Abstract
Objectives
Suggested therapeutic options for Multisystem Inflammatory Syndrome in Children (MIS‐C) include intravenous immunoglobulins (IVIG) and steroids. Prior studies have shown the benefit of combination therapy with both agents on fever control or the resolution of organ dysfunction. The primary objective of this study was to analyze the impact of IVIG and steroids on hospital and ICU length of stay (LOS) in patients with MIS‐C associated with Coronavirus Disease 2019 (COVID‐19).
Study Design
This was a retrospective study on 356 hospitalized patients with MIS‐C from March 2020 to September 2021 (28 sites in the United States) in the Society of Critical Care Medicine (SCCM) Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS) COVID‐19 Registry. The effect of IVIG and steroids initiated in the first 2 days of admission, alone or in combination, on LOS was analyzed. Adjustment for confounders was made by multivariable mixed regression with a random intercept for the site.
Results
The median age of the study population was 8.8 (Interquartile range (IQR) 4.0, 13) years. 247/356 (69%) patients required intensive care unit (ICU) admission during hospitalization. Overall hospital mortality was 2% (7/356). Of the total patients, 153 (43%) received IVIG and steroids, 33 (9%) received IVIG only, 43 (12%) received steroids only, and 127 (36%) received neither within 2 days of admission. After adjustment of confounders, only combination therapy showed a significant decrease of ICU LOS by 1.6 days compared to no therapy (exponentiated coefficient 0.71 [95% confidence interval 0.51, 0.97, p = 0.03]). No significant difference was observed in hospital LOS or the secondary outcome variable of the normalization of inflammatory mediators by Day 3.
Conclusions
Combination therapy with IVIG and steroids initiated in the first 2 days of admission favorably impacts ICU but not the overall hospital LOS in children with MIS‐C.
Keywords: corticosteroids, COVID‐19, IVIG, MIS‐C, outcomes
1. INTRODUCTION
Clinicians identified Multisystem Inflammatory Syndrome in Children (MIS‐C) as a pediatric complication of Coronavirus Disease 2019 (COVID‐19) early in the course of the pandemic. 1 , 2 Initial reports described MIS‐C as an atypical Kawasaki's disease (KD) presentation with significant inflammation occurring several weeks after a COVID‐19 infection. 3 Both KD and MIS‐C have been associated with elevated levels of cytokines and immunoglobulin concentrations. 4 , 5 Children with MIS‐C were also found to be at risk for coronary artery aneurysms. Conversely, MIS‐C was associated with a greater incidence of gastrointestinal symptoms, shock, and coagulopathy than described with KD. 3 Initial MIS‐C treatment regimens were developed from those used for KD. 1 , 6 Current MIS‐C treatment guidelines recommend a combination of intravenous immunoglobulin (IVIG) and corticosteroids as first‐line therapy. 7 , 8
Several studies have compared the therapeutic effectiveness of IVIG, steroids, or their combination in the management of MIS‐C. One study 9 reported that children given IVIG and corticosteroids were less likely to require additional treatment and had lower intensive care unit (ICU) length of stay (LOS) than those treated with IVIG alone. Another 10 reported a decreased risk of cardiovascular dysfunction and fever along with the use of fewer adjuvant agents in patients treated with IVIG and steroids but did not identify a difference in LOS with combination therapy compared to IVIG alone. A third study 11 found no difference in the composite indicator of the need for inotropic support or mechanical ventilation by day 2 or later or death between patients treated with combination therapy compared to IVIG or steroids alone. Overall, the impact of MIS‐C treatment with IVIG and corticosteroid therapy on ICU LOS, hospital LOS, and other outcomes is inconclusive.
The primary objective of this study was to compare the outcomes (hospital and ICU LOS) of children admitted to the contributing sites of the Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal study (VIRUS) COVID‐19 registry who were treated with IVIG and steroids, alone or in combination, compared to no treatment within the first 2 days of hospital admission. We hypothesized that the early initiation of the combination of IVIG and steroids would impact the disease course leading to a shorter LOS. Our secondary objectives were to compare the clinical course (fever defervescence, resolution of inflammation, cardiopulmonary support requirement) between groups.
2. METHODS
2.1. Database
The VIRUS registry is a multicenter, prospective, observational study of all eligible adult and pediatric patients admitted to the hospital with COVID‐19. 12 , 13 The VIRUS registry was approved by the Mayo Clinic institutional review board, and by all the participating sites with a waiver of informed consent. Each site collected the deidentified data through manual chart abstraction or automation and entered it in a centralized REDCap database 14 hosted by the Mayo Clinic.
2.2. Cohort
Inclusion criteria for the VIRUS registry include all patients hospitalized in the participating hospitals with COVID‐19‐related illnesses. Non–COVID‐19‐related admissions (incidental diagnosis) and repeat admissions were excluded. Data were extracted for all pediatric patients (<18 years) with COVID‐19 entered in the registry who were diagnosed with MIS‐C from March 2020 to September 2021. Patients were excluded if LOS information was missing or if they were from outside the United States. MIS‐C diagnosis was made by the participating sites utilizing Centers for Disease Control (CDC) criteria. 7 This diagnosis was not further adjudicated for this analysis.
2.3. Exposure
The primary exposure variable was the administration of IVIG or steroids or their combination within the first 2 days of hospital admission. Patients were divided into four groups, Group A (IVIG and steroids), Group B (IVIG only), Group C (steroids only), and Group D (neither IVIG nor steroids). To ensure that patient allocation was limited to the information at the time of study entry, 15 patients who did not receive IVIG (N = 10) or steroids within the first 2 days (N = 14) but received either IVIG or steroids later during hospitalization were included in the control group (Group D).
2.4. Outcomes
The primary outcome variable for the analysis was the hospital and ICU LOS (for patients admitted to the ICU). The hospital and ICU LOS for patients who died were replaced with the 99th percentile of hospital LOS (59 days) and ICU LOS (33 days). 16 The secondary outcome variables included hospital mortality, nosocomial bacterial infection (positive bacterial cultures reported on or after hospital day 2), inotrope or ventilator requirement on or after hospital day 2 (for patients for whom inotrope/ventilator was not needed at admission), the number of days of inotropes, fever defervescence by day 3 (for patients who had fever on admission), and the day of normalization of inflammatory mediators (for patients who had abnormal inflammatory mediators including white cell count, platelet count, C‐reactive protein, procalcitonin, and ferritin on admission).
2.5. Other measured variables and confounders
Data were extracted and standardized from the VIRUS registry in the following manner. Age was stratified into discrete categories: neonate (≤28 days), infant (>28 days to <2 years), child (≥2 years to <12 years), and adolescents (≥12 years). 17 The patient's weight and height were used to calculate Body Mass Index (BMI). BMI percentiles were calculated using CDC criteria for ≥2 years, and weight for height percentiles using the World Health Organization (WHO) criteria for <2 years of age. 18 The percentiles were calculated using respective SAS codes and categorized as underweight, normal weight, overweight, and obese per previously reported standards. 19 Since the obesity comorbidity variable acted as a proxy for the missing BMI, a composite variable (obesity as diagnosed with BMI or comorbidity diagnosed by a physician) was used for analysis. Race and ethnicity were self‐reported separately and included in the analysis as a socioeconomic variable for disease outcome. 20 These were later categorized as composite index as non‐Hispanic white, non‐Hispanic black, non‐Hispanic Asian, non‐Hispanic others, and Hispanic per the current CDC reporting on race/ethnicity disparities on COVID‐19. 21
Inotrope/vasopressor support included patients who received any predefined inotrope/vasopressor on any hospital day from day 0 to 14. Pediatric Risk of Mortality III (PRISM III) score 22 was determined using the online calculator if the summative score was not already entered by the individual site. 23 Patients' temperature was entered into the REDCap database on days 0‐3, 7, 14, and 21. All temperature values were converted to degrees Centigrade (°C). Fever was considered as present if the temperature at any time during the day was greater than 38°C. We utilized the previously published WHO ordinal scale, which measures illness severity from COVID‐19 over time, to stratify patients based on the degree of respiratory and hemodynamic support at hospital admission. 24 The WHO ordinal scale ranges from zero (no clinical or laboratory evidence of infection) to eight (death). A score of three indicates hospitalization without oxygen and a score of four indicates oxygen via mask or nasal cannula. Scores of 5, 6, and 7 represent increasing degrees of respiratory/hemodynamic support. 24 Besides IVIG and steroids, other medication use assessed for this study included remdesivir, hydroxychloroquine, azithromycin, therapeutic anticoagulation, vitamin C, vitamin D, zinc, aspirin, convalescent plasma, and biological agents. For descriptive analysis, medication was counted as administered if given on any day of hospitalization for which data were available in the VIRUS registry (days 0‐14, 21, or 28).
Inflammatory and other laboratory values were reviewed manually and converted into standard units. The VIRUS database allows daily lab entries for days 0‐3, 7, 14, and 21. For this analysis, the following inflammatory and other laboratory parameters were assessed: white cell count, platelet count, C‐reactive protein, pro‐calcitonin, ferritin, serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase, serum albumin, interleukin‐6, brain natriuretic peptide, pro‐brain natriuretic peptide, fibrinogen, D‐dimer, and lactate dehydrogenase. Maximum serum creatinine on days 1, 2, 3, 7, 14, and 21 was also obtained and was then categorized as normal and abnormal based on standard cutoff levels. 25 Estimated glomerular filtration rate (eGFR) was calculated utilizing the modified Schwartz equation, 26 and the Pediatric Risk, Injury, Failure, Loss, End‐stage renal disease (P‐RIFLE) score was calculated (lowest eGFR on day 0/1 was considered baseline). 27 Patients with evidence of other respiratory viral infections were categorized as "viral coinfection." Patients with concurrent blood, urine, and bacterial respiratory infections were combined into categories of "bacterial coinfection." Critical illness was defined as a composite index of in‐hospital mortality and organ support requirements as described previously by our group. 28
2.6. Missing data analysis
There were no missing data on age, race, or mortality. There was a high degree of missingness of the inflammatory mediators' variables. The missingness rate for these variables ranged from 35% (platelets) to 86% (interleukin‐6). A total of 49 (19.8%) of patients admitted to ICU were missing the PRISM III score. The R package mice was used to impute missing PRISM III scores using multiple imputations. Both values from complete case analysis and imputed PRISM III scores (Appendix S5) are presented. Up to 25 (7.0%) patients were missing obesity classification based on BMI/WHO criteria. Whether or not the patient was missing BMI was associated with obesity diagnosis as a comorbidity (fewer patients with obesity comorbidity were missing BMI [1/22, 4.6%] than patients who did not have obesity diagnosis as a comorbidity [24/334, 7.2%]). The obesity by comorbidity variable served as a surrogate for BMI, and a composite category of obesity (yes/no) was created (with preference to obesity by BMI). The remaining variables' missingness is reported as absolute numbers and percentages wherever applicable.
2.7. Statistical analysis
Standard descriptive and comparative analysis was performed using the non‐parametric Kruskal–Wallis test for the four Group comparison of continuous variables and chi‐squared/Fisher's exact test for categorical variables as appropriate. Values are represented as median (interquartile range [IQR]) or frequency (percentage). Mixed linear regression with a random intercept for the site was performed for log‐transformed hospital and ICU LOS. Parameter estimates and 95% confidence intervals (CI) were exponentiated. Other than the primary independent variable (steroid and IVIG and their combination versus none), other variables were selected based on prior evidence, expert knowledge, 29 or the potential impact on LOS (and illness severity) of patients admitted to the pediatric ICU and more specifically for COVID‐19 related illness. 28 , 30 The variables included age (in years), the number of abnormal inflammatory mediators on admission, race (dichotomized as non‐Hispanic white versus not), a composite variable of obesity and any comorbidity, a composite variable of bacterial or viral coinfection at admission, and a composite variable of WHO ordinal scale ≥ 4 on admission or ICU admission within ≤ 2 days. Collinearity was assessed using the variance inflation factor.
Mixed logistic regression was performed for the secondary outcome variable with enough sample size to conduct multivariable regression (normalization of inflammatory mediators by Day 3) using the same covariates. For other secondary outcomes, analysis was restricted to unadjusted comparisons. Statistical analysis was performed using JMP Pro v16.0 (SAS Institute, Cary, NC) and open‐source statistical program R (v 4.0.1) with a 2‐sided alternative hypothesis at a significance level of 5% (α= 0.05). This study reporting conforms to the Strengthening The Reporting of Observational Studies in Epidemiology (STROBE) statement. 31
3. RESULTS
Out of the 1627 children who entered in the VIRUS registry from March 2020 to September 2021, 409 were hospitalized with MIS‐C. Seven (1.7%) patients were excluded for missing hospital LOS, and a total of 46 patients from non‐U.S. sites were also excluded. Data from 356 patients were included in the analysis. Within the first 2 days of admission, 153 (42.9%) received both IVIG and steroids (Group A), 33 (9.2%) received only IVIG (Group B), 43 (12.1%) received only steroids (Group C), and 127 (35.6%) received neither IVIG nor steroids (Group D) (Figure 1).
FIGURE 1.
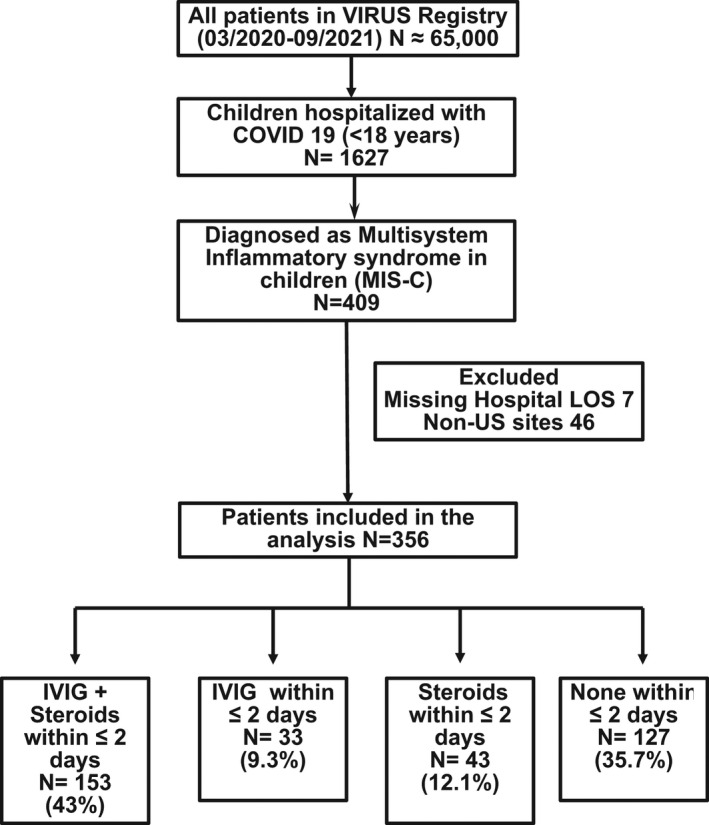
Flow diagram of children included in MIS‐C treatment analysis. Abbreviations: COVID‐19, Coronavirus Disease 2019; IVIG, intravenous immunoglobulin; LOS, length of stay; US, United States; VIRUS, viral infection and respiratory illness universal study
3.1. Demographic comparison
The median age of the study population was 8.8 (IQR 4, 13) years. There was no difference in the median age in the four groups; however, the proportion of patients in the different age categories was significantly different. Only 4.6% (7/153) of the patients were infants in Group A compared to 23.6% (30/127) of patients in Group D (p < 0.001). There was no significant difference in the racial distribution or proportion of patients with obesity in the four groups. Overall, 69% (247/356) of patients required ICU admission during their hospitalization. The proportion of patients requiring ICU admission was different among the four groups, with the highest rate in Group C (90.7% [39/43]) and the lowest in Group B (51.5% [17/33]) (p < 0.001). There was also a significant difference in the proportion of patients with critical illness and in the distribution of the admission WHO ordinal scale, with overall lower severity of illness and respiratory support in Group D. The proportion of patients with abnormal inflammatory mediators on admission was highest in Group A (85% [130/153]) and lowest in Group D (38.6% [49/127]) (p < 0.001). There was no difference in the proportion of patients with bacterial or viral coinfection. Group A had the lowest proportion of patients with positive SARS‐CoV‐2 PCR (81/153, 53%) but the highest proportion of patients with positive antibodies (137/146, 93.8%) (p = 0.001 and 0.003, respectively). (Table 1) Summary statistics (median, IQR) of the laboratory parameters and the proportion of abnormal laboratory parameters assessed in the study are provided in supplemental digital content (Appendix S4).
TABLE 1.
Demographics of patients diagnosed with multisystem inflammatory syndrome in children (MIS‐C)
| Category | Subcategory | Total Cohort N = 356 | Group A IVIG + Steroid N = 153 | Group B IVIG N = 33 | Group C Steroid N = 43 | Group D None N = 127 | p‐Value |
|---|---|---|---|---|---|---|---|
| Age (years) | 8.8 (4.0, 13) | 8.9 (5.5, 12.0) | 5.5 (3.4, 11) | 10 (5.3, 15) | 9 (1.7, 14.1) | 0.29 | |
| Age categories a | Neonate | 5 (1.4%) | 1 (0.6%) | 0 (0%) | 0 (0%) | 4 (3.2%) | <0.001 |
| Infant | 45 (12.6%) | 7 (4.6%) | 4 (12.1%) | 4 (9.3%) | 30 (23.6%) | ||
| Child | 187 (52.5%) | 104 (67.9%) | 21 (63.6%) | 21 (48.8%) | 41 (32.3%) | ||
| Adolescent | 119 (33.4%) | 41 (26.8%) | 8 (24.2%) | 18 (41.8%) | 52 (40.9%) | ||
| Sex | Male | 210 (58.9%) | 101 (66.0%) | 19 (57.6%) | 22 (51.2%) | 68 (53.5%) | 0.12 |
| Race b | White | 104 (29.2%) | 45 (29.4%) | 8 (24.2%) | 13 (30.2%) | 38 (29.9%) | 0.24 |
| Black | 115 (32.3%) | 56 (36.6%) | 7 (21.2%) | 14 (32.6%) | 38 (29.9%) | ||
| Asian | 6 (1.6%) | 4 (2.6%) | 1 (3.0%) | 0 (0%) | 1 (0.8%) | ||
| Other | 42 (11.7%) | 12 (7.8%) | 3 (9.1%) | 8 (18.6%) | 19 (14.9%) | ||
| Hispanic | 89 (25.0%) | 36 (23.5%) | 14 (42.4%) | 8 (18.8%) | 31 (24.4%) | ||
| Obesity c | Yes | 112 (31.4%) | 45 (29.4%) | 11 (33.3%) | 19 (44.2%) | 37 (29.1%) | 0.27 |
| Admit to ICU d | Yes | 247 (69.3%) | 116 (75.8%) | 17 (51.5%) | 39 (90.7%) | 75 (59.1%) | <0.001 |
| Critical Illness e | Yes | 146 (41.0%) | 76 (49.6%) | 10 (30.3%) | 22 (51.2%) | 38 (29.9%) | 0.002 |
| WHO ordinal scale f | Three | 240 (57.4%) | 91 (59.5%) | 25 (25.7%) | 17 (39.5%) | 107 (84.3%) | <0.001 |
| Four | 47 (13.2%) | 23 (15.0%) | 6 (18.2%) | 8 (18.8%) | 10 (7.9%) | ||
| Five | 37 (10.3%) | 20 (13.1%) | 1 (3.0%) | 10 (23.2%) | 6 (4.7%) | ||
| Six | 10 (2.8%) | 2 (1.3%) | 1 (3.0%) | 4 (9.3%) | 3 (2.4%) | ||
| Seven | 22 (6.1%) | 17 (11.1%) | 0 (0%) | 4 (9.3%) | 1 (0.8%) | ||
| Abnormal inflammatory mediators g | Yes | 235 (66.0%) | 130 (84.9%) | 26 (78.8%) | 30 (69.7%) | 49 (38.6%) | <0.001 |
| >3 Signs or symptoms | Yes | 326 (91.5%) | 151 (98.7%) | 32 (96.9%) | 36 (83.7%) | 107 (84.3%) | <0.001 |
| Comorbidities | Yes | 114 (32.0%) | 43 (28.1%) | 7 (21.2%) | 23 (53.4%) | 41 (32.3%) | 0.007 |
| Viral coinfection | Yes | 13 (3.6%) | 5 (3.3%) | 2 (6.1%) | 0 (0%) | 6 (4.7%) | 0.44 |
| Bacterial coinfection | Yes | 39 (10.9%) | 15 (9.8%) | 0 (0%) | 5 (11.6%) | 19 (14.9%) | 0.09 |
| SARS‐CoV‐2 PCR | Positive | 229 (64.3%) | 81 (52.9%) | 24 (72.7%) | 33 (76.7%) | 91 (71.6%) | 0.001 |
| SARS‐CoV‐2 IgG | Positive | 245/278 (88.1%) | 137/146 (93.8%) | 26/28 (92.8%) | 16/20 (80%) | 66/84 (78.6%) | 0.003 |
Note: Values represent median (interquartile range) or frequency (percentage) as appropriate.
Neonate (≤28 days); Infant (>28 days to <2 years); Child (≥2 years to 12 years); Adolescent (≥12 years).
Non‐Hispanic White, non‐Hispanic Black, non‐Hispanic Asian, non‐Hispanic Other.
By BMI or obesity listed as comorbidity.
At any time during hospitalization.
Critical disease represents invasive respiratory support (ventilator, nitric oxide) or invasive hemodynamic support (inotropes/vasopressors/ECMO) or invasive renal support (dialysis) or hospital mortality.
hospitalized, no oxygen therapy (3), oxygen by mass or nasal prongs (4), non‐invasive ventilation or high flow oxygen (5), intubation and mechanical ventilation (6), ventilation and additional organ support including pressers, renal replacement, or ECMO (7).
High or low (leukocyte count, platelets, and fibrinogen), high (CRP, pro calcitonin, ferritin, interleukin, d‐dimer), low (serum albumin) on day 0/1 of admission.
3.2. Hospital management and outcomes
About 60% (210/356) of all patients required respiratory support during admission, with the highest proportion in Group C (72.1%, 31/43) and lowest in Group B (39.4%, 13/33) (p = 0.005). A higher proportion of patients in Group C also required high‐flow nasal cannula and invasive mechanical ventilation. Inotrope requirement was highest in Group A (47.1%, 72/153) and lowest in Group D (20.5%, 26/127) (p < 0.001). There was also a significant difference in the proportion of patients receiving various therapeutic agents. Group C had the highest proportion of patients receiving remdesivir (18.6%), azithromycin (21%), therapeutic anticoagulation (30.2%), vitamin C (6.9%), vitamin D (9.3%), and zinc (6.9%). In comparison, a higher proportion of patients in Group A were prescribed aspirin (69.3%) or biological agents (18.9%). Overall median high‐flow nasal cannula duration was 1.9 (IQR 0.6, 2.9) days, with the lowest duration in Group A and highest in Group D (p = 0.02). There was no difference in noninvasive or invasive mechanical ventilation duration among the four groups. The overall median hospital LOS was 5.7 (IQR 3.8, 8.4) days and was highest in Group C at 7 (IQR 5, 11.1) days and lowest in Group D at 5.0 (IQR 3, 8) days (p = 0.04). The overall median ICU LOS was 3.8 (IQR 2.3, 6.5) days and was not different across the four groups (Table 2).
TABLE 2.
Hospital Management and outcomes
| Category | Subcategory | Total Cohort N = 356 | Group A IVIG + Steroid N = 153 | Group B IVIG N = 33 | Group C Steroid N = 43 | Group D None N = 127 | p‐Value |
|---|---|---|---|---|---|---|---|
| Categorical variables | |||||||
| Respiratory support a | Any respiratory support | 210 (58.9%) | 82 (53.6%) | 13 (39.4%) | 31 (72.1%) | 84 (56.1%) | 0.005 |
| Nasal cannula | 125 (35.1%) | 57 (37.3%) | 11 (33.3%) | 20 (45.4%) | 37 (29.1%) | 0.18 | |
| HFNC | 70 (19.6%) | 35 (22.9%) | 5 (15.2%) | 18 (41.8%) | 12 (9.4%) | <0.001 | |
| Non‐invasive ventilation | 46 (12.9%) | 20 (13.1%) | 1 (3.0%) | 10 (23.3%) | 15 (11.8%) | 0.06 | |
| Invasive ventilation | 50 (14.0%) | 21 (13.7%) | 2 (6.1%) | 13 (30.2%) | 14 (11.0%) | 0.007 | |
| NO or epoprostenol | 12 (3.3%) | 4 (2.6%) | 1 (3.0%) | 3 (6.9%) | 4 (3.2%) | 0.56 | |
| Other organ support a | Vasopressors/ inotropes | 123 (34.5%) | 72 (47.1%) | 10 (30.3%) | 15 (34.9%) | 26 (20.5%) | <0.001 |
| CRRT/HD | 4 (1.1%) | 1 (0.6%) | 0 (0%) | 1 (2.3%) | 2 (1.6%) | 0.68 | |
| ECLS | 6 (1.6%) | 1 (0.6%) | 0 (0%) | 2 (4.6%) | 3 (2.4%) | 0.24 | |
| Antiviral, Immunomodulatory and other medications b | Remdesivir c | 24 (6.7%) | 8 (5.2%) | 2 (6.1%) | 8 (18.6%) | 6 (4.7%) | 0.01 |
| Azithromycin | 16 (4.5%) | 4 (2.6%) | 0 (0%) | 9 (20.9%) | 3 (2.4%) | <0.001 | |
| Hydroxychloroquine | 5 (1.4%) | 1 (0.6%) | 0 (0%) | 1 (2.3%) | 3 (2.4%) | 0.53 | |
| Therapeutic anticoagulation | 54 (15.1%) | 28 (18.3%) | 6 (18.2%) | 13 (30.2%) | 7 (5.4%) | <0.001 | |
| Aspirin | 137 (38.5%) | 160 (69.3%) | 14 (42.4%) | 8 (18.6%) | 9 (7.1%) | <0.001 | |
| Convalescent plasma | 2 (0.5%) | 0 (0%) | 0 (0%) | 2 (4.6%) | 0 (0%) | 0.002 | |
| Biologicals | 35 (9.8%) | 29 (18.9%) | 1 (3.0%) | 2 (4.6%) | 3 (2.3%) | <0.001 | |
| Vitamin C | 6 (1.6%) | 2 (1.3%) | 0 (0%) | 3 (6.9%) | 1 (0.8%) | 0.03 | |
| Vitamin D | 13 (3.6%) | 6 (3.9%) | 2 (6.1%) | 4 (9.3%) | 1 (0.8%) | 0.05 | |
| Zinc | 8 (2.2%) | 3 (1.9%) | 1 (3.0%) | 3 (6.9%) | 1 (0.8%) | 0.12 | |
| Mortality | Yes | 2 (1.3%) | 0 (0%) | 1 (2.3%) | 4 (3.2%) | 0.58 | |
| Respiratory support duration (days) d | High Flow Nasal cannula |
1.9 (0.6, 2.9) N = 61 |
1.5 (0.38, 2.1) N = 33 |
1.6 (0.4, 2.8) N = 4 |
2.7 (1.1, 4.0) N = 14 |
2.9 (0.8, 4.7) N = 10 |
0.02 |
| Noninvasive ventilator |
1.9 (0.9, 3.2) N = 37 |
1.8 (0.6, 2.8) N = 17 |
2.1 (2.1, 2.1) N = 1 |
2.7 (1.2, 4.7) N = 8 |
1.2 (0.8, 6.3) N = 11 |
0.58 | |
| Invasive ventilator |
3.9 (1.8, 6.3) N = 42 |
3.4 (0.9, 5.8) N = 20 |
5.4 (5.1, 5.9) N = 2 |
2.1 (1.8, 5.4) N = 11 |
6.5 (2.8, 21.4) N = 9 |
0.12 | |
| Length of stay (days) e | Hospital | 5.7 (3.8, 8.4) | 5.7 (4.0, 8.3) | 5.1 (3.8, 8.2) | 7 (5, 11.1) | 5.0 (3, 8) | 0.04 |
| Intensive care unit |
3.8 (2.3, 6.5) N = 245 |
3.6 (2.1, 5.7) N = 116 |
3.9 (2.0, 6.4) N = 17 |
5 (3.2, 7.8) N = 38 |
3.3 (2.6, 6.1) N = 74 |
0.19 | |
Note: Value represents median (interquartile range) or frequency (percentage).
Abbreviations: CRRT/HD, Continuous Renal Replacement Therapy/Hemodialysis; ECLS, Extra Corporeal Life Support; HFNC, High Flow Nasal Cannula; IVIG, Intravenous Immunoglobulin; NO, Nitric Oxide.
Respiratory and other organ support represent support provided at any time during the hospitalization.
Represent medications given on either of the day 0, 1, 2, 3, 7, 14, or 21 of hospitalization. Organ support and therapeutics are not mutually exclusive.
Remdesivir is only approved by FDA for use in patients 12 years or older.
Missing HFNC duration in 9/70 (12.8%), Non‐invasive ventilator duration in 9/46 (19.5 ), Invasive ventilator duration in 8/50 (16%), ICU length of stay in 2/247 (0.8%).
Hospital and ICU length of stay of deceased patients represented as 99th percentile of total cohort (59.06 days and 33.48 days, respectively).
3.3. Primary outcome variables
On mixed linear regression of hospital LOS, no significant difference was observed between the hospital LOS among the four groups after adjusting for confounding variables as described previously. Variables of the higher number of abnormal inflammatory mediators, obesity or comorbidity, and patients with a WHO ordinal scale ≥4 or ICU admission within the first 2 days had a longer hospital stay. (Table 3) The geometric mean hospital LOS of Group A was 5.4 (95% CI 4.5‐6.6) days compared to 6.2 (95% CI 5.2‐7.5) days of Group D, with a mean difference of −0.8 days (p = 0.19, Table 4).
TABLE 3.
Regression analysis of hospital and ICU length of stay
| Variable | Exp coef | Exp 95% CI | p‐Value | Part R |
|---|---|---|---|---|
| Hospital Length of Stay | ||||
| IVIG + Steroids (within ≤2 days) vs none | 0.87 | 0.71, 1.07 | 0.19 | 0.072 |
| IVIG (within ≤2 days) vs none | 0.99 | 0.75, 1.31 | 0.94 | 0.004 |
| Steroids (within ≤2 days) vs none | 1.07 | 0.83, 1.39 | 0.61 | 0.027 |
| Age (Years) | 1.01 | 1.00, 1.03 | 0.07 | 0.095 |
| Number of abnormal inflammatory mediators | 1.07 | 1.03, 1.10 | < 0.001 | 0.203 |
| Non‐Hispanic White vs not | 1.03 | 0.87, 1.21 | 0.74 | 0.017 |
| Obesity + Any comorbidity (yes) | 1.32 | 1.14, 1.54 | < 0.001 | 0.187 |
| Bacterial or viral coinfection (yes) | 1.26 | 1.00, 1.59 | 0.05 | 0.103 |
| WHO >=4 or ICU admission (≤2 days) | 1.50 | 1.27, 1.76 | <0.001 | 0.245 |
| ICU Length of Stay | ||||
| IVIG + Steroids (within ≤2 days) vs none | 0.71 | 0.51, 0.97 | 0.03 | 0.151 |
| IVIG (within ≤2 days) vs none | 0.82 | 0.51, 1.33 | 0.43 | 0.055 |
| Steroids (within ≤2 days) vs none | 0.84 | 0.57, 1.24 | 0.38 | 0.063 |
| Age (Years) | 1.02 | 1.00, 1.04 | 0.11 | 0.111 |
| Number of abnormal inflammatory mediators | 0.99 | 0.93, 1.05 | 0.67 | 0.034 |
| Non‐Hispanic white vs not | 0.91 | 0.70, 1.19 | 0.49 | 0.049 |
| Obesity + Any comorbidity (yes) | 1.21 | 0.96, 1.52 | 0.12 | 0.110 |
| Bacterial or viral coinfection (yes) | 1.13 | 0.79, 1.61 | 0.51 | 0.047 |
| PRISM III score | 1.04 | 1.01, 1.06 | 0.004 | 0.211 |
| WHO >=4 | 1.44 | 1.09, 1.89 | 0.010 | 0.183 |
Note: Hospital and ICU LOS were log‐transformed. Exponentiated (Exp) Coefficients and 95% Confidence Intervals are shown.
TABLE 4.
Adjusted length of stay of MIS‐C treatment regimens
| Group A (IVIG + Steroid) | Group B (IVIG) | Group C (Steroid) | Group D (None) | |
|---|---|---|---|---|
| Hospital length of stay in days, geometric mean (95% CI) | 5.4 (4.5, 6.6) | 6.2 (4.7, 8.2) | 6.7 (5.1, 8.6) | 6.2 (5.2, 7.5) |
| Difference in hospital length of stay from control in days |
−0.8 p = 0.19 |
0.0 p = 0.94 |
0.5 p = 0.61 |
NA |
| ICU length of stay in days, geometric mean (95% CI) | 3.8 (3.0, 4.9) | 4.5 (2.8, 7.0) | 4.6 (3.2, 6.5) | 5.4 (4.0, 7.3) |
| Difference in ICU length of stay from control in days |
−1.6 p = 0.03 |
−0.9 p = 0.43 |
−0.8 p = 0.38 |
NA |
Note: Hospital LOS, adjusted for age, number of abnormal inflammatory mediators, race (non‐Hispanic white versus not), comorbidity (including obesity), bacterial or viral coinfection, and ICU admission (≤2 days) or WHO ordinal scale ≥ 4 on admission. ICU LOS includes PRISM and the WHO scale/ICU admission variable is replaced by WHO ordinal scale ≥ 4 on admission only.
Combined therapy with IVIG and steroids was associated with a significantly shorter ICU LOS compared to patients that received neither (exponentiated coefficient 0.71 [95% CI 0.51, 0.97], p = 0.03). The geometric mean ICU LOS of Group A was 3.8 (95% CI 3.0‐4.9) days compared to 5.4 (95% CI 4.0–7.3) days for Group D with a mean difference of −1.6 days (p = 0.03, Table 4). Among the confounding variables included in the model, only PRISM III score and WHO ordinal scale ≥ 4 at admission were associated with longer ICU LOS. (Tables 3 and 4) Linear regression of ICU LOS using imputed PRISM III scores (for patients with missing values) showed similar results (Appendix S5).
3.4. Secondary outcome variables
On unadjusted comparison, there was no significant difference between the four groups in the number of days on inotropes or ventilator requirement after day 1. The incidence of nosocomial bacterial infection, inotrope requirement after admission, and fever defervescence by day 3, differed significantly between groups, with the highest proportions in Group C (2/43, 4.6%; 5/43, 11.6%; and 27/28, 96.4%, respectively). Significant differences were also observed in the proportion of patients who had normalization of their inflammatory mediators by day 3. (Appendix S1) Multivariable logistic regression could only be conducted for inflammatory mediator normalization and did not show a significant difference between the four groups after adjustment of confounding variables (Appendix S2).
4. DISCUSSION
This VIRUS: COVID‐19 registry analysis of children admitted with MIS‐C in 28 U.S. hospitals has shown that combination therapy of IVIG and steroids within the first 2 days of admission does not impact hospital LOS but is associated with significantly shorter ICU LOS when compared to no treatment. No difference was identified in either hospital or ICU LOS when comparing individual agents to no treatment. To our knowledge, this is the first study to identify an impact of IVIG and corticosteroid combination therapy on hospital or ICU LOS when comparing across a variety of treatment options.
Our study identified a statistically significant adjusted effect size of 1.6‐day decrease in ICU LOS with combination therapy within the first 2 days of admission. Although earlier studies have assessed disease progression or recovery markers, the primary outcome variables in this analysis were the hospital and ICU LOS. These outcomes were selected because they are reliably available in all patients and are expected to be impacted by the disease course, thus providing a reliable outcome measure. Even though hospital LOS was not directly assessed in prior studies, the ICU LOS reported herein aligned with the previous reports. 9 We used regression models to adjust for confounding factors that may affect LOS in patients with MIS‐C; however, LOS is impacted by social factors, insurance coverage, and hospital resources. It is possible that these influenced the hospital LOS more than ICU LOS, which may have prevented us from observing a statistically significant difference in hospital LOS.
The Best Available Treatment Study previously compared outcomes based on treatment with IVIG, steroid, or their combination. 11 However, their primary outcome was a difference in a composite outcome of inotropic support, mechanical ventilation, or reduction in disease severity. They did not identify a significant benefit with combination therapy compared to IVIG alone. The difference in outcomes between studies may be explained by 1) comparison of combination therapy to IVIG monotherapy versus neither IVIG nor steroids, 2) MIS‐C diagnosis based on WHO versus CDC criteria, and 3) variations in patient populations. Although the VIRUS: COVID‐19 registry has international patients, these children were excluded because of the potential variability in diagnostic criteria of MIS‐C outside the United States.
Treatment of MIS‐C could have varied between institutions, clinicians, and over time. The current American College of Rheumatology guidance for MIS‐C recommends IVIG and corticosteroids. Treatment recommendations have changed throughout the pandemic and require individualization to each patient. 8 , 32 Moreover, diagnosis of MIS‐C may be difficult given the ambiguity of presentation and the rare nature of the disease. Research suggests the potential for phenotypic variation in MIS‐C. 33 Phenotypic variations could result in differences in presentation, inflammatory response, and disease progression. Differences between the four groups in this study may reflect the variability in MIS‐C natural history or management. Infants were more heavily represented in Group D than Group A. This may be because MIS‐C was first described 34 and noted to be more common in older children and adolescents, 35 potentially leading to more conservative management in the infant population. The cohort that was not treated generally had lower severity of illness metrics than the other groups, which may reflect selection bias or confounding. Age may play a role, as COVID‐19 infection and sequelae have been less severe in younger patients. 36
Many patients have an overlap in management between MIS‐C and acute COVID‐19. As noted, acute COVID therapy was more utilized in Group C, possibly because these patients were thought to have acute COVID‐19 and yet met MIS‐C criteria. In ambiguous patients, it is possible that prescribers are less likely to use IVIG, but more comfortable using corticosteroids, which can treat both conditions. Patients in Group C had higher rates of high flow nasal cannula and invasive ventilation than other groups suggesting respiratory involvement more typical of non‐MIS‐C COVID‐19. Group C patients also had a higher likelihood of being SARS‐CoV‐2 PCR positive and a lower likelihood of being SARS‐CoV‐2 IgG positive, especially when compared to Group A. The idea that Group C had a higher percentage of MIS‐C and acute COVID‐19 overlap patients could be a confounder and may partially explain this group's longer LOS, increased ICU admission rate, and a higher need for respiratory support.
There were several limitations in this retrospective registry analysis. The exposure was categorized into four groups; however, the number of patients in Groups B and C was small, potentially affecting the model's validity. The four groups had significant demographic and therapeutic variations that might reflect their different phenotypic presentations and independently impact their hospital and ICU LOS. Because of overall low numbers of other therapeutic agents, their use could not be included in the model; in particular, biological agents were utilized in 18.9% of patients in Group A compared to only 2.3% in Group D, and the results were not adjusted for the impact of biological agents. The dose/duration of IVIG and type/dose of steroids were not available, and their differences may have impacted outcomes. Additionally, 24 children received either IVIG or steroids after the first 2 days (the study inclusion period for exposure). This intervention could have resulted in disease improvement and impacted the LOS in the control group. The dataset had a high proportion of missing data in the laboratory values of inflammatory mediators. In a deidentified retrospective registry analysis, it is not possible to determine if these values were not obtained or not entered in the registry. Similarly, even with robust measures for data quality, data entry error is always a possibility with voluntary registries.
5. CONCLUSION
Combination therapy of IVIG and steroids early during hospitalization with MIS‐C leads to a shorter ICU length of stay. Treatment in the first 48 hours of admission with IVIG or steroids alone may not be as efficacious.
CONFLICT OF INTEREST
Kathleen Chiotos receives funding from the Agency for Healthcare Research and Quality (AHRQ). No COI. R.K. receives funding from the National Institutes of Health/National Heart, Lung and Blood Institute; Gordon and Betty Moore Foundation Janssen Research & Development, LLC; and royalties from Ambient Clinical Analytics. Inc. They had no influence on this manuscript's acquisition, analysis, interpretation, and reporting of pooled data. VKK receives funding from the Gordon and Betty Moore Foundation, CDC Foundation through the University of Washington, and Janssen Research & Development, LLC. They had no influence on this manuscript's acquisition, analysis, interpretation, and reporting of pooled data. AJW currently funded receives funding from the National Institutes of Health/National Heart, Lung and Blood Institute grants, Agency of Healthcare Research and Quality, Boston Biomedical Innovation Center, and royalties from UpToDate. They had no influence on this manuscript's acquisition, analysis, interpretation, and reporting of pooled data. No other authors reported any actual or potential COI related to the manuscript and associated investigation.
Supporting information
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Harthan AA, Nadiger M, McGarvey JS, et al. Early combination therapy with immunoglobulin and steroids is associated with shorter ICU length of stay in Multisystem Inflammatory Syndrome in Children (MIS‐C) associated with COVID‐19: A retrospective cohort analysis from 28 U.S. Hospitals. Pharmacotherapy. 2022;42:529‐539. doi: 10.1002/phar.2709
Funding information
The registry is funded in part by the Gordon and Betty Moore Foundation and Janssen Research & Development, LLC. They had no influence on this manuscript's acquisition, analysis, interpretation, and reporting of pooled data. This publication was supported by NIH/NCRR/NCATS CTSA Grant Number UL1 TR002377. Its contents are solely the authors' responsibility and do not necessarily represent the official views of the NIH. The registry is funded in part by the Gordon and Betty Moore Foundation and Janssen Research & Development, LLC. They had no influence on the analysis, interpretation, and reporting of pooled data.
REFERENCES
- 1. McMurray JC, May JW, Cunningham MW, Jones OY. Multisystem inflammatory syndrome in children (MIS‐C), a post‐viral myocarditis and systemic vasculitis—a critical review of its pathogenesis and treatment. Front Pediatr. 2020;8:626182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rowley AH. Understanding SARS‐CoV‐2‐related multisystem inflammatory syndrome in children. Nat Rev Immunol. 2020;8:453‐454. doi: 10.1038/s41577-020-0367-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hennon TR, Penque MD, Abdul‐Aziz R, et al. COVID‐19 associated multisystem inflammatory syndrome in children (MIS‐C) guidelines; a Western New York approach. Prog Pediatr Cardiol. 2021;62:101407. doi: 10.1016/j.ppedcard.2021.101407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Menikou S, Langford PR, Levin M. Kawasaki disease: the role of immune complexes revisited. Front Immunol. 2019;10:1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gruber CN, Patel RS, Trachtman R, et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS‐C). Cell. 2020;183(4):982‐95.e14. doi: 10.1016/j.cell.2020.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Radia T, Williams N, Agrawal P, et al. Multi‐system inflammatory syndrome in children & adolescents (MIS‐C): A systematic review of clinical features and presentation. Paediatr Respir Rev. 2021;38:51‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. CDC – Nationa Center for Immunization and Respiratory Disease . Multisystem inflammatory syndrome (MIS‐C). Centers for Disease Control and Prevention. 2021. Accessed April 21, /22. https://www.cdc.gov/mis/index.html
- 8. Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS–CoV‐2 and hyperinflammation in pediatric COVID‐19: version 3. Arthritis Rheumatol. 2022;74(4):e1‐e20. doi: 10.1002/art.42062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ouldali N, Toubiana J, Antona D, et al. Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA. 2021;325(9):855‐864. doi: 10.1001/jama.2021.0694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Son MBF, Murray N, Friedman K, et al. Multisystem inflammatory syndrome in children—initial therapy and outcomes. N Engl J Med. 2021;385(1):23‐34. doi: 10.1056/NEJMoa2102605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McArdle AJ, Vito O, Patel H, et al. Treatment of multisystem inflammatory syndrome in children. N Engl J Med. 2021;385(1):11‐22. doi: 10.1056/NEJMoa2102968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walkey AJ, Kumar VK, Harhay MO, et al. The viral infection and respiratory illness universal study (VIRUS): An international registry of coronavirus 2019‐related critical illness. Crit Care Explor. 2020;2(4):e0113. doi: 10.1097/CCE.0000000000000113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walkey AJ, Sheldrick RC, Kashyap R, et al. Guiding principles for the conduct of observational critical care research for coronavirus disease 2019 pandemics and beyond: The Society of Critical Care Medicine discovery viral infection and respiratory illness universal study registry. Crit Care Med. 2020;48(11):e1038‐e1044. doi: 10.1097/CCM.0000000000004572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377‐381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gagne J, Polinski JM, Avorn J, John D Seeger RJG. Standards for Causal Inference Methods in Analyses of Data from Observational and Experimental Studies in Patient‐Centered Outcomes Research. Patient‐Centered Outcome Research Institute Methodology Committee; 2012. Accessed April 21, 2022. https://www.pcori.org/sites/default/files/Standards‐for‐Causal‐Inference‐Methods‐in‐Analyses‐of‐Data‐from‐Observational‐and‐Experimental‐Studies‐in‐Patient‐Centered‐Outcomes‐Research1.pdf [Google Scholar]
- 16. Lin W, Halpern SD, Prasad Kerlin M, Small DS. A “placement of death” approach for studies of treatment effects on ICU length of stay. Stat Methods Med Res. 2017;26(1):292‐311. doi: 10.1177/0962280214545121 [DOI] [PubMed] [Google Scholar]
- 17. U.S Food & Drug Administration . Pediatric exclusivity study age group. FDA. 2014. Accessed April 21, 2022. https://www.fda.gov/drugs/data‐standards‐manual‐monographs/pediatric‐exclusivity‐study‐age‐group
- 18. CDC . Use and Interpretation of the WHO and CDC Growth Charts for Children from Birth to 20 years in the United States. Division of Nutrition PA, and Obesity, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention; 2014. https://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/growthchart.pdf. Accessed April 21st, 2022, DOI: 10.1021/mz400635h [DOI] [Google Scholar]
- 19. CDC‐Division of Nutrition, Physical Activity, and obestity, National Center for Chronic Disease Prevention and Health Promotion . A SAS Program for the 2000 CDC growth charts (ages 0 to <20 years). Growth Chart Training: Centers for Disease Control and Prevention; 2022. Accessed April 21, 2022. https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm [Google Scholar]
- 20. Zurca AD, Suttle ML, October TW. An antiracism approach to conducting, reporting, and evaluating pediatric critical care research. Pediatr Crit Care Med. 2022;23(2):129‐132. doi: 10.1097/PCC.0000000000002869 [DOI] [PubMed] [Google Scholar]
- 21. CDC . COVID Data Tracker. US Department of Health and Human Services, CDC; 2022. Accessed April 21, 2022. https://covid.cdc.gov/covid‐data‐tracker. [Google Scholar]
- 22. Pollack MM, Patel KM. Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24(5):743‐752. [DOI] [PubMed] [Google Scholar]
- 23. CPCCRN . PRISM III Calculator. Collaborative Pediatric Critical Care Research Network; 2020. Accessed April 21, 2022. https://www.cpccrn.org/calculators/prismiiicalculator/
- 24. World Health Organization . WHO R&D Blueprint novel Coronavirus COVID‐19 Therapeutic Trial Synopsis. WHO; 2020. Accessed April 21, 2022. https://www.who.int/publications/i/item/covid‐19‐therapeutic‐trial‐synopsis [Google Scholar]
- 25. Mayo Clinic Laboratories . Test Catalog. Mayo Clinic; 2021. Accessed April 21, 2022. https://www.mayocliniclabs.com/test‐catalog. [Google Scholar]
- 26. National Kidney Foundation . Creatinine based bedside Schwartz equation. New York, NY. 2009. Accessed April 21, 2022. https://www.kidney.org/content/creatinine‐based‐%E2%80%9Cbedside‐schwartz%E2%80%9D‐equation‐2009
- 27. Soler YA, Nieves‐Plaza M, Prieto M, García‐De Jesús R, Suárez‐Rivera M. Pediatric risk, injury, failure, loss, end‐stage renal disease score identifies acute kidney injury and predicts mortality in critically ill children: a prospective study. Pediatr Crit Care Med. 2013;14(4):e189‐e195. doi: 10.1097/PCC.0b013e3182745675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tripathi S, Gist K, Bjornstad E, Kashyap R, Boman K. Coronavirus disease 2019 associated pediatric ICU admissions: a report from the SCCM discovery network VIRUS registry. Pediatr Crit Care Med. 2021;22(7):603‐615. doi: 10.1097/PCC.0000000000002760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Horvat C. Statistical note: confounding and causality in observational studies. Pediatr Crit Care Med. 2021;22(5):496‐498. doi: 10.1097/PCC.0000000000002702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pollack MM, Holubkov R, Reeder R, et al. Pediatric intensive care unit (PICU) length of stay: factors associated with bed utilization and development of a benchmarking model. Pediatr Crit Care Med. 2018;19(3):196‐203. doi: 10.1097/PCC.0000000000001425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495‐1499. doi: 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 32. Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS–CoV‐2 and hyperinflammation in pediatric COVID‐19: version 1. Arthritis Rheumatol. 2020;72(11):1791‐1805. doi: 10.1002/art.41454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. DeBiasi RL, Harahsheh AS, Srinivasalu H, et al. Multisystem inflammatory syndrome of children: subphenotypes, risk factors, biomarkers, cytokine profiles, and viral sequencing. J Pediatr. 2021;237:125‐135. doi: 10.1016/j.jpeds.2021.06.002 [DOI] [PubMed] [Google Scholar]
- 34. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki‐like disease at the Italian epicentre of the SARS‐CoV‐2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771‐1778. doi: 10.1016/S0140-6736(20)31103-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS‐C) compared with severe acute COVID‐19. JAMA. 2021;325(11):1074‐1087. doi: 10.1001/jama.2021.2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bhalala US, Gist KM, Tripathi S, et al. Characterization and outcomes of hospitalized children with Coronavirus Disease 2019: a report from a multicenter, viral infection and respiratory illness universal study (Coronavirus Disease 2019) registry. Crit Care Med. 2022;50(1):e40‐e51. doi: 10.1097/CCM.0000000000005232 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5


