Abstract
Background
A growing proportion of transplant donors and recipients have a history of COVID‐19 infection. This study sought to characterize clinical practice after recipient or donor COVID‐19 infection.
Methods
An online survey was distributed to heart transplant clinicians through a professional society message board and social media. Responses were collected between September 29 and November 5, 2021.
Results
There were 222 health care professionals (68% transplant cardiologists, 22% transplant surgeons, 10% other) across diverse geographic regions who completed the survey. While there was significant variation in donor acceptance, as it relates to past and current COVID‐19 infection, the respondents were fairly cautious: 28% would not typically accept a donor with a history of COVID‐19 regardless of the infection course and > 80% would not accept donors who had evidence of myocardial dysfunction during past COVID‐19 infection, or who died of COVID‐19 or its complications. The timing of candidate reactivation on the waiting list after COVID‐19 infection also varied and often diverged from scenarios addressed by social guidelines. Eighty‐one percent of the respondents felt COVID‐19 vaccine should be mandatory before transplant, but this rate varied by geographic region.
Conclusion
Our results reflect evolving experience of the heart transplant field at a time of lack of high‐quality evidence. In the absence of longer‐term outcome data for donors and transplant candidates with history of COVID‐19 infection, clinicians remain cautious; however, this approach will likely need to be refined as an increasing proportion of the population will continue to be infected with COVID‐19.
Keywords: COVID vaccination, COVID‐19, heart transplantation, ISHLT
1. BACKGROUND
An increasing proportion of the general population has experienced an infection with the novel coronavirus SARS‐CoV2 (COVID‐19), 1 and the infection rate, including with new viral variants, remains high. 2 , 3 COVID‐19 infection can result in endothelial dysfunction and myocardial injury. 4 , 5 As a consequence, a significant number of patients recovered from COVID‐19 infection will continue to have abnormal left ventricular strain on echocardiography, 6 and reduction in peak oxygen uptake. 7 The evolving understanding of COVID‐19 effects on cardiovascular function represent an area of significant uncertainty, as it relates to heart donor selection, to include the risk of COVID‐19 infection transmission from the donor to the recipient and the potential risk of post‐transplant allograft dysfunction resulting from COVID‐19 myocardial injury in the donor.
Transplant societies have issued guidelines to assist clinicians in decisions regarding donor selection and recipient waitlist activation after COVID‐19 infection. 8 The recommendations are based on expert opinion and were developed by interpretation of rapidly evolving observational experiences. Using a survey instrumentation methodology, we sought to characterize clinical decision making in the following domains: a) acceptance of donors with history of COVID‐19 infection, b) timing of heart transplant candidate activation on the waiting list after a COVID‐19 infection, and c) requirement of COVID‐19 vaccination as a prerequisite for transplant listing.
2. METHODS
An initial set of questions to be used in the survey was proposed by BS and J.S, and was further evaluated and refined by a subset of authors. Questions addressing specific areas of clinical uncertainty were identified during University of Utah clinical multidisciplinary heart transplant team discussions between June and August 2021. This process led to a final set of multiple‐choice questions that were included in an online survey distributed to heart transplant clinicians through a professional societies message board (International Society for Heart and Lung Transplant, Heart Failure Society of America, and American Society of Transplantation) and social media. This approach was used to target the geographically diverse cohort of surgeons, cardiologists and other team members involved in clinical decisions who are reached by these societies. Responses were collected between September 29 and November 5, 2021, through an online survey platform (SurveyMonkey). The survey was anonymous and no identifying information was collected beyond respondent profession and geographic location. Submission of answers from a unique computer was possible only once.
The questions addressed clinical decision making regarding relevant scenarios in acceptance of donors with a history of COVID‐19 infection, timing of heart transplant candidate listing after a COVID‐19 infection, and the attitude toward requirement of COVID‐19 vaccination before transplant listing. In all the donor scenarios, the cardiac function at the time of donor assessment was presented as normal.
3. RESULTS
Among 222 respondents from diverse geographic regions, there were 152 transplant cardiologists, 50 transplant surgeons, and 20 other clinicians directly involved in the heart transplant process (infectious disease and intensive care physicians and transplant coordinators) Table 1. Two hundred nineteen responders completed the entire survey, three responders skipped one to two questions.
TABLE 1.
Baseline characteristics
| N (%) | |
|---|---|
| Respondents | 222 (100%) |
| Profession | |
| ‐ Transplant Cardiologist | 152 (68.5%) |
| ‐ Transplant Surgeon | 50 (22.5%) |
| ‐ Other Heart Transplant Clinician | 20 (9%) |
| Geographic Region | |
| ‐ North America | 101 (45.5%) |
| ‐ Europe | 52 (23.4%) |
| ‐ Central/South America | 49 (22.1%) |
| ‐ Other | 20 (9%) |
*other – Australia, Middle East, Africa, Asia Pacific.
** Infectious disease specialist, Intensive care unit specialist, transplant coordinator, nurse practitioner.
The results are presented in Figures 1 and 2. The main findings were:
Twenty‐eight percent (63/222) of respondents would not typically accept a heart from a COVID‐19 recovered donor even if the cause of death was unrelated to COVID‐19 complications and cardiac function is normal (Figure 1A);
Eighty percent (176/219) would not typically accept a heart from a donor who was considered COVID‐19 recovered but died as a result of COVID‐19 complications, without evidence of myocardial dysfunction at any point (Figure 1B);
Eighty‐six percent (190/222) of clinicians would not typically accept a heart from a COVID‐19 recovered donor where cause of death was unrelated to COVID‐19 complication and current cardiac function in normal, but the donor had evidence of myocardial dysfunction during active COVID‐19 infection (Figure 1C);
Eighty‐eight percent (195/222) would not accept a heart from a donor who died due to active COVID ‐19 infection without evidence of myocardial dysfunction at any point;
Sixty‐six percent (145/220) of clinicians follow current ISHLT consensus recommendations regarding activation on the list for heart transplant after COVID‐19 infection (clinical resolution and > 21 days from onset of symptoms and one negative PCR test), while 27% extend this period and 7% activate sooner if PCR is negative (Figure 1D).
Eighty‐one percent (179/220) felt COVID‐19 vaccine should be mandatory before transplant listing; this response varied by geographic region (Figure 2) with 65% of respondents from Europe, 79% from North America, and 94% from Central/South America, respectively, supporting this requirement (P = .001).
FIGURE 1.
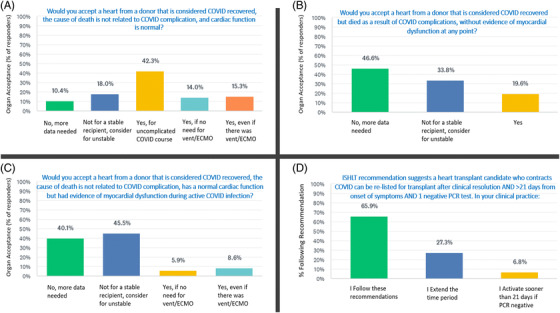
Variation in heart transplant practice after donor of recipient COVID‐19 infection
FIGURE 2.
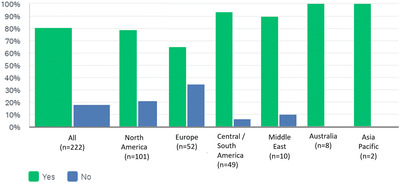
Do you believe COVID vaccination should be mandatory before listing for heart transplantation?
4. DISCUSSION
We conducted a survey among heart transplant clinicians across global geographic regions to assess clinical decision‐making with respect to common scenarios in donor acceptance, recipient COVID vaccinations, and recipient activation while awaiting heart transplantation during the COVID‐19 pandemic. We identified significant variations regarding willingness to accept donors with history of COVID‐19 infection. Across the presented scenarios, respondents were more likely to accept a heart from a donor with recent or recovered COVID‐19 infection for unstable transplant candidates, albeit in the absence of known cardiac involvement during the sentinel COVID‐19 infection.
We hypothesize that during the time period covered by this survey clinicians were primarily concerned with the risk for COVID‐19 transmission during transplantation and the possible long‐term impact of previous COVID‐19 infection on donor myocardial function.
The data available to date suggests that donor to recipient COVID‐19 infection transmission resulting in symptomatic disease in the recipient is rare 9 and limited to lung transplantation, with no evidence for COVID‐19 transmission during nonlung solid organ transplantation, including heart transplantation. Since the relatively less virulent Omicron COVID‐19 variant became predominant, some programs moved to using organs from selected COVID‐19 positive donors, and treating recipients prophylactically for COVID‐19 after transplant. This practice has so far been mostly limited to nonthoracic organ transplant. 10 , 11
Whether the cardiovascular effects of COVID‐19 infection in the donor could have short‐term or long‐term implications for the health the transplanted allograft, 12 , 13 , 14 and whether post‐transplant immunosuppression could mitigate some of the possible COVID‐19 related inflammatory changes in the myocardium is not known. There is lack of data linking donor COVID‐19 infection post‐transplant graft dysfunction.
In addition to the above considerations, institutional approach to risk acceptance or aversion, and donor availability in the respective regions, may further impact organ acceptance decisions.
We also identified variations in the approach to activation of transplant candidates on the waiting list after COVID‐19 infection, with the majority following the current ISHLT consensus guidance. Yet, 27% of respondents extend the recommended recovery period and 7% shorten it as long as the candidate's COVID‐19 test is negative. This highlights uncertainty regarding the potential for reactivation of COVID‐19 infection or reinfection after exposure to immunosuppression.
Finally, we addressed the respondent's opinions regarding the need for COVID‐19 vaccination prior to listing. In August 2021, the American Society of Transplantation (AST) and ISHLT released a statement that strongly recommended that solid organ transplant candidates be vaccinated before transplant. 15 While most respondents (81%) agreed with this recommendation, this was not unanimous. Our data suggest that beyond scientific information, societal attitudes and public discourse in the corresponding countries influence the views of clinicians regarding COVID‐19 vaccine requirements. Further it is unknown what the right duration of post vaccine observation should be prior to listing or transplant and whether post‐transplant attenuation of vaccine effect occurs.
Our studies have important limitations. As this was an online survey, we do not know the absolute response rate and the results are subject to responder bias. Multiple responses may have been obtained from within the same program and this could have entered some degree of bias in strength of a particular finding. Since respondent anonymity was maintained, we do not have a means to adjust for this limitation. Although we had a formal process for the design of the questionnaire, we did not formally test for questions for validity.
The data in this paper reflect clinician experience with the initial viral strains, including the Delta variant. As additional variants emerge and COVID‐19 transitions to become an endemic disease, it will be of interest to see how clinician practices evolve.
Some scenarios that may influence decision‐making may include the distinction between donors that died “with covid” rather than from covid, availability of new treatment options and the vaccination rate in the population. Decisions will nonetheless depend on the balance of infectivity, virulence, and increasingly available therapeutic measures.
In summary, our results reflect evolving experience of the heart transplant field at a time of lack of high‐quality evidence. In the absence of longer‐term outcome data for donors and transplant candidates with history of COVID‐19 infection, clinicians remain cautious, however this approach will likely need to be refined as an increasing proportion of the population will continue to be infected with COVID‐19.
Structured studies and large collective outcomes are needed to define the risk of SARS‐CoV‐2 transmission through organ donation, the risk of sustained myocardial injury from COVID‐19 infected heart donors, and the risk of reactivation of SARS‐CoV‐2 among recipients transplanted after recent COVID‐19 infection. Medical societies should actively support research to address these knowledge gaps.
CONFLICT OF INTEREST
Josef Stehlik reports consulting for Natera, Medtronic and Sanofi‐Aventis. Mandeep R. Mehra reports payment to his institution from Abbott for consulting. Consulting fees from Mesoblast, Janssen, Broadview ventures, Moderna, Natera, Paragonix, and Baim Institute for Clinical Research. He is an advisory board member for NuPulseCV, Leviticus and FineHeart. Kevin Shah reports Grant funding related to COVID‐19 research from the University of Utah immunology, inflammation and infectious disease (3I) initiative, outside of submitted work. None of the other authors had financial conflicts of interest to report.
None of the other authors had financial conflicts of interest to report.
Sadeh B, Ugolini S, Pinzon OW, et al. Medical decisions in organ donors and heart transplant candidates with history of COVID‐19 infection: An international practice survey. Clin Transplant. 2022;36:e14733. 10.1111/ctr.14733
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. https://covid19.who.int/
- 2. https://covid.cdc.gov/covid‐data‐tracker/#datatracker‐home#trends_totalcases_totalcasesper100k|tot_cases|incidence
- 3. https://www.cdc.gov/coronavirus/2019‐ncov/science/forecasting/forecasting‐us.html
- 4. Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID‐19). JAMA Cardiol. 2020; 5(11): 1265‐1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rajpal S, Tong MS, Borchers J, et al. Cardiovascular Magnetic Resonance Findings in Competitive Athletes Recovering From COVID‐19 Infection. JAMA Cardiol. 2021; 6(1): 116‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baruch G, Rothschild E, Sadon S, et al. Evolution of right and left ventricle routine and speckle‐tracking echocardiography in patients recovering from coronavirus disease 2019: a longitudinal study. Eur Heart J Cardiovasc Imaging. 2021. Sep 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Szekely Y, Lichter Y, Sadon S, et al. Cardiorespiratory Abnormalities in Patients Recovering from Coronavirus Disease 2019. J Am Soc Echocardiogr. 2021; 34(12): 1273‐1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. ISHLT/AST Joint Statement About Deceased donor and recipient selection for cardiothoracic transplantation during the COVID‐19 pandemic (14 April 2021) https://ishlt.org/ishlt/media/documents/COVID‐19_GuidanceDocument_Deceased‐donor‐and‐recipient‐selection‐for‐cardiothoracic‐transplantation.pdf
- 9. Kaul DR, Valesano AL, Petrie JG, et al. Donor to recipient transmission of SARS‐CoV‐2 by lung transplantation despite negative donor upper respiratory tract testing. Am J Transplant. 2021; 21(8): 2885‐2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Molnar MZ, Hall IE, Raghavan D, et al. Kidney transplantation from SARS‐CoV‐2‐positive deceased donor. Am J Transplant. 2022; 22(4): 1280‐1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jayasekera CR, Vikram HR, Rifat Z, et al. Solid Organ Transplantation From SARS‐CoV‐2‐infected Donors to Uninfected Recipients: a Single‐center Experience. Transplant Direct. 2022; 8(2): e1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petersen EL, Goßling A, Adam G, et al. Multi‐organ assessment in mainly non‐hospitalized individuals after SARS‐CoV‐2 infection: the Hamburg City Health Study COVID programme. Eur Heart J. 2022; 43(11): 1124‐1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xie Y, Xu E, Bowe B, et al. Long‐term cardiovascular outcomes of COVID‐19. Nat Med. 2022. Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Siripanthong B, Asatryan B, Hanff TC, et al. The Pathogenesis and Long‐Term Consequences of COVID‐19 Cardiac Injury: state‐of‐the‐Art Review. JACC Basic Transl Sci. 2022; 10. published online ahead of print, 2022 Feb 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. ISHLT/AST Joint Statement about COVID‐19 vaccination in organ transplant candidates and recipients. https://www.myast.org/joint‐statement‐about‐covid‐19‐vaccination‐organ‐transplant‐candidates‐and‐recipients
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


