Editor,
Primary cutaneous B‐cell lymphomas (PCBCL) are non‐Hodgkin B‐cell lymphomas that are present in the skin with no evidence of extracutaneous involvement at the time of the diagnosis. 1 According to the current World Health Organization classification, primary cutaneous follicle centre cell lymphoma is a subgroup of PCBCL that has a favourable prognosis compared to the other variants. 2 Its treatment depends on the extent of the disease and includes surgery, radiotherapy, and various immunotherapies, mainly introduced intralesionally.
In this context, we report the case of a 63‐year‐old male with no previous medical history, who was presented with a large multi‐nodular tumour extending to the forelock, mid‐scalp, and vertex (Fig. 1a). The patient consulted a general surgeon who performed a skin biopsy. The histological (Fig. 1b‐1, b‐2) and immunochemical appearance (Fig. 1b‐3, b‐4, b‐5 and b‐6) were in favour of a primary cutaneous follicle centre B‐cell lymphoma (a lymphoid proliferation in the dermis arranged in a follicular pattern with antibodies anti‐CD20 +, anti‐CD10+, anti‐Bcl6+, anti‐CD30‐, anti‐CD23‐, anti‐CD138 ‐, and anti‐Bcl2 – with weak labelling of tumour cells by anti‐Ki67 antibodies). Then, the patient was referred to our department. Two months following the biopsy, he received his first dose of anti‐SARS‐Cov2 vaccine from Oxford‐AstraZeneca, and had unexpectedly presented a spectacular tumour remission, with the persistence of non‐infiltrated erythema in the mid‐scalp (Fig. 1c). The patient's follow‐up after the second dose of the anti‐SARS‐COV2 vaccine showed a greater reduction of residual erythema. The second skin biopsy performed in the residual erythema area showed fibrous scar tissue without lymphomatous cells (Fig. 1d). The complete blood cell count, the lactate dehydrogenase, and the bone marrow biopsy were all normal, and the Borreliosis serology test was negative. However, no immunoglobulin heavy chain gene analysis was done, as it is not available in our country.
Figure 1.
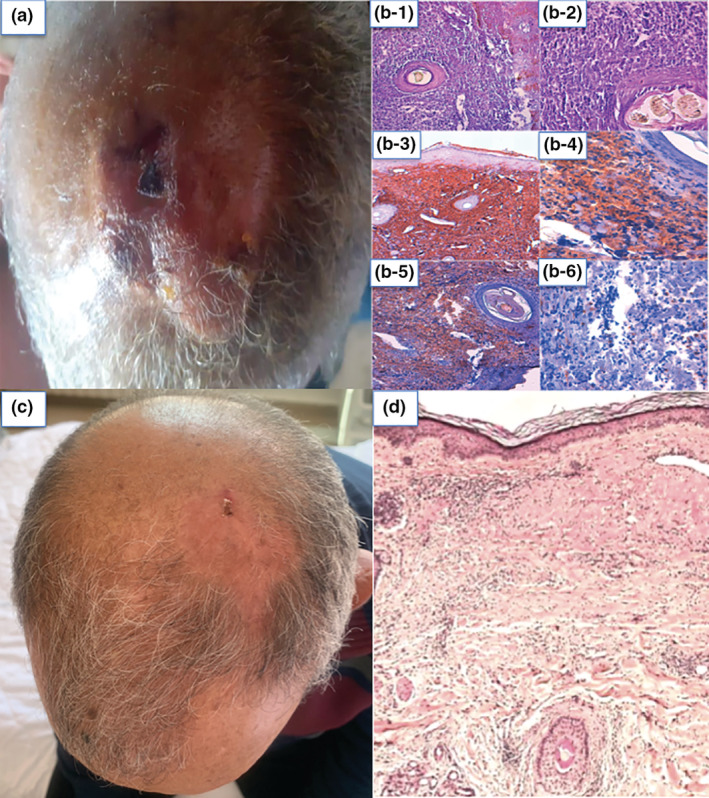
(a) A photo was taken by the patient showing a multinodular tumour of the scalp. (b) Initial histological images: (b‐1) Cutaneous tissue in which the dermis is the site of a lymphomatous proliferation showing a diffuse growth pattern, extending into the hypodermis (HE ×10). (b‐2) Mixture of main centrocytes (large cleaved cells) and centroblasts, provided with irregular nuclei, with one or more prominent nucleoli, arranged within a fibrous stroma (HE ×40). Immunochemical images: (b‐3) Immunochemical labelling CD 20+. (b‐4) Immunochemical labelling Bcl6+. (b‐5) Immunochemical labelling CD10+. (b‐6) Immunochemical labelling Bcl2−. (c) Clinical image showing the disappearance of the scalp tumour after COVID‐19 vaccine, with a residual non‐infiltrated erythema. (d) Histological image after tumour regression showing fibrous scarring.
As part of the extension assessment and to exclude a secondary cutaneous localization of a lymph node lymphoma – a cervical, thoracoabdominal, and pelvic computed tomography scan was performed, showing lobar reticulo‐micronodular infiltrate without other suspicious lesions. Bronchoscopy with biopsy was performed, but no neoplastic cells were found. Furthermore, a positron emission tomographyscan showed no sites of visceral or lymph node hypermetabolic activity. No tumour recurrence was detected after 6‐month follow‐up.
Complete remission of lymphoma has been reported following different factors, such as bacterial infections 3 or surgical trauma. 4 However, in our case, the chronology of the biopsy performance and the first dose of vaccine in relation to the tumour regression indicate that the vaccine is more likely to be incriminated.
During the COVID‐19 outbreak, a few cases of remission of lymphomatous processes were recorded following COVID‐19 infection. Federico Pasin et al. 5 reported a case of a transient remission of natural killer (NK) lymphoma during COVID19 infection, with a relapse shortly after the patient's recovery. This observation indicates the oncolytic effect of the virus, by inducing the release of a large amount of pro‐inflammatory cytokines known as ‘Cytokinic storm’ that might exhibit an anti‐tumour activity. Then, the second case of a 61‐year‐old man was reported by Sarah Challenor et al. 6 show that he has diagnosed with stage IIIs Hodgkin Lymphoma. Yet, the presented clinical and scannographic tumour was decreased after 4 months following the COVID‐19 infection.
Meanwhile, another case of a 61‐year‐old patient with follicular lymphoma showed complete remission after COVID‐19 infection, 7 supporting the hypothesis that SARS‐COV2 infection triggers an immune response that induces a local flare phenomenon which was followed by the abscopal effect.
The AstraZeneca COVID‐19 vaccine is understandably considered a replication‐deficient simian adenovirus vector, and has a stimulating role in the immune system. It is used widely in several countries with rare adverse effects. 8 It may stimulate an unspecific immune activation that induces tumour regression. This reaction is similar to the one induced by the BCG vaccine, particularly in the treatment of metastatic melanoma, as it activates the immune system and destroys the tumour cells. 9
Finally, it is important to stress here that our case report is the first to describe a complete remission of follicular B‐cell lymphoma after the COVID‐19 vaccine, which is still maintained after a follow‐up of 6 months. This report may lead to revolutionary advances in the pathophysiological understanding and treatment of lymphoma.
Conflicts of interest
None.
Funding sources
None.
Informed consent
The patients in this manuscript have given written informed consent to the publication of their case details.
Data availability statement
Data openly available in a public repository that issues datasets with DOIs.
References
- 1. Willemze R, Jaffe ES, Burg G et al. WHO‐EORTC classification for cutaneouslymphomas. Blood 2005; 105: 3768–3785. [DOI] [PubMed] [Google Scholar]
- 2. Vitiello P, Sica A, Ronchi A, Caccavale S, Franco R, Argenziano G. Primarycutaneous B‐celllymphomas: an update. Front Oncol 2020; 10: 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buckner T, Dunphy C, Fedoriw Y et al. Complete spontaneous remission of diffuse large B‐cell lymphoma of the maxillary sinus after concurrent infections. Clin Lymphoma Myeloma Leuk 2012; 12: 455–458. [DOI] [PubMed] [Google Scholar]
- 4. Aoki Y, Hasegawa S, Miyabe S, Nagao T. Spontaneous regression of malignant lymphoma of the maxillary gingiva following biopsy. Int J Oral Maxillofac Surg 2021. [DOI] [PubMed] [Google Scholar]
- 5. Pasin F, Calveri MM, Pizzarelli G, et al. Oncolytic effect of SARS‐CoV2 in a patient with NK lymphoma. Acta Bio Medica: AteneiParmensis 2020; 91: e2020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Challenor S, Tucker D. SARS‐CoV‐2‐induced remission of Hodgkin lymphoma. Br J Haematol 2021; 192: 415. [DOI] [PubMed] [Google Scholar]
- 7. Sollini M, Gelardi F, Carlo‐Stella C, Chiti A. Complete remission of follicular lymphoma after SARS‐CoV‐2 infection: from the “flare phenomenon” to the “abscopal effect”. Eur J Nucl Med Mol Imaging 2021; 48: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Knoll MD, Wonodi C. Oxford–AstraZeneca COVID‐19 vaccine efficacy. Lancet 2021; 397: 72–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benitez MLR, Bender CB, Oliveira TL, Schachtschneider KM, Collares T, Seixas FK. Mycobacteriumbovis BCG in metastaticmelanomatherapy. Appliedmicrobiol Biotechnol 2019; 103: 7903–7916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data openly available in a public repository that issues datasets with DOIs.


