Abstract
Problem
We evaluated eculizumab, a complement protein C5 inhibitor, for treatment of severe COVID‐19 in pregnant and postpartum individuals.
Method of Study
Protocol ECU‐COV‐401 (clinicaltrials.gov NCT04355494) is an open label, multicenter, Expanded Access Program (EAP), evaluating eculizumab for treatment of severe COVID‐19. Participants enrolled at our center from August 2020 to February 2021. Hospitalized patients were eligible if they had severe COVID‐19 with bilateral pulmonary infiltrates and oxygen requirement. Eculizumab was administered on day 1 (1200 mg IV) with additional doses if still hospitalized (1200 mg IV on Days 4 and 8; 900 mg IV on Days 15 and 22; optional doses on Days 12 and 18). The primary outcome was survival at Day 15. Secondary outcomes included survival at Day 29, need for mechanical ventilation, and duration of hospital stay. We evaluated pharmacokinetic and pharmacodynamic data, safety, and adverse outcomes.
Results
Eight participants were enrolled at the Cedars‐Sinai Medical Center, six during pregnancy (mean 30 ± 4.0 weeks) and two in the postpartum period. Baseline oxygen requirement ranged from 2 L/min nasal cannula to 12 L/min by non‐rebreather mask. The median number of doses of eculizumab was 2 (range 1–3); the median time to hospital discharge was 5.5 days (range 3–12). All participants met the primary outcome of survival at Day 15, and all were alive and free of mechanical ventilation at Day 29. In three participants we demonstrated that free C5 and soluble C5b‐9 levels decreased following treatment. There were no serious adverse maternal or neonatal events attributed to eculizumab at 3 months.
Conclusion
We describe use of eculizumab to treat severe COVID‐19 in a small series of pregnant and postpartum adults. A larger, controlled study in pregnancy is indicated.
Keywords: complement system proteins, COVID‐19, eculizumab, pregnancy
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), caused by infection with severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), is a pandemic with over 437 million confirmed cases and 5.9 million deaths as of March 2, 2022. 1 The complement system, one of the first lines of host defense, is critical for recognizing and eliminating novel viral pathogens including SARS‐CoV‐2. 2 Complement activation promotes opsonization of virus and virus‐infected cells and triggers an antiviral inflammatory response, which may cause bystander injury to host cells. 2 , 3 , 4 In COVID‐19, generation of the terminal complement complex C5b‐9 is associated with both parenchymal lung injury and microvascular thrombosis. 5 , 6
Adults with severe COVID‐19 have marked elevation of C5b‐9 levels compared to those with non‐severe COVID‐19 or respiratory failure of other cause. 6 , 7 Case series in non‐pregnant adults with critical COVID‐19 illness have suggested that eculizumab, a C5 inhibitory antibody that prevents formation of C5b‐9, may be effective in treating severe COVID‐19. 8 , 9 , 10 , 11 , 12 However, use of eculizumab for treatment of COVID‐19 in pregnancy has not yet been reported.
Pregnant individuals with COVID‐19 are more likely to be admitted to the intensive care unit (ICU), require mechanical ventilation, and die from their illness, compared with non‐pregnant individuals of similar age. 13 , 14 They also have high rates of cesarean delivery (60%) and preterm birth (42%), and offspring are frequently admitted to the neonatal intensive care unit (NICU, 50%). 15 Because pregnant and breastfeeding people have been excluded from most clinical trials, treatment data are limited. 16 , 17 We sought to evaluate eculizumab for treatment of pregnant or postpartum adults with severe COVID‐19.
2. METHODS
Protocol (ECU‐COV‐401) is an open label, multicenter, Expanded Access Program (EAP), which evaluated eculizumab in the treatment of severe COVID‐19. It was registered by Alexion Pharmaceuticals (now Alexion, AstraZeneca Rare Disease) on clinicaltrials.gov in April 2020 and was opened for enrollment at Cedars‐Sinai Medical Center in August 2020, after Institutional Review Board approval. This EAP was conducted in accordance with the protocol and with the Consensus ethical principles derived from international guidelines including the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines, applicable International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice (GCP) Guidelines, and applicable laws and regulations. For study reporting, we utilized Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Hospitalized adults were eligible if they were ≥18 years of age and had confirmed SARS‐CoV‐2 infection presenting as severe COVID‐19, as evidenced by symptomatic bilateral pulmonary infiltrates on chest imaging and supplemental oxygen requirement due to severe pneumonia, acute lung injury or acute respiratory distress syndrome. Exclusions were mild to moderate disease even if requiring hospitalization, life expectancy <24 h as determined by the treating provider, unresolved Neisseria meningitidis infection, or known hypersensitivity to study drug. Pregnant adults at any gestational age or breastfeeding adults were eligible to participate.
After provision of informed consent and administration of meningococcal vaccines (ACWY conjugate and serogroup B vaccines) and empiric antibiotics (oral penicillin 500 mg twice daily or azithromycin 250 mg daily) for prevention of meningococcal infection, the study drug eculizumab was given on Day 1 (1200 mg IV). Additional doses of eculizumab were given per protocol if the patient remained hospitalized (1200 mg IV on Days 4 and 8; 900 mg IV on Days 15 and 22; optional doses of 900 mg or 1200 mg IV on Days 12 and 18 per investigator decision in consultation with the medical monitor). Study drug was discontinued upon hospital discharge.
Clinical care was guided by the primary physician, who could give additional treatments for COVID‐19 as indicated; per guidelines published by the Society for Maternal Fetal Medicine, 18 the oxygen saturation goal was ≥94% for pregnant participants and ≥92% for postpartum participants. The primary outcome was survival (based on all‐cause mortality) at Day 15. Secondary outcomes included the number of days alive and free of invasive mechanical ventilation at Days 15 and 29, improvement of oxygenation from Days 1 to 15 and Day 29, number of days alive and free of supplemental oxygen at Days 15 and 29, duration of ICU stay and duration of hospitalization.
To evaluate the pharmacokinetic (PK) and pharmacodynamic (PD) profile of eculizumab in participants with COVID‐19, we assessed the change in eculizumab, free C5, and C5b‐9 levels before and after administration of study drug. Samples for PK and PD analysis were collected as feasible but could not be drawn for all participants due to limited Biobank hours for collection and storage of COVID‐19 research specimens during the pandemic. Analytes were measured at a central laboratory through Alexion Pharmaceuticals, and assays to measure serum eculizumab levels, free C5, and C5b‐9 were performed as previously described. 19 , 20 The treatment goal was to maintain eculizumab concentrations >116 μg/ml and free C5 concentrations <.5 μg/ml (defined as complete terminal complement inhibition) at all times following treatment initiation. 19 , 21 We also evaluated clinical laboratory measures before and after treatment, including absolute lymphocyte count, alanine, and aspartate transaminase (ALT, AST), C‐reactive protein (CRP), hemoglobin, lactate dehydrogenase (LDH), platelet count, and serum creatinine.
Data are presented as mean ± standard deviation (SD), median (range), or percentages. Differences in free C5 and C5b‐9 concentrations as well as CRP levels and the absolute lymphocyte count before and after treatment were evaluated by the Wilcoxon rank‐sum test, with significance at p < .05. Baseline oxygen saturation before initiation of oxygen therapy and qSOFA (Quick Sepsis Related Organ Failure Assessment) score before study entry were reported. The qSOFA score assigns 1 point for altered mental status (Glascow Coma Scale score <15), 1 point for respiratory rate ≥22, and 1 point for systolic blood pressure ≤100 mmHg; a qSOFA score of 2‐3 is associated with increased in‐hospital mortality. Safety was assessed by evaluating serious adverse events, laboratory abnormalities, and adverse maternal or neonatal outcomes. Serious adverse events were defined as any untoward medical occurrence that is life‐threatening (patient at risk of death at time of event) or that results in prolongation of hospitalization, permanent disability or death, or the occurrence of congenital anomalies (birth defects). Telephone visits were conducted monthly at 1‐, 2‐, and 3‐months following hospital discharge for long‐term follow‐up and safety assessments. For participants who were pregnant at the time of enrollment, long‐term follow up included collection of delivery and neonatal outcomes, regardless of the length of time from hospital discharge.
Data presented herein are from the Cedars‐Sinai Medical Center and were not extracted from the central study database.
3. RESULTS
Eight pregnant or postpartum participants enrolled from August 2020 to February 2021 at our center and their data are presented. No additional patients were enrolled at our site, no eligible patients declined participation, and all enrolled participants completed the study per protocol. Baseline characteristics of study participants are shown in Table 1. The mean ± SD age and BMI of participants was 32 ± 6 years and 28 ± 4 kg/m2, respectively. Six participants enrolled during pregnancy (mean 30 ± 4 weeks gestation; range 25–35 weeks) and two enrolled in the immediate postpartum period. Participant 7 presented in labor with COVID‐19 at 38 weeks gestation and were enrolled on postpartum day one when she developed severe illness. Participant 8 presented with symptomatic COVID‐19 at 38 weeks gestation and was delivered for oligohydramnios; she enrolled on postpartum Day 1 when her illness became severe. None of the participants were vaccinated against COVID‐19, and none received monoclonal antibodies prior to hospitalization.
TABLE 1.
Baseline participant characteristics
| Characteristic | Value |
|---|---|
| Gestational Age (weeks) a | 30 (25–35) |
| Maternal Age (years) | 32 ± 6 |
| BMI (kg/m2) | 28 ± 4 |
| Nulliparity | 3 (38) |
| Chronic hypertension | 0 (0) |
| Diabetes | 0 (0) |
| Race‐ethnicity | |
| Asian | 2 (25) |
| Hispanic | 3 (38) |
| White | 3 (38) |
Note: Data are median (range), mean ± SD, or n/N (%).
Abbreviations: BMI, body mass index; SD, standard deviation.
Pregnant participants (n = 6).
At baseline, all participants had chest imaging findings suggestive of viral pneumonia and all were receiving supplemental oxygen (range 2 L/min nasal cannula to 12 L/min by non‐rebreather mask). Individual participant data are shown in Table 2. Baseline oxygen saturation before oxygen supplementation ranged from 85% to 93%. The qSOFA score was 2 (high risk), due to respiratory rate ≥22 and systolic blood pressure ≤100 mmHg prior to study entry, in all eight study participants. Clinical laboratory data were consistent with severe COVID‐19, with median (range) absolute lymphocyte count (.83 109/L [.2–1.1], normal >1.0 109/L), CRP (85 mg/L [23–132], normal <5 mg/L), and D‐dimer (1.3 mg/L [.9–4.2], normal <1.0 mg/L). Prior to receiving eculizumab, all participants had abnormal elevation of total complement hemolytic (CH50) activity >60 U/ml, but normal C3 and C4 levels (mean ± SD, C3: 139 ± 26 mg/dl, and C4: 42 ± 10 mg/dl).
TABLE 2.
Baseline pregnancy status, oxygen requirement, and laboratory measures of study participants
| ID a | Pregnant or postpartum | GA or postpartum day | Baseline qSOFA score b (0‐3) | O2 Sat before oxygen therapy | Oxygen therapy at enrollment (per min) | Absolute lymphocyte count (109/L) | CRP (mg/L) | D‐dimer (mg/L) |
|---|---|---|---|---|---|---|---|---|
| 1 | Pregnant | 25w2d | 2 | 92% | 2L NC | .51 | 23.1 | 4.18 |
| 2 | Pregnant | 28w6d | 2 | 93% | 2L NC | .84 | 94.3 | .88 |
| 3 | Pregnant | 35w3d | 2 | 85% | 3L NC | .88 | 63.7 | .94 |
| 4 | Pregnant | 31w3d | 2 | 90% | 2L NC | .58 | 74.8 | 1.12 |
| 5 | Pregnant | 32w0d | 2 | 92% | 10L NRB | 1.11 | 123.9 | 3.59 |
| 6 | Pregnant | 25w3d | 2 | 89% | 12L NRB | .88 | 25.4 | 1.06 |
| 7 | Postpartum | Day 1 | 2 | 87% | 10L NRB | .81 | 132.3 | 1.39 |
| 8 | Postpartum | Day 1 | 2 | 91% | 4L NC | .21 | 129.1 | 1.48 |
Note: Normal reference ranges: absolute lymphocyte count (1.0–4.5 109/L); C‐reactive protein (<5 mg/L); D‐dimer (0–.5 mg/L); CH50 (31–60 U/ml).
Abbreviations: CRP, C‐reactive protein; GA, gestational age; NC, nasal cannula; NRB, non‐rebreather mask; qSOFA, quick sepsis related organ failure assessment.
Participant ID does not correlate with order of enrollment.
The qSOFA score assigns 1 point for altered mental status (Glascow Coma Scale score <15), 1 point for respiratory rate ≥22, and 1 point for systolic blood pressure ≤100 mmHg; a qSOFA score of 2‐3 is associated with increased in‐hospital mortality. All study participants had 2 points for respiratory rate and blood pressure parameters.
The median number of doses of eculizumab was 2 (range 1–3); one participant required a single dose on Day 1, five participants required two doses (Day 1, Day 4), and two participants required three doses (Day 1, Day 4, Day 8), Figure 1. Participants received other treatments for COVID‐19 as the standard of care, including intermittent prone positioning (or modified prone positioning for pregnancy), remdesivir (n = 7) and corticosteroids (n = 6), as detailed in Table 3. All participants who received remdesivir completed the standard 5‐day inpatient treatment course (200 mg IV × 1d, followed by 100 mg IV × 4d). Corticosteroid regimens varied slightly between participants, with dexamethasone dosing limited to 48 h in pregnant individuals to limit fetal exposure to corticosteroids. After 48 h of dexamethasone, pregnant participants received either methylprednisolone or prednisone, because of their decreased placental transfer. None of the study participants received other experimental agents such as hydroxychloroquine or tocilizumab.
FIGURE 1.
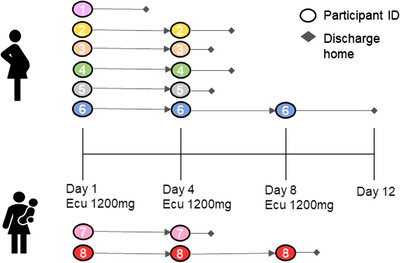
Study flow diagram among pregnant and postpartum participants. Ecu, eculizumab
TABLE 3.
Treatment for COVID‐19, disease course and final disposition of study participants
| ID a | Eculizumab doses (study day) | RDV b | Corticosteroids b | Peak oxygen requirement (per min) | IMV or ICU | Total hospital days | Final disposition |
|---|---|---|---|---|---|---|---|
| 1 | Day 1 | No | None | 2L NC | No | 6 | Home: room air |
| 2 | Day 1, 4 | Yes | None | 3L NC | No | 10 | Home: room air |
| 3 | Day 1, 4 | Yes | Dex 6 mg q12 h × 4, then MP 32 mg × 3 d | 4L NC | No | 6 | Home: room air |
| 4 | Day 1, 4 | Yes | Dex 6 mg q12 h × 4, then Prednisone 40 mg × 4 d | 3L NC | No | 7 | Home: oxygen 2 weeks |
| 5 | Day 1, 4 | Yes | Dex 6 mg q12 h × 4, then MP 32 mg × 3 d | 10L NRB | No | 6 | Home: room air |
| 6 | Day 1, 4, 8 | Yes | Dex 6 mg q12 h × 4, then MP 32 mg × 8 d | 12L NRB | No | 13 | Home: room air |
| 7 | Day 1, 4 | Yes | Dex 6 mg × 5 d | 10L NRB | No | 7 | Home: oxygen 1 week |
| 8 | Day 1, 4, 8 | Yes | Dex 6 mg × 7 d | 25L HFNC (50% FIO2) | No | 10 | Home: room air |
Abbreviations: Dex, dexamethasone; HFNC, high‐flow nasal cannula; ICU, intensive care unit; IMV, invasive mechanical ventilation; MP, methylprednisolone; NC, nasal cannula; NRB, non‐rebreather mask; RDV, remdesivir.
Participant ID does not correlate with order of enrollment.
Remdesivir (RDV) 200 mg IV × 1d, followed by 100 mg IV × 4d. All participants completed a 5‐day inpatient course of RDV except participant 1 who did not receive remdesivir. Corticosteroid regimens varied by participant, as detailed. Participant 2 started RDV and eculizumab on the same day but did not receive corticosteroids; participants 3, 4, and 6 started RDV, corticosteroids and eculizumab on the same day; participants 5 and 8 started RDV and corticosteroids one day prior to eculizumab; participant 7 started RDV and corticosteroids 2 days day prior to eculizumab.
The overall median length of hospital stay was 7 days (range 6–13) and the median time from enrollment to hospital discharge was 5.5 days (range 3–12). All eight participants met the primary outcome of survival at Day 15, as well as secondary outcomes of survival at Day 29, being free of invasive mechanical ventilation at Days 15 and 29, and improvement of oxygenation from Day 1 to Days 15 and 29. Four participants experienced a transient increase in oxygen requirement due to worsening of their COVID‐19 illness. However, none of the participants required invasive mechanical ventilation and ICU admission. Six participants were discharged home on room air, while one pregnant and one postpartum participant needed supplemental oxygen after discharge home (for 2 and 1 weeks, respectively).
Following treatment with eculizumab, participants had a decrease in CRP levels and an increase in absolute lymphocyte count, Figure 2. Compared to baseline, CRP levels decreased by treatment Day 4 (median [range] CRP, 85 [23–132] mg/L vs. 24 [6–53] mg/L, p = .02], and absolute lymphocyte count increased by treatment Day 5 (median [range] absolute lymphocyte count, .83 [.2–1.1] vs. 1.3 [1.1–1.4], p = .01). The platelet count also increased over time, from mean 217 ± 75 k/μl on Day 1 to 375 ± 115 k/μl on Day 5. Other clinical laboratories, including hemoglobin, ALT, AST, LDH, total bilirubin, and serum creatinine remained stable throughout the study. There were no adverse laboratory changes due to study drug; serum creatinine remained <1.1 mg/dl (upper limit of normal), and ALT and AST levels remained <1.5 times the upper limit of normal (55 and 34 U/L, respectively) in all participants.
FIGURE 2.
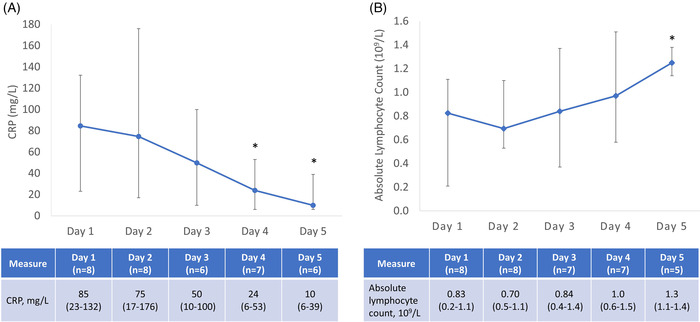
Laboratory trends following treatment with eculizumab. (A) CRP; (B) Absolute lymphocyte count. Data are median (range); CRP, C‐reactive protein; Days represent study day, with the first dose of eculizumab on Day 1 and the second dose on Day 2 per protocol. The number of study participants with laboratory data on each day are provided on the table below each plot. Laboratory data on Days 1 and 4 are pre‐treatment values. Normal range: absolute lymphocyte count (1.0–4.5 × 109/L); C‐reactive protein (<5 mg/L). *Wilcoxon rank‐sum test: CRP, Day 4 versus Day 1 (p = .02) and Day 5 versus Day 1 (p = .005); Absolute lymphocyte count, Day 5 versus Day 1 (p = .01)
PK and PD data were assessed on Day 1 in three participants. Data for other participants were not available due to limited hours for collection of research samples during the pandemic. The concentration of eculizumab increased from undetectable levels before treatment to a mean level of 321 ±13 μg/ml at 1 h after treatment. The free C5 concentration decreased after treatment with eculizumab: median (range) free C5, Day 1 pre‐dose 337 (198‐352) μg/ml versus Days 4–8 post‐dose .04 (.03–.05) μg/ml, p < .001. In two pregnant participants, we measured eculizumab levels before the second dose on Day 4; and the concentration of eculizumab remained in the therapeutic range (>116 μg/ml) in both cases (150 μg/ml, 160 μg/ml). Soluble C5b‐9 levels were also assessed in three participants and levels decreased significantly following treatment with eculizumab: median (range) C5b‐9, Day 1 pre‐dose 1172 (860–1730) ng/ml vs. Day 4‐8 post‐dose 285 (250‐420) ng/ml, p = .04.
Obstetric and neonatal outcomes were assessed for the six participants who were pregnant at enrollment, Table 4. Delivery occurred at mean gestational age 39 ± 2 weeks (range 36w3d–41w1d). Five of six participants delivered at term and the single preterm birth was a planned delivery for placenta previa. There were no cases of fetal demise or preeclampsia, or other adverse pregnancy outcomes. One participant had a cesarean delivery due to a prolonged fetal heart rate deceleration in the second stage of labor. This neonate was admitted to the NICU for transient tachypnea of the newborn and improved within 48 h; during the 4‐month pediatric follow‐up visit, a right‐eye strabismus was diagnosed. All other neonates did well and there were no other NICU admissions; all neonates were discharged home by day of life 3. One neonate had minor congenital defects (preauricular pits, dermal melanocytosis), which were considered clinically benign and unrelated to study drug.
TABLE 4.
Obstetric and neonatal outcomes among pregnant study participants
| ID | Enrollment GA | Delivery GA | Delivery Indication | Delivery Mode | Apgar Scores (1, 5 min) | Bwt (g) | NICU (days) | Neonatal course |
|---|---|---|---|---|---|---|---|---|
| 1 | 25w2d | 40w1d | Labor | NSVD | 8, 9 | 3405 | No | routine |
| 2 | 28w6d | 41w1d | Late term induction | Primary CD a | 2, 8 | 3600 | Yes (2) | TTN, NIPPV |
| 3 | 35w3d | 40w0d | Elective induction | NSVD | 8, 9 | 3204 | No | routine |
| 4 | 31w3d | 37w5d | Labor | NSVD | 8, 9 | 3220 | No | routine |
| 5 | 32w0d | 36w3d | Placenta previa | Primary CD | 9, 9 | 2410 | No | routine |
| 6 | 25w3d | 39w4d | Labor | NSVD | 9, 9 | 2920 | No | routine |
Abbreviations: bwt, birthweight; CD, cesarean delivery; GA, gestational age; NICU, neonatal intensive care unit; NSVD, normal spontaneous vaginal delivery; TTN, transient tachypnea of the newborn; NIPPV, non‐invasive positive pressure ventilation.
Cesarean delivery for prolonged fetal heart rate deceleration in 2nd stage of labor.
Follow up phone visits were completed at 1, 2, and 3 months following the last dose of study drug, with an additional visit following delivery if occurring more than 3 months after enrollment. During 3 months of phone visits, no adverse maternal, fetal, or neonatal adverse events were reported. None of the participants were readmitted to the hospital for COVID‐19, or any other non‐obstetric indication, during the 3 months of follow‐up.
4. DISCUSSION
We have described use of eculizumab, a complement C5 inhibitor, for the treatment of severe COVID‐19 in eight pregnant or postpartum adults. All eight participants improved clinically, and oxygen requirement and laboratory parameters improved within 4–5 days of receiving eculizumab. None of the participants required mechanical ventilation or ICU admission. In three participants in whom PK and PD data were available, therapeutic eculizumab levels were associated with complete terminal complement inhibition and reduced C5b‐9 levels. None of the six pregnant participants required preterm delivery for COVID‐19 and there were no serious adverse events related to eculizumab.
Our data are consistent with prior reports in non‐pregnant adults, which have demonstrated the potential benefit of eculizumab for the treatment of more critical COVID‐19 illness. 8 , 9 , 10 , 11 , 12 Annane et al. showed that patients treated with eculizumab had improved survival and more rapid improvement in serum lactate, platelet count, blood urea nitrogen levels, and oxygenation compared with patients treated without eculizumab. 9 Treatment with eculizumab led to improved 15‐day survival compared to those not treated with eculizumab (83% vs. 62%, p = .04). 9 Like pregnant participants in our series with severe COVID‐19, non‐pregnant patients with critical COVID‐19 had high C5b‐9 concentration and elevated CH50 activity prior to treatment with eculizumab. Therefore, pregnant and postpartum adults with severe COVID‐19, like non‐pregnant adults, may experience increased complement activation that can be mitigated with eculizumab.
Central to innate immunity, complement proteins are activated early in response to infection with the SARS‐CoV‐2 virus. However, complement activation is increased in COVID‐19 to a higher degree compared with other respiratory illnesses, and complement proteins have emerged as a key contributor to the development of inflammation, tissue damage, and microvascular thrombosis in COVID‐19. 2 , 4 , 5 , 6 Complement activation pathways converge to generate the potent inflammatory mediators C3a and C5a, which recruit neutrophils to the lung and stimulate cytokine release from macrophages. 2 , 3 Activated neutrophils further amplify disease severity and tissue damage through the formation of neutrophil extracellular traps. 22 , 23 Endothelial cell deposition of C5b‐9 mediates platelet aggregation and procoagulant effects, 24 , 25 and continued C5b‐9 deposition predisposes to microthrombi in the pulmonary vasculature, a characteristic feature of critical COVID‐19. 5 Pulmonary autopsy specimens from patients who died from COVID‐19 demonstrate extensive deposits of C5b‐9 localized to inter‐alveolar septal capillaries and pulmonary microvasculature. 5
In our study, participants showed improved oxygenation and clinical status, as well as reduced CRP levels and increased absolute lymphocyte count, following treatment with eculizumab in addition to standard of care (though standard of care was not defined prospectively in the study). This pattern has also been suggested in non‐pregnant adults with severe disease. Mastellos et al. showed that complement blockade, with either a C3 or C5 inhibitor, led to decreased CRP and IL‐6 concentrations and an increase in absolute lymphocyte count within 7 days of treatment. 12 While other complement inhibitors are being evaluated for treatment of COVID‐19, eculizumab has the most safety data for use in pregnant and lactating people. 26 , 27 , 28 Pregnancy safety data for eculizumab stem from its use in pregnant women with paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS). 26 , 28 Among pregnant women with PNH treated with eculizumab, Kelly et al. 26 found that eculizumab was detectable at low concentrations in 7 of 20 umbilical cord blood samples, suggesting that it crosses placenta at low levels. However, Hallstensen et al. 29 showed that treatment with eculizumab during pregnancy does not seem to alter the complement system activity of the newborn. Kelly et al. 26 also examined 10 breast milk samples among women receiving eculizumab, and the drug was not detected in any of the samples, leading the authors to conclude that breastfeeding is safe. Other complement inhibitors being evaluated for treatment of COVID‐19, such as the C3 inhibitor AMY101, 12 long‐acting C5 inhibitor ravulizumab, 30 C5a inhibitor vilobelimab, 31 and lectin pathway inhibitor narsoplimab, 32 lack safety data in pregnancy.
Our study was limited by a small sample size and lack of a comparator arm. While this EAP protocol was open to pregnant and breastfeeding adults at other sites including 6 other centers in the United States and 5 hospitals in France, 33 our center was the only site that enrolled pregnant and postpartum individuals, limiting the total number of participants in this subgroup. Data was not collected for pregnant and breastfeeding women with COVID‐19 who did not receive eculizumab, and therefore we cannot determine if eculizumab is superior to standard of care alone, including use of remdesivir and dexamethasone. The COVID‐19 landscape has changed considerably since the initial study design and COVID‐19 vaccines are now widely available. Criteria for disease severity has changed over time and our data may not apply to pregnant individuals infected with more recent viral strains (e.g., Omicron variant). However, the criteria used in our study to define severe illness is similar to the current criteria outlined by the National Institute of Health. 34
The eculizumab dosing in this study varied from existing FDA approved regimens for PNH and aHUS. Typically, dosing for eculizumab would be based on modeling and simulation; but, with COVID‐19 the dose selection was needed quickly, and clinical assessment of observed PK was used to determine dose adjustment. The dosing was empirically derived, with larger and more frequent eculizumab dosing based on earlier clinical experience suggesting complement amplification in the setting of COVID‐19 is greater than what is observed in other conditions treated with eculizumab, such as PNH and aHUS. 9 , 35
The strengths of our study include prospective data collection, dedicated assessments of safety and efficacy, clinical laboratory, and complement measurements before and after treatment, evaluation of maternal and neonatal outcomes, and 3‐month follow up from the last dose. Few COVID‐19 treatment studies have provided such data for pregnant and postpartum participants. While study participants often received remdesivir and dexamethasone as the standard of care, in addition to eculizumab, we found that C5b‐9 and CRP levels, as well as lymphocyte and platelet count, improved within 4‐5 days of study enrollment. Moreover, none of the participants progressed to critical disease and none of the pregnant participants required preterm delivery for COVID‐19. These results compare favorably to the high rate of preterm birth (40%–59%) and ICU admissions (31%–33%) reported in pregnant individuals hospitalized with COVID‐19, including those treated with remdesivir and dexamethasone. 13 , 36
There remains an unmet need for controlled clinical trials in pregnant and lactating people with severe and critical COVID‐19. A larger study comparing eculizumab to the standard of care is recommended to determine efficacy and safety of eculizumab for treatment of COVID‐19 in pregnant and breastfeeding individuals.
CONFLICT OF INTEREST
R.M.B has received honorarium from Alexion Pharmaceuticals Inc (Alexion) for participation in speaker bureaus and advisory boards. J.W. III has participated in advisory boards and received honoraria from Natera Inc. S.K. is an employee of Alexion, AstraZeneca (AZ) Rare Disease, owns unvested AZ stock, and has a patent submitted for Complement inhibition in COVID‐19. D.D. is an employee of Alexion, AZ Rare Disease and owns AZ stock options and RSUs. M.M. is an employee of Alexion, AZ Rare Disease, owns AZ stock/RSUs, and has patents with Alexion unrelated to the current manuscript. S.M. is an employee of Alexion, AZ Rare Disease and owns AZ RSUs. J.M. is an employee of Alexion, AZ Rare Disease, owns unvested AZ stock, and has patents with Alexion. S.O. is an employee of Alexion, AZ Rare Disease, owns AZ stock/RSUs and patents with Alexion unrelated to the current manuscript. S.G. has received a grant/contract from IBSA pharmaceutical. The remaining authors report no disclosures.
ACKNOWLEDGMENTS
The authors would like to thank the patients for their participation in this clinical study and Hélène Dassule, PhD for her editorial review of the manuscript (Alexion, AstraZeneca Rare Disease). This study (NCT04355494) was sponsored by Alexion Pharmaceuticals Inc (now Alexion, AstraZeneca Rare Disease). The funder was involved with study conception, design, PK/PD and biomarker studies, and review and revision of manuscript.
Burwick RM, Dellapiana G, Newman RA, et al. Complement blockade with eculizumab for treatment of severe Coronavirus Disease 2019 in pregnancy: A case series. Am J Reprod Immunol. 2022;88:e13559. 10.1111/aji.13559
Presentation information: Oral Presentation, Hot Topics Plenary Session. Society for Reproductive Investigation, 68th Annual Scientific Meeting. Boston, MA. July 6–9, 2021.
Clinical trial information: Date of registration: April 21, 2020; Clinical trial identification number: NCT04355494; URL of registration site: https://www.clinicaltrials.gov/ct2/show/NCT04355494?term=NCT04355494&draw=2&rank=1
DATA AVAILABILITY STATEMENT
Individual participant data will be made available upon reasonable request. Data will be available for the results reported in this article, after deidentification (text, tables, figures). No other documents will be available. Data will be available beginning 3 months and ending 5 years following publication. For researchers who provide a methodologically sound proposal, data will be shared to achieve aims in the proposal. Proposals should be directed to the corresponding author.
REFERENCES
- 1. WHO COVID‐19 Dashboard . Geneva: World Health Organization, 2020. [cited March 2, 2022]. https://covid19.who.int/info/
- 2. Java A, Apicelli AJ, Liszewski MK, et al. The complement system in COVID‐19: friend and foe? JCI Insight. 2020;5(15):e140711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lo MW, Kemper C, Woodruff TM. COVID‐19: complement, coagulation, and collateral damage. J Immunol. 2020;205(6):1488‐1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Skendros P, Mitsios A, Chrysanthopoulou A, et al. Complement and tissue factor‐enriched neutrophil extracellular traps are key drivers in COVID‐19 immunothrombosis. J Clin Invest. 2020;130(11):6151‐6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res. 2020;220:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma L, Sahu SK, Cano M, et al. Increased complement activation is a distinctive feature of severe SARS‐CoV‐2 infection. Sci Immunol. 2021;6(59):eabh2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holter JC, Pischke SE, de Boer E, et al. Systemic complement activation is associated with respiratory failure in COVID‐19 hospitalized patients. Proc Natl Acad Sci USA. 2020;117(40):25018‐25025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laurence J, Mulvey JJ, Seshadri M, et al. Anti‐complement C5 therapy with eculizumab in three cases of critical COVID‐19. Clin Immunol. 2020;219:108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Annane D, Heming N, Grimaldi‐Bensouda L, et al. Eculizumab as an emergency treatment for adult patients with severe COVID‐19 in the intensive care unit: a proof‐of‐concept study. EClinicalMedicine. 2020;28:100590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giudice V, Pagliano P, Vatrella A, et al. Combination of ruxolitinib and eculizumab for treatment of severe SARS‐CoV‐2‐related acute respiratory distress syndrome: a controlled study. Front Pharmacol. 2020;11:857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diurno F, Numis FG, Porta G, et al. Eculizumab treatment in patients with COVID‐19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci. 2020;24(7):4040‐4047. [DOI] [PubMed] [Google Scholar]
- 12. Mastellos DC, Pires da Silva BGP, Fonseca BAL, et al. Complement C3 vs C5 inhibition in severe COVID‐19: early clinical findings reveal differential biological efficacy. Clin Immunol. 2020;220:108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lokken EM, Huebner EM, Taylor GG, et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. 2021;225(1):77.e1‐.77.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory‐confirmed SARS‐CoV‐2 infection by pregnancy status – United States, January 22‐October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1641‐1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Metz TD, Clifton RG, Hughes BL, et al. Disease severity and perinatal outcomes of pregnant patients with Coronavirus Disease 2019 (COVID‐19). Obstet Gynecol. 2021;137(4):571‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Costantine MM, Landon MB, Saade GR. Protection by exclusion: another missed opportunity to include pregnant women in research during the Coronavirus Disease 2019 (COVID‐19) pandemic. Obstet Gynecol. 2020;136(1):26‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith DD, Pippen JL, Adesomo AA, Rood KM, Landon MB, Costantine MM. Exclusion of pregnant women from clinical trials during the Coronavirus Disease 2019 pandemic: a review of international registries. Am J Perinatol. 2020;37(8):792‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Society for Maternal‐Fetal Medicine . Management considerations for pregnant patients with COVID‐19. https://s3.amazonaws.com/cdn.smfm.org/media/2734/SMFM_COVID_Management_of_COVID_pos_preg_patients_2‐2‐21_(final).pdf. Accessed March 3, 2022.
- 19. Peffault de Latour R, Brodsky RA, Ortiz S, et al. Pharmacokinetic and pharmacodynamic effects of ravulizumab and eculizumab on complement component 5 in adults with paroxysmal nocturnal haemoglobinuria: results of two phase 3 randomised, multicentre studies. Br J Haematol. 2020;191(3):476‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mizuno M, Suzuki Y, Higashide K, et al. High levels of soluble C5b‐9 complex in dialysis fluid may predict poor prognosis in peritonitis in peritoneal dialysis patients. PLoS One. 2017;12(1):e0169111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. European Medicines Agency Summary of Product Characteristics . Annex 1, Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product‐information/soliris‐epar‐product‐information_en.pdf. Accessed March 3, 2022.
- 22. Vanderbeke L, Van Mol P, Van Herck Y, et al. Monocyte‐driven atypical cytokine storm and aberrant neutrophil activation as key mediators of COVID‐19 disease severity. Nat Commun. 2021;12(1):4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID‐19. JCI Insight. 2020;5(11):e138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peerschke EI, Yin W, Ghebrehiwet B. Complement activation on platelets: implications for vascular inflammation and thrombosis. Mol Immunol. 2010;47(13):2170‐2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sims PJ, Faioni EM, Wiedmer T, Shattil SJ. Complement proteins C5b‐9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J Biol Chem. 1988;263(34):18205‐18212. [PubMed] [Google Scholar]
- 26. Kelly RJ, Höchsmann B, Szer J, et al. Eculizumab in pregnant patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2015;373(11):1032‐1039. [DOI] [PubMed] [Google Scholar]
- 27. Sarno L, Tufano A, Maruotti GM, Martinelli P, Balletta MM, Russo D. Eculizumab in pregnancy: a narrative overview. J Nephrol. 2019;32(1):17‐25. [DOI] [PubMed] [Google Scholar]
- 28. Gupta M, Govindappagari S, Burwick RM. Pregnancy‐associated atypical hemolytic uremic syndrome: a systematic review. Obstet Gynecol. 2020;135(1):46‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hallstensen RF, Bergseth G, Foss S, et al. Eculizumab treatment during pregnancy does not affect the complement system activity of the newborn. Immunobiology. 2015;220(4):452‐459. [DOI] [PubMed] [Google Scholar]
- 30. Smith K, Pace A, Ortiz S, Kazani S, Rottinghaus S. A Phase 3 open‐label, randomized, controlled study to evaluate the efficacy and safety of intravenously administered ravulizumab compared with best supportive care in patients with COVID‐19 severe pneumonia, acute lung injury, or acute respiratory distress syndrome: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vlaar APJ, de Bruin S, Busch M, et al. Anti‐C5a antibody IFX‐1 (vilobelimab) treatment versus best supportive care for patients with severe COVID‐19 (PANAMO): an exploratory, open‐label, phase 2 randomised controlled trial. Lancet Rheumatol. 2020;2(12):e764‐e773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rambaldi A, Gritti G, Micò MC, et al. Endothelial injury and thrombotic microangiopathy in COVID‐19: treatment with the lectin‐pathway inhibitor narsoplimab. Immunobiology. 2020;225(6):152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. ClinicalTrials.Gov . Soliris (Eculizumab) Treatment of Participants with COVID‐19. [cited July 5, 2021]. https://www.clinicaltrials.gov/ct2/show/NCT04355494?term=ecu‐cov‐401&draw=2&rank=1
- 34. National Institute of Health . COVID‐19 Treatment Guidelines: Clinical Spectrum of SARS‐CoV‐2 Infection. [cited February 17, 2022]. https://www.covid19treatmentguidelines.nih.gov/overview/clinical‐spectrum/
- 35. McEneny‐King AC, Monteleone JPR, Kazani SD, Ortiz SR. Pharmacokinetic and pharmacodynamic evaluation of ravulizumab in adults with severe Coronavirus Disease 2019. Infect Dis Ther. 2021;10(2):1045‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pierce‐Williams RAM, Burd J, Felder L, et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. Am J Obstet Gynecol MFM. 2020;2(3):100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual participant data will be made available upon reasonable request. Data will be available for the results reported in this article, after deidentification (text, tables, figures). No other documents will be available. Data will be available beginning 3 months and ending 5 years following publication. For researchers who provide a methodologically sound proposal, data will be shared to achieve aims in the proposal. Proposals should be directed to the corresponding author.


