‐Value a | Multivariate analysis | p‐Value a | |||
|---|---|---|---|---|---|---|
| cOR | 95%CI | aOR | 95%CI | |||
| Age group | ||||||
| <20 years | Ref. | |||||
| 20–29 | 4.91 | 1.92–12.52 | <0.001 | 4.05 | 1.55–10.63 | 0.004 |
| 30–39 | 6.18 | 2.43–15.71 | <0.001 | 5.19 | 1.98–13.58 | <0.001 |
| ≧40 | 6.04 | 2.32–15.73 | <0.001 | 5.25 | 1.63–14.23 | 0.001 |
| Occupation | ||||||
| Housewife | Ref. | |||||
| Employee | 1.176 | 0.99–1.39 | 0.053 | 1.15 | 0.96–1.38 | 0.128 |
| Public servant | 1.362 | 1.02–1.82 | 0.035 | 1.42 | 1.04–1.94 | 0.026 |
| Self employed | 0.686 | 0.50–0.95 | 0.024 | 0.66 | 0.46–0.93 | 0.019 |
| Education | 1.392 | 0.87–2.23 | 0.168 | 1.35 | 0.83–2.20 | 0.228 |
| Health care workers | 1.652 | 1.31–2.09 | <0.001 | 1.58 | 1.23–2.03 | <0.001 |
| Part‐time | 0.889 | 0.72–1.10 | 0.283 | 0.93 | 0.74–1.18 | 0.562 |
| Others | 0.618 | 0.41–0.93 | 0.022 | 0.69 | 0.44–1.10 | 0.120 |
| Weeks of gestation b | ||||||
| Early pregnancy (≤15 weeks) | Ref. | |||||
| Mid‐pregnancy (16–27 weeks) | 1.651 | 1.40–1.95 | <0.001 | 1.65 | 1.38–1.97 | <0.001 |
| Late pregnancy (≥28 weeks) | 1.753 | 1.50–2.05 | <0.001 | 1.69 | 0.42–2.01 | <0.001 |
| Primipara | ||||||
| Yes | Ref. | |||||
| No | 0.91 | 0.80–1.03 | 0.140 | 0.90 | 0.78–1.03 | 0.131 |
| Fetal number | ||||||
| Single | Ref. | |||||
| Twins or triplets | 1.12 | 0.52–2.34 | 0.768 | 1.23 | 0.55–2.79 | 0.616 |
| Pregnancy | ||||||
| Natural | Ref. | |||||
| Artificial insemination | 1.395 | 0.95–2.05 | 0.091 | 1.30 | 0.85–1.97 | 0.226 |
| In vitro fertilization | 1.134 | 0.93–1.38 | 0.213 | 1.03 | 0.82–1.29 | 0.812 |
| Complications during pregnancy b | ||||||
| All | 1.13 | 0.97–1.31 | 0.116 | n/a | ||
| COVID‐19 | 0.29 | 0.16–0.53 | <0.001 | 0.30 | 0.14–0.62 | 0.001 |
| Gestational hypertension | 0.81 | 0.40–1.62 | 0.544 | 0.98 | 0.42–2.26 | 0.957 |
| Gestational diabetes mellitus | 1.31 | 0.92–1.87 | 0.137 | 1.23 | 0.82–1.86 | 0.317 |
| Anemia | 1.14 | 0.93–1.39 | 0.210 | 1.02 | 0.82–1.28 | 0.849 |
| Threatened premature delivery | 0.97 | 0.78–1.21 | 0.791 | 0.84 | 0.66–1.07 | 0.150 |
| Others | 1.58 | 1.01–2.48 | 0.046 | 1.54 | 0.93–2.55 | 0.095 |
| Diseases under treatment b | ||||||
| All | 1.29 | 1.07–1.55 | 0.007 | n/a | ||
| Asthma | 1.18 | 0.73–1.91 | 0.792 | 1.21 | 0.71–2.05 | 0.484 |
| Malignancy | n/a | |||||
| Thyroid disease | 1.35 | 0.92–1.98 | 0.126 | 1.23 | 0.82–1.85 | 0.318 |
| Autoimmune disease | 1.19 | 0.46–3.09 | 0.719 | 1.10 | 0.42–2.90 | 0.853 |
| Allergy | 1.65 | 1.14–2.37 | 0.007 | 1.66 | 1.14–2.42 | 0.008 |
| Inflammatory bowel disease | 1.56 | 0.47–5.22 | 0.470 | 1.65 | 0.49–5.62 | 0.421 |
| Hypertension | 0.81 | 0.30–12.17 | 0.670 | 0.87 | 0.27–2.79 | 0.813 |
| Diabetes mellitus | 1.06 | 0.41–2.78 | 0.901 | 1.16 | 0.39–3.44 | 0.788 |
| Heart disease | 0.53 | 0.10–2.74 | 0.449 | 0.70 | 0.13–3.69 | 0.673 |
| Kidney disease | 0.85 | 0.18–4.01 | 0.837 | 0.68 | 0.14–3.26 | 0.624 |
| Mental disorder | 1.36 | 0.80–2.32 | 0.254 | 1.24 | 0.72–2.13 | 0.432 |
| Others | 1.18 | 0.86–1.63 | 0.306 | 1.17 | 0.84–1.64 | 0.362 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; cOR: crude odds ratio.
Using the logistic regression model.
Missing values were excluded in the weeks of gestation (n = 10), complications during pregnancy (n = 160), and diseases under treatment (n = 482).
Age, occupation, weeks of gestation, COVID‐19 morbidity during pregnancy, and allergies were associated with the vaccination rate. Primipara, fetal number, and natural pregnancy or pregnancy with assisted reproductive technology (ART), including artificial insemination or in vitro fertilization did not affect the vaccination rates.
The pregnant women with the ages of 20–29, 30–39, and 40 years and older had higher vaccination rates than those under 20 years of age. The vaccination rate among public servants and healthcare workers was higher than that among housewives, while the vaccination rates among self‐employed pregnant women were lower. The pregnant women with a history of COVID‐19 were also less likely to be vaccinated. However, since we did not ask about the timing of COVID‐19 onset in this study, the relationship between SARS‐CoV‐2 infection and vaccination was unclear. Among the pregnant women with comorbid diseases under treatment, those with complicated allergic diseases had a low vaccination rate. The other pregnancy complications or diseases were not associated with the vaccination rates.
Adverse reactions
Table 3 shows the adverse reactions or the obstetric and gynecological symptoms of the pregnant women after vaccination.
Table 3.
Adverse reaction or obstetric and gynecological symptoms of pregnant women after vaccination
| First vaccination | Second vaccination | p‐Value a | Effect size b | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Adverse reactions | (n = 5032) | (n = 4587) | ||||
| Pain at the inoculation site | 4873 | 96.84 | 4248 | 92.61 | <0.001 | 0.095 |
| Swelling at the inoculation site | 1511 | 30.03 | 1470 | 32.05 | 0.032 | 0.022 |
| Fever | 608 | 12.08 | 2554 | 55.68 | <0.001 | 0.464 |
| Fatigue/malaise | 1504 | 29.89 | 3003 | 65.47 | <0.001 | 0.356 |
| Headache | 711 | 14.13 | 1756 | 38.28 | <0.001 | 0.276 |
| Uncomfortable/vomiting | 191 | 3.80 | 506 | 11.03 | <0.001 | 0.139 |
| Diarrhea | 103 | 2.05 | 137 | 2.99 | 0.003 | 0.030 |
| Abdominal pain | 54 | 1.07 | 114 | 2.49 | <0.001 | 0.054 |
| Arthralgia | 220 | 4.37 | 1022 | 22.28 | <0.001 | 0.267 |
| Rash | 51 | 1.01 | 47 | 1.02 | 0.957 | 0.001 |
| Sore throat | 30 | 0.60 | 53 | 1.16 | 0.003 | 0.030 |
| Anaphylactic reaction | 1 | 0.02 | 4 | 0.09 | 0.199 | 0.015 |
| Others | 159 | 3.16 | 240 | 5.23 | <0.001 | 0.052 |
| Obstetric and gynecological symptoms | (n = 5393) | (n = 4834) | ||||
| Uterine tension or contraction | 89 | 1.65 | 144 | 2.98 | <0.001 | 0.044 |
| Bleeding | 46 | 0.85 | 38 | 0.79 | 0.708 | 0.004 |
| Uterin pain | 23 | 0.43 | 51 | 1.06 | <0.001 | 0.037 |
| Decreased fetal movement | 23 | 0.43 | 25 | 0.52 | 0.503 | 0.007 |
| Swelling | 17 | 0.32 | 19 | 0.39 | 0.507 | 0.007 |
| Increased blood pressure | 5 | 0.09 | 7 | 0.14 | 0.442 | 0.008 |
| Amniorrhexis | 1 | 0.02 | 2 | 0.04 | 0.606 | 0.007 |
| Others | 98 | 1.82 | 108 | 2.23 | 0.134 | 0.015 |
Using the chi‐squared test or Fisher's exact test.
Effect size was calculated by Cramer's V (≥0.1: small, ≥0.3: medium, ≥0.5: large) for the chi‐square test.
The most frequent adverse reaction was pain at the site of vaccine administration (post‐first dose, 96.84%, post‐second dose, 92.61%). The incidence of local pain and swelling at the injection site was almost identical after the first and the second vaccinations. On the other hand, systemic reactions such as fever (post‐first dose 12.08%, post‐second dose 55.68%, p < 0.001, effect size 0.464) and fatigue/malaise (post‐first dose 29.89%, post‐second dose 65.47%, p < 0.001, effect size 0.356), and adverse reactions outside the vaccination site, such as headache (post‐first dose 14.13%, post‐second dose 38.28%, p < 0.001, effect size 0.276), and arthralgia (post‐first dose 4.37%, post‐second dose 22.28%, p < 0.001, effect size 0.267) were more frequent after the second vaccination than after the first vaccination. The occurrence of adverse reactions in the pregnant women was similar to that of the nonpregnant women of the same age, which is similar to the results that were previously reported. 2 Regarding the obstetric symptoms, uterine tension or contraction was observed in 1.65% of the pregnant women after the first vaccination and 2.98% after the second vaccination; uterine pain appeared in 1.06% of the pregnant women after the second vaccination. However, serious symptoms, such as hemorrhage, decreased fetal movement, edema, increased blood pressure, and amniorrhexis, were seen in less than 1% of vaccinated women after both the first and second vaccinations.
Discussion
Although cases of vertical transmission of COVID‐19 has been rare, the risk of maternal morbidity is increased, especially within the third trimester. 3 Vaccines are one of the gold standards for the prevention of infectious diseases, and fortunately, the COVID‐19 vaccine has been developed rapidly and has been clinically applied. However, since the COVID‐19 vaccine uses a novel mRNA vaccine platform, which has never been used before, some pregnant women tend to be reluctant to get vaccinated. Subsequently, reports from other countries, where vaccination had been promoted in advance, showed no increase in the number of adverse reactions or complications of pregnancy, and they have not shown any adverse effect on the fetus or newborn, even in pregnant women. 2 Therefore, the CDC decided to actively recommend that pregnant women be vaccinated with the COVID‐19 vaccine. In Japan, JSOG, 6 Japan Associate of Obstetricians and Gynecologist (JAOG), 7 and Japan Society for Infection Diseases in Obstetrics and Gynecology (JSIDOG) 8 have also jointly recommended that pregnant women get the COVID‐19 vaccine.
In the present study, the percentage of pregnant women who had two doses of vaccination was 73.6%, which was almost the same as the percentage of Japanese citizens who are vaccinated (79.6%) during the same period. In addition, approximately half of the unvaccinated pregnant women were planning to be vaccinated or wished to be vaccinated (including postpartum). In Japan, the widespread delta strain, which circulated from June to September 2021, caused a drastic increase in the number of infected women during pregnancy. At the fifth wave of the pandemic in Japan, the shortage of medical institutions accepting COVID‐19 pregnant women and tragic cases, including stillbirth caused by COVID‐19 infection, has been reported by various media. These social situations may have raised awareness of vaccination among pregnant women. Globally, the vaccination rate of pregnant women varied depending on countries. For instance, the vaccination rates among pregnant women are 80% in Norway (December 15, 2021), 9 67.1% in the United States (January 29, 2022), 10 and 20% in Italy (May–October 2021). 9
The pregnant women's vaccination rates also varied according to their occupation. The vaccination rate was higher among public servants and healthcare workers than among housewives and was even lower among the participants who were self‐employed. The vaccination programs at workplaces, including healthcare workers, are thought to be effective in promoting vaccination.
The vaccination rates also differed by the gestational week. The rates of vaccination were higher among the pregnant women in the second and third trimesters of pregnancy than among the pregnant women in the first trimester of pregnancy. At the beginning of the vaccination program, JSOG, JAOG, and JSIDOG initially recommended vaccination after 12 weeks of pregnancy, but later, they revised the recommendation to consider vaccination at any time during pregnancy. Therefore, at this point, the vaccination rate in early pregnancy may have been low.
The rate of vaccination was lower among pregnant women who had been affected with COVID‐19 during pregnancy. However, since the timing of COVID‐19 and vaccination were not investigated in this questionnaire, it is unclear whether they were infected with SARS‐CoV‐2 because they were not vaccinated or whether they did not consider getting the vaccination because they already contracted COVID‐19.
In this study, the most frequent adverse reaction after vaccination was pain at the vaccination site, which occurred in more than 90% of the patients who had both the first and second doses. Although the frequency of local adverse reactions did not vary significantly between the first and second vaccinations, systemic reactions such as fever, malaise/fatigue, and adverse reactions outside the vaccination site such as headache, gastrointestinal symptoms (nausea/vomiting, diarrhea, abdominal pain), and arthralgia were more common in the second vaccination than in the first vaccination. It has been reported that females were more likely to have adverse reactions to new coronavirus vaccinations than males, 11 and the frequency of adverse reactions in this study was comparable to a previous report in nonpregnant women. 12
The adverse effects on pregnancy are the most serious concern for pregnant women and obstetricians getting vaccinated. The results of this study showed that 3% of pregnant women experienced abdominal tightness and uterine pain after vaccination. However, serious symptoms, such as bleeding, decreased fetal movement, edema, increased blood pressure, and amniorrhexis, occurred in less than 1% of the patients. Since these symptoms are also experienced at a certain frequency in unvaccinated pregnant women, the causal relationship with the vaccine is unknown. Even if pregnant women who have been vaccinated have these symptoms, they are strongly recommended to contact their obstetrician immediately, and to have their routine obstetric check‐up.
It has been reported that pregnant women are not more susceptible to SARS‐CoV‐2 infection than nonpregnant women. 13 However, recent studies suggested that unvaccinated pregnant women have a higher risk of severe COVID‐19. 9 , 14 A report from Scotland indicated that 77.4% of SARS‐CoV‐2 infections, 90.9% of SARS‐CoV‐2 cases associated with hospital admission, and 98% of SARS‐CoV‐2 cases associated with critical care admission, as well as all perinatal deaths, occurred in pregnant women who were unvaccinated at the time of the COVID‐19 diagnosis. 14 These data encourage pregnant women and obstetricians to consider vaccination. Another recent meta‐analysis that used data collected from eight European studies suggested changing the governmental policy of vaccination to cover every pregnant woman because severe COVID‐19 in pregnancy was almost exclusively limited to unvaccinated women. 9
This study has several limitations. First, the study employed a web‐based online survey system. Therefore, the participation of pregnant women without access to the internet and those with low digital literacy will be restricted. In addition, participation in the survey was voluntary, the survey information might have drawn more attention from those who were concerned about the COVID‐19 pandemic and vaccine. Therefore, there might have been some level of self‐selection bias. Second, the survey was a cross‐sectional study that collected responses from the participants during a specific period. Therefore, the responses only reflect the information available at that time. Despite these limitations, we consider this study worthwhile because it is the first large‐scale study of vaccination of pregnant women in Japan. Last, the occurrence of uterine contractions and pain after vaccination increased more after the second vaccination than after the first dose (1.65% vs. 2.89%, and 0.43% vs. 1.06%, respectively) in the study. Since the COVID‐19 vaccinations were given twice with a 4‐week interval, the gestational week was also 4 weeks advanced at the second vaccination. While it is possible that the uterine contractions and pain were induced by inflammation as an adverse reaction to the vaccine, this result may be due to the fact that physiological uterine contractions, including pre‐term labor, are more frequent in late pregnancy. However, it is not possible to adjust for the number of weeks of gestation and analyze in this study. This is also one of the limitations of the survey.
In conclusion, this study clarified the actual situation of vaccination, the adverse reactions, and the obstetrical symptoms in pregnant women in Japan who had COVID‐19 vaccine up to the second dose. As a booster vaccination is currently underway, further study is needed to improve the management of pregnant women during the current pandemic.
Conflict of Interest
The authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Shihoko Komine‐Aizawa, Satoshi Hayakawa, and Hideto Yamada. Acquisition of data: Shihoko Komine‐Aizawa and Satoshi Hayakawa. Analysis and interpretation of data: Yasuo Haruyama, Gen Kobashi. Drafting of the manuscript: Shihoko Komine‐Aizawa, Yasuo Haruyama, Satoshi Hayakawa, Gen Kobashi. Critical revision of the manuscript for important intellectual content: Shihoko Komine‐Aizawa, Yasuo Haruyama, Masashi Deguchi, Satoshi Hayakawa, Kei Kawana, Gen Kobashi, Etsuko Miyagi, Hideto Yamada, and Takashi Sugiyama. Statistical analysis: Yasuo Haruyama, Gen Kobashi. Obtaining funding: Hideto Yamada, Takashi Sugiyama. Supervision: Yasuo Haruyama, Masashi Deguchi, Satoshi Hayakawa, Kei Kawana, Gen Kobashi, Etsuko Miyagi, Hideto Yamada, and Takashi Sugiyama. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This work was supported by the Ministry of Health, Labor, and Welfare of Japan (Grant Number 20CA2033) and the Subcommittee on Perinatal Infection of the Committee on the Perinatal Period of Japan Society of Obstetrics and Gynecology. The authors also thank American Journal Experts for their editorial assistance.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Reference
- 1. CDC . Evidence for conditions that increase risk of severe illness 2021 Published online: E‐Pub.
- 2. Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, et al. Preliminary findings of mRNA Covid‐19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. CDC . COVID‐19 vaccines while pregnant or breastfeeding. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html. Accessed 22 February.
- 4. WHO . The Pfizer BioNTech (BNT162b2) COVID‐19 vaccine: What you need to know. Available from: https://www.who.int/news-room/feature-stories/detail/who-can-take-the-pfizer-biontech-covid-19--vaccine-what-you-need-to-know. Accessed 22 February.
- 5. WHO . The Moderna COVID‐19 (mRNA‐1273) vaccine: what you need to know. Available from: https://www.who.int/news-room/feature-stories/detail/the-moderna-covid-19-mrna-1273-vaccine-what-you-need-to-know. Accessed 22 February.
- 6. Japan Society of Obstetrics and Gynecology. Available from: https://www.jsog.or.jp/news/pdf/20210814_COVID19_02.pdf. Accessed 22 February.
- 7. Japan Association of Obstetricians and Gynecologist. Available from: https://www.jaog.or.jp/wp/wp-content/uploads/2021/08/210814.pdf. Accessed 22 February.
- 8. Japan Society for Infection Diseases in Obstetrics and Gynecology. Available from: http://jsidog.kenkyuukai.jp/images/sys/information/20210816102601‐DCA4B5C45C25B46F8589FEF7A441A12F2E6DAD7AA278B25E18D9D1C303015C8D.pdf. Accessed 22 February.
- 9. Engjom H, van den Akker T, Aabakke A, Ayras O, Bloemenkamp K, Donati S, et al. Severe COVID‐19 in pregnancy is almost exclusively limited to unvaccinated women ‐ time for policies to change. Lancet Reg Health Eur. 2022;13:100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. CDC . COVID‐19 vaccination among pregnant people aged 18–49 years overall, by race/ethnicity, and date reported to CDC ‐ Vaccine Safety Datalink, United States. Available from: https://covid.cdc.gov/covid-data-tracker/#vaccinations-pregnant-women. Accessed on 22 February.
- 11. Vassallo A, Shajahan S, Harris K, Hallam L, Hockham C, Womersley K, et al. Sex and gender in COVID‐19 vaccine research: substantial evidence gaps remain. Front Glob Womens Health. 2021;2:761511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee YW, Lim SY, Lee JH, Lim JS, Kim M, Kwon S, et al. Adverse reactions of the second dose of the BNT162b2 mRNA COVID‐19 vaccine in healthcare workers in Korea. J Korean Med Sci. 2021;36(21):e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta‐analysis. BMJ. 2020;370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stock SJ, Carruthers J, Calvert C, et al. SARS‐CoV‐2 infection and COVID‐19 vaccination rates in pregnant women in Scotland. Nat Med. 2022;28(3):504–12. 10.1038/s41591-021-01666-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


