Abstract
Background
Lung cancer is the most common cause of cancer‐related death in the world, however lung cancer screening has not been implemented in most countries at a population level. A previous Cochrane Review found limited evidence for the effectiveness of lung cancer screening with chest radiography (CXR) or sputum cytology in reducing lung cancer‐related mortality, however there has been increasing evidence supporting screening with low‐dose computed tomography (LDCT).
Objectives
To determine whether screening for lung cancer using LDCT of the chest reduces lung cancer‐related mortality and to evaluate the possible harms of LDCT screening.
Search methods
We performed the search in collaboration with the Information Specialist of the Cochrane Lung Cancer Group and included the Cochrane Lung Cancer Group Trial Register, Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library, current issue), MEDLINE (accessed via PubMed) and Embase in our search. We also searched the clinical trial registries to identify unpublished and ongoing trials. We did not impose any restriction on language of publication. The search was performed up to 31 July 2021.
Selection criteria
Randomised controlled trials (RCTs) of lung cancer screening using LDCT and reporting mortality or harm outcomes.
Data collection and analysis
Two review authors were involved in independently assessing trials for eligibility, extraction of trial data and characteristics, and assessing risk of bias of the included trials using the Cochrane RoB 1 tool. We assessed the certainty of evidence using GRADE. Primary outcomes were lung cancer‐related mortality and harms of screening. We performed a meta‐analysis, where appropriate, for all outcomes using a random‐effects model. We only included trials in the analysis of mortality outcomes if they had at least 5 years of follow‐up. We reported risk ratios (RRs) and hazard ratios (HRs), with 95% confidence intervals (CIs) and used the I2 statistic to investigate heterogeneity.
Main results
We included 11 trials in this review with a total of 94,445 participants. Trials were conducted in Europe and the USA in people aged 40 years or older, with most trials having an entry requirement of ≥ 20 pack‐year smoking history (e.g. 1 pack of cigarettes/day for 20 years or 2 packs/day for 10 years etc.). One trial included male participants only. Eight trials were phase three RCTs, with two feasibility RCTs and one pilot RCT. Seven of the included trials had no screening as a comparison, and four trials had CXR screening as a comparator. Screening frequency included annual, biennial and incrementing intervals. The duration of screening ranged from 1 year to 10 years. Mortality follow‐up was from 5 years to approximately 12 years.
None of the included trials were at low risk of bias across all domains. The certainty of evidence was moderate to low across different outcomes, as assessed by GRADE.
In the meta‐analysis of trials assessing lung cancer‐related mortality, we included eight trials (91,122 participants), and there was a reduction in mortality of 21% with LDCT screening compared to control groups of no screening or CXR screening (RR 0.79, 95% CI 0.72 to 0.87; 8 trials, 91,122 participants; moderate‐certainty evidence). There were probably no differences in subgroups for analyses by control type, sex, geographical region, and nodule management algorithm. Females appeared to have a larger lung cancer‐related mortality benefit compared to males with LDCT screening. There was also a reduction in all‐cause mortality (including lung cancer‐related) of 5% (RR 0.95, 95% CI 0.91 to 0.99; 8 trials, 91,107 participants; moderate‐certainty evidence).
Invasive tests occurred more frequently in the LDCT group (RR 2.60, 95% CI 2.41 to 2.80; 3 trials, 60,003 participants; moderate‐certainty evidence). However, analysis of 60‐day postoperative mortality was not significant between groups (RR 0.68, 95% CI 0.24 to 1.94; 2 trials, 409 participants; moderate‐certainty evidence).
False‐positive results and recall rates were higher with LDCT screening compared to screening with CXR, however there was low‐certainty evidence in the meta‐analyses due to heterogeneity and risk of bias concerns. Estimated overdiagnosis with LDCT screening was 18%, however the 95% CI was 0 to 36% (risk difference (RD) 0.18, 95% CI ‐0.00 to 0.36; 5 trials, 28,656 participants; low‐certainty evidence).
Four trials compared different aspects of health‐related quality of life (HRQoL) using various measures. Anxiety was pooled from three trials, with participants in LDCT screening reporting lower anxiety scores than in the control group (standardised mean difference (SMD) ‐0.43, 95% CI ‐0.59 to ‐0.27; 3 trials, 8153 participants; low‐certainty evidence).
There were insufficient data to comment on the impact of LDCT screening on smoking behaviour.
Authors' conclusions
The current evidence supports a reduction in lung cancer‐related mortality with the use of LDCT for lung cancer screening in high‐risk populations (those over the age of 40 with a significant smoking exposure). However, there are limited data on harms and further trials are required to determine participant selection and optimal frequency and duration of screening, with potential for significant overdiagnosis of lung cancer. Trials are ongoing for lung cancer screening in non‐smokers.
Keywords: Adult; Female; Humans; Male; Bias; Early Detection of Cancer; Early Detection of Cancer/methods; Lung Neoplasms; Lung Neoplasms/diagnostic imaging; Lung Neoplasms/mortality; Randomized Controlled Trials as Topic; Tomography, X-Ray Computed; Tomography, X-Ray Computed/methods
Plain language summary
Impact of computed tomography (CT) on lung cancer screening
Background
Lung cancer is the most common cause of cancer‐related death worldwide. Lung cancer survival is significantly dependent on when a person is diagnosed with the disease. It is essential to detect the disease as early as possible by radiography (chest x‐ray) or by computed tomography (CT) scan, which is a more detailed type of radiography where multiple images of the lung are taken. The aim of this review was to gather information on the use of CT scan to detect lung cancer earlier and to find out if early detection of lung cancer reduces death from lung cancer. We also evaluated potential harms that can occur from using CT to screen for lung cancer, such as additional investigations and their related complications.
Description of included trials
The evidence is current to 31 July 2021. We included 11 trials, with a total of 94,445 participants. The trials came from the USA and Europe. The earliest trial started in 1991, and the most recent started in 2011. The participants were adults over the age of 40. The frequency of screening with CT ranged from yearly to more than 2.5 years.
Key findings
Eight of the trials (91,122 participants) were included in the main outcome analysis of lung cancer‐related mortality. In people over 40 years with significant smoking exposure, CT screening reduced deaths from lung cancer by 21%, with 226 people needing to undergo screening to prevent one death from lung cancer. We also found that deaths from any cause (including lung cancer) were less with CT screening. However, the effect was much lower (only 5% reduction in risk). Lung cancer was detected more frequently in the group of people who had CT screening compared with no screening. However, CT scans can induce false‐positive scans (a test that is positive or indeterminate for lung cancer, when the person does not actually have lung cancer). We found that false‐positive results were more common among people who were screened with CT than chest x‐ray. Because of that, those that underwent CT screening had more tests to investigate both cancer and non‐cancer‐related diseases. Screening also implies a risk of detecting lung cancers that may have never progressed to cause harm to the person (this is referred to as overdiagnosis). The risk of lung cancer overdiagnosis with CT screening was estimated to be 18%.
The trials were too different or did not provide enough information to look at the impact of screening on stopping smoking or quality of life. There was some evidence to suggest there were no long‐term psychological harms from screening, with some people in the CT screening group feeling less anxious compared to the control groups who were not offered screening.
Certainty of evidence
The overall certainty of evidence was moderate when it came to outcomes regarding death, with moderate‐ to low‐certainty evidence for other outcomes. The certainty rating for outcomes reflects the authors' confidence and certainty in the outcome being correct.
Summary of findings
Summary of findings 1. Low‐dose computed tomography (LDCT) screening compared to no LDCT screening for lung cancer‐related mortality.
| Low‐dose computed tomography (LDCT) screening compared to no LDCT screening for lung cancer‐related mortality | |||||
| Patient or population: healthy adults Setting: hospitals or screening centres Intervention: LDCT screening Comparison: no LDCT screening | |||||
| Outcomes | № of participants (trials) follow‐up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects*(95% CI) | |
| Risk with no screening | Risk difference | ||||
| Lung cancer‐related mortality ‐ planned time points Follow‐up: 6 years to 10 years from randomisation | 91,122 (8 RCTs) | ⊕⊕⊕⊝ Moderatea |
RR 0.79 (0.72 to 0.87) |
Trial population | |
| 21 per 1000 | 4 fewer per 1000 people screened (3 fewer to 6 fewer) | ||||
|
All‐cause mortality ‐ planned time points Follow‐up: 6 years to 10 years from randomisation |
91,107 (8 RCTs) |
⊕⊕⊕⊝ Moderatea |
RR 0.95 (0.91 to 0.99) |
Trial population | |
| 89 per 1000 | 4 fewer per 1000 people screened (1 fewer to 8 fewer) | ||||
|
Overdiagnosis Time point: ≥ 10 years from randomisation excluding CXR trials |
28,656 (5 RCTs) | ⊕⊕⊝⊝ Lowa,c |
RD 0.18 (‐0.00 to 0.36) |
Trial population | |
| 180 more lung cancers overdiagnosed per 1000 lung cancers detected (0 more to 360 more) | |||||
|
Number of invasive tests Time point: 3 years to 10 years from randomisation |
60,003 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | RR2.60 (2.41 to 2.80) | Trial population | |
| 31 per 1000 | 49 more per 1000 people screened (45 more to 55 more) | ||||
|
Any death postsurgery Time point: 6 years to 9 years from randomisation |
409 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | RR 0.68 (0.24 to 1.94) | Trial population | |
| 48 per 1000 | 15 fewer per 1000 people screened (37 fewer to 45 more) | ||||
|
Health‐related quality of life ‐ anxiety Time point: 10 months to 5 years from randomisation Measured by different scales |
8153 (3 RCT) |
⊕⊕⊝⊝ Lowa,b |
SMD ‐0.43 (‐0.59 to ‐0.27) |
Trial population | |
|
SMD 0.43 lower (0.27 to 0.59 lower ) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CXR: chest x‐ray; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio; RD: risk difference, SMD: standardised mean difference | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to high risk of "other bias" in Becker 2020, De Koning 2020, Infante 2015, and Pastorino 2012. bDowngraded one level due to indirectness: only a subset of the trial population were included for quality assessment. cDowngraded one level due to heterogeneity.
Background
Description of the condition
Lung cancer remains the most common cause of cancer‐related death in the world (Ferlay 2019), resulting in an estimated 1.76 million deaths in 2018 (WHO 2018). Whilst historically a male predominant condition, the incidence of lung cancer is now comparable in men and women in the USA, representing approximately 13% of all new cancer diagnoses (Siegel 2019). In Germany, New Zealand, Denmark, Canada, the Netherlands and the USA, age‐specific lung cancer incidence rates have declined in males with each 5‐year birth cohort, with significant transition from male to female dominance in these countries in the younger age groups (30 to 49 years old) (Fidler‐Benaoudia 2020). There is a concerning upward trend in lung cancer‐related deaths in younger women (Levi 2007), with the death rate from lung cancer expected to exceed breast cancer‐related deaths in Europe in women (Malvezzi 2017). The current 5‐year survival for lung cancer is 19% in the USA, with poorer outcomes in small cell lung cancer and in the advanced stages (Howlader 2020). In the last decade, prognosis has improved in stage III and IV non‐small cell lung cancer (NSCLC) with the introduction of immunotherapy and targeted molecular therapy (Howlader 2020; NICE 2019). However, these treatments are mostly not considered curative, with the 5‐year survival in the USA for metastatic NSCLC being 6%, compared to 61% for local NSCLC (Howlader 2020). Complete resection of early‐stage NSCLC has the greatest potential for long‐term survival (beyond 10 years) (Hubbard 2012).
Tobacco smoking is recognised as the most significant risk factor for lung cancer (Halpern 1993; Peto 1994), and as such, primary prevention is an essential component of public health campaigns. However, additional factors such as age, genetic factors, airway obstruction, infections and environmental exposure affect risk (Alberg 2007; Bach 2003), with exposure to ambient air pollution increasingly contributing to the global burden of lung cancer (WHO 2016). Particularly in females, adenocarcinomas with detectable molecular mutation are more common in never‐smokers compared to people with a tobacco‐exposure history (Subramanian 2007). A number of validated risk prediction tools have been developed which incorporate smoking history, in addition to other risk factors, to estimate lung cancer risk (Cassidy 2008; Tammemägi 2013). These risk prediction models have been suggested to improve participant selection for lung cancer screening and have already been incorporated into screening programmes (Field 2019; ten Haaf 2017).
Description of the intervention
Lung cancers are commonly diagnosed at an advanced stage, with 48% of patients in Australia and 61% of patients in Denmark having metastatic NSCLC at the time of diagnosis (Walters 2013). Hence, several trials have evaluated the role of screening for the detection of preclinical disease. Early lung cancers may be visible on plain chest radiography (CXR) or computed tomography (CT) as a pulmonary nodule. A lung nodule is defined as a focal opacity, more or less well defined, measuring up to 3 cm (Hansell 2008). The sensitivity of CXR for the detection of pulmonary nodules < 1 cm is poor (Quekel 1999). Furthermore, in people presenting with symptoms of lung cancer, the sensitivity of CXR is only 80% or less (Bradley 2019). A CT scan is a more detailed type of radiography imaging which uses a rotation x‐ray source. Multiple x‐ray attenuation measurements are taken from different angles and then processed on a computer using reconstruction algorithms to produce cross‐sectional images or virtual slices of a body. These cross‐sectional images are able to detect pulmonary nodules < 1 cm more reliably than CXR due to improved resolution and reduced obscuration from overlapping mediastinal, cardiac and chest wall structures. This is beneficial in the detection of small early‐stage lung cancers, however CT‐detected nodules are not specific to cancer, with differentials including benign nodules, such as hamartomas, granulomas, and inflammatory nodules. Additional incidental findings described with low‐dose computed tomography (LDCT) include mediastinal lymphadenopathy, coronary artery calcification, aortic aneurysm, and non‐pulmonary malignancies (Swensen 2002).
How the intervention might work
LDCT screening has been established as a more sensitive tool to detect lung cancer at an early and resectable stage compared with CXR (Diederich 2002; Nawa 2002; Sobue 2002; Sone 2001; Swensen 2002). An earlier Cochrane Review on lung cancer screening found that annual CXR did not significantly reduce lung cancer mortality (Manser 2013). The same review concluded that LDCT screening was associated with a reduction in lung cancer mortality compared with CXR among high‐risk former and current smokers. Reviewers for the 2013 US Preventive Services Task Force Evidence Synthesis also concluded that high‐certainty evidence shows that LDCT screening can significantly reduce mortality from lung cancer (Humphrey 2013). The findings of both of these systematic reviews were based largely on the results of the National Lung Screening Trial (NLST, Aberle 2011) which used the comparator of CXR in a group of high‐risk former and current smokers. In a more recent systematic review, conducted as part of a Health Technology Assessment for the National Institute for Health Research (NIHR) in the UK, the reviewers concluded that LDCT may be clinically effective in reducing lung cancer mortality, but there is considerable uncertainty (Snowsill 2018).
Why it is important to do this review
Despite multiple international guidelines recommending LDCT screening for high‐risk former and current smokers, and calls for the implementation of screening, to our knowledge a nationally co‐ordinated screening programme has not been broadly adopted, apart from in Korea (Lewin 2016; Moyer 2014; Oudkerk 2017; Zhou 2015). In the USA, the Center for Medicare and Medicaid Services has approved coverage and reimbursement for lung cancer screening for individuals who meet certain criteria (Jensen 2015). However, in the absence of a co‐ordinated programme, there have been concerns about the low up take of screening and considerable variability in false‐positive rates between different providers (Pinsky 2018).
There was an urgent need for a contemporary systematic evidence synthesis that incorporates the growing evidence base from RCTs on both benefits and harms of screening in order to better understand the potential magnitude of any benefit and to understand in which groups any benefits might outweigh the harms. False‐positive test results and overdiagnosis are both potential sources of harm from screening which may lead to unnecessary interventions with adverse psychological impacts, morbidity and mortality. Overdiagnosis refers to the detection and diagnosis of lung cancers by screening which would have never caused the person harm, such as death or symptoms, in their lifetime when left untreated (Brodersen 2018). In a recent review of RCTs in which LDCT was compared to usual care (no screening), it was estimated that 49% of lung cancers detected by screening may have been overdiagnosed (Brodersen 2020). Radiation exposure has similarly been considered, with Gierada et al. describing an estimated risk of radiation‐induced cancer mortality after 20 annual chest LDCTs of 0.1%, based on a linear no threshold model of ionising radiation effects (Berrington de González 2008; FDA 2017; Gierada 2020; Rampinelli 2017). In the UK, screening for lung cancer is part of the National Health Service (NHS) long‐term plan, and its ambition is to reach around 600,000 people over 4 years, detecting approximately 3400 cancers across the UK (NHS 2019).
The purpose of this review was to assess the evidence regarding LDCT screening methods to reduce lung cancer‐related mortality and to evaluate the possible harms associated with screening. Additionally, we estimated the incidence of lung cancer and impact on smoking behaviour following screening. Another reason for conducting this review was to involve consumer participation to allow for different perspectives on outcomes and to disseminate the review findings.
Objectives
To determine whether screening for lung cancer using LDCT of the chest reduces lung cancer‐related mortality and to evaluate the possible harms of LDCT screening.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs). Randomisation by groups, clusters or individuals was acceptable. All trials reporting mortality as an outcome were eligible for inclusion in the review; however, we did not include those with < 5 years of mortality follow‐up data in quantitative synthesis.
We excluded:
observational cohort studies; and
case‐series studies.
Types of participants
We included trials with asymptomatic adults from all backgrounds. We excluded trials in adults with previous diagnosis and treatment of lung cancer. We verified entry requirements for all included trials to include only preclinical nodules.
Types of interventions
-
Intervention
LDCT, defined as a volumetric CT dose index of ≤ 3 mGy in a standard sized patient (height 170 cm, weight 70 kg) in 2016 (Kazerooni 2016). Newer technological improvements (iterative reconstruction) have enabled further dose reductions (Willemink 2013).
-
Comparator
LDCT screening versus no screening
LDCT screening versus any non‐LDCT intervention, including (but not limited to) CXR, sputum cytology or biomarkers (alone or in any combination)
In addition, we included trials which compared different frequencies of screening with LDCT, such as annual LDCT versus biennial LDCT.
Types of outcome measures
Primary outcomes
Lung cancer‐related mortality ≥ 5 years post‐randomisation
Harms of screening at any time point, including the number of invasive tests performed in those with a false‐positive diagnosis (positive screen in the absence of lung cancer), and any complications arising from these tests, including death
Secondary outcomes
All‐cause mortality (death from any cause, including lung cancer)
Lung cancer incidence (during screening and postscreening period in those trials which have recorded the incidence postscreening, to capture data on overdiagnosis where possible). In this review, baseline screen incidence data included both incident and prevalence cases of lung cancer first detected during baseline screening.
False‐positive rates and recall rates (proportion of participants recalled for interval CT at 3 months and > 6 months for follow‐up of a nodule or suspected lung cancer)
Impact on smoking behaviour: cessation, relapse rates, smoking intensity
Health‐related quality of life (HRQoL)/psychosocial consequences. We considered all time points recorded in trials, with an analytic plan for 6 months, 12 months, and 24 months interval assessments.
We recorded, where possible, any other outcomes presented in the primary studies, including but not limited to, stage at diagnosis, histology, radiation exposure, use of biomarkers, response rate, adherence to screening, contamination, interval lung cancers, false negatives, cost, medication implications, and incidental findings.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases from inception to 31 July 2021. We performed the search in collaboration with the Information Specialist of the Cochrane Lung Cancer Group.
Cochrane Lung Cancer Group Trial Register
Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library, current issue) (Appendix 1)
MEDLINE, accessed via PubMed (Appendix 2)
Embase (Appendix 3)
We performed the MEDLINE search using the Cochrane highly sensitive search strategy, sensitivity and precision‐maximising version (2008 version) as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022).
We also conducted searches in the following clinical trials registries to identify unpublished and ongoing trials.
ClinicalTrials.gov
WHO International Clinical Trials Registry Platform (ICTRP)
We applied no restriction on language of publication.
Searching other resources
Ongoing trials and grey literature
We used the following additional resources.
Abstracts from 2018 and onwards from international lung cancer meetings, including World Conference on Lung Cancer, American Thoracic Society Conference, European Respiratory Society Conference, American Society of Clinical Oncology (ASCO) Conference, European Society of Medical Oncology (ESMO) Conference and European Conference of Clinical Oncology (ECCO)
We searched the bibliographies of identified trials and narrative reviews for additional citations.
We contacted authors of primary studies and experts in the field of lung cancer screening to determine whether they were aware of any additional relevant unpublished or published studies or works in progress.
We applied no restriction on language of publication.
Data collection and analysis
Selection of studies
We selected trials for inclusion according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022).
Two review authors (AB and CM) using Covidence (Covidence 2017) independently screened all titles and abstracts retrieved by electronic searches. Two review authors (AB and DM) then obtained the full texts for all relevant trials and independently checked the eligibility of each trial against review eligibility criteria. We pursued discordant evaluations by discussion to reach consensus. When necessary, we involved a third review author (RManser). We report the results of the trial selection process using a PRISMA flow diagram (Moher 2009).
Data extraction and management
The review authors developed a data extraction form using Covidence (Covidence 2017). Two review authors (AB and RM) independently extracted relevant data and performed a cross‐check. To reach consensus, we involved a third review author when necessary (RManser or DM). We were not blinded to the names of trial authors nor to the institutions where trials were conducted and funded. When we encountered multiple publications for the same trial, we chose the first publication dealing with the primary endpoint in this review as a study identifier (study ID).
We collected the following data.
Source: citation, trial name if applicable and contact details
Eligibility criteria and reasons for exclusion
Methods: trial design, total duration of trial, number of trial centres and locations, trial setting, date of trial and dates of first and last included participants
Characteristics of participants: number of participants, participant characteristics (age, sex, smoking status, performance status), country, ethnicity
Characteristics of interventions (e.g. frequency of scanning, dose of CT, duration of screening, interpretation of scans, criteria for significance)
Outcomes: primary and secondary outcomes (with definitions) and time points
Results: number of participants allocated to each group, and for each outcome of interest, sample size, missing participants, summary data for each group, estimate of effect with confidence interval and P value and subgroup analyses
Miscellaneous: funding source, notable conflicts of interest of trial authors
Assessment of risk of bias in included studies
Two review authors (AB and RM) independently applied the Cochrane RoB 1 tool in order to assess quality and potential biases across included trials (Higgins 2017). We rated each domain of the tool as having 'low', 'high', or 'unclear' risk of bias at trial level and for each outcome if possible, and we supported the rating of each domain with a brief description. We summarised risk of bias for each outcome within a trial by considering all domains relevant to the outcome (i.e. both trial‐level entries, such as allocation sequence concealment, and outcome‐specific entries, such as blinding). We provided a figure to summarise the risk of bias.
If the two review authors did not reach consensus, a third review author (RManser or DM) was consulted.
Using the Cochrane RoB 1 tool, we considered the following domains.
Selection bias ‐ generation of allocation sequence: we scored 'low risk' when a random component in the sequence generation process was stated, 'high risk' when a non‐random method was used such as date of birth or hospital admission and 'unclear risk' if not specified in the paper.
Selection bias ‐ allocation concealment (selection bias): we scored 'low risk' when the allocation to intervention methods were reported such as using some form of centralised randomisation scheme, an on‐site computer system or sealed opaque envelopes, we scored 'high risk' when the allocation concealment method was not appropriate and 'unclear risk' when the method was not specified in the paper.
Performance bias ‐ blinding of participants and personnel: we scored 'low risk' when the blinding of participants and key trial personnel was ensured. We scored 'high risk' when there was no blinding or incomplete blinding, for the review outcome was likely to be influenced by lack of blinding such as smoking behaviour changes. We scored 'unclear' when there was insufficient information to make this judgement.
Detection bias ‐ blinding of outcome assessors: we scored 'low risk' when the outcome assessment was blindly performed. We scored 'high risk' when there was no blinding of the other review outcome assessment. We scored 'unclear' when there was insufficient information to make this judgement.
Attrition bias ‐ incomplete outcome data: we scored 'low risk' when there were no missing data, reasons for missing data were provided, the number of missing data were balanced across the groups or when appropriate method was used to impute missing data. We scored 'high risk' when there was > 20% missing data or imbalance in numbers or reasons for missing data across the trial groups. We scored 'unclear risk' when there was insufficient information to make this judgement.
Reporting bias ‐ selective reporting: we scored 'low risk' when the trial protocol was available and all prespecified trial outcomes were reported. Moreover, when the protocol was not available, and it was clear from the published papers that all expected outcomes are reported, these trials were still rated at low risk. We scored 'high risk' when not all prespecified outcomes were reported, reported outcomes on subsets of the data, and incomplete reporting of the outcomes. We scored 'unclear risk' when there was insufficient information to make this judgement.
Other sources of bias ‐ other bias: we scored 'low risk' if the trial appeared to be free of other sources of bias. We scored 'high risk' when there was at least one important bias, for example, the risk of contamination between the intervention and the control groups.
For cluster‐RCTs we addressed the following additional issues (Higgins 2022).
Randomisation process: we reported on the number of clusters involved and whether randomisation was performed at a single time point or in batches.
Recrutment bias: we investigated bias relevant to whether the participants within the cluster were aware of the intervention, the timing of randomisation and recruitment of individuals in addition to any baseline imbalance between individuals, not clusters.
Bias due to deviations from intended interventions: we dealt with this issue similar to the individually‐randomised trials.
Bias due to missing outcome data: we reported missing data for both the participants and the cluster.
Bias in measurement of the outcome: we reported on this bias in the same way as to the individually‐randomised trials.
Other bias: we reported on this bias the same way as the individually‐randomised trials.
Measures of treatment effect
For time‐to‐event outcomes (overall survival and relapse‐free survival), we had planned to use hazard ratios (HRs) to measure intervention effects after validating the proportional hazards assumption, so far as possible. However, only a few trials reported the hazard of death from the time of the enrolment point and reported each HR along with the 95% confidence Interval (CI).
For dichotomous outcomes (i.e. lung cancer cases detected by CT screening), we used the extracted data from the original trials for both screened and unscreened controlled groups to estimate the overall incidence of newly‐diagnosed lung cancer cases.
We also calculated the risk of overdiagnosis by estimating the risk ratio (RR) of lung cancer (with 95% CIs) in the screened group compared with the control group in trials which have reported the cumulative incidence of lung cancer post the active phase of screening. The primary analysis for overdiagnosis was limited to trials in which the control group did not have any active screening; however we also estimated the risk of overdiagnosis from CT screening relative to that of CXR screening in those trials where the control group were offered CXR screening in a separate analysis.
For continuous outcomes (HRQoL), we used mean differences (MDs) between treatment arms when a similar scale was implemented to measure outcomes, and standardised mean differences (SMDs) when different scales were used to measure the same outcome. This was applied when anxiety data were pooled across the four trials reported on anxiety. If we confirmed that higher scores for continuous outcomes have the same meaning for the particular outcome, we explained the direction, and reported if directions were reversed. We analysed data on an intention‐to‐screen basis.
Unit of analysis issues
For the included RCTs, the individuals were the unit of analysis by practice.
For cluster‐RCTs we identified trials using a cluster randomisation as a way of avoiding contamination bias. Randomisation might have been performed by hospitals, centres and cities. When including data from these trials into meta‐analyses we used the effective sample size method as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022). We calculated the effective sample size of groups in each cluster trial to be the original sample size divided by the 'design effect'. We calculated the cluster design effect by 1+ (M ‐1) ICC, where M represented the average cluster size and ICC was the interclass correlation coefficient. For dichotomous data, we divided both the total number of participants and the number experiencing the event by the same design effect. For continuous data, only the sample size was reduced and the means and standard deviations (SDs) stayed the same (Higgins 2022).
Trials with multiple treatment groups
For trials with multiple comparison groups that compared two or more intervention groups with the same control group, we first tried to combine groups to create a single pair‐wise comparison. We calculated within‐study correlation as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022).
Dealing with missing data
When data were missing or unsuitable for analysis, we (DM) contacted trial authors to request further information using email addresses from trial reports, from trial registers or from trial author institutions. When data were missing to the extent that the trial could not be included in the meta‐analysis and attempts to retrieve data had been exhaustive, we presented the results in the review and discussed them in the context of trial findings. For each trial, we checked whether intention‐to‐screen analysis was applied (i.e. the number of analysed participants equalled the number of randomly‐assigned participants).
Assessment of heterogeneity
We followed Cochrane recommendations for assessment of heterogeneity (Higgins 2022). We visually investigated heterogeneity by using forest plots generated via Review Manager 5 (RevMan 5) (Review Manager 2020). We assessed statistical heterogeneity of treatment effects between pooled trials for each considered outcome by using the I² statistic to quantify the degree of heterogeneity (Higgins 2002), and we considered I² > 30% as showing moderate heterogeneity, with I² > 75% signifying substantial heterogeneity.
Assessment of reporting biases
We were unable to generate funnel plots and performed Egger's linear regression tests in order to investigate reporting biases for any of the outcomes, as the maximum number of trials included in a single meta‐analysis was insufficient (9, with at least 10 trials required). We followed recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022). We noted interpretation was difficult when small numbers of trials (< 10) were included. When we observed evidence of small‐study effects, we performed sensitivity analyses according to regression‐based adjustment methods.
Data synthesis
We used intention‐to‐screen analyses by including all randomised people who were invited to screening where possible, and have specified when intention‐to‐screen analysis was not used for a study. When there were repeated observations on participants in long‐term trials, we included outcomes at different time points in separate analyses. We combined data when outcomes from different trials were measured at similar time points.
If sufficient clinically‐similar trials were available, we performed meta‐analyses, applying both fixed‐ and random‐effects meta‐analyses according to recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022). We entered data into RevMan 5 (Review Manager 2020). A review author (RM) entered the data, and a second review author (AB) double‐checked the data for accuracy. We only included trials in the meta‐analysis for lung cancer‐specific mortality and all‐cause mortality if they had at least 5 years of follow‐up. We applied the generic inverse‐variance method and random‐effect models for all type of outcomes. For dichotomous outcomes, we applied the DerSimonian and Laird method (DerSimonian 1986).
For calculating overdiagnosis data we used the following formula for the diagnosis rate in the screened group and then bootstrapped this to obtain 95% normal based CIs.
[(Lung cancer incidence in LDCT screening group/total number of participants in screening group) ‐ (lung cancer incidence in control group/total number of participants in control group)] / (lung cancer incidence in LDCT screening group/total number of participants in screening group)]
Subgroup analysis and investigation of heterogeneity
We investigated the level of heterogeneity. When data were heterogenous we checked and identified the sources of this heterogeneity. When heterogeneity remained considerably high I2 > 75%, we reported the results narratively with no meta‐analyses.
-
We performed a number of subgroup analyses:
age
sex
smoking history or validated measures of lung cancer risk (including risk prediction model)
screening interval
geographical region
by control types ‐ usual care or CXR
Sensitivity analysis
We conducted sensitivity analyses to assess whether results were robust to assess decisions made during the review process such as our assessments about clinical heterogeneity. We looked at the impact of types of control groups. If we identified sufficient trials, we restricted the analysis to trials at low risk of bias, based on overall risk of bias judgement (Higgins 2017).
Summary of findings and assessment of the certainty of the evidence
As suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022), we presented a summary of findings table, reporting the following outcomes listed in order of priority.
Lung cancer‐related mortality, using planned follow‐up time points (predefined by trial as opposed to unplanned, post hoc, extended follow‐up)
All‐cause mortality, using planned follow‐up time points
Overdiagnosis (this replaced lung cancer incidence)
Number of invasive tests (to represent harms of screening)
Any death postsurgery (this replaced the impact on smoking behaviour with an additional harm of screening outcome)
Anxiety (to represent HRQoL and psychosocial consequences)
We followed the GRADE approach when creating our summary of findings table, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022). The GRADE approach specifies four levels of certainty (high, moderate, low, or very low) to rate the certainty of evidence in the following domains.
Risk of bias
Inconsistency
Indirectness
Imprecision
Publication bias
Results
Description of studies
Results of the search
Overall, we identified 5390 citations during our electronic search, of which we selected 43 for full‐text review. The evidence is current to 31 July 2021. Following full‐text review, we included 11 trials (reported in 182 multiple citations). We excluded 30 citations, with additional details provided in Characteristics of excluded studies. We identified an additional 47 citations for the included trials during full‐text review via searching of bibliographies and additional MEDLINE author searches. We identified two RCTs that were in keeping with our review protocol, that had not published mortality or harm data (Sagawa 2012; Yang 2018). The first is a Japanese trial that started in 2010, comparing LDCT and CXR over a 10‐year period in people with a smoking history < 30 pack years (e.g. < 1 pack of cigarettes/day for 30 years or < 1.5 packs/day for 15 years etc.) (Sagawa 2012). The second trial (Yang 2018), based in China, similarly includes participants who also do not have a strong smoking history, however participants must have at least one high‐risk factor (family history of cancer or personal history of cancer, occupational exposures to carcinogenic agents, passive or active smoking exposure, or long‐term exposure to cooking oils). This trial compares three rounds of biennial LDCT with no screening and started in 2013. These trials are described in more detail in Characteristics of ongoing studies.
The included trials were the US National Lung Screening Trial (NLST, Aberle 2011), German Lung Cancer Screening Intervention (LUSI, Becker 2020), French DEPISCAN trial (Blanchon 2007), Dutch‐Belgian Nederlands‐Leuvens Longkanker Screenings Onderzoek trial (NELSON, De Koning 2020), UK Lung Cancer Screening trial (UKLS, Field 2021), US Lung Screening Study (LSS, Gohagan 2005), Italian Detection And screening of early lung cancer by Novel imaging TEchnology trial (DANTE, Infante 2015), North American Jewish Hospital Lung Cancer Screening and Early Detection Study (LaRocca 2002), Italian Lung Cancer Screening trial (ITALUNG, Paci 2017), Multicentric Italian Lung Detection trial (MILD, Pastorino 2012), and the Danish Lung Cancer Screening Trial (DLCST, Wille 2016).
Search results are described in Figure 1.
1.
Study selection flow diagram.
Included studies
Trial design and setting
Eight of the 11 trials were phase 3 RCTs (Aberle 2011; Becker 2020; De Koning 2020; Infante 2015; LaRocca 2002; Paci 2017; Pastorino 2012; Wille 2016), whilst the LSS (Gohagan 2005) and DEPISCAN (Blanchon 2007) trials were feasibility RCTs, and UKLS was a pilot RCT (Field 2021). Three of the 11 trials were conducted in the USA (Aberle 2011; Gohagan 2005; LaRocca 2002); the remaining trials were based in Europe.
All trials were conducted via hospitals or screening centres, with the number of sites varying from 1 to 33. The NLST had the most trial sites (Aberle 2011), followed by the French DEPISCAN trial with 14 sites (Blanchon 2007).
LaRocca 2002 was the earliest trial to start, in 1991, followed by Gohagan 2005 in 2000. Wille 2016 had the latest start date (2011) of the included trials, with the remaining trials starting between 2001 and 2007.
Trial participants
Overall 94,445 people were included across the trials. The NLST had the largest sample size of the included trials with 53,456 participants (Aberle 2011). The next biggest was the NELSON trial with 15,792 (De Koning 2020). Four trials had just over 4000 participants each (Becker 2020; Field 2021; Pastorino 2012; Wille 2016), whilst LSS (Gohagan 2005) and ITALUNG (Paci 2017) had over 3000 participants each. The DANTE trial had 2450 participants (Infante 2015). DEPISCAN had the smallest reported sample size of 765 participants randomised (Blanchon 2007), and with only 621 participants continuing after 144 withdrew consent. LaRocca 2002 reported 871 participants were randomised, however did not include allocation of participants.
In the UKLS trial (Field 2021), the number of participants included in Characteristics of included studies and number of participants in some analyses differ, as 87 participants in the UKLS trial were excluded post‐randomisation from analysis of long‐term data.
Inclusion criteria
Inclusion and exclusion criteria between the trials were similar, with trials having an overlapping age range from 40 years and above. Nine of the 11 trials had a lower age limit of 50 years or above (Aberle 2011; Becker 2020; Blanchon 2007; De Koning 2020; Field 2021; Gohagan 2005; Infante 2015; Paci 2017; Wille 2016). Ten of the 11 trials had an upper age limit of 75 years or less (Aberle 2011; Becker 2020; Blanchon 2007; De Koning 2020; Field 2021; Gohagan 2005; Infante 2015; LaRocca 2002; Paci 2017; Wille 2016). All trials, except UKLS (Field 2021), had a strong smoking history requirement as part of the inclusion criteria (at least 20 pack years or more). Field 2021 was one of the few trials to use a risk prediction model; with participants requiring a 5% risk of developing lung cancer in 5 years, based on the Liverpool Lung Project (LLP) Risk Prediction Model version 2 (LLPv2). The LLPv2 is a lung cancer risk calculator that incorporates factors such as age, tobacco smoking history, personal history of pulmonary disease or cancer, family history of lung cancer and occupational exposures (Field 2016).
Of note, the DANTE trial excluded all women from the trial (Infante 2015), and the NELSON trial (De Koning 2020) only recruited women in the Belgium arm of the trial, and not for the Netherlands cohort. No included trial reported equal representation of male and female participants.
In LaRocca 2002, participants required a normal or stable CXR prior to randomisation. The DANTE trial also required a baseline CXR and sputum cytology with clinical examination in both arms of their trial (Infante 2015).
In addition to the basic demographics provided in the Characteristics of included studies, the NLST included information about education status (Aberle 2011), with 32% of participants having a college degree or higher level of education. Only 48% of their cohort were current smokers. Weight data was also collected, with 1% of their cohort underweight, 28% normal weight, 43% overweight and 28% obese. In the UKLS trial (Field 2021), 46% of the cohort had an education up to or equal to secondary level and 54% beyond secondary school. The DLCST participants had a relatively even distribution of low, middle, and high socioeconomic status (Wille 2016), with 74% of the cohort having 10 years or less of schooling.
Intervention
All trials used chest LDCT as their primary intervention, with reported settings ranging from 90 kVP to 140 kVP and 20 mA to 60 mA. The frequency and duration of LDCT varied between trials, with annual LDCT occurring in nine of the 11 trials (Aberle 2011; Becker 2020; Blanchon 2007; Gohagan 2005; Infante 2015; LaRocca 2002; Paci 2017; Pastorino 2012; Wille 2016). In the UKLS trial (Field 2021), only one LDCT was performed during the trial. The LSS trial conducted annual screening over 2 years (Gohagan 2005), whilst DEPISCAN (Blanchon 2007) and NLST (Aberle 2011) performed annual LDCT for 3 years. The ITALUNG trial performed annual LDCT for 4 years (Paci 2017), whilst four of the 11 trials performed annual LDCT screening for 5 years (Becker 2020; Infante 2015; LaRocca 2002; Wille 2016). The MILD trial had two intervention arms (Pastorino 2012), one for biennial scans and one for annual scans; over the 10‐year screening period, the biennial arm had a median of four LDCT scans whilst the annual group had a median of seven LDCT scans. The NELSON trial used incrementing intervals for the LDCT (De Koning 2020), with a baseline scan, then at 1 year, 2 years, and 2.5‐year intervals.
The majority of the trials used no screening for the control arm (Becker 2020; De Koning 2020; Field 2021; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016), however four of the 11 trials used annual CXR in the comparison arm for the duration of the screening period (Aberle 2011; Blanchon 2007; Gohagan 2005; LaRocca 2002).
Six of the 11 trials used diameter criteria and no volumetric assessment using computer‐assisted tools to determine significance of pulmonary nodules (Aberle 2011; Blanchon 2007; Gohagan 2005; Infante 2015; LaRocca 2002; Paci 2017). LUSI (Becker 2020), UKLS (Field 2021), and DLCST (Wille 2016) used both diameter and volumetric criteria to determine nodule significance. The NELSON trial (De Koning 2020) and MILD trial (Pastorino 2012) used volumetric analysis only, for evaluating nodules at baseline and calculating at 3‐month follow‐up the volume doubling time of nodules.
Outcomes and follow‐up
Of the published data, follow‐up ranged from 5 to 12 years post‐randomisation (Aberle 2011; Becker 2020; De Koning 2020; Field 2021; Gohagan 2005; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016). NLST (Aberle 2011), NELSON (De Koning 2020), ITALUNG (Paci 2017), and MILD (Pastorino 2012) all have median follow‐ups of 10 or more years. The DANTE (Infante 2015) and MILD (Pastorino 2012) trials both published mortality data before and beyond 5 years, with only the later time points included. Yang 2018 published 2‐year mortality data following the baseline scan, however this trial is ongoing.
Eight of the 11 trials used prespecified nodule follow‐up (Becker 2020; Blanchon 2007; De Koning 2020; Field 2021; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016). LaRocca 2002 did not state if any protocol was used, however NLST (Aberle 2011) and LSS (Gohagan 2005) stated they did not use a trial‐wide algorithm for nodule follow‐up. Nodule management for each trial is described in Table 2.
1. Nodule management .
| Interpretation | Management | |
| Aberle 2011 | Positive scan: findings suspicious of lung cancer, such as non‐calcified nodule ≥ 4 mm, lung consolidation, or obstructive atelectasis, nodule enlargement, and nodules with suspicious changes in attenuation | No trial‐wide algorithm |
| Becker 2020 | Positive scan: any nodule ≥ 5 mm |
On recall scans
|
| Blanchon 2007 | Positive scan: non‐calcified nodule > 5 mm |
If no change: repeat scan at 6 months, 12 months and 24 months from baseline. If growth at any time: histological diagnosis.
|
| De Koning 2020 | Classification of non‐calcified nodules:
Classification of nodules based on growth:
|
Management of non‐calcified nodules based on baseline screening
Management protocol for non‐calcified nodules at incidence screening
At year 4
At year 6
Preoperative biopsy was not routine. Suspicious nodules were removed by VATS or thoracotomy with wedge resection+frozen section. Lobectomies were performed only for central nodules that could not be approached by wedge resection. If cancer was diagnosed by VATS, the procedure was converted to an open thoracotomy with sampling of lobar, interlobar, hilar and mediastinal lymph nodes as VATS resection in lung cancer was not fully implemented at the time of trial in the Netherlands. Mediastinoscopy was performed before proceeding to VATS or thoracotomy in subjects with mediastinal lymph nodes > 10 mm in short axis and/or positive nodes. |
| Field 2021 | Classification of nodules:
|
|
| Gohagan 2005 |
Other abnormalities could also be considered suspicious for lung cancer at the discretion of the radiologist. |
No trial‐wide algorithm for management
|
| Infante 2015 |
|
No set trial‐wide algorithm for management
|
| LaRocca 2002 |
|
|
| Paci 2017 |
|
|
| Pastorino 2012 |
|
|
| Wille 2016 |
|
|
CT: computed tomography; CXR: chest x‐ray; dmean: mean diameter; dmin: minimal diameter; FDG PET: fluorodeoxyglucose positron emission tomography; FNA: fine needle aspiration; HRCT; high‐resolution computed tomography; IV: intravenous; LDCT: low‐dose computed tomography; MDM: multidisciplinary meeting; MDT: multidisciplinary team; PET: positron emission tomography; VATS: video‐assisted thoracoscopic surgery; VDT: volume doubling time.
Of the 11 trials, nine had a primary outcome that included lung cancer‐related mortality (Aberle 2011; Becker 2020; De Koning 2020; Field 2021; Infante 2015; LaRocca 2002; Paci 2017; Pastorino 2012; Wille 2016). The LSS trial had the primary outcome of feasibility to enrol participants in a lung cancer screening programme (Gohagan 2005), however it also had outcomes assessing harms of screening, such as extent of diagnostic follow‐up after abnormal screening findings. The DEPISCAN trial also had primary feasibility outcomes of enrolling participants in a lung cancer screening programme (Blanchon 2007), but also outcomes on harms and adverse events during diagnostic procedures, as well as number of futile thoracotomies for benign lesions.
Excluded studies
We excluded 30 studies for the following reasons.
Irrelevant trial design: 19 studies were not RCTs (Brodersen 2014; Dawson 2020; Favre 2003; Fink 2012; Goulart 2013; Hassannezhad 2018; Henschke 2000; Henschke 2002; Henschke 2015; Horeweg 2013; Kramer 2011; Kulaga 2007; NCT02431962; Robbins 2019; Schabath 2019; Schreuder 2021; Strauss 2012; Strauss 2015; Yip 2013).
Irrelevant intervention: seven studies did not have LDCT screening alone as an intervention (Bradley 2021; ISRCTN42704678; Marcus 2006; Spiro 2019; Sullivan 2019; Sullivan 2021; Yang 2008). Two papers related to the Yorkshire Lung Screening Trial (lung health check versus usual care) (Bradley 2021; ISRCTN42704678). Marcus 2006 compared CXR and sputum cytology to usual care. Spiro 2019 compared sputum cytology and cytometry to usual care. Two papers compared serum biomarker to usual care (Sullivan 2019; Sullivan 2021). Yang 2008 compared serum biomarker and LDCT with usual care.
Irrelevant outcomes: one trial measured feasibility of conducting a RCT for lung cancer screening (Garg 2002). Another paper evaluated the effects of computer‐aided diagnosis on lung imaging reporting (Park 2022).
One trial was a duplicate (de‐Torres 2021).
Irrelevant patient population: one trial included participants with a recent diagnosis of lung cancer (Guldbrandt 2015).
Details of these citations are provided in Characteristics of excluded studies.
Risk of bias in included studies
We performed the risk of bias assessment for all included trials with the Cochrane RoB 1 tool (Higgins 2017), and summarised the results in Characteristics of included studies, Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We deemed allocation concealment adequate in nine of the 11 trials, suggesting a low risk of bias (Aberle 2011; Becker 2020; Field 2021; Gohagan 2005; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016; De Koning 2020). The remaining two trials had unclear risk of bias (Blanchon 2007; LaRocca 2002), with insufficient information available to determine if a centralised process was used.
We judged sequence generation adequate in nine of the 11 trials, suggesting low risk of bias (Aberle 2011; Becker 2020; Field 2021; Gohagan 2005; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016; De Koning 2020). The remaining two trials had unclear risk of bias (Blanchon 2007; LaRocca 2002), with insufficient information available to determine if a random method in sequence generation was used.
Blinding
Due to the nature of the intervention, no trial participants were blinded to their trial arm in included trials. For this review, lack of blinding of participants in the primary outcomes (lung cancer‐related mortality and harms of screening) was unlikely to influence the outcomes. Blinding of assessors for the primary outcome of lung cancer‐related mortality was assessed as adequate in five of the 11 trials (Aberle 2011; Field 2021; Pastorino 2012; Paci 2017; Wille 2016). The UKLS trial (Field 2021) only assessed cause of death from registries and death certificates, without the use of a review board.
Two trials did not provide information regarding blinding of assessors (Blanchon 2007; LaRocca 2002), and we judged these at unclear risk. We also deemed Becker 2020 at unclear risk of bias; whilst the assessors in the trial were blinded to the arm when assessing lung cancer‐related mortality, the method of identification of lung cancer was not uniform, with 11 of the 67 cases in the control arm and 1 of the 85 cases in the intervention arm detected on death certificate only.
We deemed three of the 11 trials at high risk of bias (De Koning 2020; Gohagan 2005; Infante 2015): neither the LSS (Gohagan 2005) nor the DANTE trial (Infante 2015) blinded assessors; LSS (Gohagan 2005) only assessed cause of death from death certificates, without the use of a review board; and the NELSON trial (De Koning 2020) raised concerns regarding the method of assessing lung cancer as the cause of death ‐ it changed from using a death review panel to using death certificates only during active follow‐up, with assessors also unblinded in 2018.
Some outcome measurements, such as all‐cause mortality, were not likely to be influenced by lack of blinding.
Incomplete outcome data
Missing data and withdrawals were adequately described in nine of the 11 included trials (Aberle 2011; Becker 2020; De Koning 2020; Field 2021; Gohagan 2005; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016), with risk of bias deemed low risk. Of note, the NLST (Aberle 2011) included 192 participants in their analysis that were deemed ineligible for the trial post‐randomisation, and at the end of December 2009 completed active follow‐up (meaning the remaining causes of death were assessed as per the registries). The LSS (Gohagan 2005) excluded the 91 participants found to be ineligible post‐randomisation from analysis. The ITALUNG trial (Paci 2017) had moderate rates of dropout and non‐adherence (81% adherence to screening), however used intention‐to‐treat analysis. The remaining two trials had insufficient information available to make a judgement and we deemed them at unclear risk (Blanchon 2007; LaRocca 2002).
Selective reporting
We judged nine of the 11 included trials at low risk of reporting bias (Aberle 2011; Becker 2020; De Koning 2020; Field 2021; Gohagan 2005; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016). Whilst the NELSON (De Koning 2020) trial has not published their cost‐analysis data, information from the authors confirmed intention to do so. We judged the remaining two trials at unclear risk due to insufficient data available (Blanchon 2007; LaRocca 2002).
Other potential sources of bias
There were minimal deviations to protocols and balanced baselines in five of the 11 trials ‐ we deemed these at low risk of other bias (Aberle 2011; Becker 2020; Gohagan 2005; Paci 2017; Wille 2016). The DLCST (Becker 2020) reported a difference in baseline characteristics between the two groups in mean forced expiratory ratio (FER) (although no difference in mean forced expiratory volume in 1 second (FEV1)) and number of participants with > 35 pack‐year smoking history, however we judged the size of difference unlikely to have had a significant impact on outcomes. Blanchon 2007 and LaRocca 2002 both had insufficient data published to enable us to make an assessment and we judged these at unclear risk of bias. We judged four of the 11 trials at high risk of other bias (De Koning 2020; Field 2021; Infante 2015; Pastorino 2012). We deemed the NELSON (De Koning 2020) trial at high risk of bias due to a change in method of determining lung cancer‐related death during the trial, as well as additional amendments to the protocol to add a scan interval of 2.5 years after trial commencement. The trial also did not recruit any women in the Netherlands arm of the trial. Similarly, in the DANTE (Infante 2015) trial women were excluded, and there was an unbalanced baseline between trial arms with respiratory comorbidity more prevalent in the LDCT arm. The UKLS (Field 2021) trial excluded 87 participants from long‐term mortality and incidence analysis and did not use intention‐to‐screen analysis, however we judged the number of participants was likely too small to have an impact on results. It should be noted that LLPv2 was unintentionally used rather than LLPv1 as the risk prediction model in UKLS (Field 2021). The MILD (Pastorino 2012) trial had an unbalanced baseline between arms with 90% of the control arm being current smokers compared with 69% of the LDCT arm. Additionally, when the MILD (Pastorino 2012) trial commenced recruitment, there was only the annual LDCT and biennial LDCT groups, with the no‐screening control group added later.
Effects of interventions
See: Table 1
Primary outcomes
1) Lung cancer‐related mortality
Lung cancer‐related mortality using planned follow‐up time points
We pooled the latest time point for planned lung cancer‐related mortality for all available trials. We included eight trials in this analysis (Aberle 2011; Becker 2020; De Koning 2020; Field 2021; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016). Time points (median time post‐randomisation) for these trials were 6.5 years, 8.8 years, 10 years, 7 years, 8.5 years, 9.3 years, 10 years, and 10 years respectively. We did not include the LSS (Gohagan 2005) as the planned follow‐up for the trial was only 2 years. The evidence showed a difference in lung cancer‐related mortality favouring screening with LDCT, with a reduction in lung cancer‐related mortality of 21% (risk ratio (RR) 0.79, 95% confidence interval (CI) 0.72 to 0.87; 8 trials, 91,122 participants; I2 = 0%; moderate‐certainty evidence; Analysis 1.1). The number needed to screen to prevent one additional lung cancer‐related death was 226. The NLST (Aberle 2011) and NELSON (De Koning 2020) trials both had strong weighting in this analysis, with the DLCST (Wille 2016), and DANTE (Infante 2015) trials demonstrating probably no difference with LDCT screening on lung cancer‐related mortality. When we performed sensitivity analysis using three trials with low risk of bias (Aberle 2011; Paci 2017; Wille 2016), the evidence still favoured screening, with a reduction in lung cancer‐related mortality (RR 0.81, 95% CI 0.71 to 0.92; 3 trials, 60,764 participants; I2 = 0%; high‐certainty evidence; Figure 4). The number needed to screen to prevent one additional lung cancer‐related death was 296.
1.1. Analysis.

Comparison 1: Primary outcome: lung cancer‐related mortality, Outcome 1: Lung cancer‐related mortality ‐ planned time points
4.
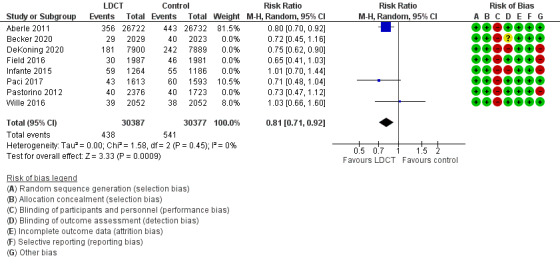
Lung cancer mortality ‐ Planned time points ‐ Sensitivity analysis
When we analysed hazard ratios (HRs) from Becker 2020, Infante 2015, and Wille 2016 at the > 8 to 10‐year planned follow‐up time point post‐randomisation, there was probably no difference for people at risk for lung cancer‐related mortality with LDCT screening (HR 0.93, 95% CI 0.72 to 1.19; 3 trials, 10,606 participants; I2 = 0%; Analysis 1.2).
1.2. Analysis.

Comparison 1: Primary outcome: lung cancer‐related mortality, Outcome 2: Lung cancer‐related mortality ‐ planned time points
Lung cancer‐related mortality using planned and unplanned follow‐up time points
We also grouped trial results by time points, including planned and unplanned extended follow‐up, as depicted in Analysis 1.3.
1.3. Analysis.

Comparison 1: Primary outcome: lung cancer‐related mortality, Outcome 3: Lung cancer‐related mortality at different follow‐up time points (including unplanned)
5 to 6 years post‐randomisation: four RCTs reported this outcome at this time point (Becker 2020; De Koning 2020; Gohagan 2005; Wille 2016). There was probably no difference between LDCT and control groups in relation to lung cancer‐related mortality (RR 0.89, 95% CI 0.64 to 1.24; 4 trials, 27,263 participants; I2 = 42%). Heterogeneity amongst trials was moderate, but within acceptable limits. On average, 466 people would have to be screened to prevent one additional lung cancer‐related death.
More than 6 to 8 years post‐randomisation: we included three RCTS (Aberle 2011; De Koning 2020; Field 2021). The evidence showed there was a difference in lung cancer‐related mortality favouring LDCT screening over no screening (RR 0.77, 95% CI 0.69 to 0.86; 3 trials, 73,211 participants; I2 = 0%). On average, 233 people would have to be screened to prevent one additional death related to lung cancer.
More than 8 to 10 years post‐randomisation: we included six RCTs (Becker 2020; De Koning 2020; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016), and once more, pooling data showed a difference favouring LDCT screening in lung cancer‐related mortality (RR 0.79, 95% CI 0.69 to 0.90; 6 trials, 33,700 participants; I2 = 0%). Of note, screening in DANTE (Infante 2015) and DLCST (Wille 2016) probably made no difference, however they were smaller trials. The MILD (Pastorino 2012) trial combined both biennial and annual trial group mortality data for the outcome. On average, 163 people would have to be screened to prevent one additional death from lung cancer.
More than 10 years post‐randomisation: we included three RCTS (Aberle 2011; De Koning 2020; Paci 2017), and the evidence showed a difference favouring LDCT screening in lung cancer‐related mortality (RR 0.86, 95% CI 0.75 to 0.98; 3 trials, 72,447 participants; I2 = 48%). Heterogeneity amongst trials was moderate, but within acceptable limits. On average, 222 people would have to be screened to prevent one additional death from lung cancer.
Lung cancer‐related mortality by time postcompletion of screening
We grouped trial results by years postcompletion of screening using planned and unplanned time point follow‐up data from all nine available trials (Aberle 2011; Becker 2020; De Koning 2020; Field 2021; Gohagan 2005; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016) in Analysis 1.5. When multiple time points were available for a trial within one bracket of time, we used the latest time point data.
1.5. Analysis.

Comparison 1: Primary outcome: lung cancer‐related mortality, Outcome 5: Lung cancer‐related mortality – by time postscreening cessation (including unplanned time points)
Zero to 1 year postscreening completion: we included four RCTs (Becker 2020; De Koning 2020; Pastorino 2012; Wille 2016). The evidence showed a difference in lung cancer‐related mortality favouring screening (RR 0.76, 95% CI 0.61 to 0.94; 4 trials, 28,044 participants; I2 = 0%). On average, 324 people would need to be screened to prevent one additional death related to lung cancer.
2 to 4.5 years postscreening completion: we included five RCTs (Aberle 2011; Becker 2020; De Koning 2020; Gohagan 2005; Infante 2015). The evidence favoured LDCT screening for lung cancer‐related morality (RR 0.82, 95% CI 0.72 to 0.93; 5 trials, 79,063 participants; I2 = 18%). On average, 262 people would need to be screened to prevent one additional death from lung cancer‐related mortality.
5 to 7 years postscreening completion: we included four RCTs (De Koning 2020; Field 2021; Paci 2017; Wille 2016). The evidence favoured screening for lung cancer‐related mortality (RR 0.78, 95% CI 0.67 to 0.90; 4 trials, 27,067 participants; I2 = 0%). On average, 149 people would need to be screened to prevent one additional death related to lung cancer.
More than 7 to 10 years postscreening completion: we included two RCTs (Aberle 2011; Paci 2017). There was probably no difference between the groups for lung cancer‐related mortality (RR 0.92, 95% CI 0.83 to 1.01; 2 trials, 56,658 participants; I2 = 6%).
Lung cancer‐related mortality by different subgroups
By screening arm
Planned time periods: we pooled lung cancer‐related mortality data from all eight available trials and divided the data into subgroups based on control arm comparator, CXR (Aberle 2011) or no screening (Becker 2020; De Koning 2020; Field 2021; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016), using the latest planned follow‐up time point for each trial in Analysis 1.4.
1.4. Analysis.

Comparison 1: Primary outcome: lung cancer‐related mortality, Outcome 4: Lung cancer‐related mortality by screening arm ‐ planned time points
For usual care: the evidence showed a difference in lung cancer‐related mortality favouring screening when compared to usual care (RR 0.78, 95% CI 0.69 to 0.88; 7 trials, 37,668 participants; I2 = 0%).
For CXR: the evidence also favoured LDCT over CXR (RR 0.80, 95% CI 0.70 to 0.92; 1 trial, 53,434 participants).
There was no difference between subgroups. Test for subgroup differences: Chi² = 0.11, df = 1 (P = 0.74), I² = 0% (Analysis 1.4).
By screening intervals
We also presented the latest planned time point data from nine available trials by screening interval in Analysis 1.6. The MILD trial (Pastorino 2012) had mortality data presented separately by intervention group (biennial and annual). The NELSON (De Koning 2020) trial, with incremental intervals, demonstrated a reduction in lung cancer‐related mortality (RR 0.75, 95% CI 0.62 to 0.90; 1 trial, 15,789 participants), while data from NLST (Aberle 2011), which had three annual screens also favoured LDCT (RR 0.80, 95% CI 0.70 to 0.92; 1 trial, 53454 participants). The overall results favoured LDCT screening for lung cancer‐related mortality (RR 0.79, 95% CI 0.72 to 0.87; 9 trials, 91,122 participants; I2 = 0), with no subgroup difference (test for subgroup differences: Chi² = 3.38, df = 6 (P = 0.76), I² = 0%).
1.6. Analysis.

Comparison 1: Primary outcome: lung cancer‐related mortality, Outcome 6: Lung cancer‐related mortality by screening interval ‐ planned time points
By sex
Five trials reported mortality due to lung cancer by sex (Aberle 2011; Becker 2020; De Koning 2020; Field 2021; Infante 2015), as depicted in Analysis 1.8 and Analysis 1.7.
1.8. Analysis.

Comparison 1: Primary outcome: lung cancer‐related mortality, Outcome 8: Lung cancer‐related mortality by sex ‐ planned time points
1.7. Analysis.

Comparison 1: Primary outcome: lung cancer‐related mortality, Outcome 7: Lung cancer‐related mortality by sex ‐ planned time points
For women: we included four RCTS (Aberle 2011; Becker 2020; De Koning 2020; Field 2021). We used data from the latest planned time point available for this analysis. The evidence showed a difference in lung cancer‐related mortality in women favouring LDCT screening, and screening reduced the risk by 29% (RR 0.71, 95% CI 0.59 to 0.86; 4 trials, 26,965 participants; I2 = 0%; Analysis 1.8). However, the pooled HRs from three RCTs showed screening reduced the risk of death by 27% compared to no screening (HR 0.73, 95% CI 0.34 to 1.56; 3 trials, 4286 participants; I2= 64%; Analysis 1.7). However, the 95% CI included 1, so there was probably no difference between the two arms. Removing Wille 2016, reduced the heterogeneity between trials without changing the finding (HR 0.50, 95% CI 0.23 to 1.07; 2 trials, 2449 participants; I2=15%) (analysis not shown).
For men: we included five RCTS (Aberle 2011; Becker 2020; De Koning 2020; Field 2021; Infante 2015). We used data from the latest planned time point available for this analysis. The evidence showed a difference in lung cancer‐related mortality in men favouring LDCT screening, and screening reduced risk by 15% (RR 0.85, 95% CI 0.76 to 0.95; 5 trials, 52,833 participants; I2 = 0%; Analysis 1.8). Analysis of HRs (HR 0.76, 95% CI 0.52 to 1.12; 2 trials, 5658 participants) demonstrated that screening could reduce the risk of death by 24% compared to no screening among men, however the 95% CI included 1, so there was probably no difference for men at risk for lung cancer‐related mortality with LDCT screening (Analysis 1.7).
There was no difference between the two subgroups. Test for subgroup differences: Chi² = 2.49, df = 1 (P = 0.11), I² = 59.9%.
By age
One trial (Aberle 2011) presented mortality data by age group for the latest planned time point Analysis 1.9.
1.9. Analysis.

Comparison 1: Primary outcome: lung cancer‐related mortality, Outcome 9: Lung cancer‐related mortality by age ‐ planned time points
For those < 65 years old: the evidence favoured LDCT screening to reduce lung cancer‐related mortality by 18% (RR 0.82, 95% CI 0.70 to 0.97; 1 trial, 39,234 participants).
For those ≥ 65 years: the evidence favoured LDCT screening to reduce lung cancer‐related mortality by 38% (RR 0.62, 95% CI 0.52 to 0.74; 1 trial, 17,218 participants).
By smoking status
Only one trial (Aberle 2011) presented lung cancer‐related mortality data by smoking status (former or current). Data from both 6.5 years and 12.3 years post‐randomisation are provided in Analysis 1.10. At both time points, the evidence showed a benefit in LDCT screening for lung cancer‐related mortality in current smokers (6.5 years: RR 0.82, 95% CI 0.70 to 0.95; 1 trial, 25,760 participants; I2 = 0%) and (12.3 years: RR 0.89, 95% CI 0.81 to 0.98; 1 trial, 25,760 participants). However, evidence suggested there was probably no difference in former smokers (6.5 years: RR 0.91, 95% CI 0.74 to 1.11; 1 trial, 27,692 participants) and (12.3 years: RR 1.01, 95% CI 0.88 to 1.15; 1 trial, 27,692 participants).
1.10. Analysis.

Comparison 1: Primary outcome: lung cancer‐related mortality, Outcome 10: Lung cancer related to smoking ‐ latest time point (including unplanned)
The DLCST (Wille 2016) presented lung cancer‐related mortality by number of pack years smoked < 35 or ≥ 35, there was probably no difference between the groups, for < 35 pack years (RR 1.26, 95% CI 0.55 to 2.90; 1 trial, 2148 participants) and ≥ 35 pack years (RR 0.92, 95% CI 0.54 to 1.54; 1 trial, 1955 participants) in this trial (Analysis 1.10).
By geographical regions
Planned time points: lung cancer‐related mortality by geographical region using the latest planned time point is presented in Analysis 1.11.
1.11. Analysis.

Comparison 1: Primary outcome: lung cancer‐related mortality, Outcome 11: Lung cancer‐related mortality by geography ‐ planned time points
Europe: we included seven trials (Becker 2020; De Koning 2020; Field 2021; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016). The evidence demonstrated a benefit in lung cancer‐related mortality with LDCT screening (RR 0.78, 95% CI 0.69 to 0.88; 7 trials, 37,668 participants; I2 = 0%).
USA: we included one trial (Aberle 2011). The evidence demonstrated a benefit in lung cancer‐related mortality with LDCT screening (RR 0.80, 95% CI 0.70 to 0.92; 1 trial, 53,454 participants).
This analysis (Analysis 1.11) is identical to Analysis 1.4, as the USA trial was the only one to use CXR as a comparison. Overall, the evidence suggested a lung cancer‐related mortality benefit with LDCT screening.
There was no difference between the groups. Test for subgroup differences: Chi² = 0.11, df = 1 (P = 0.74), I² = 0%.
By algorithms for nodule management
We also grouped trials by use of trial‐wide algorithms for nodule management (yes or no) using the latest planned time points in Analysis 1.12.
1.12. Analysis.

Comparison 1: Primary outcome: lung cancer‐related mortality, Outcome 12: Nodule management algorithm ‐ planned follow‐up time points
Yes: we included six RCTs (Becker 2020; De Koning 2020; Field 2021; Paci 2017; Pastorino 2012; Wille 2016). The evidence suggested a difference in lung cancer‐related mortality favouring screening in this group (RR 0.75, 95% CI 0.66 to 0.86; 6 trials, 35,218 participants; I2 = 0%). We also applied a fixed‐effect model for analysis and the conclusion was the same (RR 0.75, 95% CI 0.66 to 0.86; 6 trials, 35,218 participants; I2 = 0%).
No: we included two trials (Aberle 2011; Infante 2015). There was probably no difference using a random‐effects model for analysis (RR 0.84, 95% CI 0.70 to 1.01; 2 trials, 55,904 participants; I2 = 24%). However, when we applied a fixed‐effect model, the evidence showed a difference in lung cancer‐related mortality favouring screening (RR 0.83, 95% CI 0.73 to 0.94; 2 trials, 55,904 participants; I2 = 24%).
There was no difference between the groups. Test for subgroup differences: Chi² = 1.02, df = 1 (P = 0.31), I² = 2.2%.
By nodule analysis method
We grouped trials by method of nodule analysis (diameter criteria and/or volumetric criteria) using the latest planned time points in Analysis 1.13.
1.13. Analysis.

Comparison 1: Primary outcome: lung cancer‐related mortality, Outcome 13: Nodule management criteria ‐ planned follow‐up time points
Diameter criteria: we included three RCTs (Aberle 2011; Infante 2015; Paci 2017). The evidence showed a difference in lung cancer‐related mortality favouring screening (RR 0.81, 95% CI 0.72 to 0.92; 3 trials, 59,110 participants; I2 = 0%).
Volume criteria: we included two RCTs (De Koning 2020; Pastorino 2012). The evidence showed a difference in lung cancer‐related mortality favouring screening (RR 0.74, 95% CI 0.62 to 0.88; 2 trials, 19,888 participants; I2 = 0%).
Diameter and volume criteria: we included three trials (Becker 2020; Field 2021; Wille 2016). These trials demonstrated there was probably no difference between the groups (RR 0.79, 95% CI 0.60 to 1.04; 3 trials, 12,124 participants; I2 = 8%). It should be noted that all included trials had low participant numbers.
There was no difference between the groups. Test for subgroup differences: Chi² = 0.71, df = 2 (P = 0.70), I² = 0%. Nodule management pathways are detailed in Table 2.
2) Harms of screening
Number of all invasive tests performed
We grouped trial results based on time point (following baseline screening scan or at follow‐up) (Analysis 2.1).
2.1. Analysis.

Comparison 2: Primary outcome: number of non‐invasive and invasive tests ‐ all time points, Outcome 1: Number of invasive tests
At baseline: we included three RCTs (Aberle 2011; Gohagan 2005; Infante 2015). We combined invasive procedures and surgery numbers provided in Infante 2015 for this analysis. The evidence showed that more invasive tests were performed in the LDCT screening group (RR 2.90, 95% CI 2.25 to 3.75; 3 trials, 59,110 participants; I2 = 43%); 363 invasive tests were performed for every 10,000 participants screened with LDCT, with a number needed to harm (NNH) of 44. Heterogeneity was moderate, and when we removed Aberle 2011 from the analysis, there was no heterogeneity and no change to the conclusion (RR 3.56, 95% CI 2.53 to 5.01; 2 trials, 5768 participants; I2 = 0%) (analysis not shown). Both NLST (Aberle 2011) and LSS (Gohagan 2005) had CXR screening as a comparison, whilst the DANTE (Infante 2015) trial performed a CXR and sputum cytology in both groups prior to screening.
At follow‐up: we included three RCTs (Aberle 2011; Infante 2015; Pastorino 2012). The evidence showed that more invasive tests were performed in the LDCT screening group (RR 2.60, 95% CI 2.41 to 2.80; 3 trials, 60,003 participants; I2 = 0%; moderate‐certainty evidence). The data we used in the analysis for MILD trial (Pastorino 2012) was only inclusive of surgery cases; 788 invasive tests occurred for every 10,000 participants screened with LDCT (NNH = 21). The MILD trial (Pastorino 2012) was the only trial that had no CXRs performed in the control group.
There was no difference between the subgroups. Test for subgroup differences: Chi² = 0.67, df = 1 (P = 0.41), I² = 0%.
Whilst DESPICAN (Blanchon 2007) included adverse events during diagnostic procedures and number of thoracotomies for benign disease, it did not specify the participant groups when presenting results and subsequently we did not include it in our analysis of harms of screening.
Number of all non‐invasive tests performed
We grouped trial results based on time point (following baseline screening scan or at follow‐up) (Analysis 2.2).
2.2. Analysis.

Comparison 2: Primary outcome: number of non‐invasive and invasive tests ‐ all time points, Outcome 2: Non‐invasive tests
At baseline: we included three RCTs (Aberle 2011; Gohagan 2005; Infante 2015). The evidence showed that more non‐invasive tests were performed in the LDCT screening group (RR 3.28, 95% CI 2.40 to 4.48; 3 trials, 59,222 participants; I2 = 90%) (analysis not shown). Heterogeneity was high, with a slight reduction to 70% when we removed Infante 2015 (RR 2.68, 95% CI 2.30 to 3.12; 2 trials, 56,772 participants; I2 = 70%); 2154 non‐invasive tests would be performed for every 10,000 people screened with LDCT (NNH = 7). Of note, Infante 2015 was the only included trial that did not have CXR screening in the control arm, although participants did receive one at baseline. The DANTE (Infante 2015) trial included additional CT and PET scans, whilst Gohagan 2005 included pulmonary function tests, CT and CXR. The NLST (Aberle 2011) combined all additional imaging numbers, and hence heterogeneity was clinical.
At follow‐up: we included two RCTs which reported additional PET scans (Aberle 2011; Infante 2015). The evidence also showed that more non‐invasive tests were performed in the LDCT screening group (RR 3.56, 95% CI 1.81 to 7.01; 2 trials, 55,905 participants; I2 = 86%) (analysis not shown).
There was no difference between the subgroups. Test for subgroup differences: Chi² = 0.05, df = 1 (P = 0.83), I² = 0%).
Number of invasive tests performed in those with a false‐positive diagnosis (positive test in the absence of lung cancer)
We grouped trial results based on time point (following baseline screening scan or at follow‐up) (Analysis 2.3).
2.3. Analysis.

Comparison 2: Primary outcome: number of non‐invasive and invasive tests ‐ all time points, Outcome 3: Number of invasive test for false positive
At baseline: we only included one RCT (Gohagan 2005 ). The invasive interventions included bronchoscopy and biopsies, with a higher rate of intervention in the screening group (RR 3.09, 95% CI 1.57 to 6.07; 3318 participants; 1 trial; I2 = 0%); 205 invasive tests would be performed for false‐positive results for every 10,000 participants screened at baseline (NNH = 72).
At follow‐up: we included three RCTs (Aberle 2011; Infante 2015; Pastorino 2012). The NLST (Aberle 2011) included thoracotomy, bronchoscopy, and needle biopsy, whereas Infante 2015 and Pastorino 2012 included only surgery numbers for invasive procedures. The MILD (Pastorino 2012) intervention arm was the combined total of the biennial and annual screening groups. The evidence showed that more invasive tests were performed in the LDCT screening group (RR 3.91, 95% CI 3.21 to 4.76; 3 trials, 60,005 participants; I2 = 0%); 159 invasive tests would be performed in false‐positive results for every 10,000 participants screened (NNH = 85).
There was no difference between the subgroups. Test for subgroup differences: Chi² = 0.43, df = 1 (P = 0.51), I² = 0%. Invasive tests performed in non‐lung cancer‐related disease are summarised in Table 3.
2. Invasive tests in non‐lung cancer‐related disease.
| Invasive tests in non‐lung cancer‐related disease | |
| Aberle 2011 |
At 6.5 year follow‐up LDCT group: 457 procedures for non‐lung cancer‐related disease (164 thoracotomies/thoracoscopies/mediastinoscopies; 227 bronchoscopies; 66 needle biopsies) out of 17,053 positive screening results over 3 rounds with complete diagnostic information CXR group: 115 procedures for benign disease (45 thoracotomies/thoracoscopies/mediastinoscopies; 46 bronchoscopies; 24 needle biopsies) out of 4674 positive screening results over 3 rounds with complete information |
| Becker 2020 |
Baseline LDCT: 30 biopsies performed in benign disease (at least 5 thoracotomies, 2 VATS thoracoscopies, and 1 bronchoscopy) Year 1 LDCT: 19 biopsies performed in benign disease Year 2 LDCT: 12 biopsies performed in benign disease Year 3 LDCT: 16 biopsies performed in benign disease Year 4 LDCT: 13 biopsies performed in benign disease |
| Blanchon 2007 | Trial arm not specified. Baseline: 3 thoracostomies performed for benign disease |
| De Koning 2020 |
Baseline LDCT: 27.2% of invasive procedures performed in benign disease Between 2004 and 2008: 215 participants had surgery. 2/17 mediastinoscopies were in benign diease; 47/198 lung surgeries (thoracotomies+/‐ VATS) were in benign disease. |
| Field 2021 | Baseline LDCT: 7 participants had needle biopsies, 1 EBUS bronchoscopy, 4 referrals for surgery completed for benign disease |
| Gohagan 2005 |
Baseline LDCT: 16 bronchoscopies, 19 lung biopsies or resection, and 23 any invasive procedures (including biopsy/resection, bronchoscopy, thoracotomy, thoracoscopy, mediastinotomy, mediastinoscopy) performed for benign disease Baseline CXR: 5 bronchoscopies, 6 lung biopsies or resections, and 8 procedures (including biopsy/resection, bronchoscopy, thoracotomy, thoracoscopy, mediastinotomy, mediastinoscopy) performed for benign disease |
| Infante 2015 |
At 8.35 years median follow‐up LDCT group: 17 surgeries for benign disease (3 mediastinoscopies, 7 VATS wedge resections, 6 open wedge resections, 1 open segmentectomy). 7 surgeries for other conditions (reported as 1 open biopsy, 1 extrapleural pneumonectomy for mesothelioma, 2 oesophagectomies for cancer, 1 oesophageal leiomyoma VATS resection, 2 VATS thymectomies, 1 lobectomy for aspergilloma) Control arm: 5 surgeries for benign disease (2 VATS biopsies, 2 VATS wedge resection, 1 open wedge resection); 2 surgeries for other conditions (1 open lung biopsy for hilar lymphoma, 1 VATS thymectomy) |
| LaRocca 2002 | Not available |
| Paci 2017 | Baseline LDCT: 1 FNA biopsy and 1 (5.5% of all surgical resections) surgical resection for benign disease reported |
| Pastorino 2012 |
Median 6 annual LDCTs: 1 invasive diagnostic procedure (transthoracic needle aspiration, fibro bronchoscopy, transbronchial needle aspiration), 0 anatomical (lobectomy or segmentectomy) resections, 0 non‐anatomical resections (wedge resection) performed for benign disease Median 3 biennial LDCTs: 3 invasive diagnostic procedures (transthoracic needle aspiration, fibro bronchoscopy, transbronchial needle aspiration), 0 anatomical (lobectomy or segmentectomy) resections, 1 non‐anatomical resection (wedge resection) performed for benign disease |
| Wille 2016 | Baseline LDCT: 1 mediastinoscopy, 3 bronchoscopy with biopsy, 1 EUS, 2 EBUS, 2 VATS, 1 percutaneous biopsy performed for benign disease |
CXR: chest x‐ray; EBUS: endobronchial ultrasound; EUS: endoscopic ultrasound; FNA: fine needle aspirate; LDCT: low‐dose computed tomography; VATS: video‐assisted thoracoscopic surgery
There were no common time points for non‐invasive tests performed in participants without lung cancer, however we compared total numbers in both groups in two RCTs (Aberle 2011; Gohagan 2005).
Any complications arising from tests including death
Two RCTs which reported mortality rates within 60 days of surgery (Aberle 2011; Infante 2015). The NSLT (Aberle 2011) had a CXR screening comparison arm, whereas the DANTE trial (Infante 2015) had a baseline CXR and sputum cytology for all participants followed by annual clinical examinations. There was probably no difference in mortality following surgery between the groups (RR 0.68, 95% CI 0.24 to 1.94; 2 trials, 409 participants; I2 = 0%; moderate‐certainty evidence; Analysis 2.4). Another RCT also reported postsurgery mortality rates (Paci 2017), however we were unable to locate the total number of surgeries in each group and consequently, we did not include it in the analysis.
2.4. Analysis.

Comparison 2: Primary outcome: number of non‐invasive and invasive tests ‐ all time points, Outcome 4: Death postsurgery
We reported a comparison of complications arising from tests for non‐cancer‐related disease in two RCTs at different time points (Aberle 2011; Gohagan 2005).
Secondary outcomes
3) All‐cause mortality
We combined trial data from all eight available trials using the latest planned follow‐up time point for each trial (Aberle 2011; Becker 2020; De Koning 2020; Field 2021; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016; see Analysis 3.1). We excluded the LSS (Gohagan 2005) from this analysis as it did not have planned follow‐up at ≥ 5 years. The evidence showed a 5% risk reduction in all‐cause mortality with LDCR screening (RR 0.95, 95% CI 0.91 to 0.99; 8 trials, 91,107 participants; I2 = 0%; moderate‐certainty evidence); 210 people would need to be screened to prevent one death from all‐cause mortality. When we performed a sensitivity analysis using trials with low risk of bias (Aberle 2011; Paci 2017; Wille 2016), there was probably a difference between the groups for all‐cause mortality favouring LDCT (RR 0.94, 95% CI 0.89 to 0.99; 3 trials, 60,764 participants; I2 = 0%); 204 people would need to be screened to prevent one death from all‐cause mortality (Figure 5). When we analysed HRs from Becker 2020, Infante 2015 and Wille 2016 at the latest planned time points post‐randomisation, there was probably no difference for people at risk for all‐cause mortality with LDCT screening (HR 0.98, 95% CI 0.87 to 1.12; 3 trials, 10,606 participants; I2 = 0%; Analysis 3.3).
3.1. Analysis.

Comparison 3: Secondary outcome: all‐cause mortality, Outcome 1: All‐cause mortality ‐ planned time points (latest time points)
5.
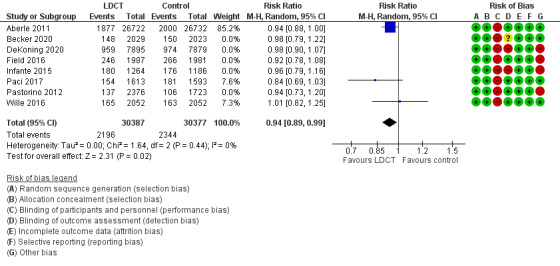
All‐cause mortality ‐ Planned time points ‐ Sensitivity analysis
3.3. Analysis.

Comparison 3: Secondary outcome: all‐cause mortality, Outcome 3: All‐cause mortality ‐ planned time points
We also grouped trial results by time points (planned and unplanned) (Analysis 3.2).
3.2. Analysis.

Comparison 3: Secondary outcome: all‐cause mortality, Outcome 2: All‐cause mortality ‐ all time points (planned and unplanned)
5 to 6 years post‐randomisation: we included three RCTS (Becker 2020; Gohagan 2005; Wille 2016). There was probably no difference between LDCT and control groups in all‐cause mortality (RR 1.14, 95% CI 0.88 to 1.47; 3 trials, 11,474 participants; I2 = 52%). There was moderate heterogeneity, which disappeared when we excluded the results from Becker 2020 (RR 1.26, 95% CI 1.03 to 1.54; 2 trials, 7422 participants; I2 = 0%).
More than 6 to 8 years post‐randomisation: we included two RCTs (Aberle 2011; Field 2021). The evidence showed there was a difference in all‐cause mortality favouring LDCT screening (RR 0.94, 95% CI 0.89 to 0.99; 2 trials, 57,422 participants; I2 = 0%).
More than 8 to 10 years post‐randomisation: we included six RCTS (Becker 2020; De Koning 2020; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016). There was probably no difference between LDCT and control groups in all‐cause mortality (RR 0.97, 95% CI 0.91 to 1.03; 6 trials, 33,685 participants; I2 = 0%).
More than 10 years post‐randomisation: we included two RCTs (Aberle 2011; Paci 2017). There was probably no difference between LDCT and control groups in all‐cause mortality (RR 0.91, 95% CI 0.76 to 1.09; 2 trials, 56,658 participants; I2 = 76%). Heterogeneity between the two trials was high, with ITALUNG (Paci 2017) favouring screening. It should be noted that the NLST (Aberle 2011) had CXR as a comparison.
All‐cause mortality by different subgroups (planned time points)
By sex
Three trials reported mortality by sex (Aberle 2011; De Koning 2020; Infante 2015); see Analysis 3.4.
3.4. Analysis.

Comparison 3: Secondary outcome: all‐cause mortality, Outcome 4: All‐cause mortality by sex ‐ planned time points
For women: we included two RCTs (Aberle 2011; De Koning 2020). The NELSON trial (De Koning 2020) results for women were not provided and so we calculated these from available data. There was probably no difference between LDCT and control groups in all‐cause mortality and heterogeneity was moderate (RR 0.89, 95% CI 0.76 to 1.03; 2 trials, 24,514 participants; I2 = 31%).
For men: we included three RCTs (Aberle 2011; De Koning 2020; Infante 2015). The evidence showed there was no difference in all‐cause mortality (RR 0.93, 95% CI 0.80 to 1.07; 3 trials, 49,162 participants; I2 = 82%) (analysis not shown). Heterogeneity was high, with Aberle 2011 having significant weight in the analysis. When we removed Aberle 2011, there was no heterogeneity, however data suggested there was probably no difference between LDCT and control groups in all‐cause mortality (RR 1.00, 95% CI 0.93 to 1.09; 2 trials, 5632 participants; I2 = 0%).
There was no difference between the two groups. Test for subgroup differences: Chi² = 1.96, df = 1 (P = 0.16), I² = 48.9%.
By cause of death: two trials reported cardiovascular mortality (Becker 2020; Paci 2017), and we grouped these by time points (Analysis 3.5).
3.5. Analysis.

Comparison 3: Secondary outcome: all‐cause mortality, Outcome 5: Cardiovascular mortality ‐ planned and unplanned
At 8 to 10 years planned time points: we included two RCTS (Becker 2020; Paci 2017) and the data was collected as part of their planned analysis. There was probably no difference between LDCT and control groups in cardiovascular‐related mortality and heterogeneity was high (RR 0.76, 95% CI 0.37 to 1.56; 2 trials, 7258 participants; I2 = 78%) (analysis not shown).
At more than 10 years using unplanned time points, we included only one RCT (Paci 2017). The evidence showed there was a difference in cardiovascular mortality favouring LDCT screening (RR 0.53, 95% CI 0.34 to 0.81; 1 trial, 3206 participants).
4) Lung cancer incidence and overdiagnosis
Lung cancer incidence
We grouped trial results by time points (planned and unplanned) (Analysis 4.1).
4.1. Analysis.

Comparison 4: Secondary outcome: lung cancer incidence, Outcome 1: Lung cancer incidence ‐ by different time points
At baseline: we included six trials (Aberle 2011; Blanchon 2007; De Koning 2020; Gohagan 2005; Infante 2015; Wille 2016). The evidence showed a higher incidence of lung cancer in the LDCT screening group, (RR 4.98, 95% CI 2.01 to 12.35; 6 trials, 79,900 participants; I2 = 87%), with high heterogeneity. Removing Aberle 2011 reduced the heterogeneity to a moderate level (RR 6.45, 95% CI 3.21 to 12.98; 5 trials, 26,448 participants; I2 = 49%). Both the NLST(Aberle 2011) and LSS (Gohagan 2005) had CXR screening in the control arm.
At 1 year post‐randomisation: we included three RCTs (Aberle 2011; De Koning 2020; Wille 2016). The evidence showed a higher incidence of lung cancer in the LDCT screening group, with high heterogeneity (RR 2.12, 95% CI 1.35 to 3.31; 3 trials, 73,345 participants; I2 = 54%). After removing Aberle 2011, there was no heterogeneity (RR 2.87, 95% CI 1.78 to 4.60; 2 trials, 19,893participants; I2 = 0%).
At two years post‐randomisation: we included two RCTs (Aberle 2011, Wille 2016). This analysis demonstrated a higher incidence of lung cancer in the LDCT screening group (RR 1.88, 95% CI 1.51 to 2.32; 2 trials, 57,556 participants; I2 = 0%).
At 3 years post‐randomisation: we included one RCT (Wille 2016). This trial suggested the possibility of no difference in the incidence of lung cancer between the groups (RR 1.71, 95% CI 0.68 to 4.35; 1 trial, 4104 participants; I2 = 0%)
At 4 years post‐randomisation: we included one RCT (Wille 2016). This trial demonstrated a higher incidence of lung cancer in the LDCT screening group (RR 2.67, 95% CI 1.05 to 6.80; 1 trial, 4104 participants; I2 = 0%).
At 5 to 7 years post‐randomisation: we included two RCTs (Aberle 2011; Becker 2020). The evidence showed a higher incidence of lung cancer in the LDCT screening group (RR 1.13, 95% CI 1.04 to 1.23; 2 trials, 57,506 participants; I2 = 0%).
At more than 7 years post‐randomisation: we included eight RCTS (Aberle 2011; Becker 2020; De Koning 2020; Field 2021; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016). Of note, only male participant data were available and included in this analysis for the NELSON trial (De Koning 2020). The MILD (Pastorino 2012) trial had both annual and biennial groups combined into the intervention arm. The evidence showed a higher incidence of lung cancer in the LDCT screening group (RR 1.17, 95% CI 1.02 to 1.33; 8 trials, 8528 participants; I2 = 65%). Heterogeneity was high, and became low when we removed Wille 2016 from the analysis (RR 1.08, 95% CI 0.99 to 1.18; 7 trials, 84,424 participants; I2 = 27%) (analysis not shown).
We grouped trials with ≥ 10 years follow‐up post‐randomisation based on control arm (Analysis 4.2).
4.2. Analysis.

Comparison 4: Secondary outcome: lung cancer incidence, Outcome 2: Lung cancer incidence ‐ by control group at ≥ 10 years
No screening comparison: we included five RCTs (Becker 2020; De Koning 2020; Paci 2017; Pastorino 2012; Wille 2016). There was possibly no difference in lung cancer incidence between the groups (RR 1.21, 95% CI 0.99 to 1.48; 5 trials, 28,656 participants; I2 = 66%). Heterogeneity was high, which was probably due to the DLCST trial (Wille 2016), which reported less lung cancer in the control group (RR 1.11, 95% CI 0.99 to 1.24; 4 trials, 24,552 participants; I2 = 0%) (analysis not shown).
CXR comparison: we included one RCT (Aberle 2011). There was probably no difference in lung cancer incidence between the groups (RR 1.01, 95% CI 0.95 to 1.08; 1 trial, 53,454 participants).
There was no difference between the subgroups. Test for subgroup differences: Chi² = 2.62, df = 1 (P = 0.11), I² = 61.8%.
Overdiagnosis
Estimates were described by five trials (Aberle 2011; Becker 2020; Field 2021; Paci 2017; Wille 2016), and ranged from ‐4% to 67% for all lung cancers (Table 4). This was in part due to some adenocarcinoma subtypes We divided overdiagnosis using definitions from the NLST (Aberle 2011) where overdiagnosis from a public health perspective used cumulated lung cancer incidence rate as the denominator, whereas the clinical perspective used screen‐detected lung cancer incidence as the denominator.
3. Recall rates, false positives and overdiagnosis .
| Recall rates at overall baseline LDCT recall rate = 18% (8078/44,920) |
False positives Overall false‐positive rate from baseline LDCT = 21% (8874/41857) |
Overdiagnosis | |
| Aberle 2011 | All chest CTs performed postbaseline LDCT: 20% (5153/26,309) All chest CTs performed postbaseline CXR in control group: 6% |
Baseline LDCT: 6911/26,309 (26%) Baseline CXR: 2243/26,035 (7%) 1‐year LDCT: 6728/24715 (27%) 1‐year CXR: 1416/24,089 (6%) 2‐year LDCT: 3838/24,102 (16%) 2‐year CXR: 1094/23, 346 (5%) |
At 6.5 years post‐randomisation:
At 11.3 years post‐randomisation:
|
| Becker 2020 | Baseline LDCT: 22% (451/2028) 1‐year LDCT: 5% 2‐year LDCT: 4% 3‐year LDCT: 6% 4‐year LDCT: 5% |
Baseline LDCT: 426/2028 (21%) 1‐year LDCT: 77/1892 (4%) 2‐year LDCT: 62/1849 (3%) 3‐year LDCT 94/1826 (5%) 4‐year LDCT: 88/1810 (5%) |
At 9.7 years post‐randomisation:
|
| Blanchon 2007 | Not available | Baseline LDCT: 73/336 (22%) Baseline CXR: 14/285 (5%) |
Not available |
| De Koning 2020 | Baseline LDCT: 19% (1438/7557) 1‐year LDCT: 19% |
Baseline LDCT 107/7557 (1%) 1‐year LDCT: 64/7295 (1%) 3‐year LDCT: 276/6922 (4%) 5.5‐year LDCT: 62/5279 (1%) |
Not available |
| Field 2021 | Baseline LDCT: 5% (103/1994) | Baseline LDCT: *909/1994 (46%) **72/1994 (4%) *when defined as needing any work‐up **when defined as referred to MDT |
Estimated 15% of all lung cancers |
| Gohagan 2005 | Baseline LDCT: 15% (232/1586) Baseline CXR: 5% (76/1550) Overall post‐LDCT: 8% Overall post‐CXR in control group: 3% |
Baseline LDCT: 286/1586 (18%) Baseline CXR: 139/1550 (9%) |
Not available |
| Infante 2015 | Baseline LDCT: 10% (128/1276) | Not available | Not available |
| LaRocca 2002 | Not available | Not available | Not available |
| Paci 2017 | Baseline LDCT: 23% 366/1406) 1‐year LDCT: 14% 2‐year LDCT: 13% 3‐year LDCT: 11% |
Not available | At 11.3 years post‐randomisation, estimated overdiagnosis rates reported as ‐4% using public health perspective and ‐10% from a clinical perspective |
| Pastorino 2012 | Baseline LDCT: 15% in annual group, 14% in (284/2303) biennial group 1‐year LDCT: 3% in annual group, 3% in biennial group 2‐year LDCT: 5% in annual group, 5% in biennial group 3‐year LDCT: 3% in annual group, 7% in biennial group 4‐year LDCT: 2% in annual group, 3% in biennial group 5‐year LDCT: 1% in annual group, 7% in biennial group 6‐year LDCT: 4% in annual group, 5% in biennial group |
Median 6 annual LDCT: 54/1152 (5%) Median 3 biennial LDCT: 34/1151 (3%) |
Not available |
| Wille 2016 | Baseline LDCT: 8% (155/2047) 1‐year LDCT: 1% 2‐year LDCT: 1% 3‐year LDCT: 1% 4‐year LDCT: 1% |
Baseline LDCT: 162/2047 (8%) 1‐year LDCT: 34/1976 (2%) 2‐year LDCT: 39/1944 (2%) 3‐year LDCT: 32/1982 (2%) 4‐year LDCT: 35/1851 (2%) |
Estimated 67.2% of lung cancers (95% CI 37.1 to 95.4) from unplanned posthoc analysis |
BAC: bronchioalveolar carcinoma; CI: confidence interval; CT: computed tomography; CXR: chest x‐ray; LDCT: low‐dose computed tomography; MDT: multidisciplinary team.
We estimated overdiagnosis based on the control arm (Analysis 4.3). We calculated estimates from the total incidence in each arm and these were not limited to screen‐detected cancers only. Based on extended follow‐up (≥ 10 years), calculated rates of overdiagnosis for the NELSON trial (De Koning 2020) was 12%, with a wide CI (95% CI ‐1% to 25%). It should be noted that extended incidence and hence overdiagnosis was only relevant to male participants in this trial. The estimated overdiagnosis rate of ITALUNG (Paci 2017) was ‐11%, with a 95% CI of ‐42% to 20%. The estimated overdiagnosis rate of MILD (Pastorino 2012) was 16%, again with a wide CI (95% CI ‐10% to 41%). The DLCST (Wille 2016) had an estimated overdiagnosis rate of 47% and had the only CI that did not cross 0 (95% CI 30% to 64%). The NLST compared LDCT to CXR and so we excluded it from the meta‐analysis, however it had an estimated overdiagnosis rate of 1% (95% CI ‐5% to 7%). The DANTE (Infante 2015) trial included CXR and sputum cytology for both trial groups pre‐screening, and had a median follow‐up of < 10 years, and so we did not include it in the meta‐analysis of overdiagnosis. The calculated overdiagnosis rate for the DANTE (Infante 2015) trial was 26% (95% CI 5 to 48%).
4.3. Analysis.

Comparison 4: Secondary outcome: lung cancer incidence, Outcome 3: Overdiagnosis at ≥ 10 years
Five trials compared LDCT screening with usual care after ≥ 10 years from randomisation (Becker 2020; De Koning 2020; Paci 2017; Pastorino 2012; Wille 2016), with an estimated overdiagnosis rate of 18% (RD 0.18, 95% CI ‐0.00 to 0.36; 5 trials, 28,656 participants; I2 = 73%; low‐certainty evidence), with a wide CI that does not meet significance (Analysis 4.3). It is estimated that 7 cases of lung cancer overdiagnosis would occur for every 1000 people screened (95% CI of 2 to 84 cases of overdiagnosis). Heterogeneity was also high, and reduced when we removed Wille 2016.
5) False‐positive, negative and recall rates
False‐positive rates were provided for eight RCTS (Aberle 2011; Becker 2020; Blanchon 2007; De Koning 2020; Field 2021; Gohagan 2005; Pastorino 2012; Wille 2016) and are detailed in Table 4. When we combined all available baseline LDCT results from seven trials (Aberle 2011; Becker 2020; Blanchon 2007; De Koning 2020; Field 2021; Gohagan 2005; Wille 2016), 21% of trial screens had a false‐positive result, with a range from 1% to 46%. The false‐positive rate of LDCT was lower in trials that used volumetric analysis alone (De Koning 2020; Pastorino 2012), which ranged from 1% to 5%, compared with diameter criteria alone (Aberle 2011; Blanchon 2007; Gohagan 2005) which ranged from 18% to 26%. The three trials that used both diameter and volumetric criteria (Becker 2020; Field 2021; Wille 2016) had false‐positive rates of 21%, 46%, and 8%, respectively. Four per cent of participants had false‐positive results reviewed in a multidisciplinary team meeting in UKLS (Field 2021). False‐positive rates also decreased with subsequent LDCT screens (Aberle 2011; Becker 2020; De Koning 2020; Wille 2016). It should be noted that false positives in the NELSON trial (De Koning 2020) did not include all participants who had an indeterminate scan, only those who had a positive follow‐up scan following an indeterminate result.
When we combined results from all available three trials comparing LDCT and CXR (Aberle 2011; Blanchon 2007; Gohagan 2005) using the latest follow‐up time point for each trial (Analysis 5.1), the evidence showed fewer false positives in the CXR groups with high heterogeneity between the trials (RR 2.82, 95% CI 1.98 to 4.01; 3 trials, 56,101 participants; I2 = 90%) (analysis not shown). Removing Gohagan 2005 reduced the heterogeneity, however the conclusion was unchanged (RR 3.31, 95% CI 2.45 to 4.47; 2 trials, 52,965 participants; I2 = 43%).
5.1. Analysis.

Comparison 5: Secondary outcome: false positives, negatives and recalls (number of screens), Outcome 1: False positive at baseline
Recall rate is the portion of participants recalled for repeat CT at 3 months and beyond 6 months for follow‐up of a nodule or suspected lung cancer. Only two RCTs (Aberle 2011; Gohagan 2005) had baseline comparison data for recall rate (Analysis 5.3). The data suggested there were probably more recalls in the LDCT screening group compared to CXR groups (RR 5.31, 95% CI 1.73 to 16.34; 2 trials, 55,480 participants; I2 = 99%) (analysis not shown). Heterogeneity was high between the groups. Both trials had no trial‐wide algorithm, however had similar definitions for a positive screen. LSS (Gohagan 2005) was a feasibility study, and there were only two screening rounds, with participants advised to seek medical follow‐up with specialists. Baseline screening recall rates from trials provided (Aberle 2011; Becker 2020; De Koning 2020; Field 2021; Gohagan 2005; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016) are summarised in Table 4, with an overall recall rate following baseline screen of 18% (range of 5% to 23%). Of note, recall rates were defined differently between trials, with most trials including scans occurring up to 12 months postscreen and the NLST (Aberle 2011) and LSS (Gohagan 2005) data including all CT scans, not specifically recall scans, performed as a result of the baseline screen.
5.3. Analysis.

Comparison 5: Secondary outcome: false positives, negatives and recalls (number of screens), Outcome 3: Recall rates at baseline
6) Smoking behaviour – cessation, relapse rates, smoking intensity
There were no common time points for assessment of quit rates. Individual trial results are presented in Analysis 6.1. Both the ITALUNG trial (Paci 2017) and the DLCST (Wille 2016) included smoking cessation as part of their programme, although Wille 2016 quantified it as minimal (< 5 minutes spent on smoking cessation per review). The ITALUNG (Paci 2017) trial and UKLS (Field 2021) confirmed smoking status via self‐reporting only, whereas the DLSCT (Wille 2016) also confirmed smoking by measuring exhaled CO2 levels.
6.1. Analysis.

Comparison 6: Secondary outcome: impact on smoking behaviour, Outcome 1: stop smoking
At 2 weeks post‐randomisation, Field 2021 showed a higher quit rate in the LDCT screening group compared with control (RR 2.16, 95% CI 1.47 to 3.18; 1 trial, 1545 participants).
At 1 year post‐randomisation, there was probably no difference in quit rates between the groups in Wille 2016 (RR 1.08, 95% CI 0.88 to 1.32; 1 trial, 3124 participants).
Within 2 years post‐randomisation, Field 2021 again showed a higher quit rate in the LDCT screening group compared with control (RR 1.51, 95% CI 1.15 to 1.97; 1 trial, 1524 participants).
At 4 years post‐randomisation, Paci 2017 demonstrated there was possibly no difference in quit rates between the groups (RR 1.17, 95% CI 0.99 to 1.37; 1 trial, 2447 participants).
Only one trial presented smoking relapse rates in both groups (Wille 2016). There was probably no difference in relapse rates between the groups in this trial (RR 0.95, 95% CI 0.65 to 1.41; 1 trial, 888 participants; Analysis 6.2).
6.2. Analysis.

Comparison 6: Secondary outcome: impact on smoking behaviour, Outcome 2: smoking relapse
7) Health‐related quality of life (HRQoL)/psychosocial consequences
HRQoL and psychosocial consequences were evaluated in four trials (Aberle 2011; De Koning 2020; Field 2021; Wille 2016). The trials measured different aspects of quality of life. Whilst small transient changes were at times reported, no long‐term adverse consequences on HRQoL were reported. The DLSCT (Wille 2016) and UKLS (Field 2021) administered questionnaires to their whole trial cohort. The NLST (Aberle 2011) initially only invited participants of the LDCT screening group from 16 of the 23 American College of Radiology Imaging Network (ACRIN) sites with positive baseline LDCTs, however later they invited participants who had significant incidental findings (SIFs) on LDCT as well. The control group matched LDCT group participants with negative results with a total number of participants of 2812 for this outcome. The NELSON (De Koning 2020) trial also only included a portion of their cohort for this analysis, taking a random sample of 733 participants from each trial group (LDCT screening and control).
The following questionnaires were used as assessments in these trials.
Lung cancer‐specific questionnaire Consequences of Screening in Lung Cancer (COS‐LC, Brodersen 2010): COS‐LC consists of nine psychosocial scales: four core scales (24 core items) and five lung cancer screening‐specific scales (25 lung cancer screening‐specific items). The four core scales measure anxiety (7 items), behaviour (7 items), dejection (6 items), sleep (4 items) and smoking (2 items). Higher scores indicate more negative psychosocial consequences.
Short form 36‐item questionnaire (SF‐36, Ware 1992) version 2: SF‐36 has a physical component summary (PCS) and a mental component summary (MCS). The score ranges from 0‐100. Higher scores indicate better HRQoL; lower scores indicate worse quality of life.
Short form 12‐item questionnaire (SF‐12, Gandek 1998; Ware 1996) is a short version of the 36‐item questionnaire which also has a PCS and a MCS, with both components having a maximum score of 50 each. As with the SF‐36, higher scores indicated better HRQoL.
EuroQol questionnaire (EQ‐5D, Essink‐Bot 1993; Kind 2005): EQ‐5D is a health questionnaire with five dimensions (mobility, self‐care, usual activities, pain/discomfort and anxiety/depression) as well as a visual analogue score (VAS). The VAS ranges from 0 (the worst imaginable health status) to 100 (the best imaginable health status).
Spielberger State‐Trait Anxiety Inventory (STAI‐6) (van der Bij 2003): STAI‐6 assesses generic anxiety, with scores ranging from 20 to 80; higher scores indicate more anxiety.
Hospital Anxiety and Depression Scale (HADS): (Zigmond 1983): HADS consists of separate anxiety and depression components, each with a score ranging from 0 to 21. Scores for each component are considered normal (0‐8), borderline abnormal (9‐11) and abnormal (12‐21).
Impact of event scale (IES, Horowitz 1979): IES measures distress caused by traumatic events (in this instance lung cancer) and consists of 15 items with total scores 0 to 75, with subscales for intrusion (0 to 35) and avoidance (0 to 40); higher scores indicated more distress.
Revised 6‐item Cancer Worry Scale (CWS‐R): CWS‐R has scores ranging from 4 to 24, with higher scores indicating more worry.
Lung cancer‐specific questionnaires
DLSCT (Wille 2016) used COS‐LC. Analysis 7.2 illustrates the change over time in mean differences (MDs) across all the core scales between the LDCT and control group at round two and five compared to baseline.
7.2. Analysis.

Comparison 7: Secondary outcome: health‐related quality of life, Outcome 2: Quality of life measures at different time points
Anxiety: at round two there was probably no difference between the groups (MD ‐0.13, 95% CI ‐0.33 to 0.07; 1 trial, 3352 participants), with higher anxiety scores in the control group compared with LDCT groups at round five (MD ‐0.51, 95% CI ‐0.76 to ‐0.26; 1 trial, 3185 participants).
Behaviour: at round two both groups had increased negative response, with probably no difference between the groups (MD ‐0.21, 95% CI ‐0.42 to 0.00; 1 trial, 3337 participants). However, scores decreased in the LDCT group by round five (MD ‐0.60, 95% CI ‐0.88 to ‐0.32; 1 trial, 3180 participants).
Dejection: at round two there was probably no difference between the groups, with elevated scores in both groups (MD ‐0.15, 95% CI ‐0.36 to 0.06; 1 trial, 3377 participants). However at round five, the LDCT group had fewer psychological consequences (MD ‐0.58, 95% CI ‐0.82 to ‐0.34; 1 trial, 3195 participants).
Negative impact on sleep: at round two there was probably no difference between the groups, with elevated scores in both groups (MD ‐0.14, 95% CI ‐0.32 to 0.04; 1 trial, 3389 participants). However, at round five there was a significant difference favouring LDCT screening (MD ‐0.70, 95% CI ‐0.95 to ‐0.45; 1 trial, 3198 participants).
Other domains (self‐blame, focusing on airway symptoms, stigmatisation, introvert and harm of smoking): the lung cancer‐specific scales tended to demonstrate more negative psychosocial consequences in the control group compared with the LDCT screening group from round two to round five.
Anxiety
When we combined standardised mean difference (SMD) in anxiety scores for the three available trials (De Koning 2020; Field 2021; Wille 2016; Analysis 7.1), the evidence favoured lower anxiety scores in the LDCT screening group (SMD ‐0.43, 95% CI ‐0.59 to ‐0.27; 3 trials, 8153 participants; I2 = 0%; low‐certainty evidence), although scores were not necessarily abnormal in either group.
7.1. Analysis.

Comparison 7: Secondary outcome: health‐related quality of life, Outcome 1: Anxiety ‐ at 10 months to 5 years (change over time and endpoints)
In the NELSON trial (De Koning 2020), there was probably no difference between the groups at baseline (MD ‐0.48, 95% CI ‐1.63 to 0.67; 1 trial, 1288 participants) and at 2 years (MD ‐0.75, 95% CI ‐1.99 to 0.49; 1 trial, 931 participants).
In the UKLS trial (Field 2021), HADS anxiety scores were in the normal range in both groups, however scores were lower in the LDCT group compared with the control groups at baseline (MD ‐0.07, 95% CI ‐0.11 to ‐0.03; 1 trial, 4037 participants) and at 10 months to 27 months (MD ‐0.36, 95% CI ‐0.57 to ‐0.15; 1 trial, 4037 participants).
We did not include the NLST trial (Aberle 2011) in this analysis as the control group consisted of participants with negative screen results and not participants from the CXR group. Participants were separated based on screening outcome (negative, true positive, false positive, and SIFs) (Analysis 7.5). There was no difference in anxiety levels between the groups for the participants with negative screens at baseline (MD ‐0.26, 95% CI ‐1.79 to 1.27; 1 trial, 1162 participants) and at 6 months (MD ‐0.33, 95% CI ‐1.91 to 1.25; 1 trial, 1019 participants). There was probably no difference between the groups with participants with true positive screens at baseline (MD 1.63, 95% CI ‐6.31 to 9.57; 1 trial, 48 participants) and at 6 months (MD ‐2.69, 95% CI ‐11.69 to 6.31; 1 trial, 42 participants). False‐positive participants also did not demonstrate a difference in anxiety levels at baseline (MD 1.77, 95% CI ‐0.04 to 3.58; 1 trial, 835 participants) or at 6 months (MD 1.31, 95% CI ‐0.61 to 3.23; 1 trial, 703 participants) between the groups.
7.5. Analysis.

Comparison 7: Secondary outcome: health‐related quality of life, Outcome 5: Anxiety by different results at 1 and 6 months
Depression
The UKLS trial (Field 2021) used the HADS score for depression. These scores were within normal limits for both groups. The LDCT group reported lower scores compared with the control group at baseline (MD ‐0.06, 95% CI ‐0.10 to ‐0.02; 1 trial, 4037 participants) and at 10 months to 27 months (MD ‐0.24, 95% CI ‐0.40 to ‐0.08; 1 trial, 4037 participants).
Stress
Both the NESLON trial (De Koning 2020) and UKLS (Field 2021) measured stress using different instruments.
The NELSON trial (De Koning 2020) used the IES score. There was probably no difference between the groups at baseline (MD 0.03, 95% CI ‐0.88 to 0.94; 1 trial, 1288 participants) and at 2 years (MD ‐0.31, 95% CI ‐1.30 to 0.68; 1 trial, 931 participants). However, they reported that people in the LDCT group who had indeterminate results had elevated distress following the result at 2 months.
The UKLS trial (Field 2021) used the CWS‐R and did not report any difference between groups in cancer distress at 10 months to 27 months. However, they did observe that those participants referred to the lung cancer multidisciplinary meeting reported more lung cancer distress in the short term (approximately 2 weeks after randomisation to the non‐screening arm or receiving results of the baseline LDCT) (mean score 11.88, 95% CI 11.10 to 12.72), although were more satisfied with their participation in the trial.
Generic HRQoL
The NELSON trial (De Koning 2020) measured HRQoL using the SF‐12 questionnaire and EQ‐5D, whilst NLST (Aberle 2011) used the SF‐36 version.
-
In the NELSON trial (De Koning 2020), there was probably no difference in the SF‐12 scores between the groups for both the PCS and MCS components, both at baseline and 2 years. There was also probably no difference between the two groups with EQ‐5D VAS at baseline and 2 years.
PCS baseline (MD 0.28, 95% CI ‐0.66 to 1.22; 1 trial, 1288 participants) and at 2 years (MD 0.88, 95% CI ‐0.34 to 2.10; 1 trial, 931 participants).
MCS baseline (MD ‐0.06, 95% CI ‐1.42 to 1.30; 1 trial, 1288 participants) and at 2 years (MD 0.81, 95% CI ‐0.65 to 2.27; 1 trial, 931 participants).
EQ‐5D VAS baseline (MD 0.69, 95% CI ‐0.98 to 2.36; 1 trial, 1288 participants) and at 2 years (MD 2.08, 95% CI 0.18 to 3.98; 1 trial, 1010 participants).
-
In the NLST trial (Aberle 2011), groups were divided again by results of screening (negative, true positive, false positive, and SIF).
-
Participants with true‐positive screens did have lower PCS and MCS scores (Analysis 7.3; Analysis 7.4), which declined at 6 months, however scores were probably not significantly different between groups.
PCS at baseline (MD ‐1.94, 95% CI ‐7.33 to 3.45; 1 trial, 63 participants) and at 6 months (MD ‐0.20, 95% CI ‐7.32 to 6.92; 1 trial, 42 participants). MCS at baseline (MD ‐1.74, 95% CI ‐6.66 to 3.18; 1 trial, 63 participants) at 6 months (MD 0.08, 95% CI ‐8.19 to 8.35; 1 trial, 42 participants).
-
There was probably no significant difference between the groups for those with negative and false‐positive screens at baseline and 6 months.
Participants with a negative screen: PCS at baseline (MD ‐1.07, 95% CI ‐2.09 to ‐0.05; 1 trial, 1381 participants) and at 6 months (MD ‐0.11, 95% CI ‐1.38 to 1.16; 1 trial, 1019 participants). MCS at baseline (MD ‐0.85, 95% CI ‐1.97 to 0.27; 1 trial, 1381 participants) and at 6 months (MD ‐0.15, 95% CI ‐1.52 to 1.22; 1 trial, 1019 participants).
Participants with false‐positive screens: PCS at baseline (MD ‐0.72, 95% CI ‐1.98 to 0.54; 1 trial, 1024 participants) and at 6 months (MD ‐0.78, 95% CI ‐2.42 to 0.86; 1 trial, 703 participants). MCS at baseline (MD ‐0.19, 95% CI ‐1.43 to 1.05; 1 trial, 1024 participants) and at 6 months (MD ‐1.02, 95% CI ‐2.67 to 0.63; 1 trial, 703 participants).
-
7.3. Analysis.

Comparison 7: Secondary outcome: health‐related quality of life, Outcome 3: SF‐36v2: PCS by different components at baseline and at 6 months
7.4. Analysis.

Comparison 7: Secondary outcome: health‐related quality of life, Outcome 4: SF‐36v2: MCS by different components at baseline and 6 months
8) Cancer stage at diagnosis
We grouped trial results by time points (Analysis 8.1; Analysis 8.2; Analysis 8.3; Analysis 8.4; Analysis 8.5). Where specified in the trials, we separated limited and extensive small cell lung cancer (SCLC) from TNM (tumour, node, metastasis) staging (Goldstraw 2016).
8.1. Analysis.

Comparison 8: Secondary outcome: lung cancer by stages at different time points, Outcome 1: baseline
8.2. Analysis.

Comparison 8: Secondary outcome: lung cancer by stages at different time points, Outcome 2: at 1 year
8.3. Analysis.

Comparison 8: Secondary outcome: lung cancer by stages at different time points, Outcome 3: At year 2
8.4. Analysis.

Comparison 8: Secondary outcome: lung cancer by stages at different time points, Outcome 4: 5 to < 10 years
8.5. Analysis.

Comparison 8: Secondary outcome: lung cancer by stages at different time points, Outcome 5: ≥ 10 years
At baseline: we included five trials (Aberle 2011; Blanchon 2007; Gohagan 2005; Infante 2015; Wille 2016; Analysis 8.1). The evidence suggested that stage 1 lung cancer was detected more in the LDCT screening group (RR 2.41, 95% CI 1.86 to 3.12; 5 trials, 64,092 participants; I2 = 0%). Analysis showed there was possibly no difference between the groups for stage 2 lung cancer (RR 1.88, 95% CI 0.99 to 3.58; 5 trials, 64,092 participants; I2 = 0%). There were fewer stage 3 cases of lung cancer in the control group, however heterogeneity between trials was high (RR 4.28, 95% CI 1.06 to 17.27; 5 trials, 64,092 participants; I2 = 59%). Heterogeneity was reduced to zero when we removed Aberle 2011, although the outcome was unchanged (RR 8.69, 95% CI 2.33 to 32.35; 4 trials, 10,637 participants; I2 = 0%). There was probably no difference between the groups with stage 4 cancer (RR 1.05, 95% CI 0.70 to 1.55; 5 trials, 64,092 participants; I2 = 0%) and unknown stage lung cancer (RR 0.99, 95% CI 0.31 to 3.13; 2 trials, 56,773 participants; I2 = 0%). The DLSCT (Wille 2016) was the only trial that reported limited and extensive stages. There were no cases in limited stage, and the extensive stage had more cases in the screening group. In the LDCT screening group, 53% of diagnosed lung cancers were stage 1, 7% of diagnosed lung cancers were stage 2, 23% of diagnosed lung cancers were stage 3, and 14% of diagnosed lung cancers were stage 4. In the control group, 40%, 7%, 28%, and 23% of diagnosed cancers, respectively were stages 1, 2, 3, and 4.
At 1 year: we included three RCTS (Aberle 2011; Gohagan 2005; Wille 2016; Analysis 8.2). The evidence suggested that stage 1 cancer was detected more in the LDCT compared with the control group (RR 2.57, 95% CI 1.24 to 5.32; 3 trials, 60,877 participants; I2 = 16%). There was probably no difference between the groups for stages 2 (RR 1.39, 95% CI 0.68 to 2.84; 3 trials, 60,877 participants; I2 = 0%) and 3 lung cancer (RR 1.22, 95% CI 0.76 to 1.95; 3 trials, 60,877 participants; I2 = 0%). The evidence showed that stage 4 lung cancer was detected more in the control group than in the LDCT screening group (RR 0.48, 95% CI 0.30 to 0.77; 3 trials, 60,877 participants; I2 = 0%). The NLST (Aberle 2011) was weighted 96% in that analysis. There was no difference between the groups for limited (RR 1.00, 95% CI 0.06 to 15.98; 1 trial, 4104 participants; I2 = 0%) and extensive (RR 3.00, 95% CI 0.12 to 73.60; 1 trial, 4104 participants; I2 = 0%) stages of lung cancer, as well as unknown stage (RR 1.35, 95% CI 0.17 to 10.75; 2 trials, 56,773 participants; I2 = 17%). In the LDCT screening group, 57% of diagnosed lung cancers were stage 1, 9% of diagnosed lung cancers were stage 2, 19% of diagnosed lung cancers were stage 3, and 13% of diagnosed lung cancers were stage 4. In the control group, 30%, 9%, 22%, and 37% of diagnosed cancers respectively, were stages 1, 2, 3, and 4.
At 2 years: we included two RCTS (Aberle 2011; Wille 2016; Analysis 8.3). The evidence suggested that stage 1 cancer was detected more in the LDCT group compared with control screening group (RR 3.53, 95% CI 1.66 to 7.53; 2 trials, 57,559 participants; I2 = 20%). There was probably no difference between the groups for stages 2, 3, and 4 lung cancer (RR 1.08, 95% CI 0.49 to 2.37; 2 trials, 57,559 participants; I2 = 0%), (RR 0.92, 95% CI 0.59 to 1.44; 2 trials, 57,559 participants; I2 = 0%) and (RR 0.80, 95% CI 0.52 to 1.24; 2 trials, 57,559 participants; I2 = 0%), respectively. The DLSCT trial (Wille 2016) did not have any events in stage 2 lung cancer. We only included one trial (Wille 2016) for each of extensive (RR 0.14, 95% CI 0.01 to 2.76; 1 trial, 4104 participants) and unknown stages (RR 7.00, 95% CI 0.86 to 56.91; 1 trial, 53,455 participants) with no limited cases. In the LDCT screening group, 63% of diagnosed lung cancers were stage 1, 5% of diagnosed lung cancers were stage 2, 14% of diagnosed lung cancers were stage 3, and 15% of diagnosed lung cancers were stage 4. In the control group, 33%, 8%, 26%, and 31% of diagnosed cancers respectively, were stages 1, 2, 3, and 4.
5 to < 10 years post‐randomisation: we included four trials (Becker 2020; Field 2021; Infante 2015; Paci 2017; Analysis 8.4). All trials used TNM staging. The NLST trial (Aberle 2011) also included an occult lung cancer stage, which we combined with stage 1 for this analysis. The evidence showed that stage 1 occurred more frequently in the LDCT screening group (RR 2.26, 95% CI 1.43 to 3.57; 4 trials, 13,676 participants; I2 = 0%). There was probably no difference between the groups for stages 2 (RR 0.78, 95% CI 0.37 to 1.66; 4 trials, 13,676 participants; I2 = 9%) and 3 lung cancer (RR 0.84, 95% CI 0.47 to 1.49; 4 trials, 13,676 participants; I2 = 27%). Heterogeneity was mild amongst the trials for stage 3. The evidence suggested fewer cases of stage 4 lung cancer detected in the LDCT screening group (RR 0.55, 95% CI 0.34 to 0.91; 4 trials, 13,676 participants; I2 = 57%). Heterogeneity was present and removing Field 2021 resulted in no heterogeneity without changing the findings (RR 0.70, 95% CI 0.50 to 0.97; 3 trials, 9708 participants; I2 = 0%). There was probably no difference between the groups with lung cancer of unknown stage (RR 0.67, 95% CI 0.41 to 1.12; 4 trials, 13,676 participants; I2 = 5%). In the LDCT screening group, 31% of diagnosed lung cancers were stage 1, 7% of diagnosed lung cancers were stage 2, 17% of diagnosed lung cancers were stage 3, and 32% of diagnosed lung cancers were stage 4. In the control group, 11%, 8%, 16%, and 46% of diagnosed cancers respectively were stages 1, 2, 3, and 4.
≥ 10 years post‐randomisation: we included four trials (Aberle 2011; Paci 2017; Pastorino 2012; Wille 2016; Analysis 8.5). As previously, we combined occult lung cancer with stage 1 lung cancer for Aberle 2011. The evidence showed that stage 1 was detected more frequently in the LDCT screening group (RR 3.28, 95% CI 1.82 to 5.90; 4 trials, 11,409 participants; I2 = 56%). The heterogeneity level was acceptable. There was probably no difference between the groups for stages 2 (RR 0.94, 95% CI 0.76 to 1.17; 4 trials, 64,864 participants; I2 = 0%) and stage 3 lung cancer (RR 1.23, 95% CI 0.79 to 1.93; 4 trials, 64,864 participants; I2 = 56%). The heterogeneity level was acceptable amongst the trials for stage 3 lung cancer. The evidence suggested there were fewer cases of stage 4 lung cancer in the LDCT screening group (RR 0.77, 95% CI 0.69 to 0.86; 4 trials, 64,864 participants; I2 = 0%) There were possibly fewer cancers at unknown stages in the LDCT group compared with the control group (RR 0.67, 95% CI 0.45 to 0.99; 3 trials, 60,765 participants; I2 = 24%). In the LDCT screening group, 40% of diagnosed lung cancers were stage 1, 8% of diagnosed lung cancers were stage 2, 18% of diagnosed lung cancers were stage 3, and 28% of diagnosed lung cancers were stage 4. In the control group, 26%, 9%, 18%, and 37% respectively, were stages 1, 2, 3, and 4.
9) Histology
We grouped trial results by time points (Analysis 9.1; Analysis 9.2; Analysis 9.3). For the purposes of this review, histological types presented are small cell lung carcinoma (SCLC), mixed SCLC and non‐small cell lung carcinoma (NSCLC), squamous cell carcinoma (SCC), adenocarcinoma (AC), bronchioalveolar carcinoma (BAC), and other. The category of 'other' is all other histological subtypes presented in trials, including sarcomatoid carcinomas, large cell carcinomas, neuroendocrine tumours and neuroendocrine carcinomas. It should be noted that BAC was reclassified as various adenocarcinoma subtypes in the lung cancer TNM classification by the World Health Organization (WHO) (Nicholson 2022), however has been included here as presented by the relevant trials.
9.1. Analysis.

Comparison 9: Secondary outcome: lung cancer histology at different time points, Outcome 1: Histology types at baseline
9.2. Analysis.

Comparison 9: Secondary outcome: lung cancer histology at different time points, Outcome 2: Histology at year 1
9.3. Analysis.

Comparison 9: Secondary outcome: lung cancer histology at different time points, Outcome 3: Histology at follow‐up
Baseline: we included four trials (Aberle 2011; Blanchon 2007; Gohagan 2005; Infante 2015; Analysis 9.1). There was probably no difference in the number of SCLC and other histology between groups (RR 0.84, 95% CI 0.45 to 1.57; 4 trials, 59,987 participants; I2 = 2%) and (RR 1.32, 95% CI 0.90 to 1.94; 4 trials, 59,987 participants; I2 = 0%), respectively. SCC, AC, and BAC were more common in the LDCT arm (RR 1.47, 95% CI 1.01 to 2.13; 4 trials, 59,987 participants; I2 = 0%), (RR 2.81, 95% CI 1.38 to 5.71; 4 trials, 59,987 participants; I2 = 46%) and (RR 4.94, 95% CI 2.41 to 10.10; 2 trials, 55,904 participants; I2 = 0%), respectively. Heterogeneity between groups was moderate in the AC analysis.
1 year: we only included one trial (Gohagan 2005; Analysis 9.2), with more cases of AC found in the LDCT screening groups and probably no difference between groups for SCLC (RR 2.00, 95% CI 0.37 to 10.89; 1 trial, 3318 participants), SCC (RR 0.83, 95% CI 0.25 to 2.72; 1 trial, 3318 participants), AC (RR 2.66, 95% CI 1.24 to 5.71; 1 trial, 3318 participants) and other histology (RR1 2.33, 95% CI 0.60 to 9.00; 1 trial, 3318 participants).
≥ 7 years post‐randomisation: we included seven RCTS in the analysis (Aberle 2011; Becker 2020; Field 2021; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016; Analysis 9.3). The latest available time point was 11.3 years post‐randomisation (Aberle 2011). For the DLCST trial (Wille 2016), the adenocarcinoma category also included mixed AC and BAC, as well as mixed AC and SCC. The MILD trial (Pastorino 2012) had both annual and biennial arms combined in the intervention group. For consistency, the latest available time point for data was taken for each trial. In the LUSI trial (Becker 2020), this meant that only data pertaining to three categories (AC, BAC, and other) were included. For SCLC, SCC, and other, there was probably no difference between the groups, with moderate heterogeneity between the trials for the other category: SCLC (RR 0.86, 95% CI 0.74 to 1.01; 6 trials, 71,281 participants; I2 = 0%), SCC (RR 1.04, 95% CI 0.81 to 1.32; 6 trials, 71,281 participants; I2 = 26%) and other (RR 0.87, 95% CI 0.68 to 1.11; 7 trials, 75,333 participants; I2 = 40%). The evidence suggested that AC and BAC were more common in the LDCT screening group, with high heterogeneity between trials in the AC category: AC (RR 1.49, 95% CI 1.05 to 2.10; 7 trials, 75,333 participants; I2 = 82%) (analysis not shown) and BAC (RR 2.73, 95% CI 1.96 to 3.81; 3 trials, 61,610 participants; I2 = 0%). When we removed NLST (Aberle 2011) from the AC analysis, heterogeneity decreased (RR 1.62, 95% CI 1.13 to 2.34; 6 trials, 21,879 participants; I2 = 69%). NLST (Aberle 2011) was the only included trial in this analysis that used CXR screening as a comparator. Mixed SCLC and NSCLC was only reported in one trial (Wille 2016) (RR 0.14, 95% CI 0.01 to 2.76; 1 trial; 4104 participants).
10) Other outcomes
Biomarkers: two trials have published data on small samples of their trial population DNA and microRNA profiles (Paci 2017; Pastorino 2012). Becker 2020 published early data on autoantibodies to tumour‐associated antigens in a subgroup of their cohort.
Response rate: eight RCTs had available data (Becker 2020; Blanchon 2007; De Koning 2020; Field 2021; Gohagan 2005; LaRocca 2002; Paci 2017; Wille 2016), which is summarised in Table 5. In larger trials that used information mail outs (Becker 2020; De Koning 2020; Field 2021; Gohagan 2005; Paci 2017), only ≤ 5% of those contacted, enrolled in the trial.
Adherence to screening: eight RCTs reported adherence to screening (Aberle 2011; Becker 2020; Blanchon 2007; De Koning 2020; Field 2021; Gohagan 2005; Paci 2017; Pastorino 2012). Overall, adherence to screening was good, with a decline noted at and after 5 years (Table 5). Three RCTs had comparative data with CXR control groups (Aberle 2011; Blanchon 2007; Gohagan 2005). Heterogeneity was high between the groups, however the evidence suggested poorer adherence in the LDCT screening group (RR 1.05, 95% CI 1.01 to 1.09; 3 trials, 57,539 participants; I2 = 85%) (analysis not shown).
Contamination: seven RCTS reported on contamination (Becker 2020; Blanchon 2007; De Koning 2020; Gohagan 2005; Infante 2015; Pastorino 2012; Wille 2016). In LUSI (Becker 2020), 13% of the control group had undergone a CT scan. In DESPICAN (Blanchon 2007), 6 participants had inadvertently undergone LDCT. In the NESLON trial (De Koning 2020) 4% of the control group in a random sample of 1460 participants had undergone chest CT. One per cent of control arm participants underwent a LDCT in the MILD trial (Pastorino 2012), and three participants in the DLCST control group (Wille 2016) had a chest CT for lung cancer screening purposes. Neither LSS (Gohagan 2005) nor DANTE (Infante 2015) clearly differentiated the number of scans performed for screening purposes only. Reported contamination rates are detailed in Table 5. Three RCTs had comparative data with control arms (Gohagan 2005; Infante 2015; Wille 2016). The evidence suggested there was no significance difference between the groups, however heterogeneity was high (RR 1.35, 95% CI 0.32 to 5.68; 3 trials, 6902 participants; I2 = 74%).
Interval lung cancers: seven trials reported rates of interval cancers at different time points (Aberle 2011; Becker 2020; De Koning 2020; Gohagan 2005; Paci 2017; Pastorino 2012; Wille 2016). These are summarised in Table 6.
False negatives: five trials reported false‐negative cases at various time points (Aberle 2011; De Koning 2020; Field 2021; Infante 2015; Pastorino 2012). In NLST (Aberle 2011), there were < 1% false negatives each round of screening for the LDCT screening group and CXR control group for the first 3 years, with more false‐negative results in the CXR screening compared with LDCT screening (Analysis 5.2). The NELSON trial (De Koning 2020) also reported small numbers of false negatives with a total of five, seven, and seven false negatives across the first, second and third round of screening, respectively. The UKLS trial (Field 2021) reported three false negatives from their baseline LDCT. The DANTE trial (Infante 2015) reported one false negative in the intervention arm. The MILD trial (Pastorino 2012) reported 17 false negatives in the annual LDCT group and nine in the biennial LDCT group.
Incidental findings: six trials reported rates of incidental findings (Aberle 2011; Blanchon 2007; De Koning 2020; Field 2021; Infante 2015; Wille 2016), and these are summarised in Table 6.
Cost: three trials reported cost data (Aberle 2011; Field 2021; Wille 2016). The lowest cost was in the UK trial (Field 2021) which, following clarification with authors, was GBP 186 per screen/per participant, including costs of recruitment. In the Danish trial (Wille 2016), the cost of a LDCT screen was EUR 282, with the total cost per year of healthcare costs per participant being EUR 3756 in the screening group. Of note, the control arm cost per participant per year in this trial was EUR 3474 (EUR 2348 without lung function or counselling). The USA trial (Aberle 2011) had a cost per participant in the LDCT screening group of USD 1130, compared with USD 336 for the CXR participant. The costs of screening included the cost of investigating clinically SIFs.
Use of anxiolytics and antidepressants: only one trial investigated this outcome and concluded that participation in the trial was not associated with a change in prescriptions of these medications (Wille 2016).
Feasibility of general practitioner (GP) enrolment to lung cancer screening trial: one trial reported this as an outcome (Blanchon 2007), with participation rate of GPs reported as 41%.
4. Response, adherence and contamination rates.
| Response rates to recruitment | Adherence to screening | Contamination | |
| Aberle 2011 | Not available | Overall adherence to all 3 screening rounds: 95% of participants completed LDCT scan, 93% in the control group completed CXR | Not available |
| Becker 2020 |
|
Baseline: almost 100% completed LDCT scan. 1‐year: 95% completed LDCT scan. 2‐year: 93% completed LDCT scan. 3‐year: 93% completed LDCT scan. 4‐year: 94% completed LDCT scan. |
10 years postrandomisation: 264 participants in the control arm had received a CT. |
| Blanchon 2007 |
|
Baseline: 86% participants completed LDCT scan, 75% in control arm completed CXR. | At baseline: 6 participants in the control arm inadvertently received a LDCT. |
| De Koning 2020 |
|
Baseline: 95% of participants completed LDCT scan 1 year: 97% of participants completed LDCT scan 3 years: 95% completed LDCT scan 5.5 years: 78% of participants completed LDCT scan |
At 2 years: 3.6% of participants in the control arm had received CT for any reason |
| Field 2021 |
|
Baseline: 98% of participants completed LDCT scan | Not available |
| Gohagan 2005 |
|
Baseline: 95% of participants completed LDCT scan, 93% of the control group completed CXR 1‐year: 86% of participants completed LDCT scan, 80% of the control group completed CXR |
Contamination assessed by random sample of participants At baseline: 5% of respondents in the intervention arm had received a CXR for medical or screening purposes. 0.9% of respondents in the control arm had received a CT for medical or screening purposes. At 1 year: 10% of participants in the intervention arm had received a CXR for medical or screening purposes and 1.3% of respondents in the control arm had received a CT for medical or screening purposes. |
| Infante 2015 | Not available | Not available | 3 years post‐randomisation:
Did not specify if for screening purposes, only outside protocol |
| LaRocca 2002 |
|
Not available | Not available |
| Paci 2017 |
|
Baseline: 87% completed LDCT scan 1 year: 85% completed LDCT scan 2 years: 82% completed scan 3 years: 80% completed scan |
Not available |
| Pastorino 2012 | Not available | Baseline: 97% of annual group completed scan, 97% of biennial group completed scan 1 year: 97% of the annual group and 97% of the biennial group completed the scan 2 years: 98% of the annual group and 95% of the biennial group completed the scan 3 years: 97% of the annual group and 97% of the biennial group completed the scan 4 years: 96% of the annual group and 92% of the biennial group completed the scan 5 years: 79% of the annual group and 98% of the biennial group completed the scan 6 years: 54% of the annual group and 77% of the biennial group completed the scan |
10 years post‐randomisation: 21 of 1723 participants in the control arm had received a LDCT |
| Wille 2016 |
|
Not available | After 5 years post‐randomisation: 0 cases of contamination in the intervention arm; 3 cases in the control arm |
CT: computed tomography; CXR: chest x‐ray; LDCT: low‐dose computed tomography
5. Interval cancers and incidental findings .
| Interval cancers | Incidental findings | |
| Aberle 2011 | Postbaseline LDCT: 18 lung cancers Post‐1 year LDCT: 10 lung cancers |
Baseline LDCT data from non‐ACRIN centres (N = 17,309): 2625 cardiovascular abnormalities, 221 thyroid abnormalities, 419 adrenal abnormalities, 780 renal abnormalities, and 1064 hepatobiliary abnormalities |
| Becker 2020 | Postbaseline LDCT: 1 lung cancer Post‐1‐year LDCT: 0 lung cancers Post‐2‐year LDCT: 2 lung cancers Post‐3‐year LDCT: 1 lung cancer Post‐4‐year LDCT: 2 lung cancers |
Not available |
| Blanchon 2007 | Not available | Baseline LDCT: 19 severe emphysema, 63 bronchiectasis, and 18 mediastinal findings Baseline CXR in control group: 5 severe emphysema, 2 bronchiectasis, and 6 mediastinal findings |
| De Koning 2020 | After 3 rounds of LDCT screening: 35 interval lung cancers | Baseline LDCT data from one centre (N = 1929): 76 liver findings, 53 kidney findings, 9 thyroid findings, 2 mediastinal findings, 1 adrenal finding, 1 breast finding, 1 colon finding, and one perineural cyst |
| Field 2021 | Not available | Baseline LDCT: 4 aortic dilatations, 5 severe aortic valva calcifications, 4 mediastinal masses, 6 mediastinal or hilar lymphadenopathy, 41 pneumonias, 5 bronchiectasis, 8 pleural thickening, 7 smoking related interstitial lung diseases, 9 severe emphysemas, 6 unspecified interstitial fibrosing lung disease, 2 nonspecific interstitial pneumonias, 12 usual interstitial pneumonias, 1 sarcoidosis, 2 oesophageal thickening or dilatation, 1 breast mass, 2 lobar collapse, 1 biliary dilatation, 3 adrenal masses, 1 liver cirrhosis, 1 hydronephrosis, 1 liver mass, 1 pancreatic cyst, 3 renal masses, 1 splenomegaly, and 1 thyroid mass |
| Gohagan 2005 | 1 year post‐randomisation: 2 lung cancers in the LDCT group and 2 lung cancers in the control group | Not available |
| Infante 2015 | Not available | Baseline LDCT: 1 lymphoma, 1 oesophageal carcinoma, 1 malignant mesothelioma, 1 colon cancer with liver metastasis, and 2 renal cancers with pulmonary metastasis Baseline CXR in the control group: 1 lymphoma |
| LaRocca 2002 | Not available | Not available |
| Paci 2017 | Overall 6 interval lung cancers reported during 4 years of screening | Not available |
| Pastorino 2012 |
|
Not available |
| Wille 2016 | 1 interval lung cancer reported in LDCT group during year 3 | After 5 rounds of screening: 140 participants had 148 significant incidental findings (1 larynx, 3 thyroid, 9 gastroesophageal, 16 breast, 5 cardiac, 12 mediastinum, 28 aorta, 18 liver/gallbladder, 6 pancreas, 1 spleen, 2 intestines, 40 kidneys, 2 skin, 3 chest wall, and 2 vertebral column) |
CXR: chest x‐ray; LDCT: low‐dose computed tomography
5.2. Analysis.

Comparison 5: Secondary outcome: false positives, negatives and recalls (number of screens), Outcome 2: False negative
Discussion
Summary of main results
Primary outcomes
For lung cancer‐related mortality, moderate‐certainty evidence showed a difference favouring LDCT screening. When we only included high‐certainty trials, the evidence still favoured LDCT screening.
The evidence showed that the number of invasive and non‐invasive interventions is higher in the LDCT screening group compared with the control group, including rates of invasive interventions for non‐lung cancer‐related disease. However, there was probably no difference in death postsurgery between groups.
Secondary outcomes
For all‐cause mortality, the evidence showed a small difference favouring screening with LDCT. The analysis still favoured screening with LDCT when only high‐certainty trials were included.
For estimated overdiagnosis at 10 or more years, the combined risk was 18%. However, the 95% CI was wide, suggesting possibly no difference between the groups, with a lower limit of the 95% CI just below 0 and an upper limit of 36%. This is in keeping with the incidence of lung cancer, demonstrating that there was possibly no difference in incidence between LDCT screening and control groups at 10 or more years.
For false‐positive results from scans, the evidence showed that these were higher in the LDCT screening group.
For recall rates, the evidence showed that these were higher in the LDCT screening group.
For smoking cessation rates, results were mixed. However, there was probably no significant difference in smoking relapse rates between LDCT screening and control groups.
For psychosocial consequences, the evidence was of low certainty due to inconsistencies in outcome measures, sample groups, and timing of assessments. Overall the limited evidence available did not suggest any long‐term adverse impact on psychosocial well‐being or HRQoL with LDCT screening.
For lung cancer staging, the evidence showed there was more stage 1 lung cancer detected in the LDCT group compared with control, across the time points. As time from randomisation increased, there was probably no difference between groups for stage 2 and 3 lung cancer. In later time points, the evidence showed there was more stage 4 lung cancer in the control groups compared to LDCT screening group.
For lung cancer histology, the evidence showed there was more squamous cell carcinoma (SCC), adenocarcinoma (AC) and bronchioalveolar carcinoma (BAC) detected in the LDCT screening group compared with control groups at baseline. AC and BAC remained more prevalent in the LDCT screening group at later time points.
Overall completeness and applicability of evidence
We identified 11 eligible RCTs and included eight for the main meta‐analysis of the primary outcome, lung cancer‐related mortality; we could not include two trials in the meta‐analysis due to data being unavailable, and we excluded one trial (Gohagan 2005) from Analysis 1.1 as it did not have any planned follow‐up time points.
The following considerations may affect the strength and completeness of the conclusions of this review.
-
Participant characteristics
Participants enrolled in these trials tended to have a strong tobacco smoking exposure history as a result of trial inclusion criteria. Trials investigating the role of LDCT for lung cancer‐screening in non‐smoking populations are still ongoing (Ongoing studies). Only one trial used a validated tool to predict lung cancer risk, with the other trials using smoking exposure as part of the inclusion criteria.
Two RCTs had either zero or a significantly underrepresented number of female participants (De Koning 2020; Infante 2015). This is significant, as the subgroup analysis of female participants demonstrated a larger lung cancer‐related mortality benefit with LDCT screening compared to the male participants (Analysis 1.8), although CIs did overlap.
All included trials were conducted in the USA or Europe. Whilst not all trials reported breakdown of ethnicity or race, the two trials that did, reported a significant majority of white participants compared to other races (Aberle 2011; Field 2021).
Seven trials had additional fitness requirements (Becker 2020; Blanchon 2007; De Koning 2020; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016), including fitness for surgery for entry into the trial.
Information regarding education level and socioeconomic status of participants was limited. Three trials described education levels amongst participants (Aberle 2011; Field 2021; Wille 2016), with 32% of NLST having a tertiary degree or higher (Aberle 2011).
We could not provide enough information about the potential harms of LDCT screening as only a few trials provided data from all trial groups (Aberle 2011; Gohagan 2005; Infante 2015; Pastorino 2012). Heterogeneity ranged from high to low amongst trials in these analyses, as trials did not have uniform reporting and categorisation of invasive and non‐invasive interventions. Additionally, trials did not have a consensus definition for significance of nodules and investigation pathways.
-
Included trials did not use consistent measures of HRQoL, which made comparison challenging.
Only two trials administered the questionnaires to their whole cohort (Field 2021; Wille 2016), with the NLST (Aberle 2011) and the NESLON (De Koning 2020) trials inviting only a small portion of their cohort. The NLST trial limited their assessment to only those in the LDCT screening group (Aberle 2011).
Whilst the exact timing of assessments varied, all trials consistently reported no long‐term adverse consequences of screening with LDCT, except for true positives (participants diagnosed with cancer as a result of screening) in NLST (Aberle 2011). The NLST trial reported lower HRQoL and more anxiety in this group.
The NELSON trial had an indeterminate category for classification of nodules (De Koning 2020), and reported an elevated cancer‐specific distress core in this group at 2 months. There was no difference between groups at 2 years.
The UKLS trial reported less decision satisfaction for participants in the control arm (Field 2021), which may reflect an increased awareness of risk of lung cancer in the control arm without the ability to participate in screening. Anxiety and lung cancer‐related distress were higher in the group referred to multidisciplinary meetings postscreening, however this group had high satisfaction rates in their decision to participate in screening.
The DLCST reported more negative responses in both the screening and no‐screening groups in the fields of behaviour, dejection and negative impact on sleep at earlier time points (Wille 2016), however the impact decreased by round five of screening. They also noted less anxiety in the screening at round five. Their response rate to questionnaires in the control group was lower (76% compared with 94% in the LDCT screening group). In their smaller cohort study, Wille 2016 reported that participants with false‐positive results had more negative psychosocial consequences of screening compared with those in the control group (no screening) or true negatives in the short term, with no significant long‐term consequences.
The DLSCT also reviewed psychosocial status and demographics of participants in the control group who did not attend their annual clinical review (Wille 2016). The trial reported that non‐attenders to visits were from more disadvantaged sociodemographic backgrounds and had lower psychosocial questionnaire scores from preceding rounds. This is an important consideration for lung cancer screening, adding to the need for more comprehensive assessments of psychosocial well‐being of potential lung cancer screening participants and acknowledging that the population who participates and engages in a trial may not be reflective of the general population.
Further research is required to assess factors affecting engagement in screening and adherence and how to manage potential short‐term adverse psychosocial outcomes.
-
Effect of screening on smoking was also limited due to available data.
Five trials involved smoking cessation counselling (Becker 2020; De Koning 2020; Paci 2017; Pastorino 2012; Wille 2016).
Motivation to quit was reported in two trials (De Koning 2020; Wille 2016), however they did not provide comparative data for trial arms.
The definition of recall rates was not consistent across all trials (Aberle 2011; Becker 2020; De Koning 2020; Field 2021; Gohagan 2005; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016). Recall rates in LUSI included all early recalls up to 12 months following LDCT (Becker 2020), whereas in the MILD trial it was defined as 3 months post‐LDCT scan (Pastorino 2012). The NLST reported all further chest CT, and did not specify recall CTs only (Aberle 2011). The NELSON trial also had an intermediate category for abnormal results (De Koning 2020), which likely resulted in an under appreciation of false‐positive screens, when defined as any result that is not a negative screen.
Incidental findings were also not well described across the included trials, with varying categories of findings reported. Incidental findings are likely common however, with the NLST reporting 15% of participants had incidental cardiovascular disease noted on baseline LDCT (N =17,309 from non‐ACRIN centres; Aberle 2011).
Some trials had limited publications. All RCT authors were contacted by a reviewer (DM) where required.
Quality of the included trials
Included trials were generally well‐designed, and all included trials were RCTs.
Due to the nature of the intervention, an open‐label design may have increased performance bias in subjective outcomes.
-
Only a few trials used blinded death panels to review lung cancer‐related mortality, which may have influenced detection bias.
Six of the included trials used a death review panel in some capacity (Aberle 2011; Becker 2020; De Koning 2020; Infante 2015; Paci 2017; Wille 2016), particularly when assessing lung cancer‐related deaths for part or all of the duration of the trial.
Three trials used registries and/or death certificates only to determine cause of death (Field 2021; Gohagan 2005; Pastorino 2012).
The NELSON trial reviewed the use of death review panels to determine cause of death compared with death certificates in the first 266 Netherlands cohort deaths (De Koning 2020); the use of the review panel reclassified 12% of cases. The NELSON trial subsequently ceased using a death review panel thereafter.
The NLST trial also reviewed use of a death review panel compared with death certificates to accurately determine cause of death (Aberle 2011). Cause of death was reclassified in 3% of cases following review by panel, with death certificates having a sensitivity of 91% for cause of death. The authors then revisited analysis of lung cancer‐related mortality using lung cancer‐related deaths provided by death certificate, and found a lung cancer mortality reduction of 18% (95% CI 4.2 to 25) compared to the 20% (95% CI 6.7 to 26.7) published.
The use of death panels in trials is expensive, and further research is required to determine whether it significantly adds to the assessment of lung cancer‐related mortality.
The use of registries alone for follow‐up and detection of mortality and lung cancer incidence was a concern, as it was limited by trial access to participant data, delays from outcome to registry notification, and assumed participants had remained in the catchment of the registry without name change or errors. In the NLST's extended follow‐up for instance (Aberle 2011), not all home state registries participated in the linkage, and some screening centres did not have access to participants' details to complete the linkage. The LUSI trial also had limitations with registry linkage (Becker 2020), with 39 participants declining data access. The UKLS trial had participants who had not consented to data linkages or had opted out of national registries (Field 2021).
Given concerns regarding completeness of follow‐up data, this review prioritised planned and/or active follow‐up in analysis over unplanned extended follow‐up, as this tended to rely more on registry data and some trials which had used the death panel (Aberle 2011; Paci 2017), ceased after planned follow‐up.
All‐cause mortality results were reliable across included trials.
Regarding risk of bias, excluding those with unclear risk (Becker 2020; Blanchon 2007; LaRocca 2002), overall most domains of importance were low risk. There were a few trials that did have high risk for certain domains (De Koning 2020; Field 2021; Gohagan 2005; Infante 2015; Pastorino 2012), however this is unlikely to have had a significant impact on the results presented.
Certainty of the evidence
See Table 1.
We graded lung cancer‐related mortality as having moderate‐certainty evidence as it included eight trials which had low to high risk of bias. It should be noted that one trial had CXR as a comparison rather than no screening (Aberle 2011). Whilst screening with CXR has not been shown to reduce lung cancer‐related mortality (Manser 2013), there may potentially be some impact from screening with CXR, which may have diluted the effect of LDCT screening in the NLST trial. However, there was no heterogeneity between the included trials. The included trials also had different definitions for positive scans (Aberle 2011; Becker 2020; De Koning 2020; Field 2021; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016).
We graded all‐cause mortality as having moderate‐certainty evidence as the eight included trials had risk of bias ratings from low to high (Aberle 2011; Becker 2020; De Koning 2020; Field 2021; Infante 2015; Paci 2017; Pastorino 2012; Wille 2016). The difference was small, with only a 5% risk reduction with screening and a 95% CI upper limit of 0.99 (1% risk reduction). CXR as a comparator in the NLST may have impacted the effect (Aberle 2011).
We graded overdiagnosis as having low‐certainty evidence due to risk of bias in the included trials and high heterogeneity between trials. The heterogeneity was largely due to the DLCST (Wille 2016), which had significantly higher lung cancer incidence in the LDCT screening group compared with no screening. There was a higher incidence of lung cancer diagnosed postcessation of screening in the screening group, which was unusual and not adequately explained by the mild overrepresentation of smoking history > 35 pack years and lower forced expiratory ratio (FER) in the screening group at baseline. We chose to present the follow‐up data at 10 or more years despite this rating as it probably provides a better estimate for overdiagnosis of lung cancer with LDCT screening. Except for the DLSCT (Wille 2016), the other trials demonstrated a reduction in estimated overdiagnosis as time from randomisation increased. The other consideration which was not well explored in all trials was the background rates of CT scans in each country for other purposes, which may conversely diminish the perceived impact of overdiagnosis.
We graded the outcomes 'number of invasive tests' and 'any death postsurgery' as having moderate‐certainty evidence as both analyses included trials with concerns regarding risk of bias.
We graded HRQoL and psychosocial consequences as having low‐certainty evidence because we judged two of the three trials contributing to this outcome at high risk of bias and the included trials were different in their assessment of this outcome (De Koning 2020; Field 2021; Wille 2016), and this limited our ability to combine results. Only two trials included the whole trial cohort in their quality of life assessments (Field 2021; Wille 2016). The UKLS trial administered the questionnaires to the whole cohort (Field 2021). The DLCST also administered their questionnaires to the whole cohort (Wille 2016), however performed an additional nested cohort trial focusing on those with a positive screen result. The NESLON trial took a random sample of 733 participants from each trial group (LDCT screening and control) (De Koning 2020). In the NLST (Aberle 2011), which we excluded from the meta‐analysis as it did not have data from the CXR group, only 16 sites invited participants to complete questionnaires. Of those 16 sites, only those with a positive scan were initially invited to participate, with the NLST subsequently inviting participants with a negative scan but SIFs on LDCT at a later time point. This cohort of participants was then matched with controls who had a negative LDCT scan. The tools used for assessment of this outcome also varied, with only one trial using a lung cancer‐specific tool to assess psychosocial consequences (Wille 2016). The NELSON trial (De Koning 2020) and UKLS trial (Field 2021) used lung cancer‐specific distress scores. The NLST (Aberle 2011) and NELSON trial (De Koning 2020) both used generic health questionnaires (Short Form‐12 and 36), as well as the STAI‐6 for anxiety. The UKLS trial also measured Hospital Anxiety and Depression Scale (HADS), Cancer Worry Scale, and used a satisfaction with decision score (Field 2021). The trials also did not evaluate outcomes at uniform time points. The NLST assessed before the scan (could be any screen interval), 1‐month postscreen, and 6 months postscreen (Aberle 2011). The NELSON trial assessed at baseline, 2 months post‐randomisation, and at 2 years (prior to the third screen) (De Koning 2020). The UKLS trial collected questionnaires before randomisation, at 2 weeks (after participants had received results of screen or been notified of allocation to control arm), and at 10 months to 27 months (Field 2021). The DLCST administered questionnaires annually for five years (Wille 2016). Their nested cohort trial collected questionnaires at baseline, 1 week (postresult of screen or annual visit), 1 month, 6 months, and 18 months. The DLSCT also reviewed psychosocial status and demographics of participants in the control group who did not attend their annual clinical review. The trial reported that non‐attenders to visits were from socioeconomically disadvantaged backgrounds and had lower psychosocial questionnaire scores from preceding rounds. This is an important consideration for lung cancer screening, as lung cancer is disproportionally represented in this group (Mao 2001).
Potential biases in the review process
This review used the Cochrane highly‐sensitive search strategy and aimed to include all trials, both published and unpublished, and conducted a wide search including ongoing trial registry databases and abstracts from major conferences. We did not apply any language restrictions, and we are confident we have included all relevant trials to date in this review. At least two review authors (AB, CM, DM, RM, RManser) reviewed search results. Multiple authors (AB, RM, DM) checked all data, both during the extraction process and analysis of data against published results. We resolved any disagreements and queries via discussion. One review author (DM) contacted trial authors when additional data were required.
Agreements and disagreements with other studies or reviews
The previous Cochrane Review on lung cancer screening (Manser 2013), evaluated multiple modalities of screening, including sputum cytology, CXR, and CT. Based on their conclusions, there was no evidence to support screening with sputum cytology or CXR, however more data were required for CT screening. As such, this review focused on lung cancer screening with LDCT and incorporated data from more RCTs (Aberle 2011; Becker 2020; Blanchon 2007; De Koning 2020; Field 2021; Gohagan 2005; Infante 2015; LaRocca 2002; Paci 2017; Pastorino 2012; Wille 2016), most of which were still ongoing in the previous review.
When comparing our review to other systematic reviews published (Hoffman 2020; Huang 2019; Jonas 2021; Rota 2019; Sadate 2020; Mazzone 2021), our review incorporated more secondary outcomes and provided analysis at more defined time points and with additional subgroups. Two of the reviews (Hoffman 2020; Huang 2019), included the AME trial (Yang 2018), which does not have complete mortality data at 5 years. Despite some differences in included trials and time points, the reviews still favoured screening to reduce lung cancer‐related mortality and did not show differences in mortality after invasive procedures between groups when reported. However, most concluded that there was probably no difference with all‐cause mortality, as their 95% CI just reached or crossed 1. Our review included data from the UKLS trial (Field 2021), and had a 95% CI upper limit of 0.99. One review (Hoffman 2020), also evaluated for diagnosis of stage 1 lung cancer, and found it to be more prevalent in the LDCT screening group.
One other review has focused on overdiagnosis in lung cancer screening RCTs (Brodersen 2020). This review included data from five RCTs (Becker 2020; De Koning 2020; Paci 2017; Pastorino 2012; Wille 2016), and estimated that 38% of screen‐detected lung cancers may be overdiagnosed in these trials (95% CI 14% to 63%). Brodersen 2020 performed a sensitivity analysis of their rated high‐quality trials (Becker 2020; Wille 2016), which had an estimated overdiagnosis rate of 49% (95% CI 11% to 89%). Of note, a recent publication by Gao 2022 investigated possible overdiagnosis of lung cancer by LDCT screening in a population of mostly non‐smoking Taiwanese women. They estimated that from 2004 to 2018, between 12% and 21% of women have been overdiagnosed with lung cancer. Women and non‐smokers were underrepresented in the trials included in our review.
Our findings were consistent with other reviews on psychosocial outcomes related to lung cancer screening (Quaife 2021; Slatore 2014; Wu 2016).
In regard to assessment of risk of bias, our review was generally consistent with other reviews, however we tended to be more conservative when evaluating 'other biases' in trials, such as protocol deviations and unbalanced baselines. We also contacted authors for clarification.
Authors' conclusions
Implications for practice.
The evidence in this review from RCTs suggests that screening with LDCT leads to a reduction in lung cancer‐related mortality in high‐risk populations, although it should be noted that the certainty of this evidence is moderate. There is also an increase in investigations associated with LDCT screening, including those unrelated to lung cancer disease. More information is required to define the ideal frequency and duration of screening, as there is a probable loss of impact of screening on lung cancer‐related mortality as time from last screening scan increases.
Whilst available data suggests there is probably no difference between LDCT screening and non‐LDCT screening groups in mortality following invasive procedures, there remains a lack of data regarding harms of screening. Quality assurance with monitoring of harms, such as complications, false positives, recall rates and follow‐up should be included as part of a screening programme with set key performance indicators.
When discussing lung cancer screening with patients, physicians can consider the relative risks and benefits for the individual prior to recommendation, and ensure that screening and subsequent follow‐up occurs in centres with experience in lung nodule and lung cancer care, particularly given concerns regarding overdiagnosis. The difference in management of solid versus subsolid nodules (Silva 2018), and incorporation of recommendations such as the European Union Position Statement on lung cancer screening (Oudkerk 2017), can be considered. Whilst there was limited use of risk prediction models in the included RCTs, these have been associated with improved participant selection (Field 2019; ten Haaf 2017), and further trials are required to evaluate and compare different models. Physicians may choose to also be mindful of potential psychosocial consequences of screening, although evidence has not demonstrated any long‐term impacts. Participants of screening programmes should receive counselling about the implications of a positive screen and significant incidental findings (SIFs).
Current smokers and former smokers with a significant pack‐year history formed the majority of the population in this review, with trials evaluating lung cancer screening with LDCT in predominantly non‐smokers still ongoing. Smoking cessation and other primary prevention strategies can be considered as part of a screening programme, although the optimal method for delivery of these strategies is still under investigation.
There have been several guidelines from the USA and Europe with favourable positions on national screening programmes for groups at high risk of lung cancer (Jonas 2021; Kauczor 2020; Mazzone 2021). Response to recruitment to screening programmes in the trial setting ranged from 1% to 5% of people approached with invitations and letters. Adherence to screening was noted to decrease over time, with only 54% of the annual screening group of the MILD trial completing their 6‐year scan (Pastorino 2012). Further consideration is required to determine the optimal way to engage with people at high risk of lung cancer, to ensure equitable access to screening when developing national screening programmes, as well as ensuring engagement with the programme once initiated.
Implications for research.
Further research is needed to determine participant selection for a lung cancer screening programme, particularly focusing on groups under‐represented in this review, women and non‐smokers, as well as other geographic regions outside the USA and Europe.
Additional research is also required for the optimal duration and frequency of screening (van der Aalst 2021), with particular attention to the optimal assessment and management of lung nodules; there is no uniform approach or guideline to nodule management. Further review of nodule classifications, such as Lung CT Screening Reporting and Data System (Lung‐RADS, Lung‐RADS 2019) and the Brock model (McWilliams 2013) for estimating lung cancer risk from nodules are needed, with particular attention to ground glass opacities and nodule management, which may contribute to overdiagnosis. None of the included trials prospectively evaluated the use of artificial intelligence in lung cancer screening.
Biomarkers are an evolving field, with most included trials only publishing very early descriptive data. Future research with biomarkers may help with the selection of participants for screening or prove useful as a screening modality.
History
Protocol first published: Issue 12, 2020
Notes
Some parts of the methods sections come from a standard template drafted by the Cochrane Lung Cancer Group.
Acknowledgements
We would like to thank the following for commenting on our review.
Virginie Westeel, Department of Pneumology, University Hospital of Besançon; INSERM UMR1098, Université de Bourgogne‐Franche‐Comté, Besançon, France
Fergus Macbeth, Centre for Trials Research, Cardiff University, UK
Dr Noelle O'Rourke, Consultant and Honorary Senior Lecturer in Clinical Oncology, West of Scotland Cancer Centre, Glasgow, UK
Prof MP Revel, Université de Paris Cité, Hôpital Cochin, Service de Radiologie, APHP Centre
Marta Roqué i Figuls, Cochrane Iberoamerica, Institut de Recerca Biomèdica Sant Pau (IIB Sant Pau)
Lars Lidgard
We would like to thank François Calais, University of Franche‐Comte, Besançon, France and Giorgio Maria Agazzi for designing the search strategies.
We also thank Franck Daval, University of Franche‐Comte, Besançon, France for his help in obtaining our full texts, and thank Charles Opondo for his help in calculating the overdiagnosis data.
We would like to thank Clare Dooley and Anne Lawson for their help copy editing the review.
We would like to thank trial authors Harry de Koning, John Field, Christine Berg, and Jamie Studts for their responses to our queries.
Appendices
Appendix 1. CENTRAL search strategy
1. MeSH descriptor: [Lung Neoplasms] explode all trees 2. "Bronchopulmonary carcino*" or "Cancer of Lung*" or "Cancer of the Lung*" or "Lung adenocarcimoma*" or "Lung Cancer*" or "Lung carcinoma*" or "Lung malignan*" or "Lung Neoplasm*" or "Lung Tumo*" or "Pulmonary adenocarcinoma*" or "Pulmonary Cancer*" or "pulmonary carcino*" or "pulmonary malignan*" or "Pulmonary Neoplasm*" or "Pulmonary tumo*" 3. MeSH descriptor: [Carcinoma, Non‐Small‐Cell Lung] explode all trees 4. "Nonsmall Cell Lung Cancer*" or "Non Small Cell Lung Cancer*" or "Nonsmall Cell Lung Carcinoma*" or "Non Small Cell Lung Carcinoma*" or NSCLC 5. MeSH descriptor: [Small Cell Lung Carcinoma] explode all trees 6. "Oat Cell Carcinoma*" or "Oat Cell Lung Cancer*" or SCLC or "Small Cell Lung Cancer*" or "Small Cell Lung Carcinoma*" 7. MeSH descriptor: [Pleural Neoplasms] explode all trees 8. mpm or "Pleural cancer*" or "pleural malignan*" or "pleural mesothelioma*" or "Pleural Neoplasm*" or "pleural tumo*" 9.#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 10. MeSH descriptor: [Tomography, X‐Ray Computed] explode all trees 11. "CT Scan*" OR "Computed Tomography" OR "Computerized Tomography" OR "CT X Ray*" OR Tomodensitometry OR "CAT Scan" OR "Cine CT" OR "Electron Beam Tomography" 12. #10 OR #11 13. #9 AND #12
Appendix 2. MEDLINE search strategy
| #21 | #10 AND #20 | |
| #20 | #18 NOT #19 | |
| #19 | animals [mh] NOT humans [mh] | |
| #18 | #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 | |
| #17 | trial [ti] | |
| #16 | ly [tiab] | |
| #15 | clinical trials as topic [mesh: noexp] | |
| #14 | placebo [tiab] | |
| #13 | randomised [tiab] | |
| #12 | controlled clinical trial [pt] | |
| #11 | randomised controlled trial [pt] | |
| #10 | #6 AND #9 | |
| #9 | #7 OR #8 | |
| #8 | CT Scan*[Title/Abstract] OR Computed Tomography[Title/Abstract] OR Computerized Tomography[Title/Abstract] OR CT X Ray*[Title/Abstract] OR Tomodensitometry[Title/Abstract] OR CAT Scan[Title/Abstract] OR Cine CT[Title/Abstract] OR Electron Beam Tomography[Title/Abstract] | |
| #7 | Tomography, X‐Ray Computed[MeSH Terms] | |
| #6 | #1 OR #2 OR #3 OR #4 OR #5 | |
| #5 | Pleural Neoplasms[MeSH Terms] OR mpm[Title/Abstract] OR Pleural cancer*[Title/Abstract] OR pleural malignan*[Title/Abstract] OR pleural mesothelioma*[Title/Abstract] OR Pleural Neoplasm*[Title/Abstract] OR pleural tumo*[Title/Abstract] | |
| #4 | Small Cell Lung Carcinoma[MeSH Terms] OR Oat Cell Carcinoma*[Title/Abstract] OR Oat Cell Lung Cancer*[Title/Abstract] OR SCLC[Title/Abstract] OR Small Cell Lung Cancer*[Title/Abstract] OR Small Cell Lung Carcinoma*[Title/Abstract] | |
| #3 | Search Carcinoma, Non‐Small‐Cell Lung[MeSH Terms] OR Nonsmall Cell Lung Cancer*[Title/Abstract] OR Non Small Cell Lung Cancer*[Title/Abstract] OR Nonsmall Cell Lung Carcinoma*[Title/Abstract] OR Non Small Cell Lung Carcinoma*[Title/Abstract] OR NSCLC [Title/Abstract] | |
| #2 | Bronchopulmonary carcino*[Title/Abstract] OR Cancer of Lung*[Title/Abstract] OR Cancer of the Lung*[Title/Abstract] OR Lung adenocarcimoma*[Title/Abstract] OR Lung Cancer*[Title/Abstract] OR Lung carcinoma*[Title/Abstract] OR Lung malignan*[Title/Abstract] OR Lung Neoplasm*[Title/Abstract] OR Lung Tumo*[Title/Abstract] OR Pulmonary adenocarcinoma*[Title/Abstract] OR Pulmonary Cancer*[Title/Abstract] OR pulmonary carcino*[Title/Abstract] OR pulmonary malignan*[Title/Abstract] OR Pulmonary Neoplasm*[Title/Abstract] OR Pulmonary tumo*[Title/Abstract] | |
| #1 | Lung Neoplasms[MeSH Terms] |
Appendix 3. Embase search strategy
| #8 | #5 AND #6 AND #7 |
| #7 | 'crossover procedure'/exp OR 'double‐blind procedure'/exp OR 'randomised controlled trial'/exp OR 'single‐blind procedure'/exp OR random* OR factorial* OR crossover* OR (cross NEXT/1 over*) OR placebo* OR (doubl* NEAR/1 blind*) OR (singl* NEAR/1 blind*) OR assign* OR allocat* OR volunteer* |
| #6 | 'ct scan*':ti,ab OR 'computed tomography':ti,ab OR 'computerized tomography':ti,ab OR 'ct x ray*':ti,ab OR 'tomodensitometry':ti,ab OR 'cat scan':ti,ab OR 'cine ct':ti,ab OR 'electron beam tomography':ti,ab OR 'x‐ray computed tomography'/exp |
| #5 | #1 OR #2 OR #3 OR #4 |
| #4 | 'pleura tumour'/exp OR 'mpm':ti,ab OR 'pleural cancer*':ti,ab OR 'pleural malignan*':ti,ab OR 'pleural mesothelioma*':ti,ab OR 'pleural neoplasm*':ti,ab OR 'pleural tumo*':ti,ab |
| #3 | 'small cell lung cancer'/exp OR 'oat cell carcinoma*':ti,ab OR 'oat cell lung cancer*':ti,ab OR 'sclc':ti,ab OR 'small cell lung cancer*':ti,ab OR 'small cell lung carcinoma*':ti,ab |
| #2 | 'non small cell lung cancer'/exp OR 'nonsmall cell lung cancer*':ti,ab OR 'non small cell lung cancer*':ti,ab OR 'nonsmall cell lung carcinoma*':ti,ab OR 'non small cell lung carcinoma*':ti,ab OR 'nsclc':ti,ab |
| #1 | 'bronchopulmonary carcino*':ti,ab OR 'cancer of lung*':ti,ab OR 'cancer of the lung*':ti,ab OR 'lung adenocarcimoma*':ti,ab OR 'lung cancer*':ti,ab OR 'lung carcinoma*':ti,ab OR 'lung malignan*':ti,ab OR 'lung neoplasm*':ti,ab OR 'lung tumo*':ti,ab OR 'pulmonary adenocarcinoma*':ti,ab OR 'pulmonary cancer*':ti,ab OR 'pulmonary carcino*':ti,ab OR 'pulmonary malignan*':ti,ab OR 'pulmonary neoplasm*':ti,ab OR 'pulmonary tumo*:ti,ab' OR 'lung tumor'/exp |
Data and analyses
Comparison 1. Primary outcome: lung cancer‐related mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Lung cancer‐related mortality ‐ planned time points | 8 | 91122 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.72, 0.87] |
| 1.2 Lung cancer‐related mortality ‐ planned time points | 3 | Hazard Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 8 to 10 years | 3 | 10606 | Hazard Ratio (IV, Random, 95% CI) | 0.93 [0.72, 1.19] |
| 1.3 Lung cancer‐related mortality at different follow‐up time points (including unplanned) | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.3.1 5 to 6 years | 4 | 27263 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.64, 1.24] |
| 1.3.2 > 6 to 8 years | 3 | 73211 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.69, 0.86] |
| 1.3.3 > 8 to 10 years | 6 | 33700 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.69, 0.90] |
| 1.3.4 > 10 years | 3 | 72447 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.75, 0.98] |
| 1.4 Lung cancer‐related mortality by screening arm ‐ planned time points | 8 | 91122 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.72, 0.87] |
| 1.4.1 usual care | 7 | 37668 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.69, 0.88] |
| 1.4.2 CXR | 1 | 53454 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.70, 0.92] |
| 1.5 Lung cancer‐related mortality – by time postscreening cessation (including unplanned time points) | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.5.1 0 to 1 year | 4 | 28044 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.61, 0.94] |
| 1.5.2 2 to 4.5 years | 5 | 79063 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.72, 0.93] |
| 1.5.3 5 to 7 years | 4 | 27067 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.67, 0.90] |
| 1.5.4 > 7 to 10 years | 2 | 56658 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.83, 1.01] |
| 1.6 Lung cancer‐related mortality by screening interval ‐ planned time points | 8 | 91122 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.72, 0.87] |
| 1.6.1 annual ‐ 1 screen | 1 | 3968 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.41, 1.03] |
| 1.6.2 annual ‐ 3 screens | 1 | 53454 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.70, 0.92] |
| 1.6.3 annual ‐ 4 screens | 1 | 3206 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.48, 1.04] |
| 1.6.4 annual ‐ 5 screens | 3 | 10606 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.73, 1.18] |
| 1.6.5 annual ‐ 7 screens | 1 | 2052 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.37, 1.28] |
| 1.6.6 biennial ‐ 4 screens | 1 | 2047 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.42, 1.40] |
| 1.6.7 incremental ‐ interval 4 screens | 1 | 15789 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.62, 0.90] |
| 1.7 Lung cancer‐related mortality by sex ‐ planned time points | 3 | 9944 | Hazard Ratio (IV, Random, 95% CI) | 0.80 [0.55, 1.17] |
| 1.7.1 females | 3 | 4286 | Hazard Ratio (IV, Random, 95% CI) | 0.73 [0.34, 1.56] |
| 1.7.2 males | 2 | 5658 | Hazard Ratio (IV, Random, 95% CI) | 0.76 [0.52, 1.12] |
| 1.8 Lung cancer‐related mortality by sex ‐ planned time points | 5 | 79798 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.73, 0.89] |
| 1.8.1 females | 4 | 26965 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.59, 0.86] |
| 1.8.2 males | 5 | 52833 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.76, 0.95] |
| 1.9 Lung cancer‐related mortality by age ‐ planned time points | 1 | 56452 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.54, 0.95] |
| 1.9.1 < 65 years old | 1 | 39234 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.97] |
| 1.9.2 ≥ 65 years old | 1 | 17218 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.52, 0.74] |
| 1.10 Lung cancer related to smoking ‐ latest time point (including unplanned) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.10.1 current smokers at 6.5 years ‐ planned | 1 | 25760 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.95] |
| 1.10.2 former smokers at 6.5 years ‐ planned | 1 | 27692 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.74, 1.11] |
| 1.10.3 current smokers at 12.3 years ‐ unplanned | 1 | 25760 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.81, 0.98] |
| 1.10.4 former smokers at 12.3 years ‐ unplanned | 1 | 27692 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.88, 1.15] |
| 1.10.5 < 35 pack‐history | 1 | 2148 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.55, 2.90] |
| 1.10.6 ≥ 35 pack‐history | 1 | 1955 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.54, 1.54] |
| 1.11 Lung cancer‐related mortality by geography ‐ planned time points | 8 | 91122 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.72, 0.87] |
| 1.11.1 Europe | 7 | 37668 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.69, 0.88] |
| 1.11.2 USA | 1 | 53454 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.70, 0.92] |
| 1.12 Nodule management algorithm ‐ planned follow‐up time points | 8 | 91122 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.72, 0.87] |
| 1.12.1 yes | 6 | 35218 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.66, 0.86] |
| 1.12.2 no | 2 | 55904 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.70, 1.01] |
| 1.13 Nodule management criteria ‐ planned follow‐up time points | 8 | 91122 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.72, 0.87] |
| 1.13.1 diameter | 3 | 59110 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.72, 0.92] |
| 1.13.2 volume | 2 | 19888 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.88] |
| 1.13.3 diameter and volume | 3 | 12124 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.60, 1.04] |
Comparison 2. Primary outcome: number of non‐invasive and invasive tests ‐ all time points.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Number of invasive tests | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1.1 at baseline | 3 | 59222 | Risk Ratio (M‐H, Random, 95% CI) | 2.90 [2.25, 3.75] |
| 2.1.2 at follow‐up | 3 | 60003 | Risk Ratio (M‐H, Random, 95% CI) | 2.60 [2.41, 2.80] |
| 2.2 Non‐invasive tests | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.2.1 at baseline | 3 | 59222 | Risk Ratio (M‐H, Random, 95% CI) | 3.28 [2.40, 4.48] |
| 2.2.2 at follow‐up | 2 | 55905 | Risk Ratio (M‐H, Random, 95% CI) | 3.56 [1.81, 7.01] |
| 2.3 Number of invasive test for false positive | 4 | 63323 | Risk Ratio (M‐H, Random, 95% CI) | 3.84 [3.18, 4.64] |
| 2.3.1 at baseline | 1 | 3318 | Risk Ratio (M‐H, Random, 95% CI) | 3.09 [1.57, 6.07] |
| 2.3.2 at follow‐up | 3 | 60005 | Risk Ratio (M‐H, Random, 95% CI) | 3.91 [3.21, 4.76] |
| 2.4 Death postsurgery | 2 | 409 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.24, 1.94] |
Comparison 3. Secondary outcome: all‐cause mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 All‐cause mortality ‐ planned time points (latest time points) | 8 | 91107 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.91, 0.99] |
| 3.2 All‐cause mortality ‐ all time points (planned and unplanned) | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.2.1 5 to 6 years | 3 | 11474 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.88, 1.47] |
| 3.2.2 > 6 to 8 years | 2 | 57422 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.89, 0.99] |
| 3.2.3 > 8 to 10 years | 6 | 33685 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.91, 1.03] |
| 3.2.4 > 10 years | 2 | 56658 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.76, 1.09] |
| 3.3 All‐cause mortality ‐ planned time points | 3 | Hazard Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.3.1 any time points | 3 | Hazard Ratio (IV, Random, 95% CI) | 0.98 [0.87, 1.12] | |
| 3.4 All‐cause mortality by sex ‐ planned time points | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.4.1 females | 2 | 24514 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.76, 1.03] |
| 3.4.2 males | 3 | 49162 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.80, 1.07] |
| 3.5 Cardiovascular mortality ‐ planned and unplanned | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.5.1 8 to 10 years | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.5.2 > 10 years ‐ unplanned | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 4. Secondary outcome: lung cancer incidence.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Lung cancer incidence ‐ by different time points | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1.1 at baseline | 6 | 79900 | Risk Ratio (M‐H, Random, 95% CI) | 4.98 [2.01, 12.35] |
| 4.1.2 at year 1 | 3 | 73345 | Risk Ratio (M‐H, Random, 95% CI) | 2.12 [1.35, 3.31] |
| 4.1.3 at year 2 | 2 | 57556 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [1.51, 2.32] |
| 4.1.4 at year 3 | 1 | 4104 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.68, 4.35] |
| 4.1.5 at year 4 | 1 | 4104 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [1.05, 6.80] |
| 4.1.6 5 to 7 years | 2 | 57506 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.04, 1.23] |
| 4.1.7 > 7 years | 8 | 88528 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.02, 1.33] |
| 4.2 Lung cancer incidence ‐ by control group at ≥ 10 years | 6 | 82110 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.99, 1.34] |
| 4.2.1 usual care | 5 | 28656 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.99, 1.48] |
| 4.2.2 CXR | 1 | 53454 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.95, 1.08] |
| 4.3 Overdiagnosis at ≥ 10 years | 6 | Risk Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.3.1 usual care | 5 | 28656 | Risk Difference (IV, Random, 95% CI) | 0.18 [‐0.00, 0.36] |
| 4.3.2 CXR | 1 | 53454 | Risk Difference (IV, Random, 95% CI) | 0.01 [‐0.05, 0.07] |
Comparison 5. Secondary outcome: false positives, negatives and recalls (number of screens).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 False positive at baseline | 3 | 56101 | Risk Ratio (M‐H, Random, 95% CI) | 2.82 [1.98, 4.01] |
| 5.2 False negative | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.2.1 baseline | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.2.2 at year 1 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.2.3 at year 2 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.3 Recall rates at baseline | 2 | 55480 | Risk Ratio (M‐H, Random, 95% CI) | 5.31 [1.73, 16.34] |
Comparison 6. Secondary outcome: impact on smoking behaviour.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 stop smoking | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1.1 at 2 weeks | 1 | 1545 | Risk Ratio (M‐H, Random, 95% CI) | 2.16 [1.47, 3.18] |
| 6.1.2 at 1 year | 1 | 3124 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.88, 1.32] |
| 6.1.3 within 2 years | 1 | 1524 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.15, 1.97] |
| 6.1.4 at year 4 | 1 | 2447 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.99, 1.37] |
| 6.2 smoking relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.2.1 at 1 year | 1 | 888 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.65, 1.41] |
Comparison 7. Secondary outcome: health‐related quality of life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Anxiety ‐ at 10 months to 5 years (change over time and endpoints) | 3 | 8153 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.59, ‐0.27] |
| 7.2 Quality of life measures at different time points | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.2.1 Physical component summary of short‐form 12 (PCS) at baseline | 1 | 1288 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐1.21, 0.87] |
| 7.2.2 Physical component summary of short‐form 12 (PCS) at 2 years | 1 | 931 | Mean Difference (IV, Random, 95% CI) | 0.88 [‐0.34, 2.10] |
| 7.2.3 Mental component summary of short‐form 12 (MCS) at baseline | 1 | 1288 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐1.42, 1.30] |
| 7.2.4 Mental component summary of short‐form 12 (MCS) at 2 years | 1 | 931 | Mean Difference (IV, Random, 95% CI) | 0.81 [‐0.65, 2.27] |
| 7.2.5 EuroQol questionnaire visual analogue scale (EQ‐5D VAS) (1‐100) at baseline | 1 | 1288 | Mean Difference (IV, Random, 95% CI) | 0.69 [‐0.98, 2.36] |
| 7.2.6 EuroQol questionnaire visual analogue scale (EQ‐5D VAS) (1‐100) at 2 years | 1 | 1010 | Mean Difference (IV, Random, 95% CI) | 2.08 [0.18, 3.98] |
| 7.2.7 Spielberger State‐Trait Anxiety Inventory (STAI‐6) at baseline | 1 | 1288 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.63, 0.67] |
| 7.2.8 Spielberger State‐Trait Anxiety Inventory (STAI‐6) at 2 years | 1 | 931 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.99, 0.49] |
| 7.2.9 Impact of event scale (IES) total at baseline | 1 | 1288 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.88, 0.94] |
| 7.2.10 Anxiety ‐ at baseline | 1 | 4037 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.11, ‐0.03] |
| 7.2.11 Impact of event scale (IES) total at 2 years | 1 | 931 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐1.30, 0.68] |
| 7.2.12 Anxiety ‐ at 10‐27 months | 1 | 4037 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.57, ‐0.15] |
| 7.2.13 Anxiety (0‐18) at round 1 to 2 | 1 | 3352 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.33, 0.07] |
| 7.2.14 Anxiety (0‐18) at round 1 to 5 | 1 | 3185 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.76, ‐0.26] |
| 7.2.15 Depression ‐ at baseline | 1 | 4037 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.10, ‐0.02] |
| 7.2.16 Depression ‐ at 10‐27 months | 1 | 4037 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.40, ‐0.08] |
| 7.2.17 Behaviour (0‐21) at round 1 to 2 | 1 | 3337 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.42, 0.00] |
| 7.2.18 Behaviour (0‐21) at round 1 to 5 | 1 | 3180 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.88, ‐0.32] |
| 7.2.19 Dejection (0‐18) at round 1 to 2 | 1 | 3377 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.36, 0.06] |
| 7.2.20 Dejection (0‐18) at round 1 to 5 | 1 | 3195 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.82, ‐0.34] |
| 7.2.21 Negative impact on sleep (0‐12) round 1 to 2 | 1 | 3389 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.32, 0.04] |
| 7.2.22 Negative impact on sleep (0‐12) round 1 to 5 | 1 | 3198 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐0.95, ‐0.45] |
| 7.3 SF‐36v2: PCS by different components at baseline and at 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.3.1 Negative at baseline | 1 | 1381 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐2.09, ‐0.05] |
| 7.3.2 Negative at 6 months | 1 | 1019 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐1.38, 1.16] |
| 7.3.3 SIFs at baseline | 1 | 344 | Mean Difference (IV, Random, 95% CI) | 0.16 [‐2.45, 2.77] |
| 7.3.4 SIFs at 6 months | 1 | 226 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐4.26, 1.76] |
| 7.3.5 False positive at baseline | 1 | 1024 | Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.98, 0.54] |
| 7.3.6 False positive at 6 months | 1 | 703 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐2.42, 0.86] |
| 7.3.7 True positive at baseline | 1 | 63 | Mean Difference (IV, Random, 95% CI) | ‐1.94 [‐7.33, 3.45] |
| 7.3.8 True positive at 6 months | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐7.32, 6.92] |
| 7.4 SF‐36v2: MCS by different components at baseline and 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.4.1 Negative at baseline | 1 | 1381 | Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.97, 0.27] |
| 7.4.2 Negative at 6 months | 1 | 1019 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐1.52, 1.22] |
| 7.4.3 SIFs at baseline | 1 | 344 | Mean Difference (IV, Random, 95% CI) | 0.63 [‐1.94, 3.20] |
| 7.4.4 SIFs at 6 months | 1 | 226 | Mean Difference (IV, Random, 95% CI) | 0.73 [‐2.27, 3.73] |
| 7.4.5 False positive at baseline | 1 | 1024 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐1.43, 1.05] |
| 7.4.6 False positive at 6 months | 1 | 703 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐2.67, 0.63] |
| 7.4.7 True positive at baseline | 1 | 63 | Mean Difference (IV, Random, 95% CI) | ‐1.74 [‐6.66, 3.18] |
| 7.4.8 True positive at 6 months | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐8.19, 8.35] |
| 7.5 Anxiety by different results at 1 and 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.5.1 Negative at 1 month | 1 | 1162 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐1.79, 1.27] |
| 7.5.2 Negative at 6 months | 1 | 1019 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐1.91, 1.25] |
| 7.5.3 SIFs at 1 month | 1 | 272 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐3.52, 3.40] |
| 7.5.4 SIFs at 6 months | 1 | 226 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐4.26, 3.06] |
| 7.5.5 False positive at 1 month | 1 | 835 | Mean Difference (IV, Random, 95% CI) | 1.77 [‐0.04, 3.58] |
| 7.5.6 False positive at 6 months | 1 | 703 | Mean Difference (IV, Random, 95% CI) | 1.31 [‐0.61, 3.23] |
| 7.5.7 True positive at 1 month | 1 | 48 | Mean Difference (IV, Random, 95% CI) | 1.63 [‐6.31, 9.57] |
| 7.5.8 True positive at 6 months | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐2.69 [‐11.69, 6.31] |
Comparison 8. Secondary outcome: lung cancer by stages at different time points.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 baseline | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1.1 stage 1 (A+B) | 5 | 64092 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [1.86, 3.12] |
| 8.1.2 stage 2 (A+B) | 5 | 64092 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [0.99, 3.58] |
| 8.1.3 stage 3 (A+B) | 5 | 64092 | Risk Ratio (M‐H, Random, 95% CI) | 4.28 [1.06, 17.27] |
| 8.1.4 stage 4 | 5 | 64092 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.70, 1.55] |
| 8.1.5 SCLC ‐ limited | 1 | 4104 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.1.6 SCLC ‐ extensive | 1 | 4104 | Risk Ratio (M‐H, Random, 95% CI) | 19.00 [1.11, 326.23] |
| 8.1.7 unknown | 2 | 56773 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.31, 3.13] |
| 8.2 at 1 year | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.2.1 stage 1 (A+B) | 3 | 60877 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [1.24, 5.32] |
| 8.2.2 stage 2 (A+B) | 3 | 60877 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.68, 2.84] |
| 8.2.3 stage 3 (A+B) | 3 | 60877 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.76, 1.95] |
| 8.2.4 stage 4 | 3 | 60877 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.30, 0.77] |
| 8.2.5 SCLC ‐ limited | 1 | 4104 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.06, 15.98] |
| 8.2.6 SCLC ‐ extensive | 1 | 4104 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.12, 73.60] |
| 8.2.7 unknown | 2 | 56773 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.17, 10.75] |
| 8.3 At year 2 | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.3.1 stage 1 (A+B) | 2 | 57559 | Risk Ratio (M‐H, Random, 95% CI) | 3.53 [1.66, 7.53] |
| 8.3.2 stage 2 (A+B) | 2 | 57559 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.49, 2.37] |
| 8.3.3 stage 3 (A+B) | 2 | 57559 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.59, 1.44] |
| 8.3.4 stage 4 | 2 | 57559 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.52, 1.24] |
| 8.3.5 SCLC ‐ limited | 1 | 4104 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.3.6 SCLC ‐ extensive | 1 | 4104 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.76] |
| 8.3.7 unknown | 1 | 53455 | Risk Ratio (M‐H, Random, 95% CI) | 7.00 [0.86, 56.91] |
| 8.4 5 to < 10 years | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.4.1 stage 1 (A+B) | 4 | 13676 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [1.43, 3.57] |
| 8.4.2 stage 2 (A+B) | 4 | 13676 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.37, 1.66] |
| 8.4.3 stage 3 (A+B) | 4 | 13676 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.47, 1.49] |
| 8.4.4 4 | 4 | 13676 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.34, 0.91] |
| 8.4.5 unknown | 4 | 13676 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.41, 1.12] |
| 8.5 ≥ 10 years | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.5.1 stage 1 (A+B) | 4 | 64864 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [1.36, 4.84] |
| 8.5.2 stage 2 (A+B) | 4 | 64864 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.76, 1.17] |
| 8.5.3 stage 3 (A+B) | 4 | 64864 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.79, 1.93] |
| 8.5.4 stage 4 | 4 | 64864 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.69, 0.86] |
| 8.5.5 unknown | 3 | 60765 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.45, 0.99] |
Comparison 9. Secondary outcome: lung cancer histology at different time points.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Histology types at baseline | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1.1 SCLC | 4 | 59987 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.45, 1.57] |
| 9.1.2 squamous cell carcinoma | 4 | 59987 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.01, 2.13] |
| 9.1.3 adenocarcinoma | 4 | 59987 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [1.38, 5.71] |
| 9.1.4 bronchoalveolar carcinoma | 2 | 55904 | Risk Ratio (M‐H, Random, 95% CI) | 4.94 [2.41, 10.10] |
| 9.1.5 other | 4 | 59987 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.90, 1.94] |
| 9.2 Histology at year 1 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.2.1 SCLC | 1 | 3318 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.37, 10.89] |
| 9.2.2 squamous cell carcinoma | 1 | 3318 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.25, 2.72] |
| 9.2.3 adenocarcinoma | 1 | 3318 | Risk Ratio (M‐H, Random, 95% CI) | 2.66 [1.24, 5.71] |
| 9.2.4 other | 1 | 3318 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [0.60, 9.00] |
| 9.3 Histology at follow‐up | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.3.1 SCLC | 6 | 71281 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.74, 1.01] |
| 9.3.2 mixed SCLC + NSCLC | 1 | 4104 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.76] |
| 9.3.3 squamous cell carcinoma | 6 | 71281 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.81, 1.32] |
| 9.3.4 adenocarcinoma | 7 | 75333 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.05, 2.10] |
| 9.3.5 bronchoalveolar carcinoma | 3 | 61610 | Risk Ratio (M‐H, Random, 95% CI) | 2.73 [1.96, 3.81] |
| 9.3.6 other | 7 | 75333 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.68, 1.11] |
Comparison 10. Secondary outcome: other outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 contamination | 3 | 6902 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.32, 5.68] |
10.1. Analysis.

Comparison 10: Secondary outcome: other outcomes, Outcome 1: contamination
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aberle 2011.
| Study characteristics | ||
| Methods |
Trial design: phase 3 RCT Duration of follow‐up: median 12 years Number of study locations: 33 |
|
| Participants |
Baseline characteristics Number of participants: LDCT arm (26,723), CXR arm (26,733) Age (no. participants)
Sex: LDCT arm (male 15,770, female 10,953); CXR arm (male 15,763, female 10,970) Smoking status: LDCT arm (current 12,869, former 13,854); CXR arm (current 12,910, former 13,823) Performance status: not published Ethnicity/race: LDCT arm (white 24,289, black 1196, Asian 559, other 516, missing 163); CXR arm (white 24,260, black 1182, Asian 536, other 546, missing 209) Environmental exposures (no. participants)
Inclusion criteria
Exclusion criteria
Preintervention investigations: nil |
|
| Interventions |
Intervention characteristics Frequency of scanning: annual LDCT setting: 120 kVp to 140 kVp and 20 mAs to 60 mAs Duration of screening: 3 years Interpretation of scans
Comparison
|
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Identification |
Sponsorship source: National Cancer Institute, Cancer Imaging Program, University of Colorado Denver, Georgetown University, Pacific Heath Research and Education Institute, Henry Ford Health System, University of Minnesota, Washington University, University of Pittsburgh, University of Utah, Marshfield Clinic Research Foundation, University of Alabama at Birmingham, Westat, Information Management Services Country: USA Setting: hospital Trial start date: August 2002 Completion of follow‐up: December 2009 Trial registration number: NCT00047385 Corresponding author's name: Denise Aberle Institution: University of California at Los Angeles Email: daberle@mednet.ucla.edu |
|
| Notes |
Conflicts of interest Denise Aberle reported grants from American College of Radiology Imaging Network (ACRIN), personal fees and other from LUNGevity Foundation, other from Siemens Medical Solutions USA. Personal fees from 2012: First Annual McLennan Lecture, University of Iowa, US Department of Veteran Affairs, Gordon Gamsu Memorial Lecture, Society of Thoracic Radiology Annual Meeting, University of Texas Southwestern Medical Center Meeting, Vanderbilt‐Ingram Cancer Center Retreat on Lung Cancer,ARRS Chest Imaging Symposium, Institute of Medicine, Affordable Care for the 21st Century Workshop, California Technology Assessment Forum Policy Symposium, Stanford University, Department of Radiology Grand Rounds Series, New York University, Head to Toe Imaging Conference, Society of Thoracic Surgeons (STS) 48th Annual Meeting, 13th International Lung Cancer Congress, European Society of Thoracic Imaging 2012 Annual Meeting. Personal fees from 2013: Glendale Memorial Hospital and Health Center’s Continuing Medical Education, Colorado Radiological Society Visiting Professor Series, University of Colorado, Denver, Grand Rounds Series, 13th International Lung Cancer Congress, International Association for the Study of Lung Cancer 15th World Conference. Non‐financial support from 2012: National Cancer Advisory Board Meeting, ACRIN Semi‐Annual Meetings, Eastern Cooperative Oncology Group‐ American College of Radiology Imaging Network Strategic Retreat, Eastern Cooperative Oncology Group‐American College of Radiology Imaging Network Imaging Workshop, AACR‐IASLC 2nd Annual Joint Conference. Non‐financial support from 2013: Institute of Medicine Affordable Care Workshop, 3rd World Congress on Thoracic Imaging (WCTI), National Cancer Institute Lung SPORE Workshop, ACRIN Semi‐Annual Meetings. Personal fees from 2014: AACR‐IASLC 3rd Joint Conference. Non‐financial support from 2014: European Congress of Radiology, University of California Dose Retreat, ECOG‐ACRIN Semi‐Annual Meetings. Personal fees and non‐financial support from 2014: American Thoracic Society. Non‐financial support from 2014: DECAMP Consortium Meeting, National Cancer Institute Lung SPORE Workshop, AACR Lung Cancer Screening Meeting, LUNGevity Science Advisory Board Meeting. Personal fees from 2014: LUNGevity Award Program Review. Personal fees from 2015: LARS Midwinter Conference. Non‐financial support from 2015: ECOG‐ACRIN Semi Annual Meeting, STR Annual Meeting, Cambridge Chest Meeting, ACRIN Semi‐Annual Meetings, LUNGevity Award Program Review, Siemens Annual UCLA Research Meeting, IASLC WCLC Annual Meeting, LUNGevity Scientific Advisory Board Meeting, AACR Annual Meeting, DECAMP Consortium Meeting, NIH SPORE. Other from 2016 Veracyte Advisory Board, American Society of Clinical Oncology (ASCO) Annual Meeting. Grants from 2015: Lung Nodule Surveillance Trail (LNST) Kick off Meeting, MCL Consortium Meeting. From 2015: Veracyte. Non‐financial support from 2016: Harvard University Chest Imaging Course, Moffitt Cancer Center, Yale University Grand Rounds, Kaiser Radiology Symposium grants, MCL Consortium Meeting,ECOG‐ACRIN Semi Annual Meeting, DECAMP Consortium Meeting, National Academy of Sciences Workshop, Siemens Annual UCLA Research Meeting. Personal fees from 2016: Torrance Medical Center Grand Rounds, Veracyte Advisory Board Meeting. Personal fees and non‐financial support from 2016: International Lung Cancer Congress (ILCC) Meeting. Grants and non‐financial support from 2016: MCL Consortium Meeting. Jolean Sicks reported a grant to the institution from NCI, U10CA18‐820‐0151, during the trial. Caroline Chiles reported a grant to the institution from NIH, CA 80098, during the trial. The remaining authors had nothing to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using 6 or 8 block groups and stratified by site, sex, and 5 year age groups |
| Allocation concealment (selection bias) | Low risk | Allocation concealment generated by a central process |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Review board was blinded when assessing the cause of death. Active follow‐up was until December 2009, with subsequent cause of death established from cancer registries |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No significant loss to follow‐up < 20%. 192 of enrolled persons who where thought to be eligible at the time of randomisation were determined to be ineligible. Reasons included: CT within 18 months (n = 68), non‐smokers or quit > 15 years before randomisation (n = 23), participation in another cancer screening trial (n = 28), recent antibiotic use (n = 17), insufficient pack years (n = 12), diagnosis of cancer in the 5 years before randomisation (n = 14), age older or younger than the required range (n = 12). These randomised but ineligible participants were included in the trial and analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported, however only selected sites measured quality of life |
| Other bias | Low risk | Minimal protocol deviations |
Becker 2020.
| Study characteristics | ||
| Methods |
Trial design: phase 3 RCT Duration of follow‐up: median follow‐up 9 years Number of trial locations: 1 |
|
| Participants |
Baseline characteristics Number of participants: LDCT (2029); control (2023) Age (no. participants)
Sex: LDCT (1315 males, 714 females); control (1307 males, 716 females) Smoking status: LDCT (1259 current smokers, 770 former smokers); control (1248 current smokers, 775 former smokers)) Performance status: not reported Ethnicity/race: not reported Environmental exposures: not reported Inclusion criteria
Exclusion criteria
Preintervention investigations: nil |
|
| Interventions |
Intervention characteristics
Interpretation of scans
Comparison
|
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Identification |
Sponsorship source: German Research Foundation, Dietmar Hopp Foundation Country: Germany Setting: hospital Trial start date: Sept 2007 Completion of follow‐up: April 2018 Trial registration number: ISRCTN30604390 Corresponding author's name: Rudolf Kaaks Institution: German Cancer Research Center, Heidelberg, Germany Email: r.kaaks@dkfz‐heidelberg.de |
|
| Notes |
Conflicts of interest Claus‐Peter Heussel reported research funding, outside the present trial, from Siemens, Pfizer, MeVis Medical Solutions, Boehringer Ingelheim, lecture fees from Gilead Sciences, Essex Pharma, Schering‐Plough, AstraZeneca, Eli Lilly and Company, Roche, Merck Sharp & Dohme, Pfizer, Bracco, MEDA Pharma, InterMune, Chiesi Farmaceutici, Siemens, Covidien, Pierre Fabre, Boehringer Ingelheim, Grifols, Novartis, Basilea, and Bayer and consultation or other fees from Schering‐Plough, Pfizer, Basilea, Boehringer Ingelheim, Novartis, Roche, Astellas, Gilead, Merck Sharp & Dohme, Eli Lilly and Company, Intermune, and Fresenius and ownership of stocks from GSK. The other authors declared no potential conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using block randomisation stratified by age < 60 versus ≥ 60 years and smoking status former versus current |
| Allocation concealment (selection bias) | Low risk | Electronic randomisation using the RANDI tool |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The lung cancer death review committee was blinded to allocation arm. However, there is a risk of bias and potential for underestimation of lung cancer as the cause of death. Cases were only reviewed by the committee if lung cancer was mentioned in the case. Furthermore, method of detection of lung cancer was not uniform, with only 1 out of 85 cases of lung cancer identified by death certificate in the intervention arm and 11 of the 67 cases in the control arm. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13 participants lost to follow‐up for mortality data (5 in intervention arm, 8 in control arm) and data linkage to registries were not available in 39 participants as they declined data access. |
| Selective reporting (reporting bias) | Low risk | The trial reported on one prespecified outcome |
| Other bias | Low risk | No protocol deviations |
Blanchon 2007.
| Study characteristics | ||
| Methods |
Trial design: feasibility RCT Duration of follow‐up: not published Number of trial locations: 14 |
|
| Participants |
Baseline characteristics Number of participants: LDCT arm (385); CXR arm (380) Age: median age LDCT arm (56 years old); CXR arm (56 years old) Sex: LDCT arm (274 males, 111 females); CXR arm (267 males, 113 females) Smoking status: LDCT arm (238 current, 129 former); CXR arm (224 current, 127 former) Performance status: not published Ethnicity/race: not published Environmental exposures: not published Inclusion criteria
Exclusion criteria
Preintervention investigations
|
|
| Interventions |
Intervention characteristics
Interpretation of scans
Comparison
|
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Identification |
Sponsorship source: Programme Hospitalier de Recherche Clinique and Pneumonologie Developpement Country: France Setting: hospital Trial start date: October 2002 Completion of follow‐up: not published Trial registration number: 2526 Corresponding author's name: Thierry Blanchon Institution: Universite Pierre and Marie Curie, Paris, France Email: blanchon@u707.jussieu.fr |
|
| Notes | Conflicts of interest: Nil declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if assessors of harm outcomes were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only baseline data published. 144 participants withdrew consent post‐randomisation; authors were contacted |
| Selective reporting (reporting bias) | Unclear risk | Only baseline data published; authors were contacted |
| Other bias | Unclear risk | Only baseline data published; authors were contacted |
De Koning 2020.
| Study characteristics | ||
| Methods |
Trial design: phase 3 RCT Duration of follow‐up: 10 years Number of trial locations: 4 |
|
| Participants |
Baseline characteristics Number of participants: LDCT arm (7900); control arm (7892) Age: median age LDCT arm (58 years old); control arm (58 years old) Sex: LDCT arm (6583 males, 1317 females); control arm (6612 males, 1277 females, 3 missing) Smoking status: LDCT arm (4415 current, 3465 former, 20 missing); control arm (4333 current, 3536 former, 23 missing) Performance status: Dutch control arm only N = 7393 (1124 had excellent/very good health, 4922 had good health, 1347 had moderate/poor health). Dutch control arm only N = 7398 (3292 had high physical activity levels, 3318 had moderate physical activity levels, 788 had low physical activity levels) Ethnicity/race: not published Environmental exposures: not published Inclusion criteria
Exclusion criteria
Preintervention investigations
|
|
| Interventions |
Intervention characteristics
Interpretation of scans
Comparison
|
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Identification |
Sponsorship source: Stichting Centraal Fonds Reserves van Voormalig Vrijwillige Ziekenfondsverzekeringen (RvvZ), Siemens Germany, G. Ph. Verhagen Stichting, Rotterdam Oncologic Thoracic Steering committee (ROTS), Erasmus Trust fund, Stichting tegen Kanker, Vlaamse Liga tegen Kanker Country: The Netherlands and Belgium Setting: hospital Trial start date: August 2003 Completion of follow‐up: December 2015 Trial registration number: ISRCTN63545820 Corresponding author's name: Harry J Koning Institution: Erasmus Medical Center, University Medical Center Rotterdam, Rotterdam, The Netherlands Email: h.dekoning@erasmusmc.nl |
|
| Notes |
Conflicts of interest Carlijn van der Aalst reported receiving supplies from Siemens. Pom A. de Jong reported receiving grant support, paid to his institution, from Philips. Mathias Prokop reported receiving fees for serving on a speakers bureau from Bayer HealthCare and Bracco Imaging and grant support and fees for serving on a speakers bureau from Canon Medical Systems and Siemens. Joachim G.J.V. Aerts reported receiving consulting fees from Amphera, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, F. Hoffmann–La Roche, Merck, and Takeda Oncology, holding pending patent #PCT/NL20/19/050636 on specific inhibition of Janus kinase 3 for modulating antitumour immune responses, and holding patent #9962433 on a method for preparing an immunogenic lysate, the lysate obtained, dendritic cells loaded with such lysate, and a pharmaceutical composition comprising the lysate or the dendritic cells. Rozemarijn Vliegenthart reported receiving fees for serving on a review committee from BTG International and grant support, paid to her institution, from Siemens. Kevin ten Haaf reported receiving supplies from Siemens. Matthijs Oudkerk reported receiving lecture fees from AstraZeneca and Siemens Medical Solutions USA. No other potential conflict of interest were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Documented 1:1 randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation concealment method used, information from author |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There were some concerns about the method of determining lung cancer‐related mortality. The 2012 publication by Horeweg et al. in Lung Cancer reviewed the preliminary 50 completed medical files of Dutch participants who were deceased and had a diagnosis of lung cancer. This trial reported a reduced specificity for death certificates (62.5%) and sensitivity of 95.2% compared with a clinical expert committee. This was subsequently followed by a larger sample of 263 participant deaths and the specificity rose to 98.8% and sensitivity of 92.6%. The committee reclassified 12.2% of causes of death. However, the remaining 163 male deaths then had cause of death determined by death certificate only. The assessors were unblinded in subsequent publication. There were no significant concerns about assessment of other outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18 persons could not be linked because a digital form could not be retrieved for national linkages, > 98% coverage |
| Selective reporting (reporting bias) | Low risk | Cost analysis will be published, information from author |
| Other bias | High risk | There was an inadequate balance of males and females included in this trial. Additionally, method of assessment of primary lung cancer‐related mortality outcome was changed during the trial. The initial trial protocol planned only 4 years of screening, however an additional scan was introduced and screening extended to 6.5 years. Information provided by author clarified that the fourth round of screening was sought in 2009, after the trial had commenced. This change to screening is unlikely to have increased risk of bias. |
Field 2021.
| Study characteristics | ||
| Methods |
Trial design: pilot RCT Duration of follow‐up: median 7 years Number of trial locations: 2 |
|
| Participants |
Baseline characteristics Number of participants: LDCT arm (2028); control arm (2027) Age: median age
Sex: LDCT arm (1529 males, 499 females); control arm (1507 males, 520 females) Smoking status: LDCT arm (2 never‐smokers, 777 current, 1249 former); control arm (0 never‐smokers, 791 current, 1236 former) Performance status: not published Ethnicity/race: LDCT arm (1992 white, 18 non‐white, 18 missing data); control arm (1992 white, 19 non‐white, 16 missing data) Environmental exposures: LDCT arm: asbestos (763); control arm: asbestos (763) Inclusion criteria
Exclusion criteria
Preintervention investigations
|
|
| Interventions |
Intervention characteristics
Interpretation of scans
Comparison
|
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Identification |
Sponsorship source: NIHR Health Technology Assessment programme, NIHR policy research program, Roy Castle Lung cancer foundation, Royal Liverpool & Broadgreen University Hospital Trust (UK) Country: England Setting: hospital Trial start date: October 2011 Completion of follow‐up: February 2020 Trial registration number: ISRCTN78513845 Corresponding author's name: John Field Institution: The University of Liverpool Cancer Research Centre, Liverpool, UK Email: j.k.field@liv.ac.uk |
|
| Notes |
Conflicts of interest John K Field reported receiving fees from AstraZeneca (Speaker's Bureau) and advisory boards of Epigenomics; NUCLEIX Ltd. AstraZeneca, iDNA; Grant Support: Janssen Research & Development, LLC. Robert C Rintoul reported being on the advisory boards of AstraZeneca and Roche. David R Baldwin reported receiving speaker remuneration from AstraZeneca, Roche, MSD, BMS, Johnson and Johnson. Kate E Brain reported receiving personal fees from Astra Zeneca outside this work. Tim Eisen reported receiving research support from AstraZeneca, Bayer, Pfizer; being employed by Roche (from March 2020) and was employed by AstraZeneca (to March 2020) and having stock in AstraZeneca and Roche; was a trustee of Macmillan Cancer Support. Arjun Nair reported having current grants and contracts with BRC, DART; Honoraria Aidence BV, AstraZeneca; Support from BLF, and as the clinical lead for NTLHC. No competing interests were reported from other co‐authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using a computer‐generated random number algorithm |
| Allocation concealment (selection bias) | Low risk | Allocation concealment via UKLS database management system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary outcome lung cancer mortality. Acknowledging the limitations of determining lung cancer mortality from death registry data without blinded committee review. All‐cause mortality and lung cancer incidence were determined without knowledge of trial allocation, since these came from routine cancer registration and death certification. Interpretation of LDCT was performed by two separate radiologists (one local and one central). The central radiologist had access to the local radiologist's report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were not significant and participants lost to follow‐up had contact attempted by site primary investigator and if unsuccessful, the trial team contacted the participant's general practitioner for follow‐up information. It should be noted however, that 87 patients were excluded due to no consent for data linkage or having censoring events after consent. Authors were contacted, and censoring events were clarified as data were unavailable via national databases. |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | High risk | Minor amendments including changing nodule protocol to include new nodules detected on subsequent scans and clarification of exclusion criteria as recent CT chest. Authors reported a computer error which used LLP risk model version 2 instead of version 1. Trial reported to use intention‐to‐treat analysis, although the 87 participants were not included in long‐term mortality and cancer incidence analysis. |
Gohagan 2005.
| Study characteristics | ||
| Methods |
Trial design: feasibility RCT Duration of follow‐up: 1 year active, median 5 years with database linkage Number of trial locations: 6 |
|
| Participants |
Baseline characteristics Number of participants: LDCT arm (1660); CXR arm (1658) Age
Sex: LDCT arm (965 males, 695 females); CXR arm (978 males, 680 females) Smoking status: LDCT arm (961 current, 699 former); CXR arm (947 current, 711 former) Performance status: not published Ethnicity/race: not published Environmental exposures: not published Inclusion criteria
Exclusion criteria
Preintervention investigations
|
|
| Interventions |
Intervention characteristics
Interpretation of scans
Comparison
|
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Identification |
Sponsorship source: National Cancer Institute Country: USA Setting: University hospitals Trial start date: August 2000 Completion of follow‐up: December 2002 Trial registration number: NCT00006382 Corresponding author's name: Paul Pinksy Institution: National Cancer Institute, Bethesda, USA Email: pp4f@nih.gov |
|
| Notes |
Conflicts of interest Jennifer M Croswell reported financial relationships involving spouse (husband owns shares in Johnson & Johnson) Barnett S Kramer reported receiving money from the Journal of the National Cancer Institute. Nil other disclosures reported for other authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation stratified for age (in 5‐year categories), sex and screening centre using blocks of varying sizes. Once eligibility was established and consent was obtained by a trial centre, participants were randomly assigned to a treatment group through a single, centralized, secure, web‐based system (which generated random code) operated by the trial co‐ordinating centre. This process ensured allocation concealment for trial site investigators. Randomisation was stratified by age group (in 5‐year categories), sex, and trial centre by using variable block sizes. |
| Allocation concealment (selection bias) | Low risk | Once eligibility was established and consent was obtained by a trial centre, participants were randomly assigned to a treatment group through a single, centralised, secure, web‐based system (which generated random code) operated by the trial co‐ordinating centre. This process ensured allocation concealment for trial site investigators. Randomisation was stratified by age group (in 5‐year categories), sex, and trial centre by using variable block sizes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to any of the outcomes. Lung cancer‐related deaths were determined by death certificate during the trial, with a registry linkage performed in 2007 for long‐term follow‐up data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 91 of initial 3409 participants initially randomised to LSS (46 participants in intervention, 45 participants in control), were subsequently found to be ineligible due to participation in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO, Oken 2011). Analysis excluded this cohort. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | There was a change in definition of positive scan between T0 and T1 scans, however this was unlikely to have impacted outcomes. |
Infante 2015.
| Study characteristics | ||
| Methods |
Trial design: phase 3 RCT Duration of follow‐up: median 8.5 years Number of trial locations: 3 |
|
| Participants |
Baseline characteristics Number of participants: LDCT arm (N = 1264); control arm (N = 1186) Age: mean age for both groups 65 years old Sex: 100% male Smoking status: LDCT arm (714 active smokers); control (681 active smokers) Performance status: not reported Ethnicity/race: not reported Environmental exposures: LDCT arm (391 participants had occupational exposures including chemical industry, insulation, construction, metallurgy, agriculture, mining); control arm (402 participants had occupational exposures including chemical industry, insulation, construction, metallurgy, agriculture, mining) Inclusion criteria
Exclusion criteria
Preintervention investigations
|
|
| Interventions |
Intervention characteristics
Interpretation of scans
Comparison
|
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Identification |
Sponsorship source: Italian Association for the fight against cancer Country: Italy Setting: hospital Trial start date: March 2001 Completion of follow‐up: May 2013 Trial registration number: NCT00420862 Corresponding author's name: Maurizio Infante Institution: Instituto Clinico Humanitas, Rozzano, Milano, Italy Email: maurizio.infante@cancercenter.humanitas.it |
|
| Notes | Conflicts of interest: nil declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation of four |
| Allocation concealment (selection bias) | Low risk | Allocation stratified by centre according to computer‐generated lists supplied by the data centre |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to cause of death nor to subjects' allocation. Cause of death was determined by death certificates which were cross‐checked with available medical records. A death review panel blinded to allocation arm was only consulted when there were several competing causes of death. Only 78% of death certificates were cross‐checked, 91% of lung cancer‐related deaths and 80% of non‐pulmonary cancer‐related deaths and 76% of non‐cancer‐related deaths. Active follow‐up was terminated in February 2012, with information regarding death being obtained from registries subsequently. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Final report was revised due to discovery of 20 duplicate registrations and 2 test records (2015 AJRCC). Compliance data‐ 1223 (97%) of participants had ≥ 3 CTs, 1184 (94%) had 5 CT scans. Used intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | High risk | Inappropriate inclusion of 10 participants (kept in trial) with a history of malignancy treated < 10 years before accrual (4 with superficial bladder cancer, 2 with prostate cancer, 1 with chronic lymphocytic leukaemia, 1 with aggressive fibromatosis, 1 with renal cancer and 1 with head and neck cancer). All male participants. Unbalanced baseline between arms with more respiratory comorbidities 35.28% (intervention) versus 31.20% (control) P = 0.032. |
LaRocca 2002.
| Study characteristics | ||
| Methods |
Trial design: phase 3 RCT Duration of follow‐up: not published Number of trial locations: not published |
|
| Participants |
Baseline characteristics Number of participants: 871 participants (allocation arm not specified) Age: not published Sex: not published Smoking status: not published Performance status: not published Ethnicity/race: not published Environmental exposures: not published Inclusion criteria
Exclusion criteria
Preintervention investigations
|
|
| Interventions |
Intervention characteristics
Interpretation of scans
Comparison
|
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Identification |
Sponsorship source: Kentucky Lung Cancer Research Board, Jewish Hospital and St. Mary's Healthcare Kentucky USA Country: USA Setting: not published Trial start date: November 1991 Completion of follow‐up: not published Trial registration number: NCT00006087 Corresponding author's name: Renato V. LaRocca Institution: Kentuckiana Cancer Institute, Louisville, Kentucky, USA Email: not available |
|
| Notes | Conflicts of interest: nil reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method was not stated |
| Allocation concealment (selection bias) | Unclear risk | Allocation method concealment method was not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information regarding the blinding status of the assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information available |
| Selective reporting (reporting bias) | Unclear risk | All outcomes either not published or incomplete data |
| Other bias | Unclear risk | Limited information available; authors were contacted |
Paci 2017.
| Study characteristics | ||
| Methods |
Trial design: phase 3 randomised control trial Duration of follow‐up: median follow‐up 11 years Number of trial locations: 3 |
|
| Participants |
Baseline characteristics Number of participants: LDCT arm (1613); control arm (1593) Age
Sex: LDCT arm (1035 males, 578 females); control arm (1039 males, 554 females) Smoking status: LDCT arm (1060 current, 553 former); control arm (1019 current, 575 former) Performance status: not published Ethnicity/race: not published Environmental exposures: not published Inclusion criteria
Exclusion criteria
Preintervention investigations
|
|
| Interventions |
Intervention characteristics
Interpretation of scans
Comparison
|
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Identification |
Sponsorship source: Local government of Tuscany, Italian Ministry of Education, University and Research Country: Italy Setting: screening centres Trial start date: September 2003 Completion of follow‐up: December 2014 Trial registration number: NCT02777996 Corresponding author's name: Eugenio Paci Institution: Prevention and Research Institute, Florence, Italy Email: paci.eugenio@gmail.com |
|
| Notes | Conflicts of interest: nil reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation using software |
| Allocation concealment (selection bias) | Low risk | Central randomisation using software |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were aware of the allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All participants followed up via cancer registry of the Tuscany region for incidence and mortality. Each CT was read independently by two radiologists on a work station with a consensus reached in case of disagreement. Independent committee reviewed and revised cause of death in a blinded fashion for those cases which met their prespecified criteria following assessment of death certificate and available hospital notes. 31 deaths out of 335 deaths (9%) underwent review with the committee by December 2014. Following cessation of active follow‐up in December 2014, deaths were determined via linkages to registries. The same prespecified algorithm was used for determining cause of death, however whether any cases were reviewed by the committee was unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Moderately significant dropouts and low adherence (81% to screening adherence), however intention‐to‐treat analysis was applied. |
| Selective reporting (reporting bias) | Low risk | All outcomes published |
| Other bias | Low risk | No protocol deviations |
Pastorino 2012.
| Study characteristics | ||
| Methods |
Trial design: phase 3 RCT Duration of follow‐up: 10 years Number of trial locations: 1 |
|
| Participants |
Baseline characteristics Number of participants: biennial LDCT arm (1186); annual LDCT arm (1190); control arm (1723) Age
Sex: biennial LDCT arm (813 males, 373 females); annual LDCT arm (814 males, 376 females); control arm (1090 males, 633 females) Smoking status: biennial LDCT arm (810 current, 376 former); annual LDCT arm (820 current, 370 former); control arm (1546 current, 177 former) Performance status: not published Ethnicity/race: not published Environmental exposures: not published Inclusion criteria
Exclusion criteria
Preintervention investigations
|
|
| Interventions |
Intervention characteristics
Interpretation of scans
Comparison
|
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Identification |
Sponsorship source: Italian Ministry of Health, Italian Association for Cancer Research, Fondazione Cariplo, National Cancer Institute Country: Italy Setting: hospital Trial start date: September 2005 Completion of follow‐up: June 2018 Trial registration number: NCT02837809 Corresponding author's name: Ugo Pastorino Institution: IRCCS Istituto Nazionale dei Tumori, Milan, Italy Email: ugo.pastorino@isitutotumori.mi.it |
|
| Notes | Conflicts of interest: nil declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation via centralised stratified randomisation using blocks of variable size. Stratification based on reference centre, age (up to 65 years or older), duration of smoking (more or less than 40 years) |
| Allocation concealment (selection bias) | Low risk | Allocation concealment via centralised system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded for the primary outcome of lung cancer‐related mortality. It should be noted there was no review panel for lung cancer‐related mortality. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 216 participants withdrew from intervention arms. Cause of death was missing in 3 cases (1 annual arm, 2 biennial arm), intention‐to‐treat analysis was applied |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Initial recruitment commenced in September 2005 with only two arms, annual and biennial screwing with LDCT. 653 participants were recruited to these arms prior to approval in December 2005 and commencement of a control no‐screening arm. 90% in the control were current smokers compared with 69% in the intervention group |
Wille 2016.
| Study characteristics | ||
| Methods |
Trial design: phase 3 randomised control trial Duration of follow‐up: At least 5 years Number of trial locations: 1 |
|
| Participants |
Baseline characteristics Number of participants: LDCT arm (2052); control arm (2052) Age
Sex: LDCT arm (1147 males, 905 females); control arm (1120 males, 932 females) Smoking status: LDCT arm (1545 current, 507 former); control (1579 current, 473 former) Performance status: not published Ethnicity/race: not published Environmental exposures: not published Inclusion criteria
Exclusion criteria
Preintervention Investigations Nil |
|
| Interventions |
Intervention characteristics
Interpretation of scans
Comparison
|
|
| Outcomes |
Primary outcome
Secondary outcome
|
|
| Identification |
Sponsorship source: Danish Ministry of Interior and Health Country: Denmark Setting: University Hospital Trial start date: October 2004 Completion of follow‐up: April 2015 Trial registration number: NCT00496977 Corresponding author's name: Mathilde Wille Institution: Nordsjaellands Hospital, Denmark Email: mathilde.winkler@gmail.com |
|
| Notes | Conflicts of interest: nil disclosures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation via computer programme using a block of 10 |
| Allocation concealment (selection bias) | Low risk | Random allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was single‐blinded; local death review board blinded to allocation arm when assessing mortality, however assessors were not blinded for other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 29 participants lost to follow‐up due to emigration (15 in screening group) at first screening and 34 patients lost to follow‐up due to emigration (20 in screening group, 14 in control group) < 1% participants lost to follow‐up (34 of 4104) 2052 in each arm = 4104 in total trials |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Minimal deviations. There were minor differences in baseline characteristics of participants with a lower mean FER in the LDCT group by 0.01, although no significant difference in the FEV1. There were also more participants with > 35 pack‐year smoking history in the LDCT group compared with control (45% versus 42%). |
CT: computed tomography; CXR: chest x‐ray; FER: forced expiratory ratio; FEV1: forced expiratory volume in 1 second; LDCT: low‐dose computed tomography; NIHR: National Institute for Health and Care Excellence; RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bradley 2021 | Irrelevant intervention ‐ lung health check versus usual care |
| Brodersen 2014 | Irrelevant trial design |
| Dawson 2020 | Irrelevant trial design |
| de‐Torres 2021 | Duplicate |
| Favre 2003 | Irrelevant trial design |
| Fink 2012 | Irrelevant trial design |
| Garg 2002 | Irrelevant outcomes ‐ feasibility of conducting a RCT for lung cancer screening among subjects with varying degrees of lung cancer risk |
| Goulart 2013 | Irrelevant trial design |
| Guldbrandt 2015 | Irrelevant patient population |
| Hassannezhad 2018 | Irrelevant trial design |
| Henschke 2000 | Irrelevant trial design |
| Henschke 2002 | Irrelevant trial design |
| Henschke 2015 | Irrelevant trial design |
| Horeweg 2013 | Irrelevant trial design |
| ISRCTN42704678 | Irrelevant intervention ‐ lung health check versus usual care |
| Kramer 2011 | Irrelevant trial design |
| Kulaga 2007 | Irrelevant trial design |
| Marcus 2006 | Irrelevant intervention ‐ sputum cytology and CXR versus usual care |
| NCT02431962 | Irrelevant trial design |
| Park 2022 | Irrelevant outcome ‐ to evaluate the effects of computer‐aided diagnosis (CAD) on inter‐reader agreement in Lung Imaging Reporting and Data System (Lung‐RADS) categorisation |
| Robbins 2019 | Irrelevant trial design |
| Schabath 2019 | Irrelevant trial design |
| Schreuder 2021 | Irrelevant trial design |
| Spiro 2019 | Irrelevant intervention ‐ sputum cytology and cytometry versus usual care |
| Strauss 2012 | Irrelevant trial design |
| Strauss 2015 | Irrelevant trial design |
| Sullivan 2019 | Irrelevant intervention‐ serum biomarker versus usual care |
| Sullivan 2021 | Irrelevant intervention ‐ serum biomarker versus usual care |
| Yang 2008 | Irrelevant intervention ‐ LDCT and serum biomarker versus usual care |
| Yip 2013 | Irrelevant trial design |
CXR: chest x‐ray; LDCT: low‐dose computed tomography; RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Sagawa 2012.
| Study name | JECS study |
| Methods |
Trial design: phase 3 RCT Duration of follow‐up: not published Number of trial locations: 6 Trial registration: UMIN000005909 |
| Participants |
Baseline characteristics Number of participants: not published Age: not published Sex: not published Smoking status: not published Performance status: not published Ethnicity: not published Environmental exposures: not published Inclusion criteria
Exclusion criteria
Preintervention investigations
|
| Interventions |
Intervention characteristics
Interpretation of scans
Comparison
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | 2010 |
| Contact information | Motoyasu Sagawa sagawam@tohoku‐mpu.ac.jp |
| Notes | Conflicts of interest: nil disclosed |
Yang 2018.
| Study name | AME Thoracic Surgery Collaborative Group |
| Methods |
Trial design: phase 3 RCT Duration of follow‐up: not published Number of trial locations: 1 Trial registration: not reported |
| Participants |
Baseline characteristics Number of participants: LDCT arm (3512); control arm (3145) Age: mean age LDCT arm (60 years old); control arm (60 years old) Sex: LDCT arm (1625 males, 1887 females); control arm (1489 males, 1656 females) Smoking status: LDCT arm (729 current, 246 former, 831 passive); control arm (701 current, 202 former, 745 passive) Performance status: not published Ethnicity: not published Environmental exposures: LDCT arm (2144 cooking oil fumes, 34 asbestos, 248 dust, 57 radiation exposure); control arm (1924 cooking oil fumes, 24 asbestos, 212 dust, 47 radiation exposure) Inclusion criteria
Exclusion criteria
Preintervention investigations
|
| Interventions |
Intervention characteristics
Interpretation of scans
Comparison
|
| Outcomes |
Primary outcome
Secondary outcome
|
| Starting date | 2013 |
| Contact information | Baohui Han; xkyyhan@gmail.com |
| Notes | Conflicts of interest: nil disclosed |
CT: computed tomography; CXR: chest x‐ray; LDCT: low‐dose computed tomography; RCT: randomised controlled trial
Differences between protocol and review
The title has been changed to better reflect the purpose of the review.
Analysis: during the review process we decided to look at all outcomes using both random‐effects and fixed‐effect models to see if there was any difference given the significance of the variables. We presented both random‐ and fixed‐effects analyses only when there was a notable difference. When results were similar, we presented only the random‐effects analysis.
Subgroup analysis: we added the subgroup 'control arm intervention'.
-
In the summary of findings table:
we removed smoking outcomes and recall rates due to limited data. Instead, we included an additional harm outcome (any death postsurgery);
we replaced lung cancer incidence with overdiagnosis;
we specifically chose to present anxiety data as an example of psychosocial consequences of screening, as it had the most robust data available.
Contributions of authors
Design of the protocol: AB, RM, CM, DM, KMF, HMM, LBI, RManser
Selection of studies: AB, CM, DM, RManser
Data extraction and management: AB, RM, DM, RManser
Assessment of risk of bias: AB, RM, DM, RManser
Dealing with missing data: DM
Data analysis: AB, RM
Manuscript preparation: AB, RM, CM, DM, KMF, HMM, LBI, RManser
Sources of support
Internal sources
No sources of support provided
External sources
-
Institut National du Cancer (INCa), France
INCa n° 2017‐186
Declarations of interest
Asha Bonney has a Postgraduate Scholarship from the Australian National Health and Medical Research Council.
Reem Malouf: none known
Corynne Marchal: none known
David Manners has received speaking honoraria from Astra Zeneca and research grant funding from Curtin Medical School, Curtin University.
Kwun M Fong: Co‐investigator on the International Lung Screening Trial. This is an international, multicentre, investigator initiated study, funded in Australia by a National Health and Medical Research Grant. The study is an observational cohort study examining low‐dose screening for lung cancer in high‐risk former and current smokers. KF also undertook the QLCSS lung cancer screening trial, a pilot one‐armed study which is not eligible as it is not a RCT ‐ Queensland Smart State Grant. Chair of the Lung Cancer Consultative Group for NGO Lung Foundation Australia (no payment). KF declares occasional speaking on lung cancer at conferences and meetings where industry may be the organiser or a sponsor. KF has an Australian Medical Research Future Fund Fellowship. KF received in‐kind support with software licences for MeVis Veolity Computer Aided Diagnosis for the ILST clinical trial. KF is a reviewer for UpToDate (not related to CT screening). KF is Editor for the Cochrane Lung Cancer Group.
Henry M Marshall is an investigator on the International Lung Screen Trial. He has received honoraria to speak on the subjects of smoking cessation, lung cancer screening and COPD.
Louis B Irving: none known
Renée Manser: none known. Co‐editor, Cochrane Lung Cancer Review Group Co‐investigator on the International Lung Screening Trial. This is an international, multicentre, investigator initiated trial, funded in Australia by a National Health and Medical Research Grant. The study is an observational cohort study examining low‐dose screening for lung cancer in high‐risk former and current smokers.
New
References
References to studies included in this review
Aberle 2011 {published data only}
- Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. New England Journal of Medicine 2011;365(5):395-409. [DOI: 10.1056/NEJMoa1102873] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberle DR, Adams AM, Berg CD, Clapp JD, Clingan KL, Gareen IF, et al. Baseline characteristics of participants in the randomized National Lung Screening Trial. Journal of the National Cancer Institute 2010;102(23):1771-9. [DOI: 10.1093/jnci/djq434] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberle DR, Berg CD, Black WC, Church TR, Fagerstrom RM, Galen B, et al. The National Lung Screening Trial: overview and study design. Radiology 2011;258(1):243-53. [DOI: 10.1148/radiol.10091808] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberle DR, DeMello S, Berg CD, Black WC, Brewer B, Church TR, et al. Results of the two incidence screenings in the National Lung Screening Trial. New England Journal of Medicine 2013;369(10):920-31. [DOI: 10.1056/NEJMoa1208962] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black WC, Gareen IF, Soneji SS, Sicks JD, Keeler EB, Aberle DR, et al. Cost-effectiveness of CT screening in the National Lung Screening Trial. New England Journal of Medicine 2014;371(19):1793-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black WC. Computed tomography screening for lung cancer in the National Lung Screening Trial: a cost-effectiveness analysis. Journal of Thoracic Imaging 2015;30(2):79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church TR, Black WC, Aberle DR, Berg CD, Clingan KL, Duan F, et al. Results of initial low-dose computed tomographic screening for lung cancer. New England Journal of Medicine 2013;368(21):1980-91. [DOI: 10.1056/NEJMoa1209120] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KW, Gierada DS, Marquez G, Moore SM, Maffitt DR, Moulton JD, et al. Collecting 48,000 CT exams for the lung screening study of the National Lung Screening Trial. Journal of Digital Imaging 2009;22(6):667-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody DD, Kim HJ, Cagnon CH, Larke FJ, McNitt-Gray MM, Kruger RL, et al. Normalized CT dose index of the CT scanners used in the National Lung Screening Trial. AJR. American Journal of Roentgenology 2010;194(6):1539-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croswell JM, Baker SG, Marcus PM, Clapp JD, Kramer BS. Cumulative incidence of false-positive test results in lung cancer screening: a randomized trial. Annals of Internal Medicine 2010;152(8):505-12. [DOI: 10.7326/0003-4819-152-8-201004200-00007] [DOI] [PubMed] [Google Scholar]
- de-Torres JP, Wisnivesky JP, Bastarrika G, Wilson DO, Celli BR, Zulueta JJ. Exploring the impact of lung cancer screening on lung cancer mortality of smokers with obstructive lung disease: analysis of the NLST-ACRIN cohort. Archivos de Bronconeumologia 2021;57(1):36-41. [DOI: 10.1016/j.arbr.2020.03.022] [DOI] [PubMed] [Google Scholar]
- Dransfield MT, Washko GR, Foreman MG, Estepar RS, Reilly J, Bailey WC. Gender differences in the severity of CT emphysema in COPD. Chest 2007;132(2):464-70. [DOI: 10.1378/chest.07-0863] [DOI] [PubMed] [Google Scholar]
- Durham D, Marcus P. Cause of death among lung cancer patients in the National Lung Screening Trial: competing causes of death as a hallmark of overdiagnosis. BMJ Evidence-Based Medicine 2018;23:A23. [DOI: 10.1136/bmjebm-2018-111070.51] [DOI] [Google Scholar]
- Gareen IF, Duan F, Greco EM, Snyder BS, Boiselle PM, Park ER, et al. Impact of lung cancer screening results on participant health-related quality of life and state anxiety in the National Lung Screening Trial. Cancer 2014;120(21):3401-9. [DOI: 10.1002/cncr.28833] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatsonis CA, Aberle DR, Berg CD, Black WC, Church TR, Fagerstrom RM, et al. The National Lung Screening Trial: overview and study design. Radiology 2010;258(1):243-53. [DOI: 10.1148/radiol.10091808] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierada DS, Garg K, Nath H, Strollo DC, Fagerstrom RM, Ford MB. CT quality assurance in the lung screening study component of the National Lung Screening Trial: implications for multicenter imaging trials. AJR. American Journal of Roentgenology 2009;193(2):419-24. [DOI: 10.2214/AJR.08.1995] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierada DS, Pilgram TK, Ford M, Fagerstrom RM, Church TR, Nath H, et al. Lung cancer: interobserver agreement on interpretation of pulmonary findings at low-dose CT screening. Radiology 2008;246(1):265-72. [DOI] [PubMed] [Google Scholar]
- Gierada DS, Pinsky PF, Duan F, Garg K, Hart EM, Kazerooni EA, et al. Interval lung cancer after a negative CT screening examination: CT findings and outcomes in National Lung Screening Trial participants. European Radiology 2017;27(8):3249-56. [DOI: 10.1007/s00330-016-4705-8] [DOI] [PubMed] [Google Scholar]
- Gohagan J, Marcus P, Fagerstrom R, Pinsky P, Kramer B, Prorok P. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs chest radiograph: the Llung Screening Study of the National Cancer Institute. Chest 2004;126(1):114-21. [DOI: 10.1378/chest.126.1.114] [DOI] [PubMed] [Google Scholar]
- Hammer MM, Palazzo LL, Kong CY, Hunsaker AR. Cancer risk in subsolid nodules in the National Lung Screening Trial. Radiology 2019;293(2):441-8. [DOI: 10.1148/radiol.2019190905] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DH, Duan F, Wu Y, Goo JM, Kim HY, Patz EF Jr. Clinical significance of lung-RADS Category 3 lesions in the National Lung Screening Trial. Journal of Thoracic Oncology 2021;16(7):1118-26. [DOI: 10.1016/j.jtho.2021.02.025] [DOI] [PubMed] [Google Scholar]
- Henderson LM, Durham DD, Tammemägi MC, Benefield T, Marsh MW, Rivera MP. Lung cancer screening with low dose computed tomography in patients with and without prior history of cancer in the National Lung Screening Trial. Journal of Thoracic Oncology 2021;16(6):980-9. [DOI: 10.1016/j.jtho.2021.02.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner J, Hossain R, Jeudy J, Dako F, Mehta V, Dalal S, et al. Lung-RADS version 1.0 versus Lung-RADS version 1.1: comparison of categories using nodules from the National Lung Screening Trial. Radiology 2021;300(1):199-206. [DOI: 10.1148/radiol.2021203704] [DOI] [PubMed] [Google Scholar]
- Katki H, Kovalchik SA, Tammemagi MC, Berg C, Caporaso N, Riley T, et al. Variation in the efficacy of low-dose computed tomographic lung screening based on risk of lung cancer mortality in the National Lung Screening Trial. American Journal of Respiratory and Critical Care Medicine 2013;187:A6073. [Google Scholar]
- Katki HA, Kovalchik SA, Tammemagi M, Berg CD, Caporaso N, Riley T, et al. Variation in the efficacy of low-dose computed tomographic lung screening based on risk of lung cancer mortality in the National Lung Screening Trial. Journal of Clinical Oncology 2013;31(15):1523. [Google Scholar]
- Kruger R, Flynn MJ, Judy PF, Cagnon CH, Seibert JA. Effective dose assessment for participants in the National Lung Screening Trial undergoing posteroanterior chest radiographic examinations. American Journal of Roentgenology 2013;201(1):142-6. [DOI: 10.2214/AJR.12.9181] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labaki WW, Xia M, Murray S, Hatt CR, Al-Abcha A, Ferrera MC, et al. Quantitative emphysema on low-dose CT imaging of the chest and risk of lung cancer and airflow obstruction: an analysis of the National Lung Screening Trial. Chest 2021;159(5):1812-20. [DOI: 10.1016/j.chest.2020.12.004] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laccarino JM, Silvestri GA, Wiener RS. Patient-level trajectories and outcomes after low-dose CT screening in the National Lung Screening Trial. Chest 2019;156(5):965-71. [DOI: 10.1016/j.chest.2019.06.016] [DOI] [PubMed] [Google Scholar]
- Marcus PM, Doria-Rose VP, Gareen IF, Brewer B, Clingan K, Keating K, et al. Did death certificates and a death review process agree on lung cancer cause of death in the National Lung Screening Trial? Clinical Trials 2016;13(4):434-8. [DOI: 10.1177/1740774516638345] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S, Washko GR, Dransfield MT, Yamashiro T, San Jose Estepar R, Diaz A, et al. Quantitative CT measurement of cross-sectional area of small pulmonary vessel in COPD: correlations with emphysema and airflow limitation. Academic Radiology 2010;17(1):93-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munden RF, Chiles C, Boiselle PM, Sicks JD, Aberle DR, Gatsonis CA. Micronodules detected on computed tomography during the National Lung Screening Trial: prevalence and relation to positive studies and lung cancer. Journal of Thoracic Oncology 2019;14(9):1538-46. [DOI: 10.1016/j.jtho.2019.05.045] [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Lung Screening Trial Research Team. Lung cancer incidence and mortality with extended follow-up in the National Lung Screening Trial. Journal of Thoracic Oncology 2019;14(10):1732-42. [DOI: 10.1016/j.jtho.2019.05.044] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00047385. National Lung Screening Trial (NLST) Screening. clinicaltrials.gov/ct2/show/NCT00047385 (first received 27 January 2003).
- Nguyen XV, Davies l, Eastwood JD, Hoang JK. Extrapulmonary findings and malignancies in participants screened with chest CT in the National Lung Screening Trial. Journal of the American College of Radiology 2017;14(3):324-30. [DOI: 10.1016/j.jacr.2016.09.044] [DOI] [PubMed] [Google Scholar]
- Park ER, Ostroff JS, Rakowski W, Gareen IF, Diefenbach MA, Feibelmann S, et al. Risk perceptions among participants undergoing lung cancer screening: baseline results from the National Lung Screening Trial. Annals of Behavioral Medicine 2009;37(3):268-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz EF, Greco E, Gatsonis C, Pinsky P, Kramer BS, Aberle DR. Lung cancer incidence and mortality in National Lung Screening Trial participants who underwent low-dose CT prevalence screening: a retrospective cohort analysis of a randomised, multicentre, diagnostic screening trial. Lancet Oncology 2016;17(5):590-9. [DOI: 10.1016/S1470-2045(15)00621-X] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz EF, Pinsky P, Gatsonis C, Sicks JD, Kramer BS, Tammemägi MC, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Internal Medicine 2014;174(2):269-74. [DOI: 10.1001/jamainternmed.2013.12738] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky PF, Bellinger CR, Miller DP. False-positive screens and lung cancer risk in the National Lung Screening Trial: Implications for shared decision-making. Journal of medical screening 2018;25(2):110-2. [DOI: 10.1177/0969141317727771] [DOI] [PubMed] [Google Scholar]
- Pinsky PF, Church TR, Izmirlian G, Kramer BS. The National Lung Screening Trial: results stratified by demographics, smoking history, and lung cancer histology. Cancer 2013;119(22):3976-83. [DOI: 10.1002/cncr.28326] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky PF, Gierada DS, Hocking W, Patz EF, Kramer BS. National Lung Screening Trial findings by age: Medicare-eligible versus under-65 population. Annals of Internal Medicine 2014;161(9):627-33. [DOI: 10.7326/M14-1484] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky PF, Gierada DS, Nath PH, Kazerooni E, Amorosa J. National Lung Screening Trial: variability in nodule detection rates in chest CT studies. Radiology 2013;268(3):865-73. [DOI: 10.1148/radiol.13121530] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabath MB, Massion PP, Thompson ZJ, Eschrich SA, Balagurunathan Y, Goldof D, et al. Differences in patient outcomes of prevalence, interval, and screen-detected lung cancers in the CT arm of the National Lung Screening Trial. PloS One 2016;11(8):e0159880. [DOI: 10.1371/journal.pone.0159880] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Gierada DS, Pinsky P, Sanders C, Fineberg N, Sun Y, et al. Reader variability in identifying pulmonary nodules on chest radiographs from the National Lung Screening Trial. Journal of Thoracic Imaging 2012;27(4):249-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner NT, Gebregziabher M, Hughes Halbert C, Payne E, Egede LE, Silvestri GA. Racial differences in outcomes within the National Lung Screening Trial. Implications for widespread implementation. American Journal of Respiratory and Critical Care Medicine 2015;192(2):200-8. [DOI] [PubMed] [Google Scholar]
- Tanner NT, Kanodra NM, Gebregziabher M, Payne E, Halbert CH, Warren GW, et al. The association between smoking abstinence and mortality in the National Lung Screening Trial. American Journal of Respiratory and Critical Care Medicine 2016;193(5):534-41. [DOI: 10.1164/rccm.201507-1420OC] [DOI] [PubMed] [Google Scholar]
- Thomas A, Szabo E, Pinsky P. Screening for small cell lung cancer: analysis of the national lung cancer screening trial data. Journal of Thoracic Oncology 2015;10(9):S221. [Google Scholar]
- White CS, Dharaiya E, Dalal S, Chen R, Haramati LB. Vancouver risk calculator compared with ACR Lung-RADS in predicting malignancy: analysis of the National Lung Screening Trial. Radiology 2019;291(1):205-11. [DOI: 10.1148/radiol.2018181050] [DOI] [PubMed] [Google Scholar]
- Wong JY, Bassig BA, Seow WJ, Hu W, Ji BT, Blair A, et al. Lung cancer risk in welders and foundry workers with a history of heavy smoking in the USA: the National Lung Screening Trial. Occupational and Environmental Medicine 2017;74(6):440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Becker 2020 {published data only}
- Becker N, Motsch E, Gross ML, Eigentopf A, Heussel CP, Dienemann H, et al. Randomized study on early detection of lung cancer with MSCT in Germany: results of the first 3 years of follow-up after randomization. Journal of Thoracic Oncology 2015;10(6):890-6. [DOI: 10.1097/JTO.0000000000000530] [DOI] [PubMed] [Google Scholar]
- Becker N, Motsch E, Gross ML, Eigentopf A, Heussel CP, Dienemann H, et al. Randomized study on early detection of lung cancer with MSCT in Germany: study design and results of the first screening round. Journal of Cancer Research and Clinical Oncology 2012;138(9):1475-86. [DOI: 10.1007/s00432-012-1228-9] [DOI] [PubMed] [Google Scholar]
- Becker N, Motsch E, Gross ML, Heussel CP, Dienemann H, Schnabel P, et al. First screening round results from the German component LUSI of the European trial on the efficacy of multislice CT for the early detection of lung cancer, and the perspective of the European trial in view of the results of the US NLST trial. Journal of Cancer Research and Clinical Oncology 2012;138:6-7. [DOI: 10.1007/s00432-011-1144-4] [DOI] [PubMed] [Google Scholar]
- Becker N, Motsch E, Trotter A, Heussel CP, Dienemann H, Schnabel PA, et al. Lung cancer mortality reduction by LDCT screening – results from the randomized German LUSI trial. International Journal of Cancer 2020;146(6):1503-13. [DOI: 10.1002/ijc.32486] [DOI] [PubMed] [Google Scholar]
- González Maldonado S, Johnson T, Motsch E, Delorme S, Kaaks R. Can autoantibody tests enhance lung cancer screening? An evaluation of EarlyCDT®-Lung in context of the German Lung Cancer Screening Intervention Trial (LUSI). Translational Lung Cancer Research 2021;10(1):233-42. [DOI: 10.21037/tlcr-20-727] [DOI] [PMC free article] [PubMed] [Google Scholar]
- González Maldonado S, Motsch E, Trotter A, Kauczor HU, Heussel CP, Hermann S, et al. Overdiagnosis in lung cancer screening: estimates from the German Lung Cancer Screening Intervention Trial. International Journal of Cancer 2021;148(5):1097-105. [DOI: 10.1002/ijc.33295] [DOI] [PubMed] [Google Scholar]
- Maldonado SG, Hynes LC, Motsch E, Heussel CP, Kauczor HU, Robbins HA, et al. Validation of multivariable lung cancer risk prediction models for the personalized assignment of optimal screening frequency: a retrospective analysis of data from the German Lung Cancer Screening Intervention Trial (LUSI). Translational Lung Cancer Research 2021;10(3):1305-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blanchon 2007 {published data only}
- Blanchon T, Bréchot JM, Grenier PA, Ferretti GR, Lemarié E, Milleron B, et al. Baseline results of the Depiscan study: a French randomized pilot trial of lung cancer screening comparing low dose CT scan (LDCT) and chest X-ray (CXR). Lung Cancer 2007;58(1):50-8. [DOI: 10.1016/j.lungcan.2007.05.009] [DOI] [PubMed] [Google Scholar]
- Blanchon T, Lukasiewicz-Hajage E, Lemarié E, Milleron B, Bréchot JM, Flahault A. DEPISCAN – a pilot study to evaluate low dose spiral CT scanning as a screening method for bronchial carcinoma. Revue des Maladies Respiratoires 2002;19(6):701-5. [PubMed] [Google Scholar]
- Brechot JM, Blanchon T, Lemarie E, Moro-Sibilot D, Milleron B, Grenier P, et al. Preliminary results of a French randomised feasibility trial of lung cancer screening with multislice-spiral CT scan (MSCT) vs chest x-ray (CXR): dépiscan. Annual Congress 2005 – Screening and imaging of lung cancer 2005;26(Suppl 49):Abstract No. 4434. [Google Scholar]
De Koning 2020 {published data only}
- Baecke E, De Koning HJ, Otto SJ, Lersel CA, Klaveren RJ. Limited contamination in the Dutch-Belgian randomized lung cancer screening trial (NELSON). Lung Cancer 2010;69(1):66-70. [DOI: 10.1016/j.lungcan.2009.08.015] [DOI] [PubMed] [Google Scholar]
- Baecke E, Klaveren RJ, Looman CW, Lammers JW, Groen HJ, Weenink C, et al. Predictors of early CT-detected lung cancers in Dutch-Belgian randomized lung cancer screening trial (NELSON). Journal of Thoracic Oncology 2011;6(6):S516-7. [Google Scholar]
- De Koning H, Aalst C, Ten Haaf K, Oudkerk M. Effects of volume CT lung cancer screening: mortality results of the NELSON randomised-controlled population based trial. Journal of Thoracic Oncology 2018;13(10):S185. [DOI: 10.1016/j.jtho.2018.08.012] [DOI] [Google Scholar]
- De Koning H, Yousaf-Khan U. NELSON emerging data. Journal of Thoracic Oncology 2015;10(9):S114. [DOI] [PubMed] [Google Scholar]
- De Koning H. The Dutch-Belgian lung cancer screening trial (NELSON). Journal of Thoracic Oncology 2017;12(11):S1611. [Google Scholar]
- De Koning HJ, Aalst CM, De Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. New England Journal of Medicine 2020;382(6):503-13. [DOI: 10.1056/NEJMoa1911793] [DOI] [PubMed] [Google Scholar]
- Gietema HA, Schilham AM, Ginneken B, Klaveren RJ, Lammers JW, Prokop M. Monitoring of smoking-induced emphysema with CT in a lung cancer screening setting: detection of real increase in extent of emphysema. Radiology 2007;244(3):890-7. [DOI: 10.1148/radiol.2443061330] [DOI] [PubMed] [Google Scholar]
- Gietema HA, Zanen P, Schilham A, Ginneken B, Klaveren RJ, Prokop M, et al. Distribution of emphysema in heavy smokers: impact on pulmonary function. Respiratory Medicine 2010;104(1):76-82. [DOI: 10.1016/j.rmed.2009.08.004] [DOI] [PubMed] [Google Scholar]
- Heuvelmans M, Smoorenburg L, Walter J, Yousaf-Khan U, Aalst C, Dorrius M, et al. Lung cancer probability in new perifissural nodules detected in a lung cancer screening study. Journal of Thoracic Oncology 2018;13(10):S567. [DOI: 10.1016/j.jtho.2018.08.822] [DOI] [Google Scholar]
- Heuvelmans M, Walter J, Yousaf-Khan U, Dorrius M, Thunnissen E, Schermann A, et al. New subsolid pulmonary nodules in lung cancer screening: the NELSON Trial. Journal of Thoracic Oncology 2018;13(10):S363-4. [DOI: 10.1016/j.jtho.2018.08.334] [DOI] [PubMed] [Google Scholar]
- Heuvelmans MA, Walter JE, Peters RB, Bock GH, Yousaf-Khan U, Aalst CM, et al. Relationship between nodule count and lung cancer probability in baseline CT lung cancer screening: the NELSON study. Lung Cancer 2017;113:45-50. [DOI: 10.1016/j.lungcan.2017.08.023] [DOI] [PubMed] [Google Scholar]
- Horeweg N, Scholten ET, Jong PA, Aalst CM, Weenink C, Lammers JW, et al. Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncology 2014;15(12):1342-50. [DOI: 10.1016/S1470-2045(14)70387-0] [DOI] [PubMed] [Google Scholar]
- Horeweg N, Aalst CM, Thunnissen E, Nackaerts K, Weenink C, Groen HJ, et al. Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. American Journal of Respiratory and Critical Care Medicine 2013;187(8):848-54. [DOI: 10.1164/rccm.201209-1651OC] [DOI] [PubMed] [Google Scholar]
- Horeweg N, Aalst CM, Vliegenthart R, Zhao Y, Xie X, Scholten ET, et al. Volumetric computed tomography screening for lung cancer: three rounds of the NELSON trial. European Respiratory Journal 2013;42(6):1659-67. [DOI: 10.1183/09031936.00197712] [DOI] [PubMed] [Google Scholar]
- Horeweg N, Klaveren RJ, Groen HJ, Lammers JW, Weenink C, Nackaerts K, et al. Blinded and uniform cause of death verification in a lung cancer CT screening trial. Lung Cancer 2012;77(3):522-5. [DOI] [PubMed] [Google Scholar]
- Horeweg N, Rosmalen J, Heuvelmans MA, Aalst CM, Vliegenthart R, Scholten ET, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncology 2014;15(12):1332-41. [DOI: 10.1016/S1470-2045(14)70389-4] [DOI] [PubMed] [Google Scholar]
- Oudkerk M, Heuvelmans MA. Screening for lung cancer by imaging: the NELSON study. Journal of the Belgian Society of Radiology 2013;96(3):163-6. [DOI: 10.5334/jbr-btr.240] [DOI] [PubMed] [Google Scholar]
- Ru Zhao Y, Xie X, Koning HJ, Mali WP, Vliegenthart R, Oudkerk M. NELSON lung cancer screening study. Cancer Imaging 2011;11(Spec No A):S79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trial NL580 (NTR636). www.trialregister.nl/trial/580 2006.
- Van't Westeinde SC, Horeweg N, De Leyn P, Groen HJ, Lammers JW, Weenink C, et al. Complications following lung surgery in the Dutch-Belgian randomized lung cancer screening trial. European Journal of Cardio-thoracic Surgery 2012;42(3):420-9. [DOI: 10.1093/ejcts/ezs081] [DOI] [PubMed] [Google Scholar]
- den Bergh KA, Essink-Bot ML, Borsboom GJ, Scholten ET, Prokop M, Koning HJ, et al. Short-term health-related quality of life consequences in a lung cancer CT screening trial (NELSON). British Journal of Cancer 2010;102(1):27-34. [DOI: 10.1038/sj.bjc.6605459] [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Bergh KA, Essink-Bot ML, Borsboom GJ, Scholten ET, Klaveren RJ, De Koning HJ. Long-term effects of lung cancer computed tomography screening on health-related quality of life: the NELSON trial. European Respiratory Journal 2011;38(1):154-61. [DOI: 10.1183/09031936.00123410] [DOI] [PubMed] [Google Scholar]
- den Bergh KA, Essink-Bot ML, Bunge EM, Scholten ET, Prokop M, Iersel CA, et al. Impact of computed tomography screening for lung cancer on participants in a randomized controlled trial (NELSON trial). Cancer 2008;113(2):396-404. [DOI: 10.1002/cncr.23590] [DOI] [PubMed] [Google Scholar]
- Aalst CM, Klaveren RJ, den Bergh KA, Willemsen MC, De Koning HJ. The impact of a lung cancer computed tomography screening result on smoking abstinence. European Respiratory Journal 2011;37(6):1466-73. [DOI: 10.1183/09031936.00035410] [DOI] [PubMed] [Google Scholar]
- de Wiel JC, Wang Y, Xu DM, Zaag-Loonen HJ, Jagt EJ, Klaveren RJ, et al. Neglectable benefit of searching for incidental findings in the Dutch-Belgian lung cancer screening trial (NELSON) using low-dose multidetector CT. European Radiology 2007;17(6):1474-82. [DOI: 10.1007/s00330-006-0532-7] [DOI] [PubMed] [Google Scholar]
- Iersel CA, Koning HJ, Draisma G, Mali WP, Scholten ET, Nackaert K, et al. Risk-based selection from the general population in a screening trial: Selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON). International Journal of Cancer 2007;120(4):868-74. [DOI: 10.1002/ijc.22134] [DOI] [PubMed] [Google Scholar]
- Klaveren RJ, Oudkerk M, Prokop M, Scholten ET, Nackaerts K, Vernhout R, et al. Management of lung nodules detected by volume CT scanning. New England Journal of Medicine 2009;361(23):2221-9. [DOI: 10.1056/NEJMoa0906085] [DOI] [PubMed] [Google Scholar]
- Walter J, Heuvelmans M, Vliegenthart R, Ooijen P, De Koning H, Oudkerk M. Management of non resolving new solid nodules after initial detection in incidence rounds of CT lung cancer screening. Journal of Thoracic Oncology 2017;12(11):S1786. [Google Scholar]
- Walter J, Heuvelmans M, Vliegenthart R, Ooijen P, De Koning H, Oudkerk M. Direct comparison of new solid nodules detected in women and men during incidence screening rounds of the NELSON trial. Journal of Thoracic Oncology 2018;13(10):S779-80. [DOI: 10.1016/j.jtho.2018.08.1348] [DOI] [Google Scholar]
- Walter J, Heuvelmans M, Vliegenthart R, Ooijen P, De Koning H, Oudkerk M. Impact of screening interval length on new nodules detected in incidence rounds of CT lung cancer screening: the NELSON trial. Journal of Thoracic Oncology 2018;13(10):S788. [DOI: 10.1016/j.jtho.2018.08.1371] [DOI] [Google Scholar]
- Walter JE, Heuvelmans MA, Jong PA, Vliegenthart R, Ooijen PM, Peters RB, et al. Occurrence and lung cancer probability of new solid nodules at incidence screening with low-dose CT: analysis of data from the randomised, controlled NELSON trial. Lancet Oncology 2016;17(7):907-16. [DOI: 10.1016/S1470-2045(16)30069-9] [DOI] [PubMed] [Google Scholar]
- Xu DM, Gietema H, Koning H, Vernhout R, Nackaerts K, Prokop M, et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer 2006;54(2):177-84. [DOI: 10.1016/j.lungcan.2006.08.006] [DOI] [PubMed] [Google Scholar]
- Yousaf-Khan U, Horeweg N, Aalst C, Ten Haaf K, Oudkerk M, Koning H. Baseline characteristics and mortality outcomes of control group participants and eligible non-responders in the NELSON lung cancer screening study. Journal of Thoracic Oncology 2015;10(5):747-53. [DOI: 10.1097/JTO.0000000000000488] [DOI] [PubMed] [Google Scholar]
- Yousaf-Khan U, Aalst C, De Jong PA, Heuvelmans M, Scholten E, Lammers JW, et al. Final screening round of the NELSON lung cancer screening trial: the effect of a 2.5-year screening interval. Thorax 2017;72(1):48-56. [DOI: 10.1136/thoraxjnl-2016-208655] [DOI] [PubMed] [Google Scholar]
- Yousaf-Khan U, Aalst C, Jong PA, Heuvelmans M, Scholten E, Walter J, et al. Risk stratification based on screening history: the NELSON lung cancer screening study. Thorax 2017;72(9):819-24. [DOI: 10.1136/thoraxjnl-2016-209892] [DOI] [PubMed] [Google Scholar]
- Zhao YR, Xie X, De Koning HJ, Mali WP, Vliegenthart R, Oudkerk M. NELSON lung cancer screening study. Cancer Imaging 2011;11(SPEC. ISS. A):S79-S84. [DOI: 10.1102/1470-7330.2011.9020] [DOI] [PMC free article] [PubMed] [Google Scholar]
Field 2021 {published data only}
- Ali N, Lifford KJ, Carter B, McRonald F, Yadegarfar G, Baldwin DR, et al. Barriers to uptake among high-risk individuals declining participation in lung cancer screening: a mixed methods analysis of the UK lung cancer screening (UKLS) trial. BMJ Open 2015;5(7):e008254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin DR, Duffy SW, Wald NJ, Page R, Hansell DM, Field JK. UK Lung Screen (UKLS) nodule management protocol: modelling of a single screen randomised controlled trial of low-dose CT screening for lung cancer. Thorax 2011;66(4):308-13. [DOI: 10.1136/thx.2010.152066] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain K, Carter B, Lifford KJ, Burke O, Devaraj A, Baldwin DR, et al. Impact of low-dose CT screening on smoking cessation among high-risk participants in the UK lung cancer screening trial. Thorax 2017;72(10):912-8. [DOI: 10.1136/thoraxjnl-2016-209690] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain K, Lifford KJ, Carter B, Burke O, McRonald F, Devaraj A, et al. Long-term psychosocial outcomes of low-dose CT screening: results of the UK lung cancer screening randomised controlled trial. Thorax 2016;71(11):996-1005. [DOI: 10.1136/thoraxjnl-2016-208283] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn CE, Edwards A, Carter B, Field JK, Brain K, Lifford KJ. The role of screening expectations in modifying short-term psychological responses to low-dose computed tomography lung cancer screening among high-risk individuals. Patient Education and Counseling 2017;100(8):1572-9. [DOI: 10.1016/j.pec.2017.02.024] [DOI] [PubMed] [Google Scholar]
- Field JK, Baldwin D, Devaraj A, Brain K, Eisen T, Holemans J, et al. United Kingdom lung cancer screening trial (UKLS): first 88897 approaches. Cancer Research 2013;73:8. [DOI: 10.1158/1538-7445.AM2013-3631] [DOI] [Google Scholar]
- Field JK, Baldwin D, Stephen DW, Devaraj A, Hansell DM. UKLS – United Kingdom Lung Cancer Screening Trial. Cancer Research 2012;72(8 Suppl):3572. [DOI: 10.1158/1538-7445.AM2012-3571] [DOI] [Google Scholar]
- Field JK, Devaraj A, Baldwin DR, Holemans J, Screaton N, Ledson M, et al. UK Lung Cancer Screening Trial (UKLS): baseline data. Journal of Thoracic Oncology 2013;8:S685. [DOI: 10.1097/01.JTO.0000438438.14562.c8] [DOI] [Google Scholar]
- Field JK, Duffy S, Baldwin D, Brain K, Eisen T, Hands CJ, et al. United Kingdom Lung Cancer Screening (UKLS) trial. Journal of Thoracic Oncology 2011;6(6):S1382-3. [Google Scholar]
- Field JK, Duffy SW, Baldwin DR, Brain KE, Devaraj A, Eisen T, et al. The UK Lung Cancer Screening Trial: a pilot randomised controlled trial of low-dose computed tomography screening for the early detection of lung cancer. Health Technology Assessment 2016;20(40):1-146. [DOI: 10.3310/hta20400] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field JK, Duffy SW, Baldwin DR, Whynes DK, Devaraj A, Brain KE, et al. UK lung cancer RCT pilot screening trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016;71(2):161-70. [DOI: 10.1136/thoraxjnl-2015-207140] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field JK, Vulkan D, Davies MP, Baldwin DR, Brain KE, Devaraj A, et al. Lung cancer mortality reduction by LDCT screening: UKLS randomised trial results and international meta-analysis. Lancet Regional Health – Europe 2021;10:100179. [DOI: 10.1016/j.lanepe.2021.100179] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, Komrower D, Murthy M, Hunt N, Holemans J, Field J, et al. Experience with suspected cancer referrals from the UK lung screen trial. Thorax 2013;68:A57-8. [DOI: 10.1136/thoraxjnl-2013-204457.117] [DOI] [Google Scholar]
- Marcus MW, Duffy SW, Devaraj A, Green BA, Oudkerk M, Baldwin D, et al. Probability of cancer in lung nodules using sequential volumetric screening up to 12 months: the UKLS trial. Thorax 2019;74(8):761-7. [DOI: 10.1136/thoraxjnl-2018-212263] [DOI] [PubMed] [Google Scholar]
Gohagan 2005 {published data only}
- Croswell JM, Baker SG, Marcus PM, Clapp JD, Kramer BS. Cumulative risk for a false-positive test using low-dose computed tomography in lung cancer screening. Journal of Clinical Oncology 2009;27(18):CRA1502. [Google Scholar]
- Doroudi M, Pinsky PF, Marcus PM. Lung cancer mortality in the Lung Screening Study feasibility trial. JNCI Cancer Spectrum 2018;2(3):pky042. [DOI: 10.1093/jncics/pky042] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohagan J, Marcus P, Fagerstrom R, Pinsky P, Kramer B, Prorok P, Writing Committee, Lung Screening Study Research Group. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs chest radiograph: the Lung Screening Study of the National Cancer Institute. Chest 2004;126(1):114-21. [DOI] [PubMed] [Google Scholar]
- Gohagan JK, Marcus PM, Fagerstrom RM, Pinsky PF, Kramer BS, Prorok PC, et al. Final results of the lung screening study, a randomized feasibility study of spiral CT versus chest X-ray screening for lung cancer. Lung Cancer 2005;47(1):9-15. [DOI: 10.1016/j.lungcan.2004.06.007] [DOI] [PubMed] [Google Scholar]
- NCT00006382. Lung Screening Study. clinicaltrials.gov/ct2/show/NCT00006382 (first received 9 June 2004).
Infante 2015 {published data only}
- Infante M, Cavuto S, Lutman FR, Brambilla G, Chiesa G, Ceresoli G, et al. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. American Journal of Respiratory and Critical Care Medicine 2009;180(5):445-53. [DOI: 10.1164/rccm.200901-0076OC] [DOI] [PubMed] [Google Scholar]
- Infante M, Cavuto S, Lutman FR, Passera E, Chiarenza M, Chiesa G, et al. Long-term follow-up results of the DANTE trial, a randomized study of lung cancer screening with spiral computed tomography. American Journal of Respiratory and Critical Care Medicine 2015;191(10):1166-75. [DOI: 10.1164/rccm.201408-1475OC] [DOI] [PubMed] [Google Scholar]
- Infante M, Lutman FR, Cavuto S, Brambilla G, Chiesa G, Passera E, et al. Lung cancer screening with spiral CT. Baseline results of the randomized DANTE trial. Lung Cancer 2008;59(3):355-63. [DOI: 10.1016/j.lungcan.2007.08.040] [DOI] [PubMed] [Google Scholar]
- Lopci E, Morenghi E, Tanzi D, Chiti A, Infante M. Cost-effectiveness of second-line diagnostic investigations in patients with suspicious lung nodules included in DANTE trial. Journal of Nuclear Medicine 2017;58(Suppl 1):1045. [Google Scholar]
- NCT00420862. The DANTE trial. A randomized study on lung cancer screening with low-dose spiral computed tomography. clinicaltrials.gov/ct2/show/NCT00420862 (first received 11 January 2007).
LaRocca 2002 {published data only}
- LaRocca RV, Falk R, Cerrito P, Lord R, Goldman S. Early results of a randomized prospective screening trial of annual low-dose spiral chest computed tomography scanning versus annual chest radiography of Kentucky patients at increased risk for lung cancer: Jewish Hospital Lung Cancer Screening. Proceedings of the American Society of Clinical Oncology 2002;21(Pt 1):306a, Abstract 1220. [Google Scholar]
- NCT00006087. Chest X-ray or chest CT scan in patients at high risk of developing lung cancer. clinicaltrials.gov/ct2/show/NCT00006087 (first received 23 December 2003).
- Studts JL, Ghate SR, Gill JL, Studts CR, Barnes CN, LaJoie AS, et al. Validity of self-reported smoking status among participants in a lung cancer screening trial. Cancer Epidemiology, Biomarkers & Prevention 2006;15(10):1825-8. [DOI: 10.1158/1055-9965] [DOI] [PubMed] [Google Scholar]
Paci 2017 {published data only}
- Carozzi FM, Bisanzi S, Carrozzi L, Falaschi F, Lopes Pegna A, Mascalchi M, et al. Multimodal lung cancer screening using the ITALUNG biomarker panel and low dose computed tomography. Results of the ITALUNG biomarker study. International Journal of Cancer 2017;141(1):94-101. [DOI: 10.1002/ijc.30727] [DOI] [PubMed] [Google Scholar]
- Carozzi FM, Bisanzi S, Pegna AL, Giusti F, Carrozzi L, Mascalchi M, et al. The contribution of biomarkers to assess the level of individual risk in the selection of high prevalence subjects for CT scan lung cancer screening. Journal of Thoracic Oncology 2011;6(6):S988-9. [Google Scholar]
- Conti B, Aquilini F, Pistelli F, De Santis M, Tavanti L, Cini S. Lung function in a group of smokers or ex smokers enrolled in a randomized controlled trial (RCT) with low-dose computed tomography (CT) for lung cancer screening (ITALUNG-CT study). European Respiratory Journal 2010;36(Suppl 54):2064. [Google Scholar]
- Lopes Pegna A, Picozzi G, Mascalchi M, Maria Carozzi F, Carrozzi L, Comin C, et al. Design, recruitment and baseline results of the ITALUNG trial for lung cancer screening with low-dose CT. Lung Cancer 2009;64(1):34-40. [DOI: 10.1016/j.lungcan.2008.07.003] [DOI] [PubMed] [Google Scholar]
- Mascalchi M, Belli G, Zappa M, Picozzi G, Falchini M, Della Nave R, et al. Risk-benefit analysis of X-ray exposure associated with lung cancer screening in the ITALUNG-CT trial. AJR. American Journal of Roentgenology 2006;187(2):421-9. [DOI: 10.2214/AJR.05.0088] [DOI] [PubMed] [Google Scholar]
- Mascalchi M, Mazzoni LN, Falchini M, Belli G, Picozzi G, Merlini V, et al. Dose exposure in the ITALUNG trial of lung cancer screening with low-dose CT. British Journal of Radiology 2012;85(1016):1134-9. [DOI: 10.1259/bjr/20711289] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascalchi M, Picozzi G, Falchini M, Vella A, Diciotti S, Carrozzi L, et al. Initial LDCT appearance of incident lung cancers in the ITALUNG trial. European Journal of Radiology 2014;83(11):2080-6. [DOI: 10.1016/j.ejrad.2014.07.019] [DOI] [PubMed] [Google Scholar]
- NCT02777996. Italian Lung Cancer Screening Trial (ITALUNG). clinicaltrials.gov/ct2/show/NCT02777996 (first received 19 May 2016).
- Paci E, Carrozzi L, Mascalchi M, Falaschi F, Giusti F, Picozzi G, et al. The Italung randomised trial: Results of the screening rounds and perspectives. Journal of Thoracic Oncology 2011;6(6):S131-2. [Google Scholar]
- Paci E, Puliti D, Carozzi FM, Carrozzi L, Falaschi F, Pegna AL, et al. Prognostic selection and long-term survival analysis to assess overdiagnosis risk in lung cancer screening randomized trials. Journal of Medical Screening 2021;28(1):39-47. [DOI: 10.1177/0969141320923030] [DOI] [PubMed] [Google Scholar]
- Paci E, Puliti D, Lopes Pegna A, Carrozzi L, Picozzi G, Falaschi F, et al. Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax 2017;72(9):825-31. [DOI: 10.1136/thoraxjnl-2016-209825] [DOI] [PubMed] [Google Scholar]
- Paci E, Puliti D, Pegna AL, Carrozzi L, Picozzi G, Falaschi F, et al. Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial (Italy). Journal of Thoracic Oncology 2017;12(1):S346-7. [DOI] [PubMed] [Google Scholar]
- Pegna AL, Picozzi G, Falaschi F, Carrozzi L, Falchini M, Carozzi FM, et al. Four-year results of low-dose CT screening and nodule management in the ITALUNG trial. Journal of Thoracic Oncology 2013;8(7):866-75. [DOI: 10.1097/JTO.0b013e31828f68d6] [DOI] [PubMed] [Google Scholar]
- Picozzi G, Mascalchi M, Falaschi F, Paci E. Initial appearance of LDCT screen-detected lung cancers in the ITALUNG trial. Journal of Thoracic Imaging 2014;29(3):W26. [DOI: 10.1097/RTI.0000000000000089] [DOI] [Google Scholar]
- Picozzi G, Paci E, Lopes Pegna A, Bartolucci M, Roselli G, De Francisci A, et al. Screening of lung cancer with low dose spiral CT: results of a three year pilot study and design of the randomised controlled trial "Italung-CT". Radiologia Medica 2005;109(1-2):17-26. [PubMed] [Google Scholar]
- Pistelli F, Aquilini F, Tavanti L, Cini S, Conti B, Falaschi F, et al. Predictors of smoking cessation within a lung cancer CT screening trial. European Respiratory Journal 2011;38(Suppl 15):4248. [Google Scholar]
- Puliti D, Mascalchi M, Carozzi FM, Carrozzi L, Falaschi F, Paci E, et al. Decreased cardiovascular mortality in the ITALUNG lung cancer screening trial: analysis of underlying factors. Lung Cancer 2019;138:72-8. [DOI: 10.1016/j.lungcan.2019.10.006] [DOI] [PubMed] [Google Scholar]
Pastorino 2012 {published data only}
- Boeri M, Pastorino U, Sozzi G. Role of microRNAs in lung cancer: microRNA signatures in cancer prognosis. Cancer Journal 2012;18(3):268-74. [EMBASE: 10.1097/PPO.0b013e318258b743] [DOI] [PubMed] [Google Scholar]
- Boeri M, Sestini S, Fortunato O, Verri C, Suatoni P, Pastorino U, et al. Recent advances of microRNA-based molecular diagnostics to reduce false-positive lung cancer imaging. Expert Review of Molecular Diagnostics 2015;15(6):801-13. [DOI: 10.1586/14737159.2015.1041377] [DOI] [PubMed] [Google Scholar]
- Boeri M, Verri C, Conte D, Roz L, Modena P, Facchinetti F, et al. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proceedings of the National Academy of Sciences of the United States of America 2011;108(9):3713-8. [DOI: 10.1073/pnas.1100048108] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortunato O, Boeri M, Verri C, Conte D, Mensah M, Suatoni P, et al. Assessment of circulating microRNAs in plasma of lung cancer patients. Molecules 2014;19(3):3038-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Boeri M, Sozzi G, Liu D, Marchianò A, Roz L, et al. Gene signatures stratify computed tomography screening detected lung cancer in high-risk populations. EBioMedicine 2015;2(8):831-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchianò A, Calabrò E, Civelli E, Di Tolla G, Frigerio LF, Morosi C, et al. Pulmonary nodules: volume repeatability at multidetector CT lung cancer screening. Radiology 2009;251(3):919-25. [DOI] [PubMed] [Google Scholar]
- Pastorino U, Boffi R, Marchianò A, Sestini S, Munarini E, Calareso G, et al. Stopping smoking reduces mortality in low-dose computed tomography screening participants. Journal of Thoracic Oncology 2016;11(5):693-9. [DOI] [PubMed] [Google Scholar]
- Pastorino U, Marchianò A, Sverzellati N, Leo F, Fabbri A, Morosi C, et al. A less intensive screening modality, such as CT every 2 years instead of annual CT, is not harmful for heavy smokers. Journal of Thoracic Oncology 2011;6(6):S518. [Google Scholar]
- Pastorino U, Rossi M, Rosato V, Marchianò A, Sverzellati N, Morosi C, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. European Journal of Cancer Prevention 2012;21(3):308-15. [DOI: 10.1097/CEJ.0b013e328351e1b6] [DOI] [PubMed] [Google Scholar]
- Pastorino U, Sabia F, Sestini S, Silva M, Boeri M, Cantarutti A, et al. Prolonged low-dose computed tomography (LDCT) screening beyond 5 years reduces overall and lung cancer specific mortality. Journal of Thoracic Oncology 2018;13(10):S363. [DOI: 10.1016/j.jtho.2018.08.333] [DOI] [Google Scholar]
- Pastorino U, Silva M, Sestini S, Sabia F, Boeri M, Cantarutti A, et al. Prolonged lung cancer screening reduced 10-year mortality in the MILD trial: new confirmation of lung cancer screening efficacy. Annals of Oncology 2019;30(7):1162-9. [DOI: 10.1093/annonc/mdz117] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino U, Sverzellati N, Sestini S, Silva M, Sabia F, Boeri M, et al. Ten-year results of the multicentric Italian lung detection trial demonstrate the safety and efficacy of biennial lung cancer screening. European Journal of Cancer 2019;118:142-8. [DOI: 10.1016/j.ejca.2019.06.009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roz L, Verri C, Conte D, Miceli R, Mariani L, Calabro E, et al. Plasma DNA levels in spiral CT-detected and clinically detected lung cancer patients: a validation analysis. Lung Cancer 2009;66(2):270-1. [DOI] [PubMed] [Google Scholar]
- Sestini S, Boeri M, Marchiano A, Pelosi G, Galeone C, Verri C, et al. Correction: circulating microRNA signature as liquid-biopsy to monitor lung cancer in low-dose computed tomography screening. Oncotarget 2019;10(57):6043. [DOI: 10.18632/oncotarget.27256] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M, Galeone C, Sverzellati N, Marchianò A, Calareso G, Sestini S, et al. Screening with low-dose computed tomography does not improve survival of small cell lung cancer. Journal of Thoracic Oncology 2016;11(2):187-93. [DOI] [PubMed] [Google Scholar]
- Silva M, Milanese G, Sestini S, Sabia F, Jacobs C, Ginneken B, et al. Lung cancer screening by nodule volume in Lung-RADS v1.1: negative baseline CT yields potential for increased screening interval. European Radiology 2021;31(4):1956-68. [DOI: 10.1007/s00330-020-07275-w] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M, Sverzellati N, Colombi D, Milanese G, La Vecchia C, Galeone C, et al. Pleural plaques in lung cancer screening by low-dose computed tomography: prevalence, association with lung cancer and mortality. BMC Pulmonary Medicine 2017;17(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozzi G, Boeri M, Rossi M, Verri C, Suatoni P, Bravi F, et al. Clinical utility of a plasma-based miRNA signature classifier within computed tomography lung cancer screening: a correlative MILD trial study. Journal of Clinical Oncology 2014;32(8):768-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozzi G, Roz L, Conte D, Mariani L, Andriani F, Lo Vullo S, et al. Plasma DNA quantification in lung cancer computed tomography screening: five-year results of a prospective study. American Journal of Respiratory and Critical Care Medicine 2009;179(1):69-74. [DOI: 10.1164/rccm.200807-1068OC] [DOI] [PubMed] [Google Scholar]
- Sverzellati N, Ingegnoli A, Calabrò E, Randi G, La Vecchia C, Marchianò A, et al. Bronchial diverticula in smokers on thin-section CT. European Radiology 2010;20(1):88-94. [DOI] [PubMed] [Google Scholar]
- Sverzellati N, Silva M, Calareso G, Galeone C, Marchianò A, Sestini S, et al. Low-dose computed tomography for lung cancer screening: comparison of performance between annual and biennial screen. European Radiology 2016;26(11):3821-9. [DOI: 10.1007/s00330-016-4228-3] [DOI] [PubMed] [Google Scholar]
Wille 2016 {published data only}
- Ashraf H, Saghir Z, Dirksen A, Pedersen JH, Thomsen LH, Døssing M, et al. Smoking habits in the randomised Danish Lung Cancer Screening Trial with low-dose CT: final results after a 5-year screening programme. Thorax 2014;69(6):574-9. [DOI: 10.1136/thoraxjnl-2013-203849] [DOI] [PubMed] [Google Scholar]
- Ashraf H, Saghir Z, Thomsen LH, Dirksen A, Dossing M, Pedersen JH, et al. Smoking habits in the Danish Lung Cancer Screening Trial (DLCST): final results after 5 year screening program. American Journal of Respiratory and Critical Care Medicine 2012;185:4. [Google Scholar]
- Ashraf H, Tønnesen P, Holst Pedersen J, Dirksen A, Thorsen H, Døssing M. Effect of CT screening on smoking habits at 1-year follow-up in the Danish Lung Cancer Screening Trial (DLCST). Thorax 2009;64(5):388-92. [DOI: 10.1136/thx.2008.102475] [DOI] [PubMed] [Google Scholar]
- Heleno B, Siersma V, Brodersen J. Estimation of overdiagnosis of lung cancer in low-dose computed tomography screening: a secondary analysis of the Danish Lung Cancer Screening Trial. JAMA Internal Medicine 2018;178(10):1420-2. [DOI: 10.1001/jamainternmed.2018.3056] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD, Siersma V, Rasmussen JF, Brodersen J. Direct and indirect healthcare costs of lung cancer CT screening in Denmark: a registry study. BMJ Open 2020;10(1):e031768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaerlev L, Iachina M, Pedersen JH, Green A, Nørgård BM. CT-Screening for lung cancer does not increase the use of anxiolytic or antidepressant medication. BMC Cancer 2012;12:188. [DOI: 10.1186/1471-2407-12-188] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmqvist J, Siersma V, Thorsen H, Heleno B, Rasmussen JF, Brodersen J. Did psychosocial status, sociodemographics and smoking status affect non-attendance in control participants in the Danish Lung Cancer Screening Trial? A nested observational study. BMJ Open 2020;10(2):e030871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00496977. Danish Lung Cancer Screening Trial (DLCST). clinicaltrials.gov/ct2/show/NCT00496977 (first received 6 July 2007).
- Pedersen JH, Ashraf H, Dirksen A, Bach K, Hansen H, Toennesen P, et al. The Danish randomized Lung Cancer CT Screening Trial – overall design and results of the prevalence round. Journal of Thoracic Oncology 2009;4(5):608-14. [DOI: 10.1097/JTO.0b013e3181a0d98f] [DOI] [PubMed] [Google Scholar]
- Pedersen JH, Dirksen A. Danish study update. Journal of Thoracic Oncology 2011;6(6):S130-1. [Google Scholar]
- Pedersen JH, Wille MW, Dirksen A. The Danish Lung Cancer Screening Trial: results 5 years after last CT screening. Journal of Thoracic Oncology 2015;10(9):S191. [Google Scholar]
- Rasmussen JF, Siersma V, Malmqvist J, Brodersen J. Psychosocial consequences of false positives in the Danish Lung Cancer CT Screening Trial: a nested matched cohort study. BMJ Open 2020;10(6):e034682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen JF, Siersma V, Pedersen JH, Brodersen J. Psychosocial consequences in the Danish randomised controlled Lung Cancer Screening Trial (DLCST). Lung Cancer 2015;87(1):65-72. [DOI: 10.1016/j.lungcan.2014.11.003] [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Frestad D, Køber L, Pedersen JH, Thomsen LH, Dirksen A, et al. Development and progression of coronary artery calcification in long-term smokers: adverse effects of continued smoking. Journal of the American College of Cardiology 2013;62(3):255-7. [DOI: 10.1016/j.jacc.2013.04.013] [DOI] [PubMed] [Google Scholar]
- Saghir Z, Ashraf H, Dirksen A, Brodersen J, Pedersen JH. Contamination during 4 years of annual CT screening in the Danish Lung Cancer Screening Trial (DLCST). Lung Cancer 2011;71(3):323-7. [DOI: 10.1016/j.lungcan.2010.06.006] [DOI] [PubMed] [Google Scholar]
- Saghir Z, Dirksen A, Ashraf H, Bach KS, Brodersen J, Clementsen PF, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax 2012;67(4):296-301. [DOI: 10.1136/thoraxjnl-2011-200736] [DOI] [PubMed] [Google Scholar]
- Saghir Z, Dirksen A, Ashraf HG, Tønnesen P, Bach KS, Hansen H, et al. CT screening of lung cancer brings forward early disease. The Danish Lung Cancer Screening Trial (DLCST): status after five years of CT screening. Journal of Thoracic Oncology 2011;6(6):S350-1. [Google Scholar]
- Saghir Z, Dirksen A, Pedersen JH. In lung cancer screening by CT incidental findings are frequent and often of clinical importance. European Respiratory Journal 2011;38:2740. [Google Scholar]
- Saghir Z, Dirksen A, Pedersen JH. Predictors of nodule malignancy in the Danish Lung Cancer Screening Trial (DLCST). Journal of Thoracic Oncology 2013;8:S678. [DOI: 10.1097/01.JTO.0000438438.14562.c8] [DOI] [Google Scholar]
- Saghir Z, Dirksen A, Rasmussen JF, Heleno BM, Brodersen J, Pedersen JH. In lung cancer screening by CT incidental findings are frequent and often of clinical importance. American Journal of Respiratory and Critical Care Medicine 2012;185:A5072. [Google Scholar]
- Thomsen LH, Dirksen A, Shaker SB, Skovgaard LT, Dahlbäck M, Pedersen JH. Analysis of FEV1 decline in relatively healthy heavy smokers: implications of expressing changes in FEV1 in relative terms. COPD 2014;11(1):96-104. [DOI: 10.3109/15412555.2013.830096] [DOI] [PubMed] [Google Scholar]
- Wille MM, Dirksen A, Ashraf H, Saghir Z, Bach KS, Brodersen J, et al. Results of the randomized Danish Lung Cancer Screening Trial with focus on high-risk profiling. American Journal of Respiratory and Critical Care Medicine 2016;193(5):542-51. [DOI: 10.1164/rccm.201505-1040OC] [DOI] [PubMed] [Google Scholar]
- Wille MM, Riel SJ, Saghir Z, Dirksen A, Pedersen JH, Jacobs C, et al. Predictive accuracy of the PanCan lung cancer risk prediction model – external validation based on CT from the Danish Lung Cancer Screening Trial. European Radiology 2015;25(10):3093-9. [DOI: 10.1007/s00330-015-3689-0] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bradley 2021 {published data only}
- Bradley C, Kennedy M, Darby M, Crosbie PA, Gabe R, Callister ME. A review of radiology reporting to date in the Yorkshire lung screening trial. Thorax 2021;76(Suppl 1):A232. [Google Scholar]
Brodersen 2014 {published data only}
- Brodersen J, Rasmussen JF, Heleno B. Screening for lung cancer with low-dose computed tomography. Annals of Internal Medicine 2014;160(3):211. [DOI: 10.7326/L14-5003] [DOI] [PubMed] [Google Scholar]
Dawson 2020 {published data only}
- Dawson Q. NELSON trial: reduced lung-cancer mortality with volume CT screening. Lancet Respiratory Medicine 2020;8(3):236. [DOI: 10.1016/S2213-2600(20)30059-X] [DOI] [PubMed] [Google Scholar]
de‐Torres 2021 {published data only}
- de-Torres JP, Wisnivesky JP, Bastarrika G, Wilson DO, Celli BR, Zulueta JJ. Exploring the Impact of lung cancer screening on lung cancer mortality of smokers with obstructive lung disease: analysis of the NLST-ACRIN cohort. Archivos de Bronconeumologia 2021;57(1):36-41. [DOI: 10.1016/j.arbres.2020.03.023] [DOI] [PubMed] [Google Scholar]
Favre 2003 {published data only}
- Favre L, Rochat T. Screening for lung cancer with low-dose chest computed tomography. Medecine et Hygiene 2003;61(2458):2197-200. [Google Scholar]
Fink 2012 {published data only}
- Fink C, Henzler T, Nitschmann S, Schönberg SO. Reduction of lung cancer-associated mortality by computed tomography screening. National Lung Screening Trial. Der Internist 2012;53(12):1505-6. [DOI: 10.1007/s00108-012-3170-y] [DOI] [PubMed] [Google Scholar]
Garg 2002 {published data only}
- Garg K, Keith RL, Byers T, Kelly K, Kerzner AL, Lynch DA, et al. Randomized controlled trial with low-dose spiral CT for lung cancer screening: feasibility study and preliminary results. Radiology 2002;225(2):506-10. [DOI: 10.1148/radiol.2252011851] [DOI] [PubMed] [Google Scholar]
Goulart 2013 {published data only}
- Goulart BH, Ramsey SD. Lung cancer screening with low-dose computed tomography. Journal of National Comprehensive Cancer Network 2013;11(4):366-7. [DOI: 10.6004/jnccn.2013.0051] [DOI] [PubMed] [Google Scholar]
Guldbrandt 2015 {published data only}
- Guldbrandt LM, Fenger-Grøn M, Rasmussen TR, Rasmussen F, Meldgaard P, Vedsted P. The effect of direct access to CT scan in early lung cancer detection: an unblinded, cluster-randomised trial. BMC Cancer 2015;15(1):934. [DOI: 10.1186/s12885-015-1941-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hassannezhad 2018 {published data only}
- Hassannezhad R, Vahed N. Prediction of the risk of malignancy among detected lung nodules in the National Lung Screening Trial. Journal of American College of Radiology 2018;15(11):1529-35. [DOI: 10.1016/j.jacr.2018.06.009] [DOI] [PubMed] [Google Scholar]
Henschke 2000 {published data only}
- Henschke CI. Early lung cancer action project: overall design and findings from baseline screening. Cancer 2000;89(11 Suppl):2474-82. [DOI: ] [DOI] [PubMed] [Google Scholar]
Henschke 2002 {published data only}
- Henschke CI, Yankelevitz DF, McCauley D, Libby D, Pasmantier M, Smith JP. CT screening for lung cancer. Cancer Chemotherapy and Biological Response Modifiers 2002;20:665-76. [PubMed] [Google Scholar]
Henschke 2015 {published data only}
- Henschke CI, Boffetta P, Ankelevitz DF, Altorki N. Computed tomography screening: the international early lung cancer action program experience. Thoracic Surgery Clinics 2015;25(2):129-43. [DOI: 10.1016/j.thorsurg.2014.12.001] [DOI] [PubMed] [Google Scholar]
Horeweg 2013 {published data only}
- Horeweg N, Nackaerts K, Oudkerk M, De Koning HJ. Low-dose computed tomography screening for lung cancer: results of the first screening round. Journal of Comparative Effectiveness Research 2013;2(5):433-6. [DOI: 10.2217/cer.13.57] [DOI] [PubMed] [Google Scholar]
ISRCTN42704678 {published data only}
- ISRCTN42704678. The Yorkshire Lung Screening Trial. www.isrctn.com/ISRCTN42704678 (first received 26 March 2018).
Kramer 2011 {published data only}
- Kramer BS, Berg CD, Aberle DR, Prorok PC. Lung cancer screening with low-dose helical CT: results from the National Lung Screening Trial (NLST). Journal of Medical Screening 2011;18(3):109-11. [DOI: 10.1258/jms.2011.011055] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kulaga 2007 {published data only}
- Kulaga S, Karp I. CT screening for lung cancer. New England Journal of Medicine 2007;356(7):744; author reply 746-7. [PubMed] [Google Scholar]
Marcus 2006 {published data only}
- Marcus PM, Bergstralh EJ, Zweig MH, Harris A, Offord KP, Fontana RS. Extended lung cancer incidence follow-up in the Mayo lung project and overdiagnosis. Journal of the National Cancer Institute 2006;98(11):748-56. [DOI: 10.1093/jnci/djj207] [DOI] [PubMed] [Google Scholar]
NCT02431962 {published data only}
- NCT02431962. Alberta Lung Cancer Screening Program. clinicaltrials.gov/ct2/show/NCT02431962 (first received 1 May 2015).
Park 2022 {published data only}
- Park S, Park H, Lee SM, Ahn Y, Kim W, Jung K, et al. Application of computer-aided diagnosis for Lung-RADS categorization in CT screening for lung cancer: effect on inter-reader agreement. European Radiology 2022;32(2):1054-64. [DOI: 10.1007/s00330-021-08202-3] [DOI] [PubMed] [Google Scholar]
Robbins 2019 {published data only}
- Robbins HA, Callister M, Sasieni P, Quaife SL, Cheung LC, Brennan P, et al. Benefits and harms in the National Lung Screening Trial: expected outcomes with a modern management protocol. Lancet Respiratory Medicine 2019;7(8):655-6. [DOI: 10.1016/S2213-2600(19)30136-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schabath 2019 {published data only}
- Schabath MB, Aberle DR. MILD trial, strong confirmation of lung cancer screening efficacy. Nature Reviews Clinical Oncology 2019;16(9):529-30. [DOI: 10.1038/s41571-019-0231-3] [DOI] [PubMed] [Google Scholar]
Schreuder 2021 {published data only}
- Schreuder A, Mets OM, Schaefer-Prokop CM, Jacobs C, Prokop M. Microsimulation modeling of extended annual CT screening among lung cancer cases in the National Lung Screening Trial. Lung Cancer 2021;156:5-11. [DOI: 10.1016/j.lungcan.2021.04.004] [DOI] [PubMed] [Google Scholar]
Spiro 2019 {published data only}
- Spiro SG, Shah PL, Rintoul RC, George J, Janes S, Callister M, et al. Sequential screening for lung cancer in a high-risk group: randomised controlled trial. European Respiratory Journal 2019;54(4):1900181. [DOI: 10.1183/13993003.00581-2019] [DOI] [PMC free article] [PubMed] [Google Scholar]
Strauss 2012 {published data only}
- Strauss GM, Dominioni L. Computed tomography (CT) and chest X-ray (CXR) screening in the national lung screening trial (NLST): do mortality differences provide an unbiased measure of the effectiveness of CT screening? American Journal of Respiratory and Critical Care Medicine 2012;185:A5063. [Google Scholar]
Strauss 2015 {published data only}
- Strauss G, Flores JP, Dominioni L. Computed tomography (CT) and chest x-ray (CXR) screening for lung cancer (LC): mortality (MORT), survival (SURV), and randomized population trials (RPTs) analysis of the Mayo lung project (MLP) and the National Lung Screening Trial (NLST). Chest 2015;148(4):554A. [DOI: 10.1378/chest.2264144] [DOI] [Google Scholar]
Sullivan 2019 {published data only}
- Sullivan F, Schembri S. PL02.03 Early detection of cancer of the lung Scotland (ECLS): trial results. Journal of Thoracic Oncology 2019;14(10):S5. [DOI: 10.1016/j.jtho.2019.08.056] [DOI] [Google Scholar]
Sullivan 2021 {published data only}
- Sullivan FM, Mair FS, Anderson W, Armory P, Briggs A, Chew C, et al. Earlier diagnosis of lung cancer in a randomised trial of an autoantibody blood test followed by imaging. European Respiratory Journal 2021;57(1):2000670. [DOI: 10.1183/13993003.00670-2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2008 {published data only}
- Yang M, Wang J, Meng LJ, Wang Y, Xu L, Liu FY, et al. Analysis of feasibility of lung cancer screening with low-radiation-dose spiral CT scan plus detection of p16 gene methylation in serum. Chinese Journal of Cancer Prevention and Treatment 2008;15(1):8-10. [Google Scholar]
Yip 2013 {published data only}
- Yip R, Yankelevitz DF, Henschke CI. CT screening for lung cancer: definition of positive test result in the national lung screening trial CT cohort compared with i-elcap. Journal of Thoracic Oncology 2013;8:S326. [DOI: 10.1097/01.JTO.0000438438.14562.c8] [DOI] [Google Scholar]
References to ongoing studies
Sagawa 2012 {published data only}
- Sagawa M, Nakayama T, Tanaka M, Sakuma T, Sobue T. A randomized controlled trial on the efficacy of thoracic CT screening for lung cancer in non-smokers and smokers of <30 pack-years aged 50-64 years (JECS study): research design. Japanese Journal of Clinical Oncology 2012;42(12):1219-21. [DOI: 10.1093/jjco/hys157] [DOI] [PubMed] [Google Scholar]
- Sagawa M. Screening in Japan – the JECS study. Journal of Thoracic Oncology 2015;10(9):S115. [Google Scholar]
Yang 2018 {published data only}
- Han B, Wang H, Teng J, Ye J, Chen Q, Zhang Y, et al. Randomized lung cancer screening with low-dose CT in China: a specific risk-based screening for Chinese population. Journal of Thoracic Oncology 2017;12(11):S1858. [Google Scholar]
- NCT02898441. Early stage lung cancer screening with low-dose computed tomographic. clinicaltrials.gov/ct2/show/NCT02898441 (first received 13 September 2016).
- Qian F, Yang W, Wang H, Teng J, Zhang Y, Chen Q, et al. Community-based lung cancer screening of high-risk population with low-dose computed tomography in China. Annals of Oncology 2017;28:v502. [DOI: 10.1093/annonc/mdx383.001] [DOI] [Google Scholar]
- Yang W, Qian F, Teng J, Wang H, Manegold C, Pilz LR, et al. Community-based lung cancer screening with low-dose CT in China: results of the baseline screening. Lung Cancer 2018;117:20-6. [DOI: 10.1016/j.lungcan.2018.01.003] [DOI] [PubMed] [Google Scholar]
Additional references
Alberg 2007
- Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidence-based medicine clinical practice guidelines (2nd edition). Chest 2007;132(3):29S-55S. [DOI: 10.1378/chest.07-1347] [DOI] [PubMed] [Google Scholar]
Bach 2003
- Bach PB, Kattan MW, Thornquist MD, Kris MG, Tate RC, Barnett MJ, et al. Variations in lung cancer risk among smokers. Journal of the National Cancer Institute 2003;95(6):470-8. [DOI] [PubMed] [Google Scholar]
Berrington de González 2008
- Berrington de González A, Kim KP, Berg CD. Low-dose lung computed tomography screening before age 55: estimates of the mortality reduction required to outweigh the radiation-induced cancer risk. Journal of Medicine Screening 2008;15(3):153-8. [DOI: 10.1258/jms.2008.00852] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradley 2019
- Bradley SH, Abraham S, Callister ME, Grice A, Hamilton WT, Lopez RR, et al. Sensitivity of chest X-ray for detecting lung cancer in people presenting with symptoms: a systematic review. British Journal of General Practice. 2019;69(689):e827-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brodersen 2010
- Brodersen J, Thorsen H, Kreiner S. Consequences of screening in lung cancer: development and dimensionality of a questionnaire. Value Health 2010;13(5):601-12. [DOI: 10.1111/j.1524-4733.2010.00697.x] [DOI] [PubMed] [Google Scholar]
Brodersen 2018
- Brodersen J, Schwartz LM, Heneghan C, O'Sullivan JW, Aronson JK, Woloshin S. Overdiagnosis: what it is and what it isn't. BMJ Evidence-Based Medicine 2018;23(1):1-3. [DOI: 10.1136/ebmed-2017-110886] [DOI] [PubMed] [Google Scholar]
Brodersen 2020
- Brodersen J, Voss T, Martiny F, Siersma V, Barratt A, Heleno B. Overdiagnosis of lung cancer with low-dose computed tomography screening: meta-analysis of the randomised clinical trials. Breathe 2020;16(1):200013. [DOI: 10.1183/20734735.0013-2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cassidy 2008
- Cassidy A, Myles JP, Tongeren M, Page RD, Liloglou T, Duffy SW, et al. The LLP risk model: an individual risk prediction model for lung cancer. British Journal of Cancer 2008;98(2):270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence 2017 [Computer program]
- Covidence. Version accessed 25 July 2022. Melbourne, Australia: Veritas Health Innovation, 2017. Available at covidence.org.
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
Diederich 2002
- Diederich S, Wormanns D, Semik M, Thomas M, Lenzen H, Roos N, et al. Screening for early lung cancer with low- dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology 2002;222(3):773-81. [DOI] [PubMed] [Google Scholar]
Essink‐Bot 1993
- Essink-Bot ML, Stouthard ME, Bonsel GJ. Generalizability of valuations on health states collected with the EuroQolc-questionnaire. Health Economics 1993;2:237-46. [DOI] [PubMed] [Google Scholar]
FDA 2017
- Food and Drug Administration. What are the radiation risks from CT? www.fda.gov/Radiation-EmittingProducts/RadiationEmittingProductsandProcedures/MedicalImaging/MedicalX-Rays/ucm115329.htm (accessed 18 November 2020).
Ferlay 2019
- Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International Journal of Cancer 2019;144(8):1941-53. [DOI] [PubMed] [Google Scholar]
Fidler‐Benaoudia 2020
- Fidler-Benaoudia MM, Torre LA, Bray F, Ferlay J, Jemal A. Lung cancer incidence in young women vs. young men: a systematic analysis in 40 countries. International Journal of Cancer 2020;147(3):811-9. [DOI: 10.1002/ijc.32809] [DOI] [PubMed] [Google Scholar]
Field 2019
- Field JK, deKoning H, Oudkerk M, Anwar S, Mulshine J, Pastorino U, et al. Implementation of lung cancer screening in Europe: challenges and potential solutions: summary of a multidisciplinary roundtable discussion. ESMO Open 2019;4:e000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gandek 1998
- Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross-validation of item selection and scoring for the SF-12 health survey in nine countries: results from the IQOLA project international quality of life assessment. Journal of Clinical Epidemiology 1998;51:1171-8. [DOI] [PubMed] [Google Scholar]
Gao 2022
- Gao W, Wen CP, Wu A, Welch HG. Association of computed tomographic screening promotion with lung cancer overdiagnosis among Asian women. JAMA International Medicine 2022;e217769:283-90. [DOI: 10.1001/jamainternmed.2021.7769] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gierada 2020
- Gierada DS, Black WC, Chiles C, Pinsky PF, Yankelevitz DF. Low-dose CT screening for lung cancer: evidence from 2 decades of study. Radiology Imaging Cancer 2020;2(2):e190058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldstraw 2016
- Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. Journal of Thoracic Oncology 2016;11(1):39-51. [DOI: 10.1016/j.jtho.2015.09.009. PMID: 26762738] [DOI] [PubMed] [Google Scholar]
Halpern 1993
- Halpern M, Gillespie BW, Warner KE. Patterns of absolute risk of lung cancer mortality in former smokers. Journal of the National Cancer Institute 1993;85:457-63. [DOI] [PubMed] [Google Scholar]
Hansell 2008
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy Jl. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook/archive/v5.2.
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Hoffman 2020
- Hoffman RM, Atallah RP, Struble RD, Badgett RG. Lung cancer screening with low-dose CT: a meta-analysis. Journal of General Internal Medicine 2020;35(10):3015-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horowitz 1979
- Horowitz M, Wilner N, Alvarez W. Impact of event scale: a measure of subjective stress. Psychosomatic Medicine 1979;41(3):209-18. [DOI: 10.1097/00006842-197905000-00004] [DOI] [PubMed] [Google Scholar]
Howlader 2020
- Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. SEER cancer statistics review, 1975–2016. seer.cancer.gov/csr/1975_2017/ (accessed 29 April 2020).
Huang 2019
- Huang KL, Wang SY, Lu WC, Chang YH, Su J, Lu YT. Effects of low-dose computed tomography on lung cancer screening: a systematic review, meta-analysis, and trial sequential analysis. BMC Pulmonary Medicine 2019;11(19):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hubbard 2012
- Hubbard MO, Fu P, Margevicius S, Dowlati A, Linden PA. Five-year survival does not equal cure in non-small cell lung cancer: a surveillance, epidemiology, and end results-based analysis of variables affecting 10- to 18-year survival. Journal of Thoracic Cardiovascular Surgery 2012;143(6):1307-13. [DOI] [PubMed] [Google Scholar]
Humphrey 2013
- Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the U.S. Preventive Services Task Force recommendation. Annals of Internal Medicine 2013;159:411-20. [DOI] [PubMed] [Google Scholar]
Jensen 2015
- Jensen TS, Chin J, Ashby L, Hemansen J, Huter JD. Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N). www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274 (accessed 4 January 2022).
Jonas 2021
- Jonas DE, Reuland DS, Reddy SM, Nagle M, Clark SD, Weber RP, et al. Screening for lung cancer with low-dose computed tomography: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2021;325(10):971-87. [DOI] [PubMed] [Google Scholar]
Kauczor 2020
- Kauczor HU, Baird AM, Blum TG, Bonomo L, Bostantzoglou C, Burghuber O, et al. ESR/ERS statement paper on lung cancer screening. European Radiology 2020;30(6):3277-94. [DOI: 10.1007/s00330-020-06727-7] [DOI] [PubMed] [Google Scholar]
Kazerooni 2016
- Kazerooni EA, Armstrong MR, Amorosa JK, Hernandez D, Liebscher LA, Nath H, et al. ACR CT accreditation program and the lung cancer screening program designation. Journal of the American College of Radiology 2016;13(2 Suppl):R30-4. [DOI] [PubMed] [Google Scholar]
Kind 2005
- Kind P, Brooks R, Rabin R, editor(s). EQ-5D Concepts and Methods: a Development History. Dordrecht (the Netherlands): Springer, 2005. [DOI: 10.1007/1-4020-3712-0] [DOI] [Google Scholar]
Levi 2007
- Levi F, Bosetti C, Fernandex E, Hill C, Lucchini F, Negri E, et al. Trends in lung cancer among young European women: the rising epidemic in France and Spain. International Journal of Cancer 2007;121(2):462-5. [DOI] [PubMed] [Google Scholar]
Lewin 2016
- Lewin G, Morissette K, Dickinson J, Bell N, Bacchus M, Singh H, et al. Canadian Task Force on preventive health care. Recommendations on screening for lung cancer. Canadian Medical Association Journal 2016;188:425-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lung‐RADS 2019
- American College of Radiology Committee on Lung-RADS. Lung-RADS Assessment Categories Version 1.1. www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/LungRADSAssessmentCategoriesv1-1.pdf (accessed 5 March 2022).
Malvezzi 2017
- Malvezzi M, Carioli G, Bertuccio P, Boffetta P, Levi F, La Vecchia C, et al. European cancer mortality predictions for the year 2017, with focus on lung cancer. Annals of Oncology 2017;28:1117-23. [DOI] [PubMed] [Google Scholar]
Manser 2013
- Manser R, Lethaby A, Irving LB, Stone C, Byrnes G, Abramson MJ, et al. Screening for lung cancer. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD001991. [DOI: 10.1002/14651858.CD001991.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mao 2001
- Mao Y, Hu J, Ugnat AM, Semenciw R, Fincham S, Canadian Cancer Registries Epidemiology Research Group. Socioeconomic status and lung cancer risk in Canada. International Journal of Epidemiology 2001;30(4):809-17. [DOI: 10.1093/ije/30.4.809] [DOI] [PubMed] [Google Scholar]
Mazzone 2021
- Mazzone PJ, Silvestri GA, Souter LH, Caverly TJ, Kanne JP, Katki HA, et al. Screening for lung cancer: CHEST guideline and expert panel report. Chest 2021;160(5):e427-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
McWilliams 2013
- McWilliams A, Tammemagi MC, Mayo JR, Roberts H, Liu G, Soghrati K, et al. Probability of cancer in pulmonary nodules detected on first screening CT. New England Journal of Medicine 2013;369(10):910-9. [DOI: 10.1056/NEJMoa1214726] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine 2009;151(4):264-9. [DOI] [PubMed] [Google Scholar]
Moyer 2014
- Moyer VA. U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Annals of International Medicine 2014;160:330-8. [DOI] [PubMed] [Google Scholar]
Nawa 2002
- Nawa T, Nakagawa T, Kusano S, Kawasaki Y, Sugawara Y, Nakata H. Lung cancer screening using low-dose spiral CT: results of baseline and 1-year follow-up studies. Chest 2002;122:15-20. [DOI] [PubMed] [Google Scholar]
NHS 2019
- National Health Service. NHS to rollout lung cancer scanning trucks across the country. www.longtermplan.nhs.uk/nhs-to-rollout-lung-cancer-scanning-trucks-across-the-country/ (accessed July 2020).
NICE 2019
- National Institute for Health and Care Excellence. Lung cancer: diagnosis and management (NICE guideline 122). www.nice.org.uk/guidance/NG122 (accessed 28 April 2020).
Nicholson 2022
- Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, et al. The 2021 WHO classification of lung tumors: impact of advances since 2015. Journal of Thoracic Oncology 2022;17(3):362-87. [DOI: 10.1016/j.jtho.2021.11.003] [DOI] [PubMed] [Google Scholar]
Oken 2011
- Oken MM, Hocking WG, Kvale PA, Andriole GL, Buys SS, Church TR et al, PLCO Project Team. Screening by chest radiograph and lung cancer mortality: the prostate, lung, colorectal, and ovarian (PLCO) randomized trial. JAMA 2011;306(17):1865-73. [DOI: 10.1001/jama.2011.1591] [DOI] [PubMed] [Google Scholar]
Oudkerk 2017
- Oudkerk M, Devaraj A, Vliegenthart R, Henzler T, Prosch H, Heussel CP, et al. European position statement on lung cancer screening. Lancet Oncology 2017;18:754-66. [DOI] [PubMed] [Google Scholar]
Peto 1994
- Peto R, Lopez AD, Boreham J, Thun M, Heath C Jr. Mortality from Smoking in Developed Countries 1950–2000. London (UK): Oxford University Press, 1994. [Google Scholar]
Pinsky 2018
- Pinsky PF. Does the evidence support the implementation of lung cancer screening with low-dose computed tomography? Expert Review of Respiratory Medicine 2018;12(4):257-60. [DOI] [PubMed] [Google Scholar]
Quaife 2021
- Quaife SL, Janes SM, Brain KE. The person behind the nodule: a narrative review of the psychological impact of lung cancer screening. Translational Lung Cancer Research 2021;10(5):2427-40. [DOI: 10.21037/tlcr-20-1179] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quekel 1999
- Quekel LG, Kessels AG, Goei R, Engelshoven JM. Miss rate of lung cancer on the chest radiograph in clinical practice. Chest 1999;115:720-4. [DOI] [PubMed] [Google Scholar]
Rampinelli 2017
- Rampinelli C, De Marco P, Origgi D, Maisonneuve P, Casirahi M, Veronesi G, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ 2017;356:j347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rota 2019
- Rota M, Pizzato M, La Vecchia C, Boffetta P. Efficacy of lung cancer screening appears to increase with prolonged intervention: results from the MILD trial and a meta-analysis. Annals of Oncology 2019;30(7):1040-3. [DOI] [PubMed] [Google Scholar]
Sadate 2020
- Sadate A, Occean BV, Beregi JP, Hamard A, Addala T, Forges H, et al. Systematic review and meta-analysis on the impact of lung cancer screening by low-dose computed tomography. European Journal of Cancer 2020;134:107-14. [DOI] [PubMed] [Google Scholar]
Siegel 2019
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a Cancer Journal for Clinicians 2019;69(1):7-34. [DOI] [PubMed] [Google Scholar]
Silva 2018
- Silva M, Prokop M, Jacobs C, Capretti G, Sverzellati N, Ciompi F, et al. Long-term active surveillance of screening detected subsolid nodules is a safe strategy to reduce overtreatment. Journal of Thoracic Oncology 2018;13(10):1454-63. [DOI: 10.1016/j.jtho.2018.06.013] [DOI] [PubMed] [Google Scholar]
Slatore 2014
- Slatore CG, Sullivan DR, Pappas M, Humphrey LL. Patient-centered outcomes among lung cancer screening recipients with computed tomography: a systematic review. Journal of Thoracic Oncology 2014;9(7):927-34. [DOI: 10.1097/JTO.0000000000000210] [DOI] [PMC free article] [PubMed] [Google Scholar]
Snowsill 2018
- Snowsill T, Yang H, Griffin E, Long L, Varley-Campbell J, Coelho H, et al. Low-dose computed tomography for lung cancer screening in high-risk populations: a systematic review and economic evaluation. Health Technology Assessment 2018;22:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sobue 2002
- Sobue T, Moriyama N, Kaneko M, Kusumoto M, Kobayashi T, Tsuchiya R, et al. Screening for lung cancer with low-dose helical computed tomography: Anti-Lung Cancer Association project. Journal of Clinical Oncology 2002;20(4):911-20. [DOI] [PubMed] [Google Scholar]
Sone 2001
- Sone S, Li F, Yang Z-G, Honda T, Maruyama Y, Takashima S, et al. Results of three-year mass screening programme for lung cancer using mobile low-dose spiral computed tomography scanner. British Journal of Cancer 2001;84(1):25-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Subramanian 2007
- Subramanian J, Govindan R. Lung cancer in never smokers: a review. Journal of Clinical Oncology 2007;25(5):561-70. [DOI] [PubMed] [Google Scholar]
Swensen 2002
- Swensen SJ, Jett JR, Sloan JA, Midthun DE, Hartman TE, Sykes AM, et al. Screening for lung cancer with low-dose spiral computed tomography. American Journal of Respiratory and Critical Care Medicine 2002;165:508-13. [DOI] [PubMed] [Google Scholar]
Tammemägi 2013
- Tammemägi MC, Katki HA, Hocking WG, Church TR, Caporaso N, Kvale PA, et al. Selection criteria for lung-cancer screening. New England Journal of Medicine 2013;368:728-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
ten Haaf 2017
- ten Haaf K, Keon J, Tammemagi MC, Han SS, Kong CY, Plevritis SK, et al. Risk prediction models for selection of lung cancer screening candidates: a retrospective validation study. PLoS Medicine 2017;14(4):e1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Aalst 2021
- Aalst CM, Ten Haaf K, Koning HJ. Implementation of lung cancer screening: what are the main issues? Translational Lung Cancer Research 2021;10(2):1050-63. [DOI: 10.21037/tlcr-20-985] [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Bij 2003
- Bij AK, Weerd S, Cikot RJ, Steegers EA, Braspenning JC. Validation of the Dutch short form of the state scale of the Spielberger State-Trait Anxiety Inventory: considerations for usage in screening outcomes. Community Genetics 2003;6:84-7. [DOI] [PubMed] [Google Scholar]
Walters 2013
- Walters S, Maringe C, Coleman MP, Peake MD, Butler J, Young N, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004–2007. Thorax 2013;68(6):551-64. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PMID: ] [PubMed] [Google Scholar]
Ware 1996
- Ware J Jr, Kosinski M, Keller SD. A 12-item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care 1996;34:220-33. [DOI] [PubMed] [Google Scholar]
WHO 2016
- World Health Organization. Ambient air pollution: a global assessment of exposure and burden of disease. www.who.int/phe/publications/air-pollution-global-assessment/en/ (accessed 10 June 2020).
WHO 2018
- World Health Organization. Cancer. www.who.int/health-topics/cancer#tab=tab_1 (accessed prior to 13 July 2022).
Willemink 2013
- Willemink MJ, Leiner T, Jong PA, Heer LM, Nievelstein RA, Schilham AM, et al. Iterative reconstruction techniques for computed tomography part 2: initial results in dose reduction and image quality. European Radiology 2013;23(6):1632-42. [DOI: 10.1007/s00330-012-2764-z] [DOI] [PubMed] [Google Scholar]
Wu 2016
- Wu GX, Raz DJ, Brown L, Sun V. Psychological burden associated with lung cancer screening: a systematic review. Clinical Lung Cancer 2016;17(5):315-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2015
- Zhou QH, Fan YG, Bu H, Wang Y, Wu N, Huang YC, et al. China national lung cancer screening guideline with low-dose computed tomography. Thoracic Cancer 2015;6:812-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67(6):361-70. [DOI: 10.1111/j.1600-0447.1983.tb09716.x] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bonney 2021
- Bonney A, Malouf R, Marchal C, Manners D, Fong KM, Marshall HM, et al. Low‐dose computed tomography (LDCT) screening for lung cancer‐related mortality. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD013829. [DOI: 10.1002/14651858.CD013829] [DOI] [PMC free article] [PubMed] [Google Scholar]


