To the Editor,
In Germany, SARS‐CoV‐2 infections in fall 2021 were caused by the Delta (B.1.617.2) variant of concern (VOC), which was completely replaced by the Omicron (BA.1, B.1.529.1/BA.2, B.1.529.2) VOC in winter. Meanwhile, the BA.2 sublineage dominates, apparently having a selection advantage. 1
We studied the kinetics of anti‐spike (S) protein IgG, Delta neutralizing antibodies (NA), and the release of interferon‐gamma (IFN‐γ) from stimulated T‐cells in 152 individuals (117/35 women/men, median age 41 years) who received two doses of vector vaccine (AstraZeneca, AZD, N = 34), mRNA vaccine (BioNTech/Moderna, mRNA, N = 62), or a combination of both (N = 56) followed by an mRNA vaccine booster (N = 81). Delta and Omicron BA.1/BA.2 NAs and T‐cell reactivity were analyzed in a subset of 15 age‐ and gender‐matched vaccinees and in 10 triple‐vaccinated and two unvaccinated individuals after BA.1 infection. The presence of Delta and Omicron BA.1‐NA was assessed in unvaccinated convalescents after Alpha (N = 10) or Beta (N = 1) VOC infection. For more information, see the Appendix S1.
Within 279 days after the second dose, a decrease in anti‐S IgG concentrations (Figure S1A–C) and Delta NA titers (Figure 1A) was measured regardless of the immunization regimen. The mRNA booster led to an increase of anti‐S IgG concentrations (Figure S1D‐F) and of Delta NA titers (Figure 1B). The IgG levels and Delta NAs reached 4 weeks after this booster were 1.3–1.7‐fold higher than after the second mRNA dose (Figures 1A, C, S1A–C, G–I). During the entire period (≤279 days) before mRNA booster vaccination, SARS‐CoV‐2‐specific T‐cells were detectable in the majority of subjects, as shown by measurement of IFN‐γ release after stimulation with antigens presumably derived from a Wuhan‐like virus. Their concentrations were not affected by the underlying vaccination regimen and increased 5.5–10.5‐fold after the mRNA booster (Figure 1D, E). Thereafter, adaptive immunity parameters decreased again over time (Figures 1C, F, S1G‐I).
FIGURE 1.
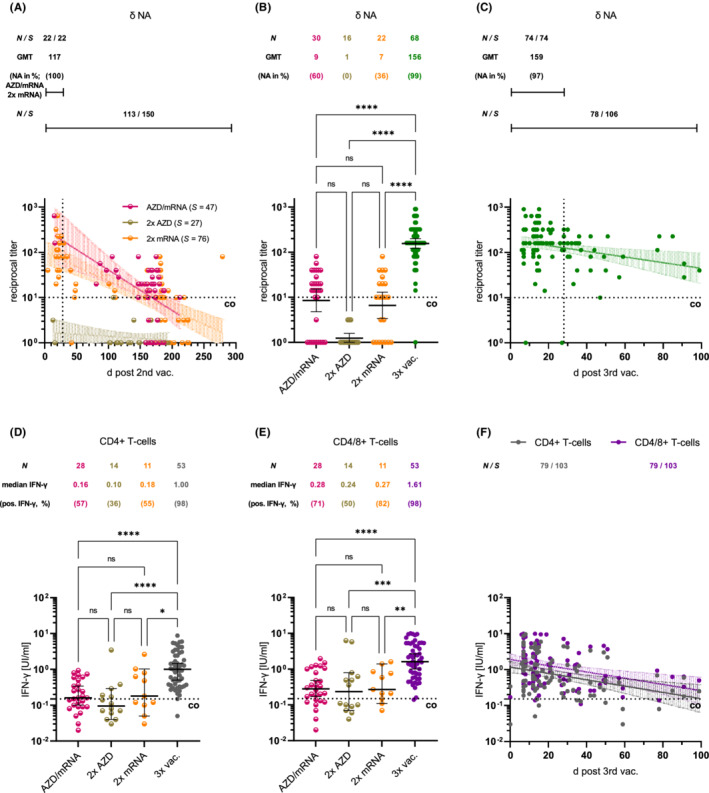
(A, B) Decrease in Delta (δ)‐neutralizing antibody (NA) titers after double vaccination followed by mRNA booster‐induced increase. Geometric mean titers (GMT) and prevalence of titers >1:10 are tabulated. (C) Renewed titer decline. Increase of δ‐NAs four weeks after second (homologous/heterologous) and third mRNA dose (vertical dotted lines; p = 0.04; Mann–Whitney). (D, E) Increase in CD4+ and CD4/CD8+ T‐cell reactivity after mRNA booster. (F) Renewed decrease in T‐cell reactivity. Indicated are the number of individuals (N)/samples (S) tested, and cut‐off values (co). ****p < 0.0001; ***p < 0.001; **p < 0.01; ns: not significant (Kruskal—Wallis)
As reported by others, 1 , 2 , 3 NAs to Omicron BA.1 were induced by the mRNA vaccine booster, but also against the BA.2 sublineage, which was previously unclear (Figure 2A). With respect to the results presented in Figures 1C and 2B, we suspect that NAs against the Omicron VOC will decline rapidly after booster vaccination alone. In triple‐vaccinated individuals, Omicron breakthrough infection resulted in 1.9–5.3 higher BA.1/BA.2 and Delta NA titers (ca. 3 weeks post‐infection) than after mRNA booster vaccination alone (Figure 2A). This indicates broadened immunity covering additional viral variants and may also explain why few symptomatic BA.2 infections have occurred such individuals till date. 4 Whether this also implies some cross‐reactivity to the current BA.4 and BA.5 sublineages is unclear. Because Omicron is proposed to be a distinct serotype, 5 only NAs against this VOC were detectable in two unvaccinated BA.1‐infected individuals (Figure 2A), while unvaccinated Alpha‐ and Beta VOC patients presented isolated NAs against the antigenically more related Delta VOC (Figure S2A), as previously reported. 6 Accordingly, both BA.1 patients had very low IgG levels against the receptor‐binding domain of a Wuhan‐like virus (Figure S2B), whereas IgGs against the higher preserved nucleocapsid‐protein were barely affected (Figure S2C, D). Results of a surrogate neutralization assay confirmed very limited humoral immunity after Omicron infection alone (Figure S2E). Reliable conclusions about the extent to which Omicron breakthrough infection leads to increased IFN‐γ release cannot be drawn due to the small number of samples (Figure 2C).
FIGURE 2.

(A) Increase of Delta (δ)‐ and Omicron (ο)‐ BA.1/BA.2 neutralizing antibody (NA) titers after mRNA booster in age‐ and gender‐matched individuals pre‐vaccinated with AZD/mRNA (N = 5), AZD/AZD (N = 5), and mRNA/mRNA (N = 5). δ‐ and ο‐BA.1/BA.2‐NA titers after BA.1 (breakthrough) infection in triple‐vaccinated and unvaccinated individuals. (B, C) δ‐ and ο‐BA.1 NA titers and CD4+ or CD4/CD8+ T‐cell reactivities before/after BA.1 (breakthrough) infection. Indicated are geometric mean titers (GMT), prevalence of NAs >1:10, number of individuals (N)/samples (S) tested, cut‐off values (co). ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; ns, not significant
In conclusion, booster vaccination with the conventional mRNA vaccine resulted in measurable BA.1/BA.2 NAs whose titers increased after breakthrough infection. This suggests that a variant‐adapted vaccine or even a multivalent vaccine may be beneficial.
AUTHOR CONTRIBUTIONS
A.K. involved in conceptualization. A.K., F.N., and R.R. involved in methodology, formal analysis, and writing—original draft. C.B., F.N., F.S., M.S., R.R., and S.M. involved in investigation. A.K., D.W., H.F., J.R., O.G., S.S., and T.L. involved in resources. All authors involved in writing—review and editing. A.K. and R.R. involved in visualization. A.K., H.F., O.G., and T.L. involved in supervision.
CONFLICT OF INTEREST
The authors have no conflict of interest in relation to this work.
FUNDING INFORMATION
This research received no external funding.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
The authors thank Abbott GmbH, Diasorin Deutschland GmbH, Mikrogen GmbH, and QIAGEN GmbH for free or discounted test kits. The contribution of all volunteers participating in this study is kindly acknowledged. The authors would thank Corina Bahr, Mohsen Hegab, Jasper Maack, and Christina Martínez Christophersen (all Labor Krause MVZ GmbH) for their assistance with the sequence analysis. Open Access funding enabled and organized by Projekt DEAL.
Rose and Neumann first authors contributed equally.
REFERENCES
- 1. Yu J, Collier AY, Rowe M, et al. Neutralization of the SARS‐CoV‐2 Omicron BA.1 and BA.2 variants. N Engl J Med. 2022;386:1579‐1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng SMS, Mok CKP, Leung YWY, et al. Neutralizing antibodies against the SARS‐CoV‐2 Omicron variant following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat Med. 2022;28:486‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wratil PR, Stern M, Priller A, et al. Three exposures to the spike protein of SARS‐CoV‐2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat Med. 2022;28:496‐503. [DOI] [PubMed] [Google Scholar]
- 4. Stegger M, Edslev SM, Sieber RN, et al. Occurrence and significance of Omicron BA.1 infection followed by BA.2 reinfection. medRxiv. 2002;2022(2022):2019‐22271112. [Google Scholar]
- 5. Simon‐Loriere E, Schwartz O. Towards SARS‐CoV‐2 serotypes? Nat Rev Microbiol. 2022;20(4):187‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rossler A, Riepler L, Bante D, von Laer D, Kimpel J. SARS‐CoV‐2 Omicron Variant Neutralization in Serum from Vaccinated and Convalescent Persons. N Engl J Med. 2022;386(7):698‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


