Abstract
Objective
Immunogenicity and safety following receipt of the standard SARS–CoV‐2 vaccination regimen in patients with immune‐mediated inflammatory diseases (IMIDs) are poorly characterized, and data after receipt of the third vaccine dose are lacking. The aim of the study was to evaluate serologic responses and adverse events following the standard 2‐dose regimen and a third dose of SARS–CoV‐2 vaccine in IMID patients receiving immunosuppressive therapy.
Methods
Adult patients receiving immunosuppressive therapy for rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, Crohn's disease, or ulcerative colitis, as well as healthy adult controls, who received the standard 2‐dose SARS–CoV‐2 vaccination regimen were included in this prospective observational study. Analyses of antibodies to the receptor‐binding domain (RBD) of the SARS–CoV‐2 spike protein were performed prior to and 2–4 weeks after vaccination. Patients with a weak serologic response, defined as an IgG antibody titer of ≤100 arbitrary units per milliliter (AU/ml) against the receptor‐binding domain of the full‐length SARS–Cov‐2 spike protein, were allotted a third vaccine dose.
Results
A total of 1,505 patients (91%) and 1,096 healthy controls (98%) had a serologic response to the standard regimen (P < 0.001). Anti‐RBD antibody levels were lower in patients (median 619 AU/ml interquartile range [IQR] 192–4,191) than in controls (median 3,355 AU/ml [IQR 896–7,849]) (P < 0.001). The proportion of responders was lowest among patients receiving tumor necrosis factor inhibitor combination therapy, JAK inhibitors, or abatacept. Younger age and receipt of messenger RNA–1273 vaccine were predictors of serologic response. Of 153 patients who had a weak response to the standard regimen and received a third dose, 129 (84%) became responders. The vaccine safety profile among patients and controls was comparable.
Conclusion
IMID patients had an attenuated response to the standard vaccination regimen as compared to healthy controls. A third vaccine dose was safe and resulted in serologic response in most patients. These data facilitate identification of patient groups at risk of an attenuated vaccine response, and they support administering a third vaccine dose to IMID patients with a weak serologic response to the standard regimen.
INTRODUCTION
The ongoing COVID‐19 pandemic is a global health emergency. Vaccines are important in resolving this crisis, having been proven to be efficacious and safe in the general population (1, 2, 3, 4). Vaccines, however, rely on a functional immune system. Patients with immune‐mediated inflammatory disease (IMID), including inflammatory joint and bowel diseases, have impaired immune systems due to treatment with immunosuppressive medications. There is a concern that immune responses to SARS–CoV‐2 vaccines are attenuated in this large patient population, which is also at risk of severe COVID‐19 (5, 6). Patients with IMIDs were prioritized for vaccination to mitigate their COVID‐19 risk, but because they were excluded from initial vaccine trials, there is a paucity of data on the efficacy and safety of SARS–CoV‐2 vaccines in this population (1, 2, 7), as well as concerns regarding the risk of disease flares (5, 8).
Rheumatoid arthritis (RA), spondyloarthritis (SpA), psoriatic arthritis (PsA), Crohn's disease (CD), and ulcerative colitis (UC) are different IMIDs, but they share several key features and are treated with many of the same immunosuppressive medications, such as tumor necrosis factor inhibitors (TNFi), non‐TNFi biologics, metabolite inhibitors, and targeted small molecule drugs (9). It is important to identify which patients are at risk of a reduced vaccine response, due to either immunosuppression or underlying disease, yet it is still unclear whether the serologic response to vaccine among IMID patients should be monitored. In addition, no consensus currently exists on whether it would be beneficial to delay specific treatments in patients receiving vaccination (7). Observational studies of response to SARS–CoV‐2 vaccine among IMID patients have been published recently, but they have generally involved few patients within each medication group (5, 10, 11, 12, 13, 14, 15).
The utility of 3 or more SARS–CoV‐2 vaccine doses in immunosuppressed patients, as well as in the general population, is an urgent question in the global medical community and for policy makers (16, 17). Findings of a recent study suggested that immunocompromised recipients of a solid organ transplant benefited from a third vaccine dose (18). Apart from a study of a third dose of vaccine in rituximab‐treated RA patients, only a case report and small studies (involving 33 or 17 participants) have been published regarding the immunogenicity and safety of a third dose in IMID patients who were receiving other therapies and had no response to the 2‐dose vaccination regimen (19, 20, 21, 22, 23, 24). The prospective, observational Norwegian Study of Vaccine Response to COVID‐19 (Nor‐vaC) includes patients with any of 5 different IMIDs who are receiving any approved immunosuppressive medication. In this study, we evaluate the immunogenicity and safety of the standard 2‐dose SARS–CoV‐2 vaccination regimen in these groups and examine the response to a third vaccine dose in patients with a weak serologic response to the standard regimen.
PATIENTS AND METHODS
Participants, setting, and study design
Nor‐vaC is an ongoing longitudinal observational study conducted at 2 Norwegian IMID referral centers: the Division of Rheumatology at Diakonhjemmet Hospital and the Department of Gastroenterology at Akershus University Hospital. Adult patients (age ≥18 years) with RA, SpA, PsA, UC, or CD who used any of the immunosuppressive medications of interest (Supplementary Materials, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.42153) and intended to receive a SARS–CoV‐2 vaccine were consecutively recruited into the study. All patients identified by hospital records as eligible for enrollment, based on a diagnosis of an IMID of interest, received an invitation to participate in the study prior to the initiation of the national vaccination program in February 2021. Healthy controls were either volunteer health care workers from Diakonhjemmet Hospital, Akershus University Hospital, and Oslo University Hospital or blood donors from Oslo University Hospital. In the present analyses, we included patients and healthy controls who provided blood specimens for serologic testing 2–4 weeks after receiving the second vaccine dose (Supplementary Figure 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.42153). Patients with COVID‐19 diagnosed before the second dose received only 1 dose of the standard vaccination regimen and were also included in the study.
Patients receiving CD20‐depleting therapy were not included in the present analyses (Supplementary Figure 1). The study (ClinicalTrials.gov identifier: NCT04798625) was approved by an independent ethics committee (Regional Committees for Medical Research Ethics South East Norway, reference numbers 235424, 135924, and 204104) and by appropriate institutional review boards. All participants provided written informed consent.
During the Nor‐vaC study, patients with a weak serologic response >3 weeks after completing the standard 2‐dose regimen were recruited into a separate intervention study (EudraCT database no. 2021‐003618‐37) and allotted a third vaccine dose in July–August 2021. The cutoff for a weak serologic response (i.e., an IgG antibody level of ≤100 arbitrary units per milliliter [AU/ml] against the receptor‐binding domain [RBD] of the full‐length SARS–Cov‐2 spike protein) when selecting patients qualifying for a third vaccine dose was based on discussions within the study group and with the Norwegian Institute of Public Health, with the aim of including not only patients with no response (i.e., an antibody level of <70 AU/ml) but also those with an impaired response (i.e., an antibody level of ≤100 AU). In the present observational study, the serologic response following receipt of a third dose is reported for 153 such patients. Those with inflammatory joint diseases (i.e., RA, SpA, and PsA), but not those with inflammatory bowel diseases (IBDs) (i.e., CD and UC), were asked to pause their medication from 1 week before through 2 weeks after receipt of the third vaccine dose.
Exposures
All patients and controls received SARS–CoV‐2 vaccines according to the Norwegian national vaccination program, administered by the Norwegian Institute of Public Health. Three SARS–CoV‐2 vaccine types were available: ChAdOx1 and the messenger RNA (mRNA) vaccines BNT162b2 and mRNA‐1273. The 2 mRNA vaccines were given with an interval of 3–6 weeks between the 2 doses. ChAdOx1 was withdrawn from the Norwegian national vaccination program in March 2021, and all persons who had received 1 dose of this vaccine received one of the mRNA vaccines as the second dose. According to the program, persons with COVID‐19 diagnosed before the second dose received only 1 dose of the standard vaccination regimen.
Assessments
Patients and controls were asked to provide serum samples prior to the first vaccine dose and 2–4 weeks after the second and third vaccine doses, respectively. Assessments of immunogenicity were performed at the Department of Immunology at Oslo University Hospital. The samples were first screened for antibodies to RBD at the full‐length spike protein by using an in‐house bead‐based method, with seroconversion defined as an anti‐RBD antibody level ≥5 AU (25, 26). Measurement of the World Health Organization international standard for anti‐RBD antibody showed that the screening assay has a lower detection limit of 1 binding antibody unit per milliliter (BAU/ml) and an upper dynamic range of ~100 BAU/ml. For quantification of antibody levels, most patient samples and a representative selection of control samples (Supplementary Table 1) were thereafter analyzed using a second assay, with a dynamic range of 300–10,000 BAU (25). In this assay, effects of sera on binding of angiotensin‐converting enzyme 2 to RBDs from SARS–CoV‐2 variants were measured as a proxy for neutralizing antibody activity (25).
The cutoff for response was preset to an anti‐RBD antibody level of 70 AU/ml, based on results obtained from healthy individuals, of whom 98% had levels >70 AU/ml after receipt of 2 vaccine doses (27). Moreover, calibration to the World Health Organization international standard showed that 70 AU/ml corresponds to ~40 BAU/ml. Using a SARS–CoV‐2 (Wuhan) microneutralization assay, we have determined that 200 BAU/ml is the lower threshold for detection of neutralizing antibodies (28).
The Norwegian Immunization Registry and Norwegian Surveillance System for Communicable Diseases provided information on the date of vaccination, the type of vaccine received, and, when applicable, the date of COVID‐19 (29, 30). Additionally, information regarding COVID‐19 was also obtained from patient questionnaires.
Electronic data collection at Diakonhjemmet Hospital was conducted using the Services for Sensitive Data platform (University of Oslo), and by Viedoc, version 4 (Viedoc Technologies), at Akershus University Hospital. Demographic data were collected at baseline only, while data on medication use, patient‐reported disease activity, and responses to COVID‐19–related questions were also collected during follow‐up. For healthy controls, age and sex were recorded. Disease activity scores (i.e., the Disease Activity Score in 28 joints [DAS28] for patients with RA and patients with PsA, the Ankylosing Spondylitis Disease Activity Score for patients with SpA, the Harvey‐Bradshaw Index for CD, and the Partial Mayo Scoring Index for patients with UC) (31, 32, 33, 34) were obtained at the baseline visit for patients with IBD and retrieved from the medical records for patients with inflammatory joint disease (i.e., from a clinic visit within 3 months before or after receipt of the first vaccine dose). Adverse events were reported ~14 days after receipt of the first, second, and third doses in all patients and in a subset (n = 245) of the healthy controls (i.e., health care workers from Diakonhjemmet Hospital and Akershus University Hospital).
Objectives and outcomes
The 2 main objectives of this study were 1) to assess humoral responses to standard SARS–CoV‐2 vaccination in IMID patients receiving immunosuppressive therapy as compared to that in healthy controls, and 2) to assess changes in humoral responses after a third vaccine dose given to IMID patients with weak serologic responses to standard vaccination. Other objectives were to assess the safety of the standard regimen and the third dose and to identify predictors of serologic response in patients. The main end points were 1) the proportion of participants with a serologic response (i.e., an anti‐RBD antibody level >70 AU/ml) and the anti‐RBD antibody level following the standard regimen and third dose and 2) the change in levels of anti‐RBD antibody after receipt of the third dose. Other end points included adverse events and predictors of the serologic response to the standard regimen and the third dose.
Statistical analysis
Demographic data, adverse events, and serologic response according to medication group were summarized using descriptive statistics. Comparisons of the serologic response between patients and controls were performed by logistic regression. Adjustments were made for sex, age, and vaccine type. Comparisons of anti‐RBD antibody level between patients and healthy controls were performed using the Mann‐Whitney U test. Prevaccination and postvaccination samples collected from patients receiving a third dose were compared by the Wilcoxon's signed rank test for paired samples. There were no missing data for the main variables. Predictors of response among patients were assessed by univariable and multivariable logistic regression. All tests were 2‐sided, and P values of less than 0.05 were considered statistically significant. All analyses were performed using R, release 4.0.3.
RESULTS
Patient and control characteristics
Between February 2, 2021, and June 11, 2021, a total of 2,178 patients were included in the Nor‐vaC study. A total of 1,647 eligible patients (566 with RA, 305 with SpA, 295 with PsA, 280 with CD, and 195 with UC; median age 52 years [interquartile range (IQR) 40–63]; female sex, 899 [55%]) and 1,114 healthy controls (median age 43 years [IQR 32–55]; female sex, 854 [77%]) underwent serologic testing after receipt of the standard 2‐dose vaccination regimen and were included in the present analyses. Patient disposition is summarized in Supplementary Figure 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.42153. Baseline characteristics of patients and controls are shown in Table 1 and Supplementary Tables 1 and 2, available at http://onlinelibrary.wiley.com/doi/10.1002/art.42153. The most common immunosuppressive medications were TNFi (n = 1,082 patients) and methotrexate monotherapy (n = 348). Seventy percent of patients and 56% of controls received BNT162b2 for doses 1 and 2. In total, 23 patients (1%) had COVID‐19 before the second dose and received only the first of 2 doses in the standard vaccination regimen. Controls were included in this study only if they had received 2 vaccine doses and had no signs or symptoms consistent with clinical COVID‐19.
Table 1.
Baseline characteristics of IMID patients and healthy controls who received a standard 2‐dose SARS–CoV‐2 vaccination regimen and IMID patients who received a third dose*
| Characteristic | Patients | Healthy controls (n = 1,114) | |
|---|---|---|---|
| Overall (n = 1,647) | Third‐dose recipients (n = 153) | ||
| Age, median years (IQR) | 52 (40–63) | 57 (46–67) | 43 (32–55) |
| Sex | |||
| Female | 899 (55) | 80 (52) | 854 (77) |
| Male | 748 (45) | 73 (48) | 260 (23) |
| CRP level, median mg/dl (IQR) | 1 (1–3) | 1 (1–4) | No data |
| BMI, median kg/m2 (IQR) | 26 (23–29) | 26 (24–29) | No data |
| IMID | |||
| Joint | |||
| Rheumatoid arthritis | 566 (34) | 52 (34) | NA |
| Psoriatic arthritis | 295 (18) | 21 (14) | NA |
| Spondyloarthritis | 305 (19) | 16 (10) | NA |
| Bowel | |||
| Ulcerative colitis | 195 (12) | 17 (11) | NA |
| Crohn's disease | 280 (17) | 47 (31) | NA |
| Medication | |||
| TNFi† | |||
| Monotherapy | 696 (42) | 46 (30) | NA |
| Combination therapy | 386 (23) | 52 (34) | NA |
| Methotrexate | 348 (21) | 27 (18) | NA |
| Vedolizumab | 55 (3) | 7 (5) | NA |
| JAK inhibitor | 50 (3) | 11 (7) | NA |
| Ustekinumab | 34 (2) | 3 (2) | NA |
| Tocilizumab | 32 (2) | 2 (1) | NA |
| Abatacept | 15 (1) | 4 (3) | NA |
| Secukinumab | 13 (1) | 1 (1) | NA |
| Other‡ | 18 (1) | 0 | NA |
| Prednisolone comedication | |||
| Overall | 71 (4) | 16 (10) | NA |
| Dose ≤7.5 mg | 61/71 (86) | 13/16 (81) | NA |
| Vaccine related§ | |||
| BNT162b2 regimen, 2 doses | 1,152 (70) | 131 (86) | 625 (56) |
| mRNA‐1273 regimen, 2 doses | 401 (24) | 14 (9) | 246 (22) |
| Combination regimen, 2 doses | 71 (4) | 4 (3) | 243 (22) |
| COVID‐19 and 1 of any mRNA vaccine | 23 (1) | 4 (3) | 0 |
Except where indicated otherwise, values are no. (%) of patients or controls. IMID = immune‐mediated inflammatory disease; IQR = interquartile range; CRP = C‐reactive protein; BMI = body mass index; NA = not applicable.
Monotherapy consisted of infliximab, etanercept, adalimumab, golimumab, or certolizumab pegol. Combination therapy consisted of methotrexate, sulfasalazine, leflunomide, or azathioprine, in addition to any tumor necrosis factor inhibitor (TNFi).
Data are for sulfasalazine, leflunomide, azathioprine, risankizumab, and prednisolone monotherapy, each of which was received by <10 patients.
BNT162b2 and mRNA‐1273 are messenger RNA (mRNA) vaccines. Combination regimen was defined as ChAdOx1 (first dose) + BNT162b2 or mRNA‐1273 (second dose) or as BNT162b2 + mRNA‐1273 in any sequence.
Humoral response to the standard regimen
A total of 1,628 patients (98.8%) receiving immunosuppressive therapy and 1,110 healthy controls (99.6%) had detectable antibodies to SARS–CoV‐2 (level, >5 AU/ml) after receiving the standard 2‐dose vaccination regimen (Supplementary Figures 1A and B, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.42153). In this population, 1,493 patients (91%) as compared to 1,096 healthy controls (98%) had anti‐RBD antibody levels ≥70 AU/ml and were considered serologic responders (P < 0.001) (Table 2 and Supplementary Figures 1A and 1B, available at http://onlinelibrary.wiley.com/doi/10.1002/art.42153). Response was detected in ≥90% of patients receiving methotrexate, TNFi monotherapy, ustekinumab, tocilizumab, or vedolizumab, in 80–90% of patients receiving TNFi combination therapy or secukinumab, and in ≤80% receiving JAK inhibitors (78%) or abatacept (53%) (Table 2). To obtain more precise information about antibody levels, samples were reanalyzed using a quantitative assay (Supplementary Figures 1C and D, available at http://onlinelibrary.wiley.com/doi/10.1002/art.42153). Patients had significantly lower levels of anti‐RBD antibody as compared to healthy controls (median 619 AU/ml [IQR 192–4,191] and 3,355 AU/ml [IQR 896–7,849]) (Figure 1).
Table 2.
Serologic response to the standard 2‐dose SARS–CoV‐2 vaccination regimen among healthy controls and among IMID patients overall and by clinical and demographic characteristic*
| Population, characteristic | Response, proportion (%) | OR (95% CI) | P | Anti‐RBD IgG level, median AU/ml (IQR) |
|---|---|---|---|---|
| Healthy controls | 1,096/1,114 (98) | 1 | – | 3,355 (896–7,849) |
| Patients, characteristic | ||||
| Overall | 1,504/1,647 (91) | 0.19 (0.11–0.32) | <0.001 | 619 (192–4,191) |
| IMID | ||||
| Joint | ||||
| Rheumatoid arthritis | 503/566 (89) | 0.16 (0.08–0.29) | <0.001 | 548 (194–4,311) |
| Psoriatic arthritis | 286/295 (97) | 0.19 (0.09–0.41) | <0.001 | 652 (215–4,501) |
| Spondyloarthritis | 271/305 (89) | 0.17 (0.08–0.36) | <0.001 | 689 (225–3,893) |
| Bowel | ||||
| Ulcerative colitis | 184/195 (94) | 0.13 (0.06–0.26) | <0.001 | 1,403 (219–5,940) |
| Crohn's disease | 255/280 (91) | 0.19 (0.08–0.45) | <0.001 | 409 (155–2,262) |
| Medication | ||||
| TNFi† | ||||
| Monotherapy | 664/696 (95) | 0.3 (0.15–0.57) | <0.001 | 726 (225–4,293) |
| Combination therapy | 332/386 (86) | 0.08 (0.04–0.15) | <0.001 | 312 (120–2,178) |
| Methotrexate | 317/348 (91) | 0.2 (0.09–0.42) | <0.001 | 709 (206–4,670) |
| Vedolizumab | 52/55 (95) | 0.31 (0.08–1.21) | 0.091 | 2,415 (412–10,177) |
| JAK inhibitor | 39/50 (78) | 0.05 (0.02–0.12) | <0.001 | 361 (45–4,204) |
| Tocilizumab | 32/32 (100) | – | – | 956 (356–4,578) |
| Ustekinumab | 32/34 (94) | 0.19 (0.04–0.99) | 0.049 | 3,286 (281–8,097) |
| Abatacept | 8/15 (53) | 0.01 (0–0.04) | <0.001 | 70 (38–138) |
| Secukinumab | 11/13 (85) | 0.2 (0.03–1.25) | 0.086 | 1,165 (276–1,456) |
| Other‡ | 16/18 (89) | – | – | 2,907 (391–8,981) |
| Vaccine related§ | ||||
| BNT162b2 regimen, 2 doses | 1,026/1,152 (89) | – | – | 408 (170–2,205) |
| mRNA‐1273 regimen, 2 doses | 391/401 (98) | – | – | 2,308 (377–8,812) |
| Combination regimen, 2 doses | 65/71 (92) | – | – | 699 (272–4,253) |
| COVID‐19 and 1 of any mRNA vaccine | 22/23 (96) | – | – | 6,969 (878–10,768) |
| Other | ||||
| Age, years | ||||
| <30 | 169/176 (96) | – | – | 2,247 (418–7,536) |
| 30–65 | 1,070/1,155 (93) | – | – | 667 (192–4,175) |
| >65 | 265/316 (84) | – | – | 329 (155–1,838) |
| Female sex | 826/899 (92) | – | – | 682 (197–4,639) |
| Current smoker | 143/157 (91) | – | – | 446 (168–1,809) |
Response was defined as an IgG antibody level of ≥70 AU/ml against the receptor‐binding domain (RBD) of SARS–CoV‐2 spike protein, and it was evaluated using logistic regression analysis (adjusted for age, sex, and vaccine type), with healthy controls as the reference group. OR = odds ratio; 95% CI = 95% confidence interval; AU = arbitrary units (see Table 1 for other definitions).
Monotherapy consisted of infliximab, etanercept, adalimumab, golimumab, or certolizumab pegol. Combination therapy consisted of methotrexate, sulfasalazine, leflunomide, or azathioprine, in addition to any TNFi.
Data are for sulfasalazine, leflunomide, azathioprine, risankizumab, and prednisolone monotherapy, each of which was received by <10 patients.
BNT162b2 and mRNA‐1273 are mRNA vaccines. Combination regimen was defined as ChAdOx1 (first dose) + BNT162b2 or mRNA‐1273 (second dose) or as BNT162b2 + mRNA‐1273 in any sequence.
Figure 1.
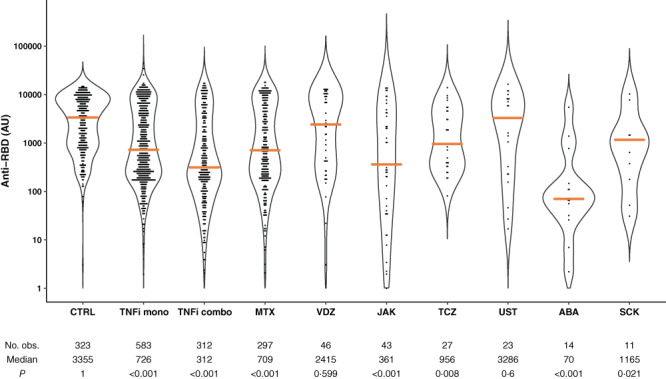
Violin plots of probability densities, smoothed by a kernel density estimator, of IgG antibody levels against the receptor‐binding domain of SARS–CoV‐2 spike protein (anti‐RBD) after the standard 2‐dose SARS–CoV‐2 vaccination regimen among healthy controls (CTRL) and among patients with immune‐mediated inflammatory disease (IMID) stratified by immunosuppressive therapy. Points denote participants, and solid orange lines show group medians. P values show comparisons to CTRL and were calculated by Mann‐Whitney U test. TNFi mono = tumor necrosis factor inhibitor monotherapy; TNFi combo = TNFi combination therapy; MTX = methotrexate; VDZ = vedolizumab; TCZ = tocilizumab; UST = ustekinumab; ABA = abatacept; SCK = secukinumab. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.42153/abstract.
Predictors of response
Age (odds ratio [OR] 0.96, 95% confidence interval [95% CI] 0.94–0.98]) and vaccination with mRNA‐1273 as compared to BNT162b2 (OR 4.45, 95% CI 1.66–11.92) were identified as predictors of a serologic response following receipt of the standard 2‐dose vaccination regimen (Table 3). A total of 98% of patients receiving mRNA‐1273 as compared to 89% receiving BNT162b2 were responders, with median anti‐RBD antibody levels of 2,308 AU/ml (IQR 377–8,812) and 408 AU/ml (IQR 170–2,205), respectively. Patients receiving TNFi combination therapy (OR 0.27, 95% CI 0.14–0.52), JAK inhibitors (OR 0.18, 95% CI 0.05–0.64), or abatacept (OR 0.01, 95% CI 0.01–0.13) were less likely to have a response following receipt of the standard regimen, compared to patients receiving TNFi monotherapy (Table 3). Pausing treatment did not improve vaccine response (Table 3). The same predictors (i.e., age, mRNA‐1273 receipt, and comedication use) were identified in a subanalysis of patients receiving TNFi monotherapy or combination therapy (Supplementary Table 3, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.42153).
Table 3.
Univariable and multivariable analyses to determine predictors of a serologic response among IMID patients after receipt of the standard 2‐dose SARS–CoV‐2 vaccination regimen*
| Potential predictor | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Demographic | ||||
| Age, years | 0.96 (0.95–0.98) | <0.001 | 0.95 (0.93–0.97) | <0.001 |
| Male sex | 0.92 (0.62–1.37) | 0.68 | 0.70 (0.41–1.22) | 0.199 |
| IMID | ||||
| Joint | ||||
| Rheumatoid arthritis | 1 | – | 1 | – |
| Spondyloarthritis | 1.53 (0.83–2.69) | 0.16 | 0.39 (0.14–1.09) | 0.066 |
| Psoriatic arthritis | 1.89 (0.99–3.63) | 0.05 | 1.436 (0.47–3.91) | 0.562 |
| Bowel | ||||
| Crohn's disease | 1.36 (0.81–2.28) | 0.242 | 0.34 (0.13–0.89) | 0.026 |
| Ulcerative colitis | 2.22 (1.11–4.45) | 0.021 | 0.54 (0.18–1.58) | 0.25 |
| Medication | ||||
| TNFi† | ||||
| Monotherapy | 1 | – | 1 | – |
| Combination therapy | 0.38 (0.23–0.64) | <0.001 | 0.27 (0.14–0.52) | <0.001 |
| Methotrexate | 0.61 (0.34–1.09) | 0.089 | 0.36 (0.13–1.04) | 0.286 |
| Vedolizumab | 1 (0.29–3.49) | 0.998 | 1.17 (0.28–4.93) | 0.824 |
| JAK inhibitor | 0.21 (0.09–0.49) | <0.001 | 0.18 (0.05–0.64) | 0.007 |
| Tocilizumab‡ | Not done | 0.978 | Not done | 0.983 |
| Ustekinumab | 0.92 (0.2–4.17) | 0.917 | 0.36 (0.13–8.06) | 0.528 |
| Abatacept | 0.02 (0.01–0.10) | <0.001 | 0.01 (0–0.013) | <0.001 |
| Secukinumab | 0.35 (0.04–3.11) | 0.334 | 0.1 (0.01–1.21 | 0.064 |
| Prednisolone | 0.27 (0.14–0.51) | <0.001 | 0.41 (0.13–1.24) | 0.106 |
| Vaccine related§ | ||||
| BNT162b2 regimen, 2 doses | 1 | – | 1 | – |
| mRNA‐1273 regimen, 2 doses | 5.06 (2.29–11.18) | <0.001 | 4.45 (1.66–11.92) | 0.002 |
| Combination regimen, 2 doses | 1.11 (0.46–2.69) | 0.814 | 0.72 (0.24–2.12) | 0.54 |
| COVID‐19 and 1 of any mRNA vaccine§ | – | 0.977 | – | 0.995 |
| Other | ||||
| IBD or IJD duration | 1 (0.98–1.02) | 0.945 | 1.01 (0.99–1.04) | 0.389 |
| CRP level | 0.97 (0.96–0.99) | 0.01 | 0.97 (0.95–1.0) | 0.018 |
| BMI | 1.01 (0.98–1.05) | 0.474 | 1.03 (0.98–1.08) | 0.292 |
| Pause in medication¶ | 1.8 (0.81–4.03) | 0.142 | 1.59 (0.5–5.07) | 0.428 |
Response was defined as an IgG antibody level of ≥70 AU/ml against the RBD of SARS–CoV‐2 spike protein. IBD = inflammatory bowel disease; IJD = inflammatory joint disease (see Table 2 for other definitions).
Monotherapy consisted of infliximab, etanercept, adalimumab, golimumab, or certolizumab pegol. Combination therapy consisted of methotrexate, sulfasalazine, leflunomide, or azathioprine.
Because of the low number of tocilizumab recipients, analysis was not performed.
BNT162b2 and mRNA‐1273 are mRNA vaccines. Combination regimen was defined as ChAdOx1 (first dose) + BNT162b2 or mRNA‐1273 (second dose) or as BNT162b2 + mRNA‐1273 in any sequence.
Patient‐reported pause in medication from 1 week before through 2 weeks after receipt of a vaccine dose.
Response to a third vaccine dose
A total of 153 patients (median age 57 years [IQR 46–67]; 80 female patients [52%]) with weak responses to the standard 2‐dose regimen (anti‐RBD antibody levels ≤100 AU/ml) were allotted a third vaccine dose a median of 70 days (IQR 56–90) after the second vaccine dose. An increase in antibody levels was observed in 129 (94%) of 153 patients (P < 0.001), with a median change of 362 AU/ml (IQR 48–2,501) (Figure 2). Median antibody levels were 45 AU/ml (IQR 17–105) and 544 AU/ml (IQR 143–4,543) before and 2–4 weeks after receipt of the third vaccine dose, respectively (Figure 2). Percentages of responders, stratified by therapy, were as follows: 89% (41 of 46) among TNFi monotherapy recipients, 84% (44 of 52) among TNFi combination therapy recipients, 75% (21 of 28) among methotrexate recipients, 63% (7 of 11) among JAK inhibitor recipients, and 100% (4 of 4) among abatacept recipients. Except for age, no predictors of response to the third vaccine dose were identified (Supplementary Table 4, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.42153).
Figure 2.
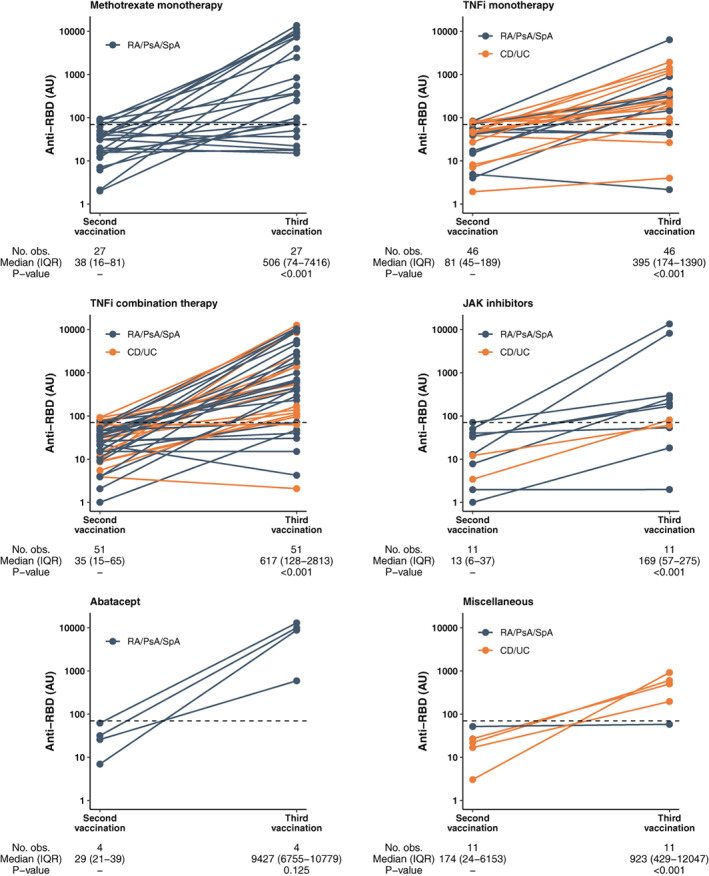
Anti‐RBD levels after receipt of a third SARS–CoV‐2 vaccine dose among IMID patients with a weak response to the standard 2‐dose vaccination regimen. Levels were measured 2–4 weeks after the second and third vaccine doses. Horizontal dotted lines indicate the serologic response cutoff (70 arbitrary units per milliliter [AU/ml]). Orange dots and lines indicate anti‐RBD levels in individual patients with inflammatory bowel disease; blue dots and lines indicate levels in individual patients with inflammatory joint disease. P values were calculated by Wilcoxon paired test. RA = rheumatoid arthritis; PsA = psoriatic arthritis; SpA = spondyloarthritis; obs. = observations; IQR = interquartile range; CD = Crohn's disease; UC = ulcerative colitis; miscellaneous = vedolizumab, ustekinumab, tocilizumab, secukinumab, or azathioprine (see Figure 1 for other definitions).
Adverse events
Among recipients of the standard 2‐dose vaccination regimen, adverse events were reported in 810 (50%) of 1,516 patients and 191 (78%) of 244 healthy controls, with a comparable safety profile (Figure 3 and Supplementary Table 5, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.42153). Following receipt of the third dose, 70 patients (44%) reported adverse events; no new safety issues emerged, except for an increase in disease flares, which were reported by 26 patients (16%), all of whom had inflammatory joint disease. After receipt of the first and second doses, disease flare was reported by 78 patients (6%) and 88 patients (6%), respectively.
Figure 3.
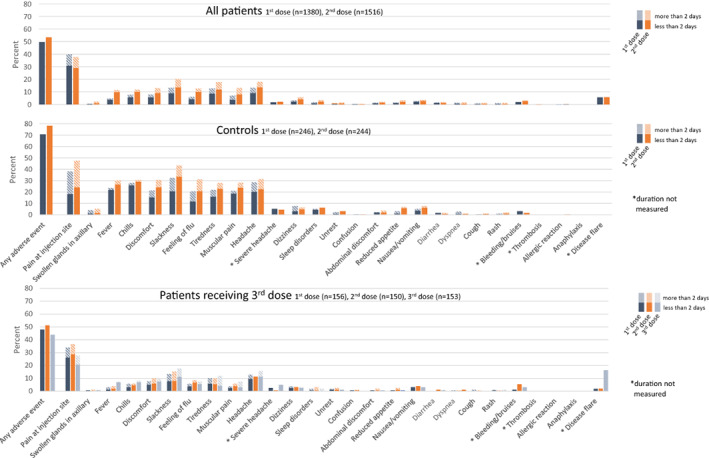
Type and duration of adverse events reported after doses 1 (blue bars) and 2 (orange bars) of SARS–CoV‐2 vaccine among patients with immune‐mediated inflammatory disease (IMID) and healthy controls and after dose 3 (gray bars) among IMID patients who had a weak serologic response (defined as <70 arbitrary units per milliliter) to doses 1 and 2. Adverse events were reported for all patients and a subset of 246 healthy controls described in Patients and Methods. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.42153/abstract.
DISCUSSION
This study, the largest to date on response to the standard 2‐dose SARS–CoV‐2 vaccination regimen in IMID patients receiving immunosuppressive therapy, demonstrated that the percentage of responders and the anti‐RBD antibody level were lower in 1,647 patients as compared to 1,114 healthy controls. Adverse reactions were comparable in the 2 groups. Among patients with a weak serologic response after the standard 2‐dose regimen, the third dose was safe and resulted in a response in most recipients.
The study provides detailed information regarding the impact of commonly used immunosuppressive drugs for inflammatory joint diseases and IBDs on the serologic response to SARS–CoV‐2 vaccines. A difference among the medications was shown, with the lowest proportion of responders observed among recipients of abatacept (50%), JAK inhibitors (78%), TNFi used in combination with methotrexate or azathioprine (86%), and sekukinumab (88%), suggesting a rationale for postvaccination serologic monitoring in patients using these medications. Prior studies regarding the effect of abatacept and JAK inhibitors on the immunogenicity of SARS–CoV‐2 vaccines differ in their conclusions, which may be due to the limited number of patients they evaluated (n = 8–16) (11, 13, 35). Data regarding the effect of TNFi on the immunogenicity of SARS–CoV‐2 vaccines have also been conflicting (5, 10, 11, 12, 13, 35). The Nor‐vaC study included >1,000 TNFi recipients, roughly the same total number previously described across several smaller studies (35). In the present study, attenuated immunogenicity was mainly seen in TNFi recipients receiving combination therapy with azathioprine or methotrexate. These synthetic drugs are known to reduce antidrug antibody responses to the TNF inhibitor itself, and it is reasonable to assume similar effects on vaccine immunogenicity (36).
Despite the relatively high response rates in most medication groups, the median anti‐RBD antibody levels were significantly lower among patients, compared to healthy controls. There is increasing evidence that antibody levels correlate to the degree of clinical protection against breakthrough COVID‐19 (37) and that anti‐RBD antibody levels correlate to SARS–CoV‐2 neutralization levels, with higher levels needed for neutralizing novel virus strains (28, 38). As antibody levels decay over time, it seems likely that patients who attain a weak antibody response after vaccination will have a less durable response (39). Patients with a weak response may also have developed less robust immunologic memory responses (40). Further studies are needed to elucidate whether IMID patients receiving immunosuppressive therapy lose their protective immunity more quickly than the general population.
In addition to medication type, lower age and receipt of mRNA‐1273 were predictors of a serologic response. Prior studies have suggested that mRNA‐1273 may be more immunogenic than BNT162b2 in healthy subjects (41). To our knowledge, this is the first study presenting findings on the immunogenicity of different vaccine types in IMID patients. Subanalyses in TNFi recipients showed similar results.
In the 153 patients receiving a third vaccine dose, a response was induced in the majority of patients. The effectiveness of additional vaccine doses for immunocompromised patients, as well as the utility of booster shots for healthy people, is now being debated in the scientific community (16). Prior data on the immunogenicity of 3 SARS–CoV‐2 vaccine doses in IMID patients who were receiving immunosuppressive drugs other than rituximab and had no response to the standard 2‐dose vaccination regimen consist of case series and small studies (n = 33 and n = 17) and indicated a moderate additional humoral response following receipt of the third dose (19, 23, 24). The present data show a clear benefit in terms of serologic response, while the frequency and profile of reported adverse events were comparable to those observed after receipt of the standard 2‐dose regimen. We did not find that pausing medication benefited vaccine immunogenicity. The humoral response to the third dose was comparable in patients with inflammatory joint diseases, for whom a pause in medication was recommended, and in patients with IBDs, who did not receive this recommendation. Further, self‐reported pausing of medication was not associated with a humoral response to the standard vaccination regimen. These results must be interpreted with caution, however.
There are limited data on the safety of SARS–CoV‐2 vaccines in IMID patients (13, 42). This study supports that these vaccines are safe in an immunosuppressed population, and it demonstrates that the frequency of reported adverse events was lower among IMID patients than among controls, with the same range of adverse events reported in both groups. This finding suggests that immunosuppressive medication might reduce the frequency of adverse events due to SARS–CoV‐2 vaccines and might also reduce the vaccines’ immunogenicity. A major concern has been whether the mRNA SARS–CoV‐2 vaccines may cross‐react with human proteins and aggravate autoimmunity (43). The Nor‐vaC results are reassuring in this regard, as hardly any patients reported a disease flare after receiving the standard 2‐dose vaccination regimen. However, we found a clear increase in disease flares among inflammatory joint disease patients following receipt of the third dose. This was not seen in patients with IBDs. Among patients with inflammatory joint diseases, the increase may have been due to the recommended pause in medication from 1 week before through 2 weeks after receipt of the third dose.
Strengths of this study include the prospective study design, the broad inclusion criteria, the well‐characterized population of patients, and the large sample sizes of patients and controls. A further strength is that the study population was drawn from both gastroenterology and rheumatology settings, enabling assessment of patients across a range of diseases who are being treated with the same medical compounds.
This study has some limitations. First, we did not measure cellular immune responses. The adaptive immune response to SARS–CoV‐2 depends not only on virus‐specific antibodies but also on T cell–mediated responses (44). Further studies are needed to determine if the serologic responses are predictive of protection against severe disease. Second, some medication groups included a low number of patients. Third, controls or patients with a normal antibody response to the standard 2‐dose vaccination regimen were not given a third dose; hence, we could not evaluate the response to and safety of a third dose in these groups. Fourth, the patients were generally older than the controls, raising the possibility of biased results. However, we have corrected for age in all analyses comparing patients and controls. Fifth, full data on comorbidity were not available. Sixth, we cannot exclude the possibility that some of the participants may have had a subclinical SARS–CoV‐2 infection. However, the rate of SARS–CoV‐2 infection in Norway during the relevant period was very low.
The proportion of responders and the anti‐RBD antibody levels were lower among IMID patients as compared to controls following receipt of the standard vaccination regimen. These data facilitate identification of patient groups who are at risk of an attenuated vaccine response and therefore should be considered for postvaccination serologic monitoring. Receipt of a third vaccine dose by patients with a weak response was safe and resulted in a response in most. These results will aid health care systems in the planning and implementation of SARS–CoV‐2 vaccine programs aimed at IMID patients treated with immunosuppressive medication and will aid clinical decision‐making regarding revaccinations and tailoring of medication to keep this vulnerable population protected against severe COVID‐19.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revised in critically for important intellectual content, and all authors approved the final version to be published. Dr. Goll had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Syversen, Jyssum, Warren, Kvien, Munthe, Haavardsholm, Vaage, Lund‐Johansen, Jørgensen, Goll.
Acquisition of data
Syversen, Jyssum, Tveter, Tran, Grødeland, Nissen‐Meyer, Ricanek, Chopra, Andersson, Jahnsen, Munthe, Vaage, Lund‐Johansen, Jørgensen, Goll.
Analysis and interpretation of data
Syversen, Jyssum, Tveter, Sexton, Provan, Mjaaland, Grødeland, Kro, Jahnsen, Munthe, Vaage, Lund‐Johansen, Jørgensen, Goll.
Supporting information
Disclosure Form
Appendix S1 Supporting Information
ACKNOWLEDGMENTS
We thank the patient representatives in the study group—Kristin Isabella Kirkengen Espe, and Roger Thoresen—for their contributions. We also thank all study personnel, laboratory personnel, and other staff involved in the participating clinical departments and at Department of Immunology at Oslo University Hospital, particularly Synnøve Aure (Akershus University Hospital) and May Britt Solem and Kjetil Bergsmark (Diakonhjemmet Hospital).
ClinicalTrials.gov identifier: NCT04798625. EudraCT database no. 2021‐003618‐37.
The Norwegian Study of Vaccine Response to COVID‐19 (Nor‐vaC) was an investigator‐initiated study with no initial funding. During its conduct, study grants were received from the Coalition for Epidemic Preparedness Innovations (CEPI), the K. G. Jebsen Foundation, Dr. Trygve Gythfeldt og frues Foundation, the Karin Fossum Foundation, Diakonhjemmet Hospital Research Foundation, Oslo University Hospital, the University of Oslo, and the Southeastern Norway Regional Health Authority.
Drs. Syversen and Jyssum contributed equally to this work. Drs. Vaage, Lund‐Johansen, Jørgensen, and Goll contributed equally to this work.
A deidentified patient data set can be made available to researchers upon reasonable request. The data will only be made available after submission of a project plan outlining the reason for the request and any proposed analyses, and it will have to be approved by the Nor‐vaC steering group. Project proposals can be submitted to the corresponding author. Data sharing will have to follow appropriate regulations.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Fart.42153&file=art42153‐sup‐0001‐Disclosureform.pdf.
REFERENCES
- 1. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med 2021;384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS‐CoV‐2 infections and COVID‐19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021;397:1819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, et al. Interim findings from first‐dose mass COVID‐19 vaccination roll‐out and COVID‐19 hospital admissions in Scotland: a national prospective cohort study. Lancet 2021;397:1646–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friedman MA, Curtis JR, Winthrop KL. Impact of disease‐modifying antirheumatic drugs on vaccine immunogenicity in patients with inflammatory rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021;80:1255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ungaro RC, Brenner EJ, Gearry RB, Kaplan GG, Kissous‐Hunt M, Lewis JD, et al. Effect of IBD medications on COVID‐19 outcomes: results from an international registry. Gut 2021;70:725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ, Baden LR, Bass AR, et al. American College of Rheumatology guidance for COVID‐19 vaccination in patients with rheumatic and musculoskeletal diseases: version 3. Arthritis Rheumatol 2021;73:e60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. D'Amico F, Rabaud C, Peyrin‐Biroulet L, Danese S. SARS‐CoV‐2 vaccination in IBD: more pros than cons. Nat Rev Gastroenterol Hepatol 2021;18:211–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schett G, McInnes IB, Neurath MF. Reframing immune‐mediated inflammatory diseases through signature cytokine hubs. N Engl J Med 2021;385:628–39. [DOI] [PubMed] [Google Scholar]
- 10. Kennedy NA, Goodhand JR, Bewshea C, Nice R, Chee D, Lin S, et al. Anti‐SARS‐CoV‐2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut 2021;70:865–75. [DOI] [PubMed] [Google Scholar]
- 11. Deepak P, Kim W, Paley MA, Yang M, Carvidi AB, Demissie EG, et al. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS‐CoV‐2: a prospective cohort study. Ann Intern Med 2021;174:1572–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boekel L, Steenhuis M, Hooijberg F, Besten YR, van Kempen ZL, Kummer LY, et al. Antibody development after COVID‐19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol 2021;3:e778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID‐19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021;80:1330–8. [DOI] [PubMed] [Google Scholar]
- 14. Kappelman MD, Weaver KN, Boccieri M, Firestine A, Zhang X, Long MD. Humoral immune response to messenger RNA COVID‐19 vaccines among patients with inflammatory bowel disease. Gastroenterology 2021;161:1340–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Melmed GY, Botwin GJ, Sobhani K, Li D, Prostko J, Figueiredo J, et al. Antibody responses after SARS‐CoV‐2 mRNA vaccination in adults with inflammatory bowel disease. Ann Intern Med 2021;174:1768–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bar‐On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, et al. Protection of BNT162b2 vaccine booster against Covid‐19 in Israel. N Engl J Med 2021;385:1393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patalon T, Gazit S, Pitzer VE, Prunas O, Warren JL, Weinberger DM. Odds of testing positive for SARS‐CoV‐2 following receipt of 3 vs 2 doses of the BNT162b2 mRNA vaccine. JAMA Intern Med 2021;182:179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Del Bello A, Abravanel F, Marion O, Couat C, Esposito L, Lavayssière L, et al. Efficiency of a boost with a third dose of anti‐SARS‐CoV‐2 messenger RNA‐based vaccines in solid organ transplant recipients. Am J Transplant 2021;22:322–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Connolly CM, Teles M, Frey S, Boyarsky BJ, Alejo JL, Werbel WA, et al. Booster‐dose SARS‐CoV‐2 vaccination in patients with autoimmune disease: a case series. Ann Rheum Dis 2021;81:291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Felten R, Gallais F, Schleiss C, Chatelus E, Javier RM, Pijnenburg L, et al. Cellular and humoral immunity after the third dose of SARS‐CoV‐2 vaccine in patients treated with rituximab. Lancet Rheumatol 2021;4:e13–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baker MC, Mallajosyula V, Davis MM, Boyd SD, Nadeau KC, Robinson WH. Effective viral vector SARS–CoV‐2 booster vaccination in a patient with rheumatoid arthritis after initial ineffective messenger RNA vaccine response. Arthritis Rheumatol 2021;74:541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jyssum I, Kared H, Tran TT, Tveter AT, Provan SA, Sexton J, et al. Humoral and cellular immune responses to two and three doses of SARS‐CoV‐2 vaccines in rituximab‐treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol 2021;4:e177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmiedeberg K, Vuilleumier N, Pagano S, Albrich WC, Ludewig B, Kempis JV, et al. Efficacy and tolerability of a third dose of an mRNA anti‐SARS‐CoV‐2 vaccine in patients with rheumatoid arthritis with absent or minimal serological response to two previous doses. Lancet Rheumatol 2022;4:e11–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simon D, Tascilar K, Fagni F, Schmidt K, Krönke G, Kleyer A, et al. Efficacy and safety of SARS‐CoV‐2 revaccination in non‐responders with immune‐mediated inflammatory disease. Ann Rheum Dis 2022;81:1023–7. [DOI] [PubMed] [Google Scholar]
- 25. Tran TT, Vaage EB, Mehta A, Chopra A, Kolderup A, Anthi AK, et al. Multiplexed measurement of binding‐ and neutralizing antibodies to SARS‐CoV‐2 variants in 12.000 post‐vaccine sera [preprint]. BioRxiv 2022. doi: 10.1101/2022.03.26.484261:2022.03.26.484261. E‐pub ahead of print. [DOI] [Google Scholar]
- 26. Holter JC, Pischke SE, de Boer E, Lind A, Jenum S, Holten AR, et al. Systemic complement activation is associated with respiratory failure in COVID‐19 hospitalized patients. Proc Natl Acad Sci U S A 2020;117:25018–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. König M, Lorentzen ÅR, Torgauten HM, Tran TT, Schikora‐Rustad S, Vaage EB, et al. Humoral immunity to SARS‐CoV‐2 mRNA vaccination in multiple sclerosis: the relevance of time since last rituximab infusion and first experience from sporadic revaccinations. J Neurol Neurosurg Psychiatry 2021. doi: 10.1136/jnnp-2021-327612. E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nguyen D, Simmonds P, Steenhuis M, Wouters E, Desmecht D, Garigliany M, et al. SARS‐CoV‐2 neutralising antibody testing in Europe: towards harmonisation of neutralising antibody titres for better use of convalescent plasma and comparability of trial data. Euro Surveill 2021;26:2100568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Norwegian Institute of Public Health . Norwegian Immunisation Registry (SYSVAK), 2021. URL: https://www.fhi.no/en/hn/health-registries/norwegian-immunisation-registry-sysvak/. [Google Scholar]
- 30. Norwegian Institute of Public Health . Norwegian Surveillance System for Communicable Diseases (MSIS), 2019. URL: https://www.fhi.no/en/hn/health-registries/msis/. [Google Scholar]
- 31. Prevoo ML, van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty‐eight–joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 32. Lukas C, Landewé R, Sieper J, Dougados M, Davis J, Braun J, et al, for the Assessment of Spondyloarthritis International Society. Development of an ASAS‐endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:18–24. [DOI] [PubMed] [Google Scholar]
- 33. Harvey RF, Bradshaw JM. A simple index of Crohn's‐disease activity. Lancet 1980;315:514. [DOI] [PubMed] [Google Scholar]
- 34. Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis 2008;14:1660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jena A, Mishra S, Deepak P, Kumar MP, Sharma A, Patel YI, et al. Response to SARS‐CoV‐2 vaccination in immune mediated inflammatory diseases: systematic review and meta‐analysis. Autoimmun Rev 2021;21:102927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Atiqi S, Hooijberg F, Loeff FC, Rispens T, Wolbink GJ. Immunogenicity of TNF‐inhibitors [review]. Front Immunol 2020;11:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gilbert PB, Montefiori DC, McDermott AB, Fong Y, Benkeser D, Deng W, et al. Immune correlates analysis of the mRNA‐1273 COVID‐19 vaccine efficacy clinical trial. Science 2022;375:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cromer D, Steain M, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralising antibody titres as predictors of protection against SARS‐CoV‐2 variants and the impact of boosting: a meta‐analysis. Lancet Microbe 2022;3:e52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Okamoto M, Kawada S, Fujii N, Matsukawa K, Shimagami H, Ishikawa N, et al. Rapid attenuation of anti–SARS–CoV‐2 antibody in patients with musculoskeletal diseases who reinitiated intensive immunosuppressive therapies after COVID‐19. Arthritis Rheumatol 2022;74:726–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS‐CoV‐2 assessed for up to 8 months after infection. Science 2021;371:eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS‐CoV‐2 antibody response following vaccination with BNT162b2 and mRNA‐1273. JAMA 2021;326:1533–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Botwin GJ, Li D, Figueiredo J, Cheng S, Braun J, McGovern DPB, et al. Adverse events after SARS‐CoV‐2 mRNA vaccination among patients with inflammatory bowel disease. Am J Gastroenterol 2021;116:1746–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vojdani A, Kharrazian D. Potential antigenic cross‐reactivity between SARS‐CoV‐2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol 2020;217:108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ni L, Ye F, Cheng ML, Feng Y, Deng YQ, Zhao H, et al. Detection of SARS‐CoV‐2‐specific humoral and cellular immunity in COVID‐19 convalescent individuals. Immunity 2020;52:971–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Appendix S1 Supporting Information


