Abstract
We aimed to analyze the efficacy and safety of an inactivated SARS‐CoV‐2 vaccine in people living with HIV (PLWH). A total of 143 PLWH and 50 healthy individuals were included in this study. A commercially available magnetic chemiluminescence enzyme immunoassay kit was used to detect serum IgG and IgM antibodies against SARS‐CoV‐2. Serum levels of SARS‐CoV‐2‐specific IgG were significantly higher in the control group than in the PLWH group (p = 0.001). Overall, 76% of individuals in the control group were detected with seropositivity IgG against SARS‐CoV‐2 compared to 58% in the PLWH group (p = 0.024). In PLWH with IgG seropositivity, CD4+ T‐cell counts before antiretroviral therapy (ART) was higher (p = 0.015). Multivariable analysis indicated that CD4+ T cells at IgG detection (odds ratio [OR] = 1.004, p = 0.006) and time after vaccination (OR = 0.977, p = 0.014) were independently associated with seropositivity IgG against SARS‐CoV‐2 in PLWH. Neutralizing antibody (nAb) titers in PLWH against wild‐type SARS‐CoV‐2 were similar to those in the control group (p = 0.160). The proportion of seropositive nAbs against wild‐type SARS‐CoV‐2 was also similar (95% in the control group vs. 97% in the PLWH group, p = 0.665). Similar results were obtained when nAb was detected against the delta variants with similar titers (p = 0.355) and a similar proportion of seropositive nAbs were observed (p = 0.588). All the side effects observed in our study were mild and self‐limiting. The inactivated COVID‐19 vaccine appears to be safe with good immunogenicity in Chinese PLWH.
Keywords: COVID‐19, human immunodeficiency virus, neutralizing antibody, SARS‐CoV‐2 vaccination, side effects
Abbreviations
- ALB
albumin
- ALT
alanine aminotransferase
- ART
antiretroviral therapy
- AUROC
area under the receiver operating characteristic
- COVID‐19
Coronavirus disease 2019
- FBS
fetal bovine serum
- HBsAg
hepatitis B surface antigen
- INSTI
integrase strand transfer inhibitor
- nAb
neutralizing antibody
- PLWH
people living with HIV
- SD
standard deviation.
- S/CO
signal to the cut‐off value
1. INTRODUCTION
The goal of antiretroviral therapy (ART) in people living with HIV (PLWH) is to reduce the morbidity and mortality associated with HIV infection and prevent HIV transmission. 1 Although ART has significantly reduced the mortality rate of PLWH, improving their long‐term prognoses remains a clinical challenge. Infections, including opportunistic infections, are important factors that accelerate the natural history of HIV infection and cause morbidity and death in PLWH. Previous studies have shown that vaccination is an effective strategy for reducing infections. 2 However, due to the compromised immune system of PLWH, the immune response after vaccination is not ideal. 3
Since the coronavirus disease 2019 (COVID‐19) pandemic, there has been great interest in developing vaccines to provide immunity against SARS‐CoV‐2 infection. 4 , 5 Several vaccines have been rapidly developed and approved in different countries worldwide, and mass vaccination programs are underway. To date, the evidence does not suggest that PLWH have a markedly higher susceptibility or worse prognosis following SARS‐CoV‐2 infection, although a large, population‐based study in South Africa reported both HIV and current tuberculosis were independently associated with increased COVID‐19 mortality. 6 , 7 , 8 Moreover, some of the risk factors for severe COVID‐19, such as cardiovascular and pulmonary diseases, are more prevalent in PLWH. 9 , 10 , 11 , 12 Therefore, vaccinating PLWH against SARS‐CoV‐2 in a timely manner is vital. Nonetheless, the humoral response to vaccination has been found to be inadequate in PLWH, especially in those with low CD4+ T‐cell counts. 13 Thus, it is critical to explore the effectiveness and safety of COVID‐19 vaccines in PLWH.
Therefore, we conducted a noninterventional cross‐sectional study enrolling PLWH who received two doses of inactivated SARS‐CoV‐2 vaccine and a similarly vaccinated control group of healthy people. Our aim was to analyze the levels of IgG against SARS‐CoV‐2 and the safety of the SARS‐CoV‐2 vaccine in PLWH.
2. SUBJECTS AND METHODS
2.1. Patient population
To study the proportion of PLWH with seropositivity IgG against SARS‐CoV‐2 after receiving the inactivated vaccine, we enrolled 169 PLWHs. Of these, seven individuals had received only one dose of the inactivated vaccine and were excluded from the study. Fifteen people were excluded because they had received their second vaccination within 5 days of the start of the study. In addition, four people were excluded because of missing key clinical data. Ultimately, 143 PLWHs were included in the analysis.
The retention in care of all PLWHs in our study was as follows: all patients were confirmed to have HIV‐1 infection by Western blot analysis at the Guangzhou Disease Control Center, and all were regularly followed up at our research center. Clinical data, including demographics, biochemical markers, blood cell counts, virological markers, and comorbidities, were extracted from the electronic medical system and recorded. The exclusion criteria were as follows: (i) presence of fever, cough, and other symptoms of upper respiratory tract infection 1 month prior; (ii) treatment with oral hormones or immunosuppressive drugs; (iii) previous infection with SARS‐CoV‐2; (iv) recently received plasma replacement therapy, and (v) pregnancy. We also enrolled 50 healthy controls who were vaccinated with two doses of inactivated vaccine and at least 5 days after the second dose of the vaccine.
Among these 143 PLWHs, 54 were vaccinated with the Sinopharm SARS‐CoV‐2 vaccine (Sinopharm Inactivated Whole Virus), and a total of 65 people were vaccinated with the SinoVac SARS‐CoV‐2 vaccine (SinoVac Inactivated Whole Virus), a total of 15 people had received the Sinopharm vaccine for the first vaccination and SinoVac SARS‐CoV‐2 inactivated vaccine for the second. Nine people received the SinoVac vaccine, followed by the Sinopharm vaccine. Among the healthy controls, 28 and 22 were vaccinated with the Sinopharm and SinoVac vaccines, respectively.
To further study the neutralizing ability of HIV‐infected patients against wild‐ type and novel SARS‐CoV‐2 delta variants following vaccination with the inactivated vaccine, we analyzed 67 PLWH and 20 healthy controls with seropositivity IgG against SARS‐CoV‐2. The ethics committee of Nanfang Hospital approved this study, and all the enrolled patients provided informed consent. This study was been registered in the Chinese Clinical Trial Registry (ChiCTR2100051956).
2.2. Laboratory testing
Blood cell counts were analyzed using a Sysmex SE9000 automatic blood cell analyzer. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), and other serum biochemical parameters were measured using an Olympus AU5400 automatic biochemical analyzer. The CD4+/CD8+ lymphocyte counts were determined using a flow cytometer. HIV RNA was detected by polymerase chain reaction. All detections and evaluations were performed according to the manufacturer's instructions at the Department of the Clinical Laboratory of Nanfang Hospital.
2.3. Detection of IgG and IgM recognizing SARS‐CoV‐2
Serum samples were collected from the Department of Infectious Diseases at the Nanfang Hospital. All samples were inactivated at 56°C for 30 min and stored at −20°C before testing. We used a magnetic chemiluminescence enzyme immunoassay kit to detect serum IgG and IgM levels against SARS‐CoV‐2, according to the manufacturer's instructions (Darui Biotechnology Co., Ltd.). The detection procedure was described in a previous report. 14 The protein detected by IgG is the receptor‐binding domain of spike protein, and the protein detected by IgM is the nucleocapsid protein of SARS‐CoV‐2. The antibody level was expressed as the ratio of the chemiluminescence signal to the cut‐off value (S/CO). An IgG or IgM S/CO value higher than 1.0 was considered positive. The time after vaccination in our study referred to the length of time between vaccinations and IgG against SARS‐CoV‐2 got detected when patients enrolled.
2.4. Micro‐neutralization test
A micro‐neutralization test was performed at the BSL‐3 laboratory of the Guangdong Provincial Center for Disease Control and Prevention. VERO‐E6 cells were seeded in 96‐well plates for 2−3 days to proliferate into monolayer cells. Serum samples from patients were inactivated at 56°C for 30 min and then serially diluted fourfold (1:4 to 1:1024). Second, a total of 120 μl diluted serum was preincubated with the same volume of the SARS‐CoV‐2 suspension (100 TCID50/50 μl) for 120 min at 37°C in a 5% CO2 incubator. Third, 100 μl/well of virus‐serum mixtures were added to the prepared Vero ‐E6 cells at 37°C in a 5% CO2 incubator. After 5 days of culture, the cytopathic effect (CPE) of each well was observed and recorded under a microscope by two independent researchers (Dr. Lirong Zou and Dr. Huan Zhang). The highest dilution that protected >50% of the cells from CPE was regarded as the neutralizing antibody (nAb) titer. An nAb titer > 1:4 was defined as positive. 15 , 16 Representative images are presented in Supporting Information: Figure 1. The SARS‐COV‐2 virus 20SF014/vero‐E6/3 (WT strain) was isolated from a SARS‐COV‐2 infected patient in Shenzhen in February 2020. The SARS‐CoV‐2 delta strain is isolated from a SARS‐CoV‐2 infected patient in Guangzhou in May 2021.
2.5. Statistical analysis
Measurement units are expressed as mean ± standard deviation (SD) for normally distributed data. Categorical data were expressed as percentages. Differences in the proportion of patients with seropositivity IgG against SARS‐CoV‐2 between the PLWH and control groups were detected by the χ 2 test from crosstabulation. The differences in sex ratio, ART treatment, the proportion of patients with syphilis, HBV infection, and tuberculosis were also calculated using the χ 2 test from crosstabulation. The t test and Pearson's correlation analysis were used to compare differences in demographic and clinical data, including age, BMI, time after vaccination, CD4+ T cells, HIV RNA viral load, ART duration, ALT level, AST level, ALB level, globulin level, lymphocyte count and platelets (PLT) level. The area under the receiver operating characteristic (AUROC) curve was used to calculate the optimal cut‐off value for IgG levels. Multivariate logistic regression was performed to evaluate the independent risk factors. All analyses were performed using SPSS (version 26.0) with an alpha level of 0.05.
3. RESULTS
3.1. Baseline demographics and clinical characteristics
In total, 143 PLWHs (PLWH group) and 50 healthy controls (control group) were included. The demographic and clinical characteristics of the patients are compared and shown in Table 1. CD4+ T‐cell counts and CD4+/CD8+ ratios were significantly higher in the control group (all p < 0.001).
Table 1.
Demographics and clinical characteristics in people enrolled
| Characteristic | Control group | PLWH group | p value |
|---|---|---|---|
| Sample size, n | 50 | 143 | — |
| Sex | 0.466 | ||
| Male | 48 (96.0%) | 140 (97.9%) | |
| Female | 2 (4.0%) | 3 (2.1%) | |
| Age, years | 29.84 ± 8.51 | 32.55 ± 8.69 | 0.058 |
| Time after vaccination, days | 64.46 ± 41.22 | 35.78 ± 27.99 | <0.001 |
| CD4+ T cells, cells/μl | 916.24 ± 281.26 | 398.96 ± 202.31 | <0.001 |
| CD4+/CD8+ ratio | 1.19 ± 0.46 | 0.62 ± 0.41 | <0.001 |
Abbreviation: PLWH, people living with HIV.
3.2. Proportion of seropositive SARS‐CoV‐2‐specific IgG in PLWH
The serum level of SARS‐CoV‐2‐specific IgG was significantly higher in the control group than in the PLWH group (p = 0.001) (Figure 1A). Overall, 76% of the control group was detected seropositive SARS‐CoV‐2‐specific IgG compared to 58% in the PLWH group (p = 0.024) (Figure 1B). However, similar levels of serum IgM against SARS‐CoV‐2 were observed in both groups (p = 0.346) (Figure 1C), and we found no significant difference in the proportion of patients with seropositive SARS‐CoV‐2‐specific IgM (2.0% in the control group vs. 3.5% in the PLWH group, p = 0.60) (Figure 1D).
Figure 1.
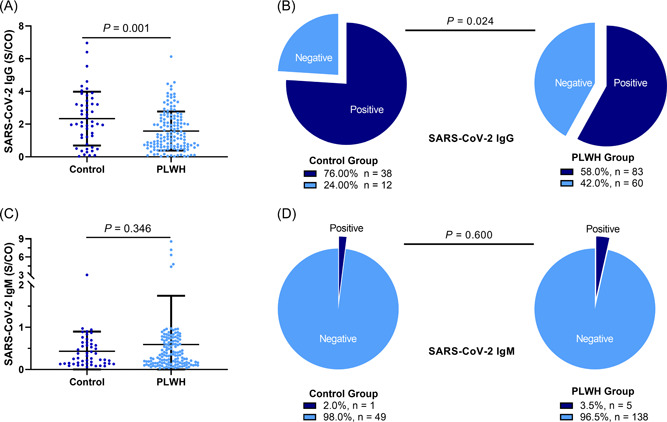
Proportion of patients with seropositivity IgG. (A) Serum levels of SARS‐CoV‐2‐specific IgG were significantly higher in the control group than in the people living with HIV (PLWH) group (2.33 ± 1.65 vs. 1.58 ± 1.19, p = 0.001). (B) The proportion of people with seropositivity IgG against SARS‐CoV‐2 in the control group was higher than in the PLWH group (76% vs. 58%, p = 0.024). (C) Levels of serum IgM against SARS‐CoV‐2 were similar in both groups (0.43 ± 0.47 vs. 0.59 ± 1.15, p = 0.346). (D) The proportion of people with IgM positivity was similar in both groups (2.0% vs. 3.5%, p = 0.60). PLWH, people living with HIV.
3.3. Clinical variables associated with seropositive SARS‐CoV‐2‐specific IgG in PLWH
Several variables differed between PLWH who were IgG seropositive or seronegative. The time after vaccination in the seronegative group was significantly longer (43.38 ± 34.96 days vs. 30.27 ± 20.12 days, p = 0.005). In PLWH with seropositive SARS‐CoV‐2‐specific IgG, CD4+ T‐cell counts before ART (p = 0.015) were higher (Table 2).
Table 2.
Characteristics in PLWH with or without SARS‐CoV‐2 IgG seropositive
| Variable | People live with HIV | ||
|---|---|---|---|
| IgG seropositive | IgG seronegative | p value | |
| Sample size | 83 | 60 | |
| Age, year | 31.56 ± 8.05 | 33.90 ± 9.41 | 0.114 |
| Sex | 0.760 | ||
| Male | 81 (97.6) | 59 (98.3) | |
| Female | 2 (2.4) | 1 (1.7) | |
| BMI, kg/m2 | 21.93 ± 3.35 | 20.99 ± 4.86 | 0.189 |
| Time after vaccination, days | 30.27 ± 20.12 | 43.38 ± 34.96 | 0.005 |
| CD4+ T cells before ART, cells/μl | 311.10 ± 174.04 | 237.67 ± 167.55 | 0.015 |
| CD4/CD8 ratio before ART | 0.35 ± 0.24 | 0.08 ± 0.23 | 0.091 |
| CD4+ T cells at IgG detection, cells/μl | 457.68 ± 197.68 | 319.33 ± 181.51 | <0.001 |
| CD4/CD8 ratio at IgG detection | 0.73 ± 0.41 | 0.48 ± 0.35 | <0.001 |
| HIV RNA before ART, IU/ml | 4.24 ± 0.72 | 4.32 ± 0.79 | 0.581 |
| ART duration, months | 22.01 ± 22.15 | 14.19 ± 17.09 | 0.024 |
| ALT level, U/L | 34.7 ± 31.45 | 30.89 ± 20.37 | 0.415 |
| AST level, U/L | 26.17 ± 19.01 | 24.85 ± 12.17 | 0.639 |
| ALB level, g/L | 47.21 ± 3.05 | 44.76 ± 6.37 | 0.003 |
| Globulin level, g/L | 30.02 ± 4.98 | 32.33 ± 7.69 | 0.032 |
| Lymphocyte counts, 109/L | 1.99 ± 0.56 | 1.78 ± 0.55 | 0.031 |
| PLT level, 109/L | 244.69 ± 61.18 | 223.14 ± 65.89 | 0.047 |
| ART treatment | 0.043 | ||
| No | 11 (13.3) | 16 (26.7) | |
| Yes | 72 (86.7) | 44 (73.3) | |
| Syphilis | 0.864 | ||
| Negative | 66 (79.5) | 47 (78.3) | |
| Positive | 17 (20.5) | 13 (21.7) | |
| HBV infection | 0.023 | ||
| Negative | 77 (92.8) | 48 (80.0) | |
| Positive | 6 (7.2) | 12 (20.0) | |
| Tuberculosis | 0.311 | ||
| Negative | 79 (95.2) | 59 (98.3) | |
| Positive | 4 (4.8) | 1 (1.7) | |
Abbreviations: ALB, albumin; ALT, alanine aminotransferase; ART, antiretroviral therapy; AST, aspartate aminotransferase; BMI, body mass index; HBV, hepatitis B virus; PLT, platelets; PLWH, people living with HIV.
To further evaluate the relationship between clinical variables and serum IgG levels against SARS‐CoV‐2 in PLWH, correlation analysis was conducted (Figure 2). Body mass index (BMI), CD4+/CD8+ ratio before ART, ART duration, CD4+ T‐cell count at the time when IgG detected, CD4+/CD8+ ratio at the time when IgG detected, and ALB levels were positively correlated with IgG levels while time after vaccination and globulin levels were negatively correlated with IgG levels against SARS‐CoV‐2. Moreover, as shown in Supporting Information: Figure 2, we found that serum IgG levels were significantly lower in PLWH who were seropositive for hepatitis B surface antigen (HBsAg) (p = 0.012). PLWH receiving ART had significantly higher IgG levels than those not receiving ART (p = 0.01). Nonetheless, the ART regimen did not affect the status of serum SARS‐CoV‐2‐specific IgG in our study. A total of 56.5% of people treated with integrase strand transfer inhibitor (INSTI)‐based ART were detected with seropositive SARS‐CoV‐2‐specific IgG compared to 63.4% of individuals treated without INSTI‐based ART (p = 0.54).
Figure 2.
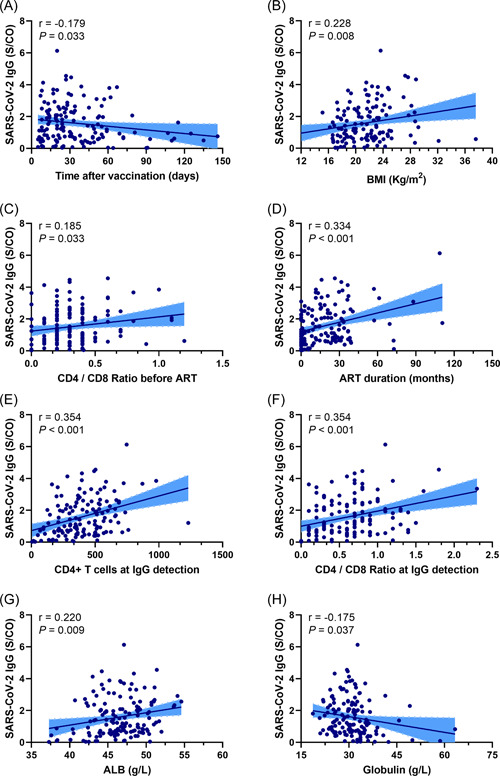
Correlation between variables and SARS‐CoV‐2‐specific IgG in people living with HIV. (A) Correlation between time after vaccination and SARS‐CoV‐2‐specific IgG (r = −0.179, p = 0.033). (B) Correlation between body mass index and SARS‐CoV‐2‐specific IgG (r = 0.288, p = 0.008). (C) Correlation between CD4+/CD8+ ratio before ART and SARS‐CoV‐2‐specific IgG (r = 0.185, p = 0.033). (D) Correlation between ART duration and SARS‐CoV‐2‐specific IgG (r = 0.334, p < 0.001). (E) Correlation between CD4+ T‐cell count at IgG detection and SARS‐CoV‐2‐specific IgG (r = 0.354, p < 0.001). (F) Correlation between CD4+/CD8+ ratio at IgG detection and SARS‐CoV‐2‐specific IgG (r = 0.354, p < 0.033). (G) Correlation between ALB levels and SARS‐CoV‐2‐specific IgG (r = 0.220, p = 0.009). (H) Correlation between globulin levels and SARS‐CoV‐2‐specific IgG (r = −0.175, p = 0.037). ALB, albumin; ART, antiretroviral therapy.
3.4. Independent factors associated with seropositivity IgG against SARS‐CoV‐2
Multivariate analysis was conducted to evaluate the factors associated with seropositivity IgG against SARS‐CoV‐2 (Table 3). When we evaluated the demographic variables of PLWH (adjusted Model 1), we found that only the time after vaccination was a factor associated with seropositivity IgG against SARS‐CoV‐2 (OR = 0.98, p = 0.004). When we included demographic and biochemical variables (adjusted Model 2), we found that serum ALB levels (OR = 1.159, p = 0.016), PLT levels (OR = 1.006, p = 0.045), and time after vaccination (OR = 0.982, p = 0.009) were independent factors. When we evaluated all variables, including HIV‐related markers, we found that only CD4+ T cells at the time when IgG detected (OR = 1.004, p = 0.006) and time after vaccination (OR = 0.977, p = 0.014) were independently associated with seropositivity IgG against SARS‐CoV‐2 in PLWH.
Table 3.
Factors associated with IgG seropositive in PLWH
| OR | 95%CI | p value | |
|---|---|---|---|
| Adjusted Model 1 | |||
| Time after vaccination, days | 0.980 | 0.967−0.994 | 0.004 |
| Adjusted Model 2 | |||
| Serum ALB level, g/L | 1.159 | 1.028−1.307 | 0.016 |
| PLT level, 109/L | 1.006 | 1.000−1.012 | 0.045 |
| Time after vaccination, days | 0.982 | 0.968−0.995 | 0.009 |
| Adjusted Model 3 | |||
| CD4+ T cells at IgG detection, cells/μl | 1.004 | 1.001−1.007 | 0.006 |
| Time after vaccination, days | 0.977 | 0.960−0.995 | 0.014 |
Abbreviations: ALB, albumin; PLT, platelets; PLWH, people living with HIV.
We also evaluated the risk factors associated with seropositivity IgG against SARS‐CoV‐2 in all individuals including healthy controls and PLWH. The results are shown in Supporting Information: Table 1. Age (OR = 0.943, p = 0.007), time after vaccination (OR = 0.976, p < 0.001), and CD4+/CD8+ ratio (OR = 7.544, p < 0.001) were independent risk factors associated with seropositivity IgG against SARS‐CoV‐2.
3.5. nAb titers in PLWH with seropositivity IgG against SARS‐CoV‐2
We further evaluated nAb titers in 67 PLWH and 20 healthy controls with IgG seropositivity against SARS‐CoV‐2. Baseline variables are presented in Supporting Information: Table 2. The nAb titers in PLWH against wild‐type SARS‐CoV‐2 were similar to those in the control group (p = 0.160) (Figure 3A). The proportion of nAb seropositivity against wild‐type SARS‐CoV‐2 was also similar (95% in the control group vs. 97% in the PLWH group, p = 0.665) (Figure 3B). Similar results were observed when we compared nAb seropositivity against delta variants with similar titers (p = 0.355) (Figure 3C) and the proportion of nAb seropositivity (p = 0.588) (Figure 3D).
Figure 3.
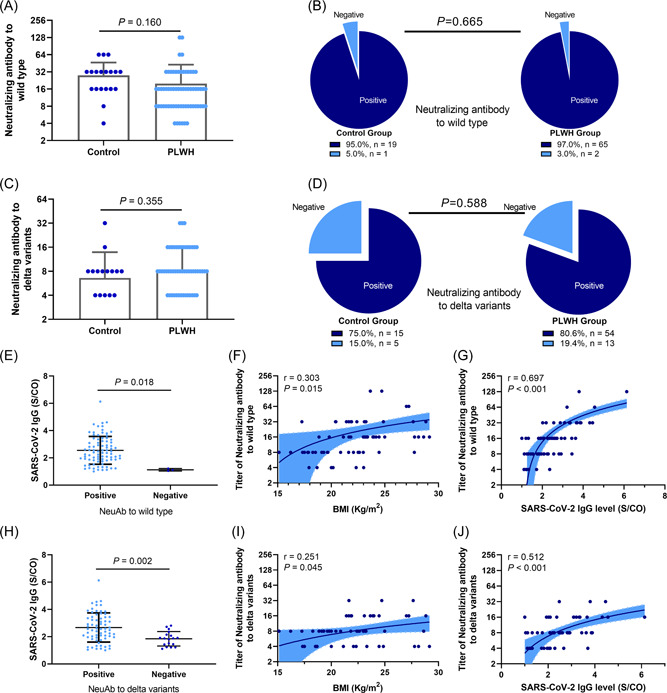
Neutralizing antibody titers in people living with HIV and control group. (A) The neutralizing antibody (nAb) titers in people living with HIV (PLWH) against wild‐type SARS‐CoV‐2 were similar compared with those of the control group (27.8 ± 18.69 vs. 19.82 ± 22.95, p = 0.160). (B) The proportion with nAb seropositivity against the wild‐type was similar (95% vs. 97%, p = 0.665). (C) The nAb titers in PLWH against delta variants were similar compared with those of the control group (6.60 ± 7.26 vs. 8.36 ± 7.46, p = 0.355). (D) Proportion of nAb seropositivity against delta variants in the two groups was similar (75% vs. 80.6%, p = 0.588). (E) In patients with seropositive IgG, IgG levels were significantly higher in individuals with seropositive nAb against the wild‐type than those with seronegative nAb (1.12 ± 0.09 vs. 2.55 ± 1.02, p = 0.018). (F) Correlation between BMI and titers of nAb against the wild‐type (r = 0.303, p = 0.015). (G) Correlation between SARS‐CoV‐2‐specific IgG levels and titers of nAb against the wild‐type (r = 0.697, p < 0.001). (H) In patients with seropositive IgG, IgG levels were significantly higher in individuals with seropositive nAbs against delta variants than those with seronegative nAb (1.84 ± 0.53 vs. 2.67 ± 1.06, p = 0.002). (I) Correlation between BMI and titers of nAb against delta variants (r = 0.251, p = 0.045). (J) Correlation between SARS‐CoV‐2‐specific IgG levels and titers of nAb against delta variants (r = 0.512, p < 0.001). BMI, body mass index; PLWH, people living with HIV.
We found that, in patients with seropositivity IgG against SARS‐CoV‐2, IgG levels were significantly higher in individuals seropositive for nAbs against the wild‐ type than those seronegative for nAbs (P=0.018) (Figure 3E). BMI (p = 0.015) (Figure 3F) and SARS‐CoV‐2‐specific IgG levels (p < 0.001) were positively correlated with nAb titers against the wild‐type SARS‐CoV‐2. Similarly, for nAb against delta variants, individuals with positive nAb against delta variants achieved higher IgG levels against SARS‐CoV‐2 (p = 0.002) (Figure 3H). BMI (p = 0.045) (Figure 3I) and IgG levels against SARS‐CoV‐2 (p < 0.001) (Figure 3J) were positively correlated with nAb titers against delta variant titers.
The ROC curve was plotted and is shown in Supporting Information: Figure 3. For all individuals, the AUROC of IgG levels able to predict positive nAbs against wild‐type SARS‐CoV‐2 was 0.966 (p = 0.006) (Supporting Information: Figure 3A). When IgG levels were ≥1.226 S/CO, sensitivity and specificity were 92.9% and 100%, respectively. For PLWH, the AUROC was 0.981 (p = 0.021) (Supporting Information: Figure 3B). When IgG levels were ≥1.112 S/CO, sensitivity and specificity were 96.9% and 100%, respectively. Similarly, for all individuals included, the AUROC of IgG levels predicting positive nAb against delta variants was 0.744 (p = 0.002) (Supporting Information: Figure 3C), When IgG levels ≥1.923 S/CO, the sensitivity was 76.8% and specificity was 66.7%. The AUROC for PLWH was 0.709 (p = 0.020) (Supporting Information: Figure 3D). When IgG levels were ≥1.923 S/CO, sensitivity and specificity were 72.2% and 69.2%, respectively.
Further, we conducted multivariate analyses to evaluate factors associated with the nAb seropositivity against delta variants in all included individuals. The results showed that serum IgG levels against SARS‐CoV‐2 were the only independent factors associated with nAb seropositivity against delta variants (OR = 2.798, p = 0.016) (Supporting Information: Table 3).
3.6. Safety of inactivated vaccines
In our study, we found no significant difference in the occurrence of side effects between the control and PLWH groups. As shown in Figure 4, only mild symptoms, including red swelling, skin nodules, pain, fatigue, dizziness, and diarrhea, were observed. No serious adverse events, including allergies, were identified in either group. No patient experienced a life‐threatening event after vaccination. All uncomfortable symptoms disappeared within 48 h as self‐reported by the enrolled participants.
Figure 4.
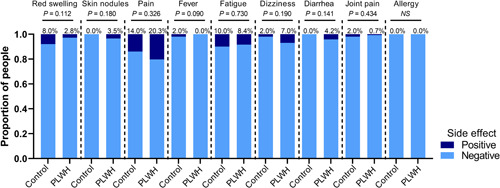
Safety of inactivated vaccines in people living with HIV. No significant differences in the occurrence of side effects between the control and people living with HIV groups were observed. No patients experienced life‐threatening events after vaccination in this study.
4. DISCUSSION
In this study, we found that inactivated vaccines effectively protected against SARS‐CoV‐2 infection in PLWH. Although the proportion of PLWH with seropositivity IgG against SARS‐CoV‐2 is lower than that of the general population, titers of nAbs against SARS‐CoV‐2 wild‐type or delta variants in the PLWH population were similar to those in the general population. CD4+ T‐cell counts and time after vaccination were associated with seropositivity IgG against SARS‐CoV‐2 in PLWH. No serious side effects were observed in the PLWH population following vaccination with inactivated vaccine.
The effectiveness of the SARS‐CoV‐2 inactivated vaccine was confirmed. 17 , 18 Currently available inactivated vaccines in the clinic include vaccines produced by Sinopharm and Sinovac. Data from a Phase III clinical study suggested that the effectiveness of the two inactivated vaccines was 72.8%–83.5%. 17 , 18 Inactivated vaccines can also be effective for patients with underlying diseases, but their effectiveness may vary. 19 , 20 , 21 In patients with autoimmune rheumatic diseases receiving the CoronaVac vaccine produced by Sinovac, 19 a lower anti‐SARS‐CoV‐2‐specific IgG seroconversion rate was observed (70.4% vs. 95.5% in the control group) (p < 0.001). The seropositivity rate for nAb was 56.3% versus 79.3% in the control group (p < 0.001). IgG titers (12.1 vs. 29.7, p < 0.001) and median neutralization activity (58.7 vs. 64.5%, p = 0.013) were also lower in patients with autoimmune rheumatic diseases. Similar results were observed in this study. We found that inactivated vaccines effectively induced SARS‐CoV‐2‐specific IgG seroconversion in PLWH. However, the IgG levels of PLWH were lower than those of healthy controls. In the present study, the time postvaccination of the control group was 64.46 ± 41.22 days, while that of the PLWH group was 35.78 ± 27.99 days. Anti‐SARS‐CoV‐2 antibody titers wane substantially over time. However, the antibody positivity rate in the control group with a longer postvaccination time was still higher than that in the PLWH group. Further improving the effectiveness of vaccination in the PLWH population is an important issue. While previous studies have suggested that a mixed approach or a third vaccination may further enhance protection against SARS‐CoV‐2, 22 , 23 , 24 , 25 these interventions require further evidence before they can be recommended for the PLWH population.
A study evaluating the long‐term kinetics of antibody responses in convalescent patients after SARS‐CoV‐2 infection showed that approximately 90% of recovered patients had detectable SARS‐CoV‐2‐specific IgG after 1 year, whereas neutralizing activity was only detectable in 43% of patients. 26 Here, we also found that IgG levels in PLWH were negatively correlated with the time after vaccination. Dan et al. 27 recently showed that SARS‐CoV‐2‐specific IgG antibodies can be maintained for up to 8 months. Whether the duration of IgG and nAb in PLWH is different from that in the general population is unknown and requires further exploration. For most immune responses, the IgM response wanes rapidly. In our study, IgM detection was relatively low. This may be because the samples were collected on average 1−2 months postvaccination.
In this study, we also included ALT and AST levels in the analysis. The liver is vital in human immunity. 28 Interestingly, a recent study also suggested that the liver plays an important role in regulating antibody titers. 29 However, we failed to identify ALT and AST levels as independent factors associated with seropositivity IgG against SARS‐CoV‐2 in PLWH in the present study.
The immunogenicity achieved by vaccines may be less effective in PLWH than in the general population. 30 , 31 , 32 Previous studies have explored the effectiveness of vaccination in PLWH to prevent tuberculosis infection. For HIV, a single MVA85A vaccination was well tolerated but the T‐cell response magnitude was lower in HIV‐infected people who had received the vaccine than in vaccinated people without HIV. 31 Similarly, another study found that M72/AS01 was well tolerated and induced a polyfunctional M72‐specific CD4+ T‐cell response, which was higher in patients receiving ART. 33 In our study, we confirmed a similar result. The inactivated COVID‐19 vaccine appeared to be safe and achieved suitable immunogenicity in PLWH. However, its effectiveness is slightly lower than that in the general population. The proportion of seropositivity IgG against SARS‐CoV‐2 in PLWH treated with ART was higher than that of ART naïve patients. Moreover, we found that the CD4+ T‐cell count was an independent factor that affected seropositivity IgG against SARS‐CoV‐2 in PLWH.
Most SARS‐CoV‐2 vaccines aim to induce sustained IgG responses to mount potent nAb responses, which are considered to correlate with protection. 4 In our study, we found that nAb responses were robust in PLWH who had completed the vaccination program. No serious adverse reactions were observed in any of the enrolled patients. Similar to the general population, the side effects of the COVID‐19 vaccine in PLWH were mild and self‐limiting. Most PLWH with SARS‐CoV‐2‐specific IgG seropositivity exhibit nAbs against wild‐type and delta variants after vaccination. Interestingly, we found that the nAb titers in PLWH were comparable to those in the general population with seropositive SARS‐CoV‐2‐specific IgG. The SARS‐CoV‐2‐specific IgG level was the only factor associated with the titer of nAbs. However, the duration of these nAb in serum remains unclear and requires further exploration.
The proportion of men in our study population was relatively high. This is because the proportion of men among Chinese PLWHs is high. The patients in our study were enrolled consecutively. An epidemiological study in China analyzed the HIV‐infected Chinese population from 2001 to 2020. 34 They found that, in the past two decades, male patients accounted for 84.36% of all patients with Stage 1 population. The reason for the imbalance in the sex ratio is that HIV infections from men who have sex with men have increased sharply as a proportion of all causes of infection in China. Our findings may be more suitable for Chinese male PLWH than other cohorts. Whether inactivated vaccines have a similar effect in female PLWH requires further studies that include only female patients.
Our study had several limitations. First, it was a single‐center study with limited sample size. Second, although the inactivated vaccine produced nAb responses in the most PLWH, whether this could protect against SARS‐CoV‐2 infection in the real world remains unknown. Third, the nature of the cross‐sectional study may have resulted in some individuals being miscategorized as humoral nonresponders, such as those that have not yet produced specific IgG after vaccination or those who lost specific IgG long after vaccination. A larger prospective multicenter study evaluating its efficacy is warranted.
5. CONCLUSIONS
The inactivated COVID‐19 vaccine appears to be safe with good immunogenicity in Chinese PLWH. Titers of nAbs against SARS‐CoV‐2 wild‐type or delta variants in the PLWH population with IgG seropositivity against SARS‐CoV‐2 are similar to those in the general population. CD4+ T‐cell counts and the time after vaccination were associated with seropositivity IgG against SARS‐CoV‐2 in PLWH.
AUTHOR CONTRIBUTIONS
Jie Peng, Yingsong Wu, and Baisheng Li conceived and designed the study. Shaohang Cai and Guichan Liao analyzed and interpreted the data, drafted and finalized the manuscript. Qiging Gao, Xuwen Xu, Juanjuan Chen, Aili Lu, and Tao Yu participated in the recruitment of patients, data collection, and critical revision of the article. Lirong Zou and Huan Zhang conducted the virus micro‐neutralization test. All the authors critically reviewed the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was performed in accordance with the Declaration of Helsinki and was approved by the Institutional Ethics Committee of Nanfang Hospital (NFEC‐2021‐178). All patient provided written informed consent, agreed to follow the protocol and take specimens, and was willing to anonymously publish details of the medical record.
Supporting information
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
The authors would like to thank all the patients included in this study, and the nurses who assisted in patient management and collection of serum samples. This study was supported by the Clinical Research Startup Program of Southern Medical University by the High‐Level University Construction Funding of Guangdong Provincial Department of Education (no. LC2016PY003) and by Guangdong Science and Technology Program Project (no. 2021B1212030007). The funding sources did not have any influence on the study design, data collection, analysis and interpretation of the data, writing of the manuscript, or decision to submit for publication.
Cai S, Liao G, Yu T, et al. Immunogenicity and safety of an inactivated SARS‐CoV‐2 vaccine in people living with HIV: a cross‐sectional study. J Med Virol. 2022;94:4224‐4233. 10.1002/jmv.27872
Shaohang Cai and Guichan Liao contributed equally to this study.
Contributor Information
Yingsong Wu, Email: wg@smu.edu.cn.
Baisheng Li, Email: libsn@126.com.
Jie Peng, Email: pjie138@163.com.
DATA AVAILABILITY STATEMENT
Authors can confirm all relevant data are included in the article and materials are available on reasonable request from the authors.
REFERENCES
- 1. Ryom L, Cotter A, De Miguel R, et al. 2019 update of the European AIDS Clinical Society Guidelines for treatment of people living with HIV version 10.0. HIV Med. 2020;21(10):617‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58(3):309‐318. [DOI] [PubMed] [Google Scholar]
- 3. Lobermann M, Borso D, Hilgendorf I, Fritzsche C, Zettl UK, Reisinger EC. Immunization in the adult immunocompromised host. Autoimmun Rev. 2012;11(3):212‐218. [DOI] [PubMed] [Google Scholar]
- 4. Krammer F. SARS‐CoV‐2 vaccines in development. Nature. 2020;586(7830):516‐527. [DOI] [PubMed] [Google Scholar]
- 5. Gao Q, Bao L, Mao H, et al. Development of an inactivated vaccine candidate for SARS‐CoV‐2. Science. 2020;369(6499):77‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gervasoni C, Meraviglia P, Riva A, et al. Clinical features and outcomes of patients with human immunodeficiency virus with COVID‐19. Clin Infect Dis. 2020;71(16):2276‐2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Byrd KM, Beckwith CG, Garland JM, et al. SARS‐CoV‐2 and HIV coinfection: clinical experience from Rhode Island, United States. J Int AIDS Soc. 2020;23(7):e25573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boulle A, Davies MA, Hussey H, et al. Risk factors for COVID‐19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2020;73(7):e2005‐e2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hsue PY, Waters DD. HIV infection and coronary heart disease: mechanisms and management. Nat Rev Cardiol. 2019;16(12):745‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barnes RP, Lacson JC, Bahrami H. HIV infection and risk of cardiovascular diseases beyond coronary artery disease. Curr Atheroscler Rep. 2017;19(5):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hemkens LG, Bucher HC. HIV infection and cardiovascular disease. Eur Heart J. 2014;35(21):1373‐1381. [DOI] [PubMed] [Google Scholar]
- 12. Fitzpatrick ME, Kunisaki KM, Morris A. Pulmonary disease in HIV‐infected adults in the era of antiretroviral therapy. AIDS. 2018;32(3):277‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nemes E, Scriba TJ, Hatherill M. Prospects for a vaccine to prevent HIV‐related tuberculosis. Curr Opin HIV AIDS. 2018;13(6):522‐527. [DOI] [PubMed] [Google Scholar]
- 14. Xu X, Sun J, Nie S, et al. Seroprevalence of immunoglobulin M and G antibodies against SARS‐CoV‐2 in China. Nat Med. 2020;26(8):1193‐1195. [DOI] [PubMed] [Google Scholar]
- 15. Marklund E, Leach S, Axelsson H, et al. Serum‐IgG responses to SARS‐CoV‐2 after mild and severe COVID‐19 infection and analysis of IgG non‐responders. PLoS One. 2020;15(10):e241104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang J, Xing S, Liang D, et al. Differential antibody response to inactivated COVID‐19 vaccines in healthy subjects. Front Cell Infect Microbiol. 2021;11:791660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS‐CoV‐2 vaccines on symptomatic COVID‐19 infection in adults: a randomized clinical trial. JAMA. 2021;326(1):35‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanriover MD, Doğanay HL, Akova M, et al. Efficacy and safety of an inactivated whole‐virion SARS‐CoV‐2 vaccine (CoronaVac): interim results of a double‐blind, randomised, placebo‐controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Medeiros‐Ribeiro AC, Aikawa NE, Saad C, et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med. 2021;27:1744‐1751. [DOI] [PubMed] [Google Scholar]
- 20. Karacin C, Eren T, Zeynelgil E, et al. Immunogenicity and safety of the CoronaVac vaccine in patients with cancer receiving active systemic therapy. Future Oncol. 2021;17:4447‐4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang J, Hou Z, Liu J, et al. Safety and immunogenicity of COVID‐19 vaccination in patients with non‐alcoholic fatty liver disease (CHESS2101): a multicenter study. J Hepatol. 2021;75(2):439‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Del BA, Abravanel F, Marion O, et al. Efficiency of a boost with a third dose of anti‐SARS‐CoV‐2 messenger RNA‐based vaccines in solid organ transplant recipients. Am J Transplant. 2021;22(1):322‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del BA. Three doses of an mRNA Covid‐19 vaccine in solid‐organ transplant recipients. N Engl J Med. 2021;385(7):661‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS‐CoV‐2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174:1330‐1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spencer AJ, McKay PF, Belij‐Rammerstorfer S, et al. Heterologous vaccination regimens with self‐amplifying RNA and adenoviral COVID vaccines induce robust immune responses in mice. Nat Commun. 2021;12(1):2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xiang T, Liang B, Fang Y, et al. Declining levels of neutralizing antibodies against SARS‐CoV‐2 in convalescent COVID‐19 patients one year post symptom onset. Front Immunol. 2021;12:708523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS‐CoV‐2 assessed for up to 8 months after infection. Science. 2021;371(6529). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang X, Lu D, Zhuo J, Lin Z, Yang M, Xu X. The gut−liver axis in immune remodeling: new insight into liver diseases. Int J Biol Sci. 2020;16(13):2357‐2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. James BH, Papakyriacou P, Gardener MJ, Gliddon L, Weston CJ, Lalor PF. The contribution of liver sinusoidal endothelial cells to clearance of therapeutic antibody. Front Physiol. 2021;12:753833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mizusawa M, Perlman DC, Lucido D, Salomon N. Rapid loss of vaccine‐acquired hepatitis B surface antibody after three doses of hepatitis B vaccination in HIV‐infected persons. Int J STD AIDS. 2014;25(3):201‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scriba TJ, Tameris M, Smit E, et al. A phase IIa trial of the new tuberculosis vaccine, MVA85A, in HIV‐ and/or Mycobacterium tuberculosis‐infected adults. Am J Respir Crit Care Med. 2012;185(7):769‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Katzenstein TL. Pneumococcal vaccination of HIV‐infected individuals: safety and efficacy. AIDS Patient Care STDS. 1997;11(4):269‐275. [DOI] [PubMed] [Google Scholar]
- 33. Kumarasamy N, Poongulali S, Bollaerts A, et al. A randomized, controlled safety, and immunogenicity trial of the M72/AS01 candidate tuberculosis vaccine in HIV‐positive Indian adults. Medicine. 2016;95(3):e2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin Q, Deng B, Rui J, et al. Epidemiological characteristics and transmissibility of human immunodeficiency virus in Nanning City, China, 2001−2020. Front Public Health. 2021;9:689575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
Authors can confirm all relevant data are included in the article and materials are available on reasonable request from the authors.


