Abstract
Background and Objective
The SARS‐CoV‐2 Omicron variant displays increased infectiveness as well as mutations resulting in reduced neutralizing activity of antibodies acquired after vaccination or infection involving earlier strains. To assess the ability of vaccinated COVID‐19 convalescent plasma (CCP‐V) collected before November 2021 to seroneutralize Omicron, we compared neutralizing antibody (nAb) titres of 63 samples against Omicron and earlier B.1 (D614G) strains.
Methods and Findings
Relationship between anti‐Omicron titres and IgG anti‐S1 levels (binding arbitrary unit: BAU/ml) was studied. Although correlated, anti‐Omicron titres were significantly lower than anti‐B.1 titres (median = 80 [10–1280] vs. 1280 [160–10,240], p < 0.0001). Omicron nAb titres and IgG anti‐S1 levels were correlated (Spearman's rank correlation coefficient = 0.67). Anti‐S1 IgG threshold at 7000 BAU/ml may allow to discard CCP‐V without anti‐Omicron activity (nAb titre <40). Conversely, only those with highest titres (≥160) had systematically anti‐S1 IgG levels >7000 BAU/ml.
Conclusion
A fraction of CCP‐V collected before November 2021 retains anti‐Omicron seroneutralizing activity that may be selected by quantitative anti‐IgG assays, but such assays do not easily allow the identification of ‘high‐titre’ CCP‐V. However, collecting plasma from vaccinated donors recently infected with Omicron may be the best option to provide optimal CCP‐V for immunocompromised patients infected with this variant.
Keywords: convalescent plasma, neutralizing antibodies, Omicron, SARS‐CoV‐2, vaccination
Highlights.
Omicron neutralizing antibody titres were significantly lower than anti‐B.1 titres in vaccinated COVID‐19 convalescent plasma (CCP‐V) collected before November 2021.
A fraction of CCP‐V collected before November 2021 retains anti‐Omicron seroneutralizing activity that may be selected by quantitative anti‐IgG assays.
Providing CCP with potent anti‐Omicron activity may require collecting CCP from vaccinated donors who have recovered from an Omicron infection.
INTRODUCTION
The emergence and swift spreading of the Omicron variant (B.1.1.529) of the SARS‐CoV‐2 virus in November 2021 have raised concern due to the high number of mutations (>30) deletions and insertions in its genome when compared to the D614G strain [1]. Most of these mutations are concentrated on functional epitopes of the spike receptor binding domain (RBD), resulting in a significant risk of immune evasion [2]. Furthermore, the infectivity of the Omicron has been estimated to be 13 and 2.8 times higher than that of the original and delta strains, respectively [3]. Accordingly, the number of Omicron infections has skyrocketed worldwide since the end of 2021. In France, at the end of January 2022, more than 95% of new infections were caused by Omicron [4]. COVID‐19 convalescent plasma (CCP), notably high‐titre CCP may improve clinical outcomes, in particular when administered to high‐risk patients early after symptoms onset [5], as well as to immunosuppressed patients throughout their disease [6, 7]. Assessing the Ab‐mediated neutralizing activity in CCP collected prior to the Omicron wave is critical when considering the use of such CCP to treat patients infected with the Omicron variant.
MATERIALS AND METHODS
CCP donors
The strategy for selecting CCP donors has been described recently [8]. The sample panel was drawn from 63 plasmas collected between 10 June and 21 September 2021 from donors (sex ratio M/F = 2.15; mean age = 41.2 years old [19–65]) who had been infected with SARS‐CoV‐2 and subsequently vaccinated (CCP‐V). All had a complete vaccination schedule (at the time, one dose of vaccine for individuals with a history of SARS‐CoV‐2 infection) and none had received a booster dose. The mean time from clinical onset to donation was 325 days (n = 53, median = 321 days [45–554]). The mean time from vaccination to donation was 76.6 days (n = 63, median = 70 days [13–215]).
Anti‐SARS‐CoV‐2 IgG testing
Samples were tested for specific anti‐SARS‐CoV‐2 antibodies using three ELISA assays according to the manufacturer's instructions. Anti‐S1 IgG screening was performed using the ‘ELISA SARS‐CoV‐2 IgG’ Euroimmun test [8], anti‐S1 IgG were quantified with the Euroimmun Anti‐SARS‐CoV‐2 QuantiVac ELISA (IgG) assay and IgG antibodies targeting the nucleocapsid (anti‐N) were tested by the Euroimmun SARS‐CoV‐2‐NCP (IgG) ELISA.
Seroneutralization testing
Neutralizing antibodies (nAbs) were detected using a virus neutralization test (VNT) as previously described [9]. We used VeroE6 cells expressing the human transmembrane serine protease 2 (TMPRSS2) cultured in 96‐well microplates, 100 TCID50 of SARS‐CoV‐2 and serial dilutions of plasma (1/10 to 1/20,480). Virus strains included the ancestral D614G BavPat1 European strain (B.1 lineage) and a French clinical strain of Omicron. Specimens with a VNT titre ≥40 were considered positive for both strains.
Statistical analysis
Paired data (VNT titres against B.1 and Omicron strains) were compared using the Wilcoxon signed‐rank test, and Spearman's rank correlation coefficients were calculated between titres against Omicron and titres against B.1, anti‐SARS‐CoV‐2 IgG anti‐S1 and time since vaccination. Other comparisons were made using the Wilcoxon test. Statistical analyses were conducted in SAS, version 9.4 (SAS Institute, Cary, NC). The cut‐off for significant difference was p < 0.05.
RESULTS
Neutralizing activity of anti‐SARS‐CoV‐2 Abs
The nAb titres against Omicron and against B1 were correlated (Spearman test = 0.73, p > 0.0001, Figure 1a). However, serum neutralization titres of CCP‐V were significantly (p < 0.0001) lower against the Omicron strain (median = 80 [10–1280]) than against the B.1 strain (median = 1280 [160–10,240]) (Table 1, Figure 1b), corresponding to a mean 4 log2 reduction. Notably, 8 (12.7%) of the 63 tested samples had no anti‐Omicron neutralizing activity (titre <40), although they neutralized B.1 strain at titres ranging from 160 to 1280. Among the 55 samples with anti‐Omicron neutralizing activity (titre ≥40), 22 (40%) had titres ≥160, the lowest anti‐B.1 titre observed in the studied panel. The highest nAb titres were 1280 and 10,240 for Omicron and B.1 strains, respectively.
FIGURE 1.
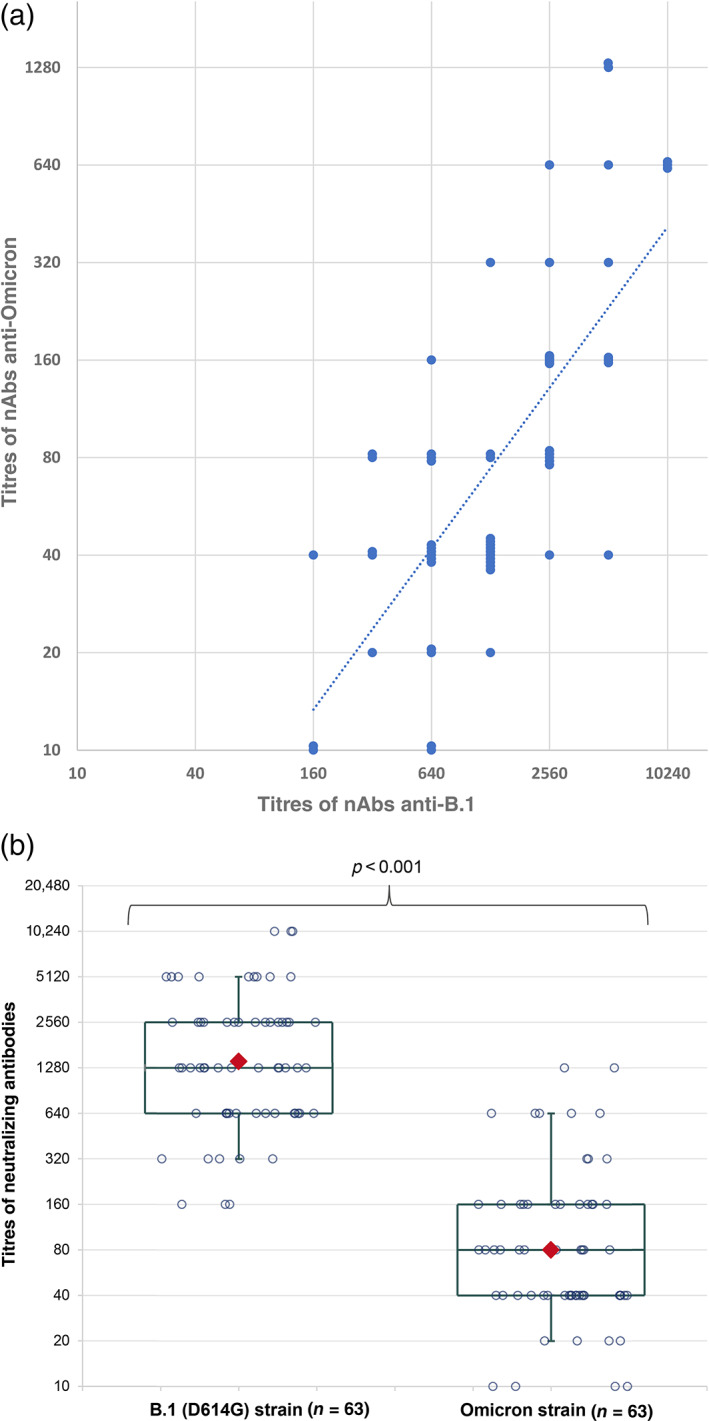
Neutralizing activity of vaccinated COVID‐19 convalescent plasma (CCP‐V) antibodies against B.1 and Omicron strains. (a) Titres correlation between both strains. Titres are expressed in log2 scale, and the power trend line is provided (y = 0.2029x 0.8248, R 2 = 0.54). (b) Distribution of titres (in log2 scale) against B.1 and Omicron strain. The boxes represent the median and interquartile range. The whiskers represent the 10th and 90th percentiles. The red diamonds indicate the geometric mean
TABLE 1.
Titres in CCP‐V assessed by VNT for B.1 and Omicron strains and quantitative levels of anti‐S‐1 antibodies: geometric mean, median and range
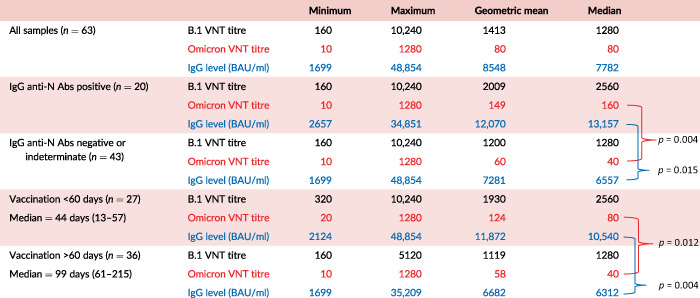
|
Note: Median values in subpopulations were compared using the Wilcoxon test.
Abbreviations: Abs, antibodies; CCP‐V, vaccinated COVID‐19 convalescent plasma; VNT, virus neutralization test.
Association between anti‐Omicron titres and anti‐SARS‐CoV‐2 IgG
Anti‐S1 IgG levels of CCP‐V averaged 11,480 BAU/ml [1699–48,854 BAU/ml]. Anti‐Omicron titres and quantitative anti‐S1 IgG were correlated as depicted in Figure 2a (Spearman's rank correlation coefficient = 0.67, p < 0.001). The eight samples with no anti‐Omicron seroneutralizing activity (titre <40) ranged from 3447 to 6290 BAU/ml. A receiver operating characteristic (ROC) plot analysis conducted to predict anti‐Omicron titres ≥40 from anti‐S1 IgG levels revealed a ROC plot area under curve at 0.8636. Using the Youden index, the best threshold was at ≥6300 BAU/ml (sensitivity, 39/55 = 0.71%; specificity, 8/8 = 100%). Considering a 7000 BAU/ml threshold, we observed that all samples (n = 28) with more than 7000 BAU/ml exhibited anti‐Omicron nAb titres ≥40, but above this threshold, the distribution of titres was heterogeneous with a poor correlation between BAU levels and nAb titres. Only samples with anti‐Omicron titres ≥160 (n = 22) (160: n = 12; 320: n = 3; 640: n = 5 and 1280: n = 2) had systematically IgG anti‐S1 levels >7000 BAU/ml. Below 7000 BAU/ml, 20 samples had anti‐Omicron nAb titres between 40 and 80.
FIGURE 2.
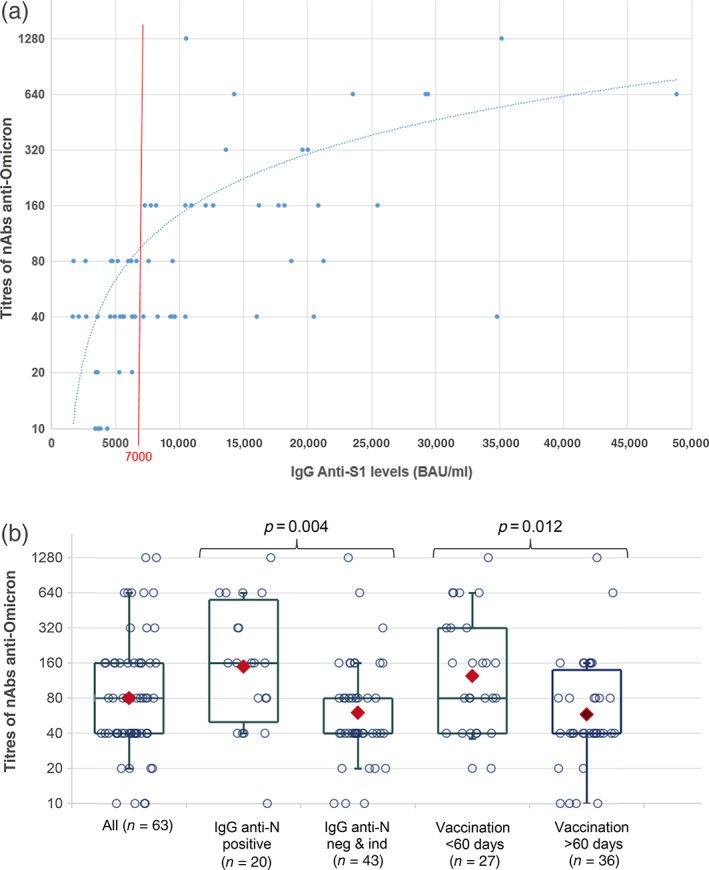
Distribution of SARS‐CoV‐2 anti‐Omicron neutralizing antibody (nAb) titres (log2 scale). (a) According to the IgG anti‐S1 levels (BAU/ml), trend curve is provided (y = 0.016x − 16.563, R 2 = 0.3364); (b) according to the presence of IgG anti‐N and a delay < or ≥60 days between vaccination and plasma donation. The boxes represent the median and interquartile range. The whiskers represent the 10th and 90th percentiles. The red diamonds indicate the geometric mean
In addition, median anti‐Omicron nAb titre was significantly higher (p = 0.004) in anti‐N positive samples (median = 160, n = 20) than in anti‐N negatives or indeterminates (median = 40, n = 43) (Table 1, Figure 2b).
Association between anti‐Omicron titres and time between vaccination and donation
We observed a weak negative correlation between anti‐Omicron titres and the number of days between vaccination and plasma donation (Spearman's rank correlation coefficient = −0.38, p = 0.002). Anti‐Omicron nAb titres were significantly (p = 0.012) higher among donors who reported a vaccination <60 days prior to donation (median titre = 80) versus those with a vaccination delay >60 days prior to donation (median titre = 40) (Table 1, Figure 2b). The anti‐S1 IgG levels of donors vaccinated less than 60 days prior to donation were significantly higher (p = 0.004) than those vaccinated more than 60 days prior to donation (Table 1).
DISCUSSION
The impact of the mutations observed in the genome of the SARS‐CoV‐2 Omicron variant, especially those described in the RBD of the spike viral protein, and its increased contagiousness compared to the previous circulating strains required a re‐evaluation of the anti‐SARS‐CoV‐2 potency of available vaccines and therapeutic antibodies to treat patients with COVID‐19. It has been reported that most available therapeutic antibodies lose their activity against the Omicron variant [10, 11]. Moreover, there was a substantial decrease in neutralizing titres after primary course vaccination with some vaccinated exhibiting undetectable neutralization activity against Omicron [12]. Similar findings were reported in convalescent patients infected before the Omicron wave [10, 13]. However, individuals who had received a booster vaccination [10, 13, 14, 15] or who have been vaccinated after being previously infected [13, 16, 17] exhibited lesser decreases of anti‐Omicron titres when compared to titres in individuals after two vaccines doses or in non‐vaccinated convalescent patients. As therapeutic resources are limited to treat patients infected with Omicron, availability of CCP active against this variant may provide a therapeutic solution in immunocompromised patients, notably B‐cell depleted patients [6, 7, 18, 19].
Anti‐Omicron neutralizing activity of CCP‐V collected prior to the circulation of the Omicron variant was found significantly diminished (by approximately a factor of 10) compared to the wild‐type strain. This is in agreement with recently reported 8.4‐ to 53‐fold decrease of anti‐Omicron neutralizing activity when compared to the D614G strain, including in vaccinated convalescent individuals [13, 20, 21].
Therefore, the selection criteria for CCP should be revised. Our data suggest that an anti‐S1 IgG threshold set at 7000 BAU/ml may help to discard some samples without anti‐Omicron activity (all samples with titres <40 had less than 7000 BAU/ml). Conversely, among samples with anti‐S1 IgG levels >7000 BAU/ml, anti‐Omicron activity was present with nAb titres ranging from 40 to 1280. In our experience, only those with titre ≥160 had systematically anti‐S1 IgG levels >7000 BAU/ml. Overall, despite a correlation between anti‐Omicron nAb titres and anti‐S1 IgG levels (Figure 2a), an anti‐S1 IgG threshold with a strong predictive value for selecting plasmas with high anti‐Omicron nAb titres remain difficult to identify. In addition, although anti‐Omicron titres were overall higher in anti‐N positive individuals or in those who have been vaccinated within the 60 days prior to donation, these parameters could not be used to complement biological selection in order to discriminate CCP‐V with or without anti‐Omicron activity. Our study had some limitations. First, the number of tested CCP‐V was limited and they were likely not fully representative of all collected CCP‐V. Secondly, we have considered a seroneutralization titre of ≥40 as reflecting significant anti‐Omicron activity in vitro, but this may not be the case in vivo after transfusion. Careful assessment of potential relationships between in vitro assessment of CCP‐V potency, volume of plasma transfused, patient status and clinical outcome is of paramount importance. CCP‐V serological screening using quantitative assays calibrated to international standards should be implemented to allow for comparability between studies. While detection of nAbs to Omicron remains the golden standard to quantify Ab‐mediated activity, it remains tedious and unsuitable for large series. Therefore, the development of alternative methods such as anti‐RBD serological tests specific to the Omicron variant should be encouraged. Neutralizing activity of CCP‐V must be verified upon emergence of SARS‐CoV‐2 variants with high propensity to escape the immune response, while taking into account booster(s) vaccine that may enhance CCP‐V potency.
In conclusion, despite the increased seroneutralization titres and widened immune spectrum provided by the vaccination of convalescent donors [22], the activity of the nAbs contained in currently available CCP‐V is considerably diminished against the Omicron variant. Only the fraction CCP‐V with the highest anti‐B.1 nAb titres or anti‐S1 levels (BAU/ml) retain anti‐Omicron seroneutralization activity. It is therefore necessary to evolve the biological and clinical selection criteria to ensure the most appropriate production of CCP for evaluating the treatment of immunocompromised patients infected with the Omicron variant. Providing CCP with potent anti‐Omicron activity may require collecting CCP from vaccinated donors having recovered from an Omicron infection.
CONFLICT OF INTEREST
C.I., P.G., S.L., S.L.C., N.B., L.M., P.R., P.M. and P.T. are employed by the French transfusion public service (Etablissement Français du Sang) in charge of the manufacturing and issuing of blood components in France.
ACKNOWLEDGEMENTS
S.L.C., C.I., N.B., B.P., A.A. and E.N. performed the research; X.L. and P.G. wrote the first draft of the manuscript; S.L., P.R. and P.M. designed the research study; P.G. and L.M. acquired and analysed the data; S.L., X.L., P.M. and P.T. supervised the research and reviewed the manuscript. The authors thank the blood donors for their motivation to contribute to the CCP collection, and the EFS medical staff involved in this project from blood collection to plasma supply management.
Gallian P, Amroun A, Laperche S, Le Cam S, Brisbarre N, Malard L, et al. Reduced neutralizing antibody potency of COVID‐19 convalescent vaccinated plasma against SARS‐CoV‐2 Omicron variant. Vox Sang. 2022;117:971–975.
Funding information Emergency Support Instrument (ESI) of the European Commission, Grant/Award Number: 101021793
REFERENCES
- 1. Callaway E. Heavily mutated Omicron variant puts scientists on alert. Nature. 2021;600:21. [DOI] [PubMed] [Google Scholar]
- 2. Dejnirattisai W, Huo J, Zhou D, Zahradník J, Supasa P, Liu C, et al. SARS‐CoV‐2 Omicron‐B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185:467–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen J, Wang R, Gilby NB, Wei GW. Omicron variant (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. J Chem Inf Model. 2022;62:412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. COVID‐19 Point épidémiologique 27 janvier 2022. N°100. Santé Publique France. Available from: https://www.santepubliquefrance.fr/maladies‐et‐traumatismes/maladies‐et‐infections‐respiratoires/infection‐a‐coronavirus/documents/bulletin‐national/covid‐19‐point‐epidemiologique‐du‐27‐janvier‐2022
- 5. Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, et al. Early high‐titer plasma therapy to prevent severe Covid‐19 in older adults. N Engl J Med. 2021;384:610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thompson MA, Henderson JP, Shah PK, Rubinstein SM, Joyner MJ, Choueiri TK, et al. Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID‐19. JAMA Oncol. 2021;17:1167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hueso T, Godron AS, Lanoy E, Pacanowski J, Levi LI, Gras E, et al. Convalescent plasma improves overall survival in patients with B‐cell lymphoid malignancy and COVID‐19: a longitudinal cohort and propensity score analysis. Leukemia. 2022;36:1025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gallian P, Le Cam S, Brisbarre N, Pastorino B, Amroun A, Malard L, et al. COVID‐19 convalescent plasma: evolving strategies for serological screening in France. Vox Sang. 2021. 10.1111/vox.13228 [DOI] [PubMed] [Google Scholar]
- 9. Gallian P, Pastorino B, Morel P, Chiaroni J, Ninove L, de Lamballerie X. Lower prevalence of antibodies neutralizing SARS‐CoV‐2 in group O French blood donors. Antiviral Res. 2020;181:104880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lusvarghi S, Pollett SD, Neerukonda SN, Wang W, Wang R, Vassell R, et al. SARS‐CoV‐2 Omicron neutralization by therapeutic antibodies, convalescent sera, and post‐mRNA vaccine booster. bioRxiv . 2021; 2021.12.22.473880.
- 11. Wilhelm A, Widera M, Grikscheit K, Toptan T, Schenk B, Pallas C, et al. Reduced neutralization of SARS‐CoV‐2 Omicron variant by vaccine sera and monoclonal antibodies. medRxiv . 2021; 2021.12.07.21267432.
- 12. Dejnirattisai W, Shaw RH, Supasa P, Liu C, Stuart AS, Pollard AJ, et al. Reduced neutralisation of SARS‐CoV‐2 Omicron B.1.1.529 variant by post‐immunisation serum. Lancet. 2022;399:234–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Planas D, Saunders N, Maes P, Guivel‐Benhassine F, Planchais C, Buchrieser J, et al. Considerable escape of SARS‐CoV‐2 variant Omicron to antibody neutralization. Nature. 2022;602:671–5. [DOI] [PubMed] [Google Scholar]
- 14. Garcia‐Beltran WF, St Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML, et al. mRNA‐based COVID‐19 vaccine boosters induce neutralizing immunity against SARS‐CoV‐2 Omicron variant. Cell. 2022;185:457–466.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeng C, Evans JP, Qu P, Faraone J, Zheng YM, Carlin C, et al. Neutralization and stability of SARS‐CoV‐2 Omicron variant. bioRxiv . 2021; 2021.12.16.472934.
- 16. Cele S, Jackson L, Khan K, Khoury D, Moyo‐Gwete T, Tegally H, et al. SARS‐CoV‐2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv . 2012; 2012.2008.21267417.
- 17. Rössler A, Riepler L, Bante D, von Laer D, Kimpel J. SARS‐CoV‐2 Omicron variant neutralization in serum from vaccinated and convalescent persons. N Engl J Med. 2022;386:698–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hueso T, Pouderoux C, Péré H, Beaumont AL, Raillon LA, Ader F, et al. Convalescent plasma therapy for B‐cell‐depleted patients with protracted COVID‐19. Blood. 2020;136:2290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Writing Committee for the REMAP‐CAP Investigators . Effect of convalescent plasma on organ support–free days in critically ill patients with COVID‐19: a randomized clinical trial. JAMA. 2021;326:1690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang L, Li Q, Liang Z, Li T, Liu S, Cui Q, et al. The significant immune escape of pseudotyped SARS‐CoV‐2 variant Omicron. Emerg Microbes Infect. 2022;11:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aggarwal A, Alberto Ospina S, Walker G, Akerman A, Milogiannakis V, Brilot F et al. SARS‐CoV‐2 Omicron: evasion of potent humoral responses and resistance to clinical immunotherapeutics relative to viral variants of concern. medRxiv . 2021; 2021.12.14.21267772. [DOI] [PMC free article] [PubMed]
- 22. Stamatatos L, Czartoski J, Wan YH, Homad LJ, Rubin V, Glantz H, et al. mRNA vaccination boosts cross‐variant neutralizing antibodies elicited by SARS‐CoV‐2 infection. Science. 2021. 10.1126/science.abg9175 [DOI] [PMC free article] [PubMed] [Google Scholar]


