Abstract

Agricultural practices in modern society have a detrimental impact on the health of the ecosystem, environment, and consumers. The significantly high usage rate of chemicals causes serious harm, and the sector demands the development of innovative materials that can foster improved food production and lessen ecological impacts. The majority of layered double hydroxides (LDH) are synthetic. At the same time, some of them occur in the form of natural minerals (hydrotalcite), which have recently emerged as favorable materials and provided advanced and ingenious frontiers in various fields of agriculture through practical application possibilities that can replace conventional agricultural systems. LDH can exchange anions intercalated between the layers in the interlayer structure, and there is evidence that atmospheric carbon dioxide and moisture can completely break down LDH over time. Due to certain unique properties such as tunable structure, specific intercalation chemistry, pH-dependent stability, as well as retention of the guest molecules within interlayers and their subsequent controlled release, LDHs are increasingly investigated as materials to enhance yield, quality of crops, and soil in recent times. This review aims to present the current research progress in the design and development of LDH-based materials as nanoscale agrochemicals to illustrate its relevance in making agro-practices more sustainable and efficient. Specific emphasis is given to the functionality of these materials as effective materials for the slow release of fertilizers and plant growth factors as well as adsorption of toxic agrochemical residues and contaminants. Relevant research efforts have been briefly reviewed, and the potential of LDH as new generation green materials to provide solutions to agricultural problems for improving food productivity and security has been summarized.
1. Introduction
The agricultural sector is facing new challenges to meet food demand and product quality as the population of the world is increasing day by day. It is reported that by 2050, the world population will be around 9.2 billion,1a and the demand for food will subsequently increase by 98%. Consequently, crop production must be increased by boosting productivity to reduce the mismatch between demand and supply. From a materials point of view, the current agricultural practices heavily depend on chemicals in the form of fertilizers, herbicides, and pesticides to maximize agricultural production. Increased utilization of chemicals in agriculture is regularly justified based on financial benefits and increased efficiencies in terms of yield. However, it is well recognized that disproportionate and insensitive usage of fertilizers and pesticides has heightened residues and toxins in the soil, ground/surface waters, and air, confronting the cost of increased fertilization, irrigation, and energy to retain productivity, health costs, and ultimate desertion of some of the best farming lands all over the world.1b−1l Hence, it is crucial to develop and implement environmentally and human-friendly materials-based strategies to increase crop production rate while conserving our mother nature and protecting the health of the consumers.
Nanotechnology, the science of designing materials in the nanometer size range, has provided an innovative and resourceful platform to improve the competence of agricultural materials and processes for improving food productivity and security while offering solutions to agricultural and environmental problems. For instance, small quantities of nutrient-rich nanomaterials can make soils more capable of efficient nutrient uptake for greater productivity and better environmental security.2a The use of certain nanomaterials for environmental remediation is widely researched these days, given the removal potential of organic compounds and heavy metals. Great opportunities are realized for nanotechnology in genetic improvement of plants, genes, active ingredient delivery, and sensors. Advanced delivery systems for agrochemicals like fertilizers and pesticides and integration of smart systems for food processing, packaging, and monitoring of agricultural and food system security are part of the emerging technologies.2b With nanofertilizers as substitutes to conventional fertilizers, accumulation of nutrients in the soil and thus eutrophication and pollution of drinking water may be diminished as they allow the release of nutrients in a controlled manner in required amounts.2c
Most recently, 2-dimensional inorganic nanoparticles, namely, layered double hydroxides (LDHs), are receiving increased attention from the agricultural sector as a sustainable and prospective material with multifunctional properties. Research groups were able to prepare LDH as an eco-friendly material that is very useful to increase crop yield as well as reduce environmental pollution.3a,3b A significant number of possible arrangements and metal as well as anion intercalation make LDH a versatile material with unique characteristics such as high chemical stability, basicity, pH-dependent solubility, and bio and environmental compatibility.3c,3d LDH have been demonstrated as sources of micronutrients as well as carriers of water, diverse anions such as nitrate, phosphor ions, and antimicrobial agents.
This review highlights the recent opportunities realized for LDH in agricultural science in the context of increasing consumer concerns over approaches unfavorable to health used for crop production. The review presents the functionality characteristics of LDH. It explores the competence of the LDH as a soil friendly slow-release matrix for fertilizer and agrochemicals in addition to its use for decontamination of soil and water, soil conditioning, and nutrient delivery. The review aims to unveil the emerging value propositions of LDH as a green agro-product and demonstrate its performance in soil remediation, soil conditioning, as well as crop production. Reviews of LDH summarizing the complete state of the art in the agricultural field are very scarce. We expect this review to support future endeavors to integrate LDH as a sustainable material for various facets of crop production practices.
2. Structure and Multifunctionality of Layered Double Hydroxides
Layered double hydroxides are referred to as a class of anionic layered materials with a two-dimensional layered structure and an ion-exchanging power.4a,4bFigure 1 shows the molecular structure of M2+/M3+-A–-LDH. Although LDH is derived from the structure of the mineral brucite, Mg(OH)2, unlike the brucite structure, some of the divalent metal ions are replaced with trivalent metal ions in the LDH structure. LDH has the chemical formula [M2+1–xM3+x(OH2)]x+(An–)x/n·yH2O (where M2+ is the divalent metal ions (e.g., Mg2+, Zn2+, Mn2+); M3+ is the trivalent metal ions (e.g., Al3+, Fe3+, Cr3+); An–, is the intercalated anions (e.g., NO3–, CO32–, Cl–), and x is the fractional aluminum substitution in the layers, which is the metal ratio M3+/(M2+ + M3+)]. The value of x varies preferably between 0.2 and 0.33, with the corresponding proportion of M2+/M3+ within 2:1 to 4:1.4c Other LDH are also reported; for instance, LDH with monovalent and trivalent (Li+ and Al3+),4d metal ions as well as trimetallic LDH with more than one divalent or trivalent metal ion (Ni2+, Fe2+, Cr3+).4e The chemical formulas of these LDH hence show variation according to the metal compositions and intercalated anions, for example, [Mn6Al3(OH)18][(HPO42–)2A+]·yH2O (A+ = Li, Na, or K).4f The combinations of metal-anion and the incredible number of feasible arrangements are the highlights of LDH among layered materials. Due to the excess positive charge from the trivalent metal ions, LDH layers carry a net positive charge, which is balanced by negative ions intercalated in interlayer galleries. Along with water molecules, these anions assist in stacking layers of LDH with an interlayer domain weakly organized. The layers are not just connected by hydrogen bonds, such as in brucite, but also with the electrostatic interaction between the positively charged plates and interlayer inorganic anions (Figure 1).4g
Figure 1.

Basic structure of a generic 3R polytype layered double hydroxides. Reproduced from ref (4g). This is an open-access article distributed under the Creative Commons Attribution License.
Layered double hydroxides are excellent anion exchange materials. A great variety of anionic species can either be intercalated between the layers or adsorbed on the surface during the formation of the layered structure or by the other anionic exchanges. The ratio of M3+ and M2+ influences the anion exchange capacity of LDH, which could range from 200 to 450 cmolc kg–1 for obtaining a relatively stable LDH structure.4h Their extensive intercalation chemistry has widespread applications as absorbents and controlled release agents in areas including medicine due to their unique host–guest type structure. LDH exhibits unique properties such as flexibility of chemical composition, swelling properties, extreme affinity to carbonate ions, pH-dependent solubility, and biodegradability. Its preference for carbonate ions as the interlayer anion and acid lability allow LDH to be compatible with various biological systems and environments. The crystallinity and the textural and structural properties can also be tailored by varying the type of the interlayer anions, the method of preparation technique, and the reaction conditions.
Due to the high surface charge and hydrophilic properties of LDHs, the particles of conventionally synthesized LDH are generally highly aggregated, resulting in a low specific surface area of typically 5–50 m2/g.5 However, higher surface areas can be obtained by changing the synthesis conditions.6a,6b Another remarkable property of LDH is that it can reconstruct its primary structure through what is known as a memory effect after calcination under heating conditions (400–500 °C).7 The small size of LDH is significant for colloidal properties crucial for developing LDH-based hydrogel structure.8 The LDH structure, when decomposed in the environment,9 can provide necessary nutrients to plants. The high-water retention capacity and acid-neutralizing capacity also make them ideal materials for soil conditioning.10a,10b The usage of LDHs in agriculture was minimal due to the abundance and focused on natural clay minerals. However, in recent years a lot of attention has been given to developing LDH-based agro-products considering the multifunctionality of the material.10b LDHs quickly release the nutrient ions from the external surfaces. Afterward, the intercalated nutrient ions release slowly. A couple of research studies already show that LDH releases nutrients much slower than other commercial fertilizers.11a−11c Because of the positively charged brucite-like layers with relatively poor interlayer bonding, LDH has an excellent capability to capture the organic and inorganic anions. Utilizing this property, LDHs are used as adsorbents to uptake the anionic contaminants such as phosphate,12a nitrates,12b fluoride,12c arsenate,12d pesticides,12e and organic pollutants,12f etc., from water or wastewater or soil. According to Benício et al.,3b LDH can enhance the nutrient availability in the soil solution by increasing the soil pH, which increases the negative charge on the soil matrix and decreases the adsorption of anions. LDH is entirely biodegradable, and the main advantage is that it can degrade over time and then completely release the intercalated micronutrient anions. Some studies concluded that LDH degradation occurs gradually under atmospheric CO2 and moisture.13,14 Many review articles can be found in the literature that provide details of various synthesis and characterization methods of LDH.15a−15c
3. Layered Double Hydroxides in Agricultural Applications
The characteristics of LDH that are routinely investigated for agricultural applications include slow-release ability, excellent adsorption, and ion exchange capacity as well as soil conditioning. Ion exchange properties can be utilized for the adsorption of fertilizer residues and as well as for releasing agrochemical ingredients. LDH can also form hydrogels, and the high basicity is ideal for neutralizing the acidity of the soil. The following sections will explore the use of LDH in various fields of crop production.
3.1. Layered Double Hydroxides in Soil Remediation
Nitrogen and phosphorus are essential nutrients in crop production. Nitrate and orthophosphates, dihydrogen phosphate, and hydrogen phosphate are the primary forms of nitrogen and phosphorus absorbed by plants. Generally, soil particles do not retain excess nitrates but are carried downward through water percolation. That is one of the reasons for environmental pollution as well as health hazards.16a Similarly, crops can absorb only 5–30% of the phosphorus that is used in the field due to the attraction of orthophosphate to clay fraction in soils. LDH with positively charged layered structure and comparatively weak interlayer bonding demonstrate an excellent ability to adsorb several groups of anions such as halides, oxyanions, anionic metal complexes, organic anions, and anionic polymers.16b
Torres-Dorante et al.17a investigated the properties of Mg/Al-LDH intercalated with chloride anions to evaluate the nitrate leaching from the soil. The study was conducted in both soil and aqueous solutions to explore the adsorption and selectivity, anion exchange capacity, as well as the diffusion of nitrate into LDH nanomaterials from the soil. While LDH showed high selectivity toward nitrate than other anions (bicarbonate, sulfate, chloride, and dihydrogen phosphate), the adsorption capacity was 1.54 cmolc kg–1 in aqueous solutions. The nitrate adsorption and release by LDH were successfully demonstrated by 15 cycle experiments, although a considerable decrement of exchange capacity was observed at the end of the 15th cycle. During 1 week of testing, the reduction of nitrate concentration up to 8 cm deep in soil layers was observed, which means a higher adsorption capacity of LDH toward nitrate from the soil. The result thus indicated that LDH nanomaterial could be used as nitrate absorbers in agriculture to minimize the nitrate leaching into the groundwater. In a subsequent study,17b the authors also investigated the performance of Mg/Al-LDH as a buffer for nitrate in the soil. The experiments were performed using soil and plant (Triticum aestivum cv) within the greenhouse environments to assess the nitrate adsorption capacity by the LDH as well as its capability to maintain mineralized nitrate throughout the growth period of the crop. Furthermore, the capacity of the LDH to exchange nitrate with other anions in a farming environment was also studied. The results indicated that throughout the growth process of the plant, the uptake of nitrate from the soil by LDH never influenced the uptake of nitrate by the plant. The nitrate concentration in the soil was 10 times lower in the presence of LDH when compared to the soil without LDH nanomaterial. LDH was able to absorb nitrate and hence decrease the leaching up to 80%. Moreover, the same LDH (10 g LDH kg–1) was used to release nitrate back into the soil during the next harvesting seasons. The nitrate buffer capacity of the soil (generally defined as b = ΔCT/ΔCL) is the relation between the total amount of an ion in soil (ions in soil solution plus those adsorbed to the solid phase = ΔCT) and the concentration of the ion in the soil solution (ΔCL) and was enhanced to 2.7 after 15 months, while the normal soil without LDH showed a buffer capacity of 0.3. The study thus indicated that LDH nanomaterial could be utilized as a long-term buffering agent to manage the nitrate movement in the soil and decrease the leaching of nitrate to the groundwater.17b It is understood that the selectivity of LDH toward nitrate is affected by the presence of other anions as LDH, in general, has greater affinities for anions with higher charge density.17c Halajnia et al.12b prepared Mg/Al and Mg/Fe-LDH intercalated with chloride by the coprecipitation method with a molar ratio of M2+/M3+ 3:1 and 4:1 and used them for nitrate removal. Nitrate adsorption isotherm and kinetics with various pH and temperatures, thermodynamic parameters, anion exchange capacity, and nitrate selectivity of the prepared nanomaterials in soil solutions were also examined. The results validated that 4:1 Mg/Al-LDH was a better performer for nitrate adsorption (with the rate of 0.623 g mmol–1 min–1 by the pseudo-second-order model) in the presence of other anions (phosphor, sulfate, and bicarbonate) in solution. Outcomes showed that adsorbents did not perform efficiently for nitrate removal in soil solutions (due to the presence of multiple anions). They also observed a decrease in nitrate adsorption at higher solution temperatures. However, Ito and co-workers17d synthesized Mg/Fe-LDH cost-effectively from the MgO source and demonstrated its higher exchange selectivity toward nitrate ions in the presence of other anions. The anion exchange capacity (AEC) of the best-performing composition of PA1.5 was 166 cmolc kg–1, which corresponded to 23 g of N per kg of LDH. The authors tried to explain these contradictory results by stating that the nitrate ions having a planar structure could be better stacked in the interlayers.17e Other studies are showing high adsorption capacity and selectivity of LDH to nitrate adsorption.17f,17g Very recently, Mohammadi and co-workers17g used Mg/Al-LDH loaded with chloride anions for four crops (bell pepper, mentheae, cherry tomato, and wheat) for a 16 month period (four fallows) to examine the sorption capacity of nitrate adsorption and also to examine the effect of LDH on plant growth. Results showed that 2, 4, 8, and 16 g/kg of LDH removed 34%, 44%, 58%, and 69% of nitrate compared to the control while significantly increasing the plant height (cherry tomato) up to 80% in the case 16 g/kg LDH. The prospect of LDH as a long-term nitrate exchanger, thereby reducing nitrate leaching, was highlighted in this study. However, the study was conducted in controlled laboratory environmental conditions.
Although various LDHs have been reported for phosphor anion removal from aqueous solutions,17h studies on the adsorption characteristics of the LDH from soil solutions are very scarce. Strategic removal of phosphor ions from wastewater using Mg/Fe-LDH was reported by Ashekuzzaman et al.17i The optimal removal of phosphor ions was observed under pH of 3–7.5 and they could achieve about 95% of phosphor ions reduction after 6 cycles of repeated use of 2 g/L of LDH. Azimzadeh et al.17j worked on Mg/Al-LDH and Mg/Al- LDH-biochar/hydrochar as a phosphor ions removal agent from aqueous solutions as well as in calcareous soils and further used phosphate-LDH-biochar/hydrochar as a nutrient source for maize cultivation. Using a 0.03 mol/L KCl solution, the percentage of phosphor ions uptake was measured as 18, 22, and 27% for LDH, LDH-biochar, and LDH-hydrochar, respectively. In a recent study, Liao and colleagues17k demonstrated competitive adsorption of phosphor ions and nitrate by calcined Mg/Al-LDH. LDH showed preferential adsorption toward phosphor ions while nitrate was adsorbed on redundant adsorption sites. Synthetic wastewater prepared by adding KH2PO4 and KNO3 in distilled water was used as the medium. Furthermore, after adsorption, adsorbed nitrate was released more readily than phosphor ions anions from LDH. Calcination of LDH is not energy efficient and can negate the green status and cost-effectiveness of the material. The performance of the material for soil remediation was not investigated in this work.
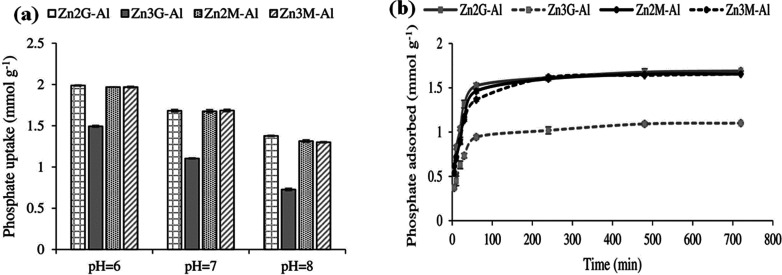
Yong-Un et al.17l developed LDH containing alginate beads and used them for phosphor ions adsorption. Results in the batch experiment revealed that between the solution pH 5.0 and 9.0, there was no strong effect for phosphor ions removal and 8% LDH-alginate beads showed the best removal capacity when compared to other percentages of LDH-alginate beads (0%, 2%, 4%, 6%, and 10%). Column experiments indicated that the phosphor ions removal capacity of 8% LDH alginate beads was 2 orders of magnitude greater than that of pure alginate beads. This research did not demonstrate the impact of the materials as an adsorbent in the agriculture field studies. Thereafter, Hatami et al.12a used nitrate and carbonate intercalated Zn/Al-LDH with the ratio of 2:1 and 3:1 using the conventional and modified urea hydrolysis methods for phosphor ions adsorption and desorption in soil solution. Of the various samples prepared 3:1 Zn/Al-LDH prepared by the general urea hydrolysis method, which demonstrated the coexistence of nitrate and carbonate anions in the interlayer, showed the minimal adsorption of phosphor ions. Nitrate intercalated LDH absorbed the highest amount of phosphor ions while uptake decreased in the presence of other anions (sulfate, bicarbonate, and chloride). The study also explains the role of pH in phosphor ions adsorption, which showed that as the pH increases, the adsorption decreases. The decrease in uptake efficiency with an increase in pH is expected for inorganic sorbents, which remove anions via exchange of their surface OH-groups as the initiating step (see Figure 2a). Recently Qiao et al.17m examined the capacity of release and adsorption of phosphor ions from prepared magnetic layered double hydroxide (Fe3O4/Zn-Al-Fe-La-LDH), and results showed that 1.2 g L–1 of LDH could adsorb the highest number of about 169.5 mg g–1 within 24 h while the initial concentration of phosphor ions and solution pH was 200 mg L–1 and 4, respectively. The adsorption capacity of LDH was 31 mg g–1 after four adsorption–desorption cycles, which proved that the materials were reusable. LDH did not release the metal ions into the water, which was confirmed by inductively coupled plasma-optical emission spectrometry (ICP-OES) analysis. The magnetic LDH demonstrated better results for phosphor ions removal, showed a good release behavior, and increased plant height by 13.46% higher than TSP.
Figure 2.
Effect of (a) pH in phosphor ions adsorption by LDH and (b) time on phosphor ions adsorption from phosphor ions solution. Reproduced with permission from ref (12a). Copyright 2017 Elsevier Science Ltd.
3.2. Layered Double Hydroxides as Slow-Release Fertilizers
The growing requirement for food and ever-increasing world population leaves no option to increase crop production with the intensive use of synthetic fertilizers. Unfortunately, there are negative impacts of applying larger quantities of fertilizers to the soil. The focus has now shifted to slow-release fertilizers that release a small, steady amount of nutrients over time. Several studies have indicated the prospects of LDH as effective slow-release substrates for various fertilizer ingredients.3a,11a
Silva et al.18a prepared Mg/Al-LDH-NO3 by the coprecipitation method. The release of nitrate was examined at pH 6.5 in NaHCO3 buffered solution and distilled water. The release profiles indicated two types of release from the material: initial fast release, then a slow and gradual release. The outcome of this study highlighted that the material was useful as a slow-release nitrate fertilizer. The authors observed the experiment under laboratory conditions at a constant pH and did not attempt to see the effect of LDH material on soil or different buffer solutions, so the usability of the material on soil or agricultural condition as a slow-release matrix was not demonstrated. Berber et al.18b developed Mg/Al-LDH-NO3 by the coprecipitation method and applied it as a controlled release nitrate fertilizer in the soil. One acidic and one basic soil solutions were prepared and used to study the release characteristics at various temperatures (15, 25, and 35 °C). The results indicated that at 15 °C, the nitrate was released for 16 days in an acidic soil solution; however, in basic soil solution, nitrate release was observed for 20 days. The study also demonstrated an increased release rate for nitrate when the soil temperature was increased. The investigation revealed the prospects of LDH as a pH and temperature-responsive nitrogen fertilizer. In the following study, Berber et al.3a prepared Mg/Al-LDH-NO3 by the reconstruction method, which showed a higher number of intercalated nitrate anions. The release was conducted in artificially prepared acidic agriculture soil as well as basic soil at pH 4 and 7. According to the results, about 90 wt % of the intercalated nitrate anions were released in acid soil in 10 days, but in basic soil, it took 20 days.
Kotlar et al.18c synthesized Mg/Fe-LDH-NO3 with the ratio of 2:1 by the coprecipitation method to evaluate the nitrate release. Batch experiments and soil columns were used to measure the exchange properties of prepared materials. 0.5, 0.1, or 0.01 M of KCl, K2SO4, or CaCl2 solutions were employed to release nitrate in batch experiments which indicated that within the first few hours, almost 100% of nitrate was exchanged with other anions through a burst release. Soils from temperate (Denmark) and tropical regions (Brazil) were used in column studies. Sandy loam temperate soils showed the optimum result with decreasing the nitrate leaching up to 22%. The maximum exchange capacity of LDH remained at the surface of the tropical soil, which was demonstrated through the strength of Mg and Al signals measured by X-ray fluorescence spectroscopy (XRF). Fe in LDH dissolved in pH between 2.5 and 5, whereas 25% more of Mg could be dissolved within this range. All the experiments were conducted using the medium (water or soil) in a controlled environment; as a result, the capability of the used material in a cultivation environment was not projected. Recently Nunes et al.18d prepared Zn/Al-LDH with interlayer nitrate, then encapsulated in alginate, and employed in powder and bead forms. To evaluate the release rate of nitrate, KNO3 was used as a reference in water as well as in soil for growing pearl millet (Pennisetum glaucum L). The study also evaluated nitrogen uptake efficiency by plants in water. The results indicated that the nitrate release rate significantly decreased by using beads in contrast to powder material. However, similar results were obtained in the growth chamber due to the physicochemical obstruction having high buffering and cation-exchange capacities in bead shaped material. So, according to the experiments, it was demonstrated that alginate encapsulation magnified the slow-release ability of LDH and gradually maintained the release rate of nitrate. However, the performance of the encapsulated LDH was not compared to any commercial slow-release nitrate fertilizers to validate the industrial application prospects.
To prepare the phosphor ions intercalated LDH through the coprecipitation method is difficult due to the competitive formation of metal phosphates instead of LDH. In addition, phosphor ions can resist the changes in pH value of the solvent on the addition of a small amount of an acid or a base.18e Badreddine et al.18e used indirect method-ion exchange to prepare phosphor ions intercalated Zn/Al-LDH. In this process, already prepared low valence anions (Cl–) intercalated LDH is utilized for the synthesis, and it was confirmed by FTIR spectroscopy that different phosphor ions species were intercalated within the LDH interlayer. The focus of the research was only on the fundamental understanding of the exchange of phosphor anion by the prepared materials, and the author did not use the material for other lab or field trials. Subsequently, Everaert et al.11a employed Mg/Al-LDH with various Mg/Al ratios to prepare phosphor ion intercalated LDH with different AEC by the ion-exchange method. It was evident that the amount of intercalated phosphor ions in the LDH layer was high if the positive charge density of the LDH structure was high. The release experiments were done using prepared material with KH2PO4, and the release profile was compared to that of granular triple superphosphate in an acid weathered soil and calcareous soil. The prepared LDH material showed 4.5 times higher phosphor ions availability than the reference source in acidic soil. Although the amount of KH2PO4 and the concentration of intercalated phosphor ions were similar, the plants showed better phosphor ions uptake from LDH. Results from the pot trial indicated that the plant grown using prepared LDH material showed 1.2 times higher phosphorus in the shoot. Later in another study, Everaert et al.11b utilized Mg/Al-LDH phosphor (Mg/Al-LDH-P) as powdered or granulated fertilizer to compare the performance with water-soluble mono ammonium phosphate (MAP) fertilizer and struvite, an eco-friendly fertilizer. Wheat (Triticumaestivum) was used in pot trials along with acid and calcareous soil. To evaluate the release properties, a 100-day incubation experiment was employed. Mg/Al-LDH-P showed 74–90% of phosphor ions still intercalated in the interlayer after 100 days of experiments, showing slow-release properties. For granular fertilizers in pot trials, high phosphor ions adsorption by wheat was observed when MAP was used. On the other hand, in the powder form, MAP was shown less effective than granular MAP. Mg/Al-LDH-P and struvite were utilized in waste streams for phosphor ion recycling and as a slow-release and eco-friendly fertilizer. However, this study did not find higher agronomic effectiveness for granulated LDH and struvite as slow-release fertilizers than for a common soluble phosphorus fertilizer (MAP) in two soils that strongly sorbed soluble phosphorus (acid weathered and calcareous soil).
In a subsequent study, Everaert et al.11c utilized MAP, granulated Mg/Al-LDH-P to evaluate the performance of reducing dissolved phosphorus runoff under rainfall simulation experiments. All the three fertilizers were used at equal total phosphorus doses on soil packed in trays (5% slope) and covered with perennial ryegrass (Lolium perenne) under four different rainfall simulation events of 30 min at 1, 5, 15, and 30 days after the fertilizer application. The analyses of the runoff water showed that the runoff losses of dissolved P in LDH and struvite trials were 1.9% and 1.5%, respectively (due to the low solubility and anion exchange properties of these materials, respectively). In contrast, the total runoff loss was 42% for MAP where the losses were high in the first two rain events and leveled off in later rain events. The effectiveness of LDH for slow-release of phosphor ion (through ion exchange with HCO3– and lower surface in contact with soil) to lower the P content of wastewater and thereby surface water eutrophication was highlighted in this study.
Benício et al.3b used K2HPO4 as a source of phosphor ions exchanging with intercalated nitrate anions to form Mg/Al-LDH-P. The release of phosphor ions was evaluated in the tropical soil through a kinetic study using a continuous stirred-flow system with deionized water as well as bioassays. Maize (Zea mays) was used in a growth chamber with favorable conditions of humidity, temperature, and light. In bioassays, the authors used sandy and clayey soils, furthermore, with commercial fertilizer triple superphosphate (TSP) to compare phosphor ion release with prepared LDH. The kinetic release of phosphorus indicated that LDH presented an accumulative release rate lower than that of TSP; 60% of the total amount of phosphorus was delivered to the solution after 150 min of flow, while the TSP sample released 100% after about 50 min. Mg/Al-LDH-P showed the best results and enhanced the plant height, productivity, and amount of phosphorus in the plant. Moreover, prepared materials were found to be useful to enhance the soil pH and reduce phosphorus contamination, which helps plants to take up more phosphor ions. Subsequently, the same research group prepared phosphor ion intercalated LDH using Mg/Al and Mg/Fe with a ratio of 2:1 and 3:1 and Mg/Al/Fe-LDH with a ratio of 2:0.5:0.5.18f They also studied the kinetics of phosphor ion release of the prepared materials in water through the stirred-flow method. According to the results, a Mg/Al 2:1 ratio and Mg/Al 3:1 ratio demonstrated a slower release of phosphor ions with 60% and 84% of phosphor ion release after 150 min. On the other side, Mg/Al/Fe-LDH released the phosphor ion after 130 min. According to the author, the release of phosphor ions from LDH depends on the layer components, charge density, crystallinity, and a different molar ratio of metal cations. Results also suggest that fast release occurs in low crystalline material compared to the other better structure materials, and Al3+ holds more phosphor ions in interlayer space than Fe3+.18f Bernardo et al.18g prepared Mg/Al-LDH-P by the reconstruction method and investigated the released property. A high amount of phosphor ion around 40 mg g–1 was intercalated into the LDH. The release experiments were conducted in water and soil using wheat (Triticumaestivum) as a model crop, and the results are reported in Figure 3. The kinetic release of phosphor ions from LDH in water was compared to KH2PO4 and MAP, and the data (Figure 3a) indicated that LDH released 90% of phosphor ions in ∼53 min. In contrast, an equal amount of phosphor ion was released by soluble phosphorus sources KH2PO4 and MAP during the first 5 min, indicating a 10 times slower release from LDH. Phosphor ion was mainly associated with Fe3+ and prevented its availability for the plant for reutilization; however, no significant interaction of phosphor ion with Al3+ was noted in the soil experiments. After sowing wheat for 30 days, the data showed (Figure 3b) that the LDH materials were capable of supplying phosphor ions in a controlled way keeping the availability of the phosphor ion throughout the growth period. Although the researchers screened the performance of the prepared materials in water, soil, and pot trials using various plants, the materials utility in different pH and temperature conditions was not investigated. Borges et al.18h suggested a separate method (mechanochemical process) to prepare LDH from Mg2Al-CO3 or Mg2Fe-CO3 and K2HPO4 by varying molar ratios and the milling times. They examined the impact of the process on phosphor ion and potassium release. To evaluate the release of phosphorus and potassium, testing was conducted through the isothermal process for up to 31 days at various temperatures. MgAl/K2HPO4 with a molar ratio of 2:1 showed the optimum result that released 75% of phosphor ion in 28 days. Similarly, the intercalation of carboxymethylcellulose with LDH/K2HPO4 also enhanced the slow-release of phosphor ions.
Figure 3.
Kinetics of P release into the (a) water: (A) KH2PO4, (B) MAP, and (C) LDH-P and (b) phosphorus uptake by wheat. Reproduced with permission from ref (18g). Copyright 2018 American Chemical Society.
Recently, Onishi et al.18i reported phosphor ion sorption followed by kinetic release from Mg/Al-LDH-P prepared by the reconstruction method (after calcination at 500 °C for 4 h) in soil solution. The adsorption of phosphor ions in LDH was 52.56 mg g–1. To imitate the natural environment, soil solution was used as a released medium for LDH and MAP (commercial fertilizer). Results demonstrated slower phosphor ion release from LDH. After the release experiments (45 days), the release of phosphor ions in the clayey soil solution and the sandy soil solution was 11% and 5.5%, respectively, whereas MAP was released 91.5% and 94.8% within 24 h. The release of phosphor ions in clayey soil was perfectly explained by the intraparticle diffusion pattern, but in sandy soil, the pattern followed second-order kinetics. According to the authors, the phosphor ion release was extremely pH-dependent. In contrast, LDH released nutrients slowly, which is a distinct concept about phosphor ion release from material in an aqueous solution for an extended period. The practical application prospects of materials prepared involving a high-temperature step are questionable in terms of the material cost, and the efficiency of the materials in a real application scenario was not proposed. Azimzadeh et al.17j used P-LDH-biochar/hydrochar as a nutrient source for maize cultivation. After 5 desorption cycles in 0.1 mol L–1 Na2CO3 solution, 52%, 63%, and 66% of phosphor ion release was registered for the LDH-biochar composite. A direct relation was established between the solution pH and the desorption rate of phosphor ions. Results showed that the release of phosphor ions from LDH-biochar and LDH-hydrochar increased the phosphorus content in maize shoots and roots, dry matter, soil pH, AEC, as well as the soil available phosphorus. Lohmousavi et al.18j developed an eco-friendly banana peel cellulose (BPC)-g-poly(acrylic acid)/ poly(vinyl alcohol)/LDH(Mg/Al-LDH-Cl)/ NP nanocomposite to use as a slow-release matrix of nitrogen, phosphorus fertilizers, and water. The pH of the solution impacted the water uptake capacity; the amount of water uptake increased between the pH range of 7–10. Although these ranges had a reverse impact on the fertilizer slow-release rate, the nitrogen release increased in acidic pH while the basic pH range reduced the nitrogen release rate. The highest amount of phosphorus was released at pH 7. Schott’s second-order equation was used to measure the swelling kinetic of the prepared material. Based on the results, the prepared materials were able to improve the water holding capacity of soil for an extended period while exhibiting slow-release properties. Some researchers have prepared coated fertilizer that can release nitrogen, phosphorus, and potassium in a controlled manner; the purpose of the research was also similar, but the utility of the chloride intercalated LDH for this material was not explored enough. Furthermore, the applicability of the materials under the cropping conditions was unmapped.18k Buates et al.18l also investigated the feasibility of using biochar, functionalized with LDH, as a fertilizer after its use in phosphate treatment (P-BC-Mg/Al-LDHs). Lettuce cultivated under a controlled environment using P-BC-LDH, at an application rate of 2.5% (w/w), showed superior growth quality to those grown under nontreated conditions. The length of lettuce shoots and roots from this optimal dosage were increased by at least 24% compared to untreated samples due to the slow release of phosphor ions, thereby increasing the long-term nutrient availability for plants.
3.3. Layered Double Hydroxides as Slow-Release Substrate for Agrochemicals
Excessive use of agrochemicals such as pesticides and herbicides can contaminate the soil, surface, and groundwater. Most of these agrochemicals, in free forms, are released into the environment directly causing environmental pollution. Especially anionic herbicides that are poorly attached to the soil components can easily be dissolved in the soil solution and pollute nature through volatilization, leaching, runoff, etc.19 To reduce the effect of agrochemicals in nature, the new strategy is to release these agrochemicals in a sustained, eco-friendly manner while improving agriculture production.
Chiriac et al.20a prepared Mg/Al, Cu/Al, and Ba/Al-LDH intercalated with pesticide and plant growth regulator (chlorothalonil, salicylate anion, and atonik). Chlorothalonil was only stable at pH 7, and hence the reconstruction method was used to develop the LDH with chlorothalonil and chlorothalonil-Fe3O4. Corn (Zea mays) and wheat (Triticum sativum) were used as model plants to examine the germination rate, elongation, and root growth. Seeds of the cereal grain were applied on Petri dishes and maintained the ideal climate (20 °C) for 3 days in dark conditions. The spectrophotometer was used to measure photosynthetic pigments as well as an amino acid. Results indicated that pesticide was released in a controlled way. Subsequently, in another study, Cardoso et al.20b used three processes (i) direct synthesis, (ii) regeneration, and (iii) ion exchange for preparing Mg/Al-LDH intercalated with anions of 2,4-D, 4-chloro-2-methylphenoxyacetic acid (MCPA), and picloram. The final anionic herbicide intercalated LDH product was analyzed in water, soil, as well as garden cress (Lepidiumsativum) by using the batch method, column leaching method, and bioassay. The results demonstrated that the release performance was not considerably reliant on the synthesis process used for intercalation of the herbicide anions within the LDH layers or on the nature of the anions. LDH materials were able to reduce herbicide leaching and diminish groundwater contamination; however, the herbicide intercalated LDH could enhance the risk of molecular degradation in the environment. Moreover, the efficacy of the materials under various climatic conditions of temperatures and pH was unexplored. Hashim et al.20c successfully prepared Zn/Al-LDH intercalated with anions of phenoxy herbicide 2-(3-chlorophenoxy) propionic acid (cloprop), which was confirmed by XRD where the basal distance increased to 21.0 and 22.7 Å from 8.9 Å. For the release of phenoxy herbicide anions, phosphate solution was chosen at different initial concentrations (0.002 0.005, and 0.008 mol/L), and results demonstrated that crystalline cloprop-Zn-LDH was moderately better for controlled release. According to the authors, the release of the intercalated guest from nanocomposites with high crystallinity and larger particle size would be slower than the ones with lower crystallinity and smaller particle size.
Recently, Phuong et al.20d utilized glyphosate (GLY) and 2,4-dichlorophenoxyacetic acids (2,4D) for intercalation with Zn/Al-LDH. Decarbonated distilled water, carbonate, and chloride solutions at various concentrations were used as exchange media at 28 ± 0.5 °C to initiate the release of the anions. Because the attraction of chloride anion in the interlayer is less than carbonate and hydroxide, the release rate of herbicide was higher in carbonate solution and water compared to chloride solution. Results indicated that in between 6.5 to 18.6 h and 10 to 21.5 h, 50% of herbicides were released from GLY-LDH and 2,4D-LDH. Although the experiments successfully demonstrated the potential of LDH nanomaterials as controlled release agents for agrochemicals and thereby decrease pollution by preventing the herbicide from leaching in agricultural soil, the feasibility of the materials under agronomic conditions was not considered.
3.4. Derivatives of Layered Double Hydroxides as Adsorbents of Polar Organic Pollutants and Metal Cations
Various agrochemicals such as pesticides, herbicides, and plant growth regulators are also extensively applied in crop production. The unused part of agrochemicals contributes to contaminated soil, surface, and groundwater through the leaching process.21aVia drinking water, the food chain, or maybe by direct contamination, these pollutants can spread into the human body. So, pollutant concentrations need to be controlled to reduce the pollution level in nature as well as to protect human health. For an ideal adsorbent material, good surface properties such as large surface area, porosity, and interaction with the pollutants are the essential requirements. LDH is considered an excellent material as an adsorbent due to its smaller size, intercalation chemistry, and surface characteristics.21b−21d LDHs are anion exchange materials, and they are expected to have a low affinity toward neutral organic molecules and metal cations. However, the sorption properties can be modified by using derivatives of LDH as adsorbent and polar organic molecules as adsorbate. To decrease the pollution caused by the excessive use of agrochemicals, several researchers were able to use LDH-based materials as new generation adsorbents as described below.
Inacio and co-workers22a produced carbonate, nitrate, and chloride-loaded LDH to adsorb the phenoxy herbicides (MCPA (4-chloro-2-methylphenoxyacetic acid)), and the adsorption profile was obtained through adsorption isotherms and kinetic study. The effect of pH, Mg2+/Al3+ ratio, anion exchange capacity, character of the intercalated anion, as well as the morphology of the adsorbent on the amount of adsorption were also determined. The S-type tending to the L-type of adsorption isotherms at high equilibrium concentrations of MCPA was well explained by the Freundlich model, which indicated low sorption capacity of the material. The nature of the anions had an impact on the capacity of the adsorption in solution, and the following order was observed in terms of the affinity toward incoming anion, nitrate < chloride < carbonate. Khenifi et al.22b used nitrate intercalated Ni/Al-LDH to adsorb glyphosate and glufosinate anion herbicides from an aqueous solution. The adsorption experiment was performed at 25 °C using the batch equilibrium method to measure the impact of several attributes, for example, the initial concentration and contact time. The results showed that the adsorption of herbicides happened in two steps: the herbicides were adsorbed in outer crystallites; subsequently, the herbicide anions were intercalated within interlayer through the exchange between anions. The pseudo-second-order model was used to calculate the adsorption kinetics of the two-herbicide, whereas the adsorption isotherms were well described by the Langmuir model. Hence, the study indicated that LDH nanomaterial might function as an adsorbent for organophosphate or organo-phosphonate pollutant removal in water.
Chaara et al.12e developed Mg/Al-LDHs intercalated with dodecyl sulfate and sebacate, which enhanced the uptake of the nonionic pesticides (alachlor and metolachlor). Several initial adsorbents concentrations, pH, as well as contact times were used in the adsorption process. For the elimination of the herbicide, prepared materials were very suitable due to the range of the basal spacing, which was 1.5–3.8 nm. Results indicated that organo/LDHs adsorbed significant amounts of pesticides from water. Sebacate intercalated LDH showed controlled release properties better than dodecyl sulfate intercalated LDH and decreased the metolachlor leaching in soil columns. A very recent mini-review by Mao et al.22c describes the prospects of LDH materials as amendments for heavy metal contaminants through in situ immobilization. The capacity of modified LDH to remove metal cations (through the formation of precipitates based on chemical precipitation and/or surface complexation mechanism), as well as heavy metal oxoanions, were highlighted in this review. Wang et al.22d examined hierarchical MoS42– intercalated Ni/Fe-LDH immobilized on carbon foam as an adsorbent for Hg2+, Pb2+, and Cu2+ suggesting a significant contribution of MoS42– (soft Lewis bases) in the removal process. The uptake order Cu2+ < Pb2+ < Hg2+ was well interpreted by soft–hard acid–base, where Hg2+ is softer than the other two ions, thus more desirable to attach to the MoS42– group. In another study, Ma et al.,22e reported the selective removal of metal oxoanions (CrO42–, HAsO32–, and HAsO42–) by MoS42– intercalated Mg/Al-LDH. The oxoanions removal mechanisms were suggested as fast coordination between S–Cr and S–As and the simple anion exchange in the interlayer spaces. Another review by Sharma et al.22f also describes various studies on LDH materials as adsorbents for heavy metal ions and metal oxoanions. The results showcased LDH-based materials as eco-friendly and cost-effective, adsorbents of organic and metal residues.
3.5. Layered Double Hydroxides in Other Fields of Agriculture
Recently, LDH has been explored in various other fields of agriculture to increase crop production, as evident from the following section. Rayo et al.23a developed Zn-doped Mg/Fe-LDHs intercalated with nitrate and carbonate to understand the release of Zn in buffered solutions and evaluate the uptake of Zn as a nutrient by barley. These experiments were conducted in quartz and calcareous soil for 8 weeks, 11 weeks, and 28 weeks. At pH 5.2, materials showed the highest release of Zn, almost 45% (46% of nitrate intercalated LDH and 41% of carbonate intercalated LDH, respectively), following first-order release kinetics. According to the results, the prepared Zn doped materials could supply Zn in plants 2–9.5 times better than undoped LDH, and also results show that the dissolving rate of Mg was higher than Zn at alkaline conditions. In a recent study, Mitter et al.14 established that LDH (prepared by modified nonaqueous precipitation, pursued by heat treatment) intercalated with double-stranded RNA (dsRNA) could protect plant leaves from viral infections. According to the results reported in Figure 4, after 5 days of application, LDH could still be spotted on leaves. Results also demonstrated sustained release of dsRNA that could provide up to 20 days of virus protection for plant leaves compared with unprotected leaves. Subsequently, Songkhum et al.23b developed borate-loaded two Zn/Al-LDH through different methods (ion-exchange process and in situ coprecipitation). According to the XRD results, only the monoborate anion was intercalated within the interlayer. The release experiment was conducted in water, soil, as well as in a pot trail using grape tomatoes (Solanum Lycopersicum), where both prepared materials showed controlled release of boron and zinc. Boron release was facilitated via ion exchange and desorption, whereas Zn originated from the displacement of zinc ions during the structural rearrangement of LDH to Zn(OH)2 and ZnO. Prepared materials also had a good impact on pot trails which enhanced the plant growth.
Figure 4.
BioClay (dsRNA–LDH) spray protects against viruses in local lesion assays. (a) Local lesions caused by CMV inoculation on cowpea. Plants at the two-leaf stage were sprayed with LDH, CMV2b-dsRNA, and CMV2b-BioClay on day 0 (n = 8–16 leaves per treatment group). Plants were mechanically inoculated with CMV at 1- or 5-days post-treatment. Lesions were counted 10 days pvc. (b) Local lesions caused by PMMoV inoculation on N. tabacum cv. Xanthi. Plants were sprayed with either water, LDH, PMMoVIR54-dsRNA, or PMMoVIR54-BioClay on day 0 (n = 10–25 leaves per treatment group). Plants were mechanically inoculated with PMMoV at either 5 or 20 days post-treatment and necrotic lesions were counted 10 days pvc. (c,d) Images showing the extent of necrotic lesions on N. tabacum cv. Xanthi leaves challenged with PMMoV 5 days postspray treatment (c) and 20 days postspray treatment (d). *P < 0.05, **P < 0.01, and ***P < 0.001 are significant using the Kruskal–Wallis test with posthoc Nemenyi test for multiple comparisons between samples compared with LDH. Data represent mean ± s.e.m. Reproduced with permission from ref (14). Copyright 2017 Springer Nature.
Berber et al.23c used an in situ polymerization process and prepared poly(acrylic acid)–LDH as a high adsorbent material to increase soil water-holding capacity along with water-use efficiency. The prepared materials showed good results in terms of increased soil moisture as well as performance as a water carrier.
Bendinelli et al.23d investigated the efficiency of LDH for molybdenum release, which is crucial for the process of symbiotic nitrogen fixation by Rhizobia bacteria in legume root modules. Plants take up molybdenum as the MoO42– ions. In this study, Mg/Al-LDH intercalated with MoO42– anions was prepared by various synthesis methods. To evaluate the release rate, sodium chloride solution in deionized water was used as a release medium at a controlled temperature under a nitrogen atmosphere. According to their observation, the synthesis method of the prepared materials plays a vital role in the release kinetics. It also observed that the amount of anions released depends not only on the surface area but also on the bonding forces between molybdate and layered structure and outer walls. In conclusion, materials demonstrated excellent slow-release properties.
Using gibberellic acid (GA-plant phytohormone) as an intercalated anion, Hafez et al.23e developed Mg/Al-LDH. The researchers used soil solutions with various pH (3, 7) to release the GA in two different soils (sandy and sandy clay loam). Outcomes indicated that GA released from LDH after 1 day was 80% and 60%, respectively, at pH 3 and 7 while maintaining the slow release property even after 6 days. In comparison to GA in pure form, GA intercalated to LDH showed better stability in soil samples. This research mainly focused on the proficiency of the GA intercalated LDH in solubility, controlled release rate, the biodegradability. However, the ability of the prepared materials to enhance the growth and development of plants in a controlled manner was not explored, which can give a better understanding of the materials performance in agronomical conditions.
Soil existing microbes convert the released nutrients into simpler plant-useable forms (e.g., nitrogen fixation and converting insoluble inorganic phosphorus compounds that plant roots cannot take)24a and indiscriminate use of chemical fertilizers and heavy metal contamination can affect the health of soil microbial consortia. Therefore, the preparation and application of environment-friendly slow-release multifunctional fertilizers are of paramount importance for sustainable crop production. Xiang et al.24b proposed the use of humic acid-modified magnetic Fe3O4/Mg/Al-LDH (HA-LDH) for remediation of contaminated soil from the mining area. Greenhouse experiments of the Artemisia ordosica plant showed increased plant growth, reduced metal residue, and increased enzyme activity, indicating good soil biota at a 5% optimum level of LDH. The uptake of heavy metals, which can inhibit the microbial activity by LDH, is proposed as the reason for increased enzyme activity.
Shafigh et al.24c used sand culture experiments with Zn/Mg/Fe-LDH intercalated with nitrate (LDH-N) and phosphor (LDH-P) anions in the absence and presence of plant growth-promoting rhizobacteria (PGPR) to determine Zn phyto-availability for maize plants. The LDH appreciably improved the Zn content of plants, irrespective of the sources applied. However, the concentration and uptake of Zn in the root and shoot of maize plants grown in the LDH-N-sand system were higher than those grown in the LDH-P-sand culture. All three Pseudomonas strains increased the Zn content of roots and shoots. The ability of bacteria in cumulative Zn released increased by increasing the time of incubation. Among the three strains, the highest cumulative Zn release from LDH was recorded in the presence of p. putida P19, followed by Pseudomonas sp. A5 and P. fluorescens P52 strains.
Bernardo et al.24d prepared phosphor anion-loaded Ca/Al-LDH using various phosphor anions by ion exchange and the reconstruction method to examine the effect of LDH on the growth of nitrogen-fixing bacterium Bradyrhizobium elkanii. According to the results, LDH prepared with 33.10 mmol L–1 by the ion exchange process notably increased bacterial growth, and the higher content of phosphor ions could slowly release as the microbial mass increased. The authors used a single strain of bacteria in traditional culture media for the experiments, and hence the effectiveness of the material in a diverse microbial environment such as soil could not be assessed.
4. Summary and Outlook
Mankind has searched for a solution to grow productivity in agricultural practices throughout history. The development of nanotechnology and nanomaterials have shown promising potential in creating sustainable strategies in crop production with low ecological impact. The use of sustainable materials and processes where one can maintain public health using “green chemistry “principles is very much advocated these days. In the agroindustry arena, nanomaterials in fertilizers, herbicides, pesticides, fungicides, and nanosensors are increasingly being developed currently. Green nanomaterials such as layered double hydroxides are gaining attention in reducing the amount of agrochemicals, lessening nutrient losses in fertilization, and heightened yield through plant/soil health and nutrient management.
Layered double hydroxides have emerged as a sustainable material for agricultural applications such as nutrient storage, nanofertilizers with sustained-release property or for removal of agrochemicals, and postharvest disease control in recent years. However, most of its prospects are limited to laboratory investigations of characterization, synthesis, and kinetics released in an aqueous or buffered solution. The studies and conclusions are based on experiments performed under controlled conditions, whereas insufficient data are available concerning their efficacy in field trials and practical applications. More knowledge at the field level would be highly useful for the large-scale implementation of LDH-based strategies. Though few studies have demonstrated that LDH can undoubtedly be more successful at the commercial scale as slow-release fertilizer with minimal harm to the environment, more commercial-scale trials are required to establish these materials as an effective multifunctional candidate in agriculture. It is paramount that LDH-based technologies are meticulously assessed against available products presently in the market and that the efficacy comparisons are judiciously analyzed and communicated to the patrons (including the public and regulators).
Acknowledgments
The authors are grateful to the CSIR and the Department of Science and Innovation (Grant C6EEM68) South Africa. A.S.R. is thankful to the University of Johannesburg for its financial support (Student No. 219053157).
Biographies
Abhinandan Singha Roy is currently pursuing a Ph.D. degree at the University of Johannesburg, Department of Chemical Sciences and is associated with the Centre for Nanostructures and Advanced Materials as a researcher, DSI-CSIR Nanotechnology Innovation Centre, Council for Scientific and Industrial Research, Pretoria, South Africa. His main research interest centers on the development of sustainable slow-release fertilizers based on layered double hydroxide for soil conditioning and the application of nano clay-based nanostructured materials in the area of agriculture. This is an essential field for food security and wishes to understand how to apply fertilizer in a controlled manner that can be beneficial for the agroindustry as well as the environment.
Sreejarani Kesavan Pillai is a Principal Researcher at the Centre for Nanostructures and Advanced Materials, Council for Scientific and Industrial Research (CSIR), Pretoria, South Africa. She obtained her Ph.D. in Physical Chemistry from Cochin University of Science and Technology, Kerala, India, in 2003. Her current research interests are in nanostructures, delivery systems, and emulsions for topical and active packaging applications.
Suprakas Sinha Ray is a Chief Researcher at the Council for Scientific and Industrial Research with a Ph.D. in Physical Chemistry from the University of Calcutta in 2001, Manager of the Centre for Nanostructures and Advanced Materials, and Director of the DSI-CSIR Nanotechnology Innovation Centre. He is also associated with the University of Johannesburg as a Distinguished Visiting Professor of Chemical Sciences. Ray’s current research focuses on polymer-based advanced nanostructured materials and their applications.
Author Contributions
The manuscript was written through the contributions of all the authors.
The authors declare no competing financial interest.
References
- a Alexandratos N.; Bruinsma J.. World Agriculture Towards 2030/2050, the 2012 Revision, 2012; 10.22004/ag.econ.288998. [DOI]; b Alvarez A.; Saez J. M.; Davila Costa J. S.; Colin V. L.; Fuentes M. S.; Cuozzo S. A.; Benimeli C. S.; Polti M. A.; Amoroso M. J. Actinobacteria: current research and perspectives for bioremediation of pesticides and heavy metals. Chemosphere. 2017, 166, 41–62. 10.1016/j.chemosphere.2016.09.070. [DOI] [PubMed] [Google Scholar]; c Udeigwe T. K.; Teboh J. M.; Eze P. N.; Hashem Stietiya M.; Kumar V.; Hendrix J.; Mascagni H. J.; Ying T.; Kandakji T. Implications of leading crop production practices on environmental quality and human health. J. Environ. Manage. 2015, 151, 267–279. 10.1016/j.jenvman.2014.11.024. [DOI] [PubMed] [Google Scholar]; d Gilliom R. J. Pesticides in US streams and groundwater. Environ. Sci. Technol. 2007, 41, 3408–3414. 10.1021/es072531u. [DOI] [PubMed] [Google Scholar]; e Carpenter S. R.; Caraco N. F.; Correll D. L.; Howarth R. W.; Sharpley A. N.; Smith V. H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. 10.1890/1051-0761(1998)008[0559:NPOSWW]2.0.CO;2. [DOI] [Google Scholar]; f Relyea R. A. The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities. Ecol. Appl. 2005, 15, 618–627. 10.1890/03-5342. [DOI] [PubMed] [Google Scholar]; g Ikoyi I.; Fowler A.; Schmalenberger A. One-time phosphate fertilizer application to grassland columns modifies the soil microbiota and limits its role in ecosystem services. Sci. Total Environ. 2018, 630, 849–858. 10.1016/j.scitotenv.2018.02.263. [DOI] [PubMed] [Google Scholar]; h Pimentel D.; Acquay H.; Biltonen M.; Rice P.; Silva M.; Nelson J.; Lipner V.; Giordano S.; Horowitz A.; D'Amore M. Environmental and economic costs of pesticide use. BioScience. 1992, 42, 750–760. 10.2307/1311994. [DOI] [Google Scholar]; i van der Werf H. M. Assessing the impact of pesticides on the environment. Agric. Ecosyst Environ. 1996, 60, 81–96. 10.1016/S0167-8809(96)01096-1. [DOI] [Google Scholar]; j Tscharntke T.; Clough Y.; Wanger T. C.; Jackson L.; Motzke I.; Perfecto I.; Vandermeer J.; Whitbread A. Global food security, biodiversity conservation and the future of agricultural intensification. Biol. Conserv. 2012, 151, 53–59. 10.1016/j.biocon.2012.01.068. [DOI] [Google Scholar]; k Elfvendahl S.; Mihale M.; Kishimba M. A.; Kylin H. Pesticide pollution remains severe after cleanup of a stockpile of obsolete pesticides at Vikuge, Tanzania. AMBIO: J. Hum. Environ. Stud. 2004, 33, 503–508. 10.1579/0044-7447-33.8.503. [DOI] [PubMed] [Google Scholar]; l Zhang L.; Yan C.; Guo Q.; Zhang J.; Ruiz-Menjivar J. The impact of agricultural chemical inputs on environment: global evidence from informetrics analysis and visualization. Int. J. Low Carbon Technol. 2018, 13, 338–352. 10.1093/ijlct/cty039. [DOI] [Google Scholar]
- a Mukhopadhyay S. S. Nanotechnology in agriculture: prospects and constraints. Nanotechnol. Sci. Appl. 2014, 7, 63. 10.2147/NSA.S39409. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Tilman D.; Cassman K. G.; Matson P. A.; Naylor R.; Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002, 418, 671–677. 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]; c Paramo L. A.; Feregrino-Pérez A. A.; Guevara R.; Mendoza S.; Esquivel K. Nanoparticles in agroindustry: Applications, toxicity, challenges, and trends. Nanomaterials. 2020, 10, 1654. 10.3390/nano10091654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Berber M. R.; Hafez I. H. Synthesis of a new nitrate-fertilizer form with a controlled release behavior via an incorporation technique into a clay material. Bull. Environ. Contam. Toxicol. 2018, 101, 751–757. 10.1007/s00128-018-2454-x. [DOI] [PubMed] [Google Scholar]; b Benício L. P. F.; Constantino V. R. L.; Pinto F. G.; Vergütz L.; Tronto J.; da Costa L. M. Layered double hydroxides: new technology in phosphate fertilizers based on nanostructured materials. ACS Sustain.Chem. Eng. 2017, 5, 399–409. 10.1021/acssuschemeng.6b01784. [DOI] [Google Scholar]; c Kuthati Y.; Kankala R. K.; Lee C. H. Layered double hydroxide nanoparticles for biomedical applications: Current status and recent prospects. Appl. Clay Sci. 2015, 112, 100–116. 10.1016/j.clay.2015.04.018. [DOI] [Google Scholar]; d Rives V.; del Arco M.; Martín C. Intercalation of drugs in layered double hydroxides and their controlled release: A review. Appl. Clay Sci. 2014, 88, 239–269. 10.1016/j.clay.2013.12.002. [DOI] [Google Scholar]
- a Cavani F.; Trifiro F.; Vaccari A. Hydrotalcite-type anionic clays: Preparation, properties, and applications. Catal. Today. 1991, 11, 173–301. 10.1016/0920-5861(91)80068-K. [DOI] [Google Scholar]; b Forano C.; Hibino T.; Leroux F.; Taviot-Gue’Ho C. Layered Double Hydroxides. In Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; pp 1019–1128. [Google Scholar]; c Konta J. Clay and man: clay raw materials in the service of man. Appl. Clay Sci. 1995, 10, 275–335. 10.1016/0169-1317(95)00029-4. [DOI] [Google Scholar]; d Britto S.; Kamath P. V. Polytypism in the Lithium-Aluminum Layered Double Hydroxides: The [LiAl2 (OH)6]+ Layer as a Structural Synthon. Inorg. Chem. 2011, 50, 5619–5627. 10.1021/ic200312g. [DOI] [PubMed] [Google Scholar]; e Yang Y.; Dang L.; Shearer M. J.; Sheng H.; Li W.; Chen J.; Xiao P.; Zhang Y.; Hamers R. J.; Jin S. Highly active trimetallic NiFeCr layered double hydroxide electrocatalysts for oxygen evolution reaction. Adv. Energy Mater. 2018, 8, 1703189. 10.1002/aenm.201703189. [DOI] [Google Scholar]; f Gomez N. A. G.; Sotiles A. R.; Wypych F. Layered double hydroxides with the composition [Mn6Al3 (OH)18][(HPO42–)2A+]. yH2O (A+= Li, Na or K) obtained by topotactic exchange reactions. Appl. Clay Sci. 2020, 193, 105658. 10.1016/j.clay.2020.105658. [DOI] [Google Scholar]; g Li T.; Miras H. N.; Song Y. F. Polyoxometalate (POM)-layered double hydroxides (LDH) composite materials: design and catalytic applications. Catalysts. 2017, 7, 260. 10.3390/catal7090260. [DOI] [Google Scholar]; h Leroux F.; Besse J. P. Layered double hydroxide/polymer nanocomposites. In Interface Science and Technology, Vol. 1; Elsevier, 2004; pp 459–495. [Google Scholar]
- Adachi-Pagano M.; Forano C.; Besse J. P. Delamination of layered double hydroxides by use of surfactants. Chem. Commun. 2000, 91–92. 10.1039/a908251d. [DOI] [Google Scholar]
- a Chubar N.; Gerda V.; Megantari O.; Micusik M.; Omastova M.; Heister K.; Man P.; Fraissard J. Applications versus properties of Mg–Al layered double hydroxides provided by their syntheses methods: Alkoxide and alkoxide-free sol–gel syntheses and hydrothermal precipitation. Chem. Eng. J. 2013, 234, 284–299. 10.1016/j.cej.2013.08.097. [DOI] [Google Scholar]; b Wang Q.; Wu Z.; Tay H. H.; Chen L.; Liu Y.; Chang J.; Zhong Z.; Luo J.; Borgna A. High temperature adsorption of CO2 on Mg–Al hydrotalcite: effect of the charge compensating anions and the synthesis pH. Catal. Today 2011, 164, 198–203. 10.1016/j.cattod.2010.10.042. [DOI] [Google Scholar]
- Chibwe K.; Jones W. Intercalation of organic and inorganic anions into layered double hydroxides. J. Chem. Soc. Chem. Commun. 1989, 14, 926–927. 10.1039/c39890000926. [DOI] [Google Scholar]
- Zhao Y.; Li F.; Zhang R.; Evans D. G.; Duan X. Preparation of layered double-hydroxide nanomaterials with a uniform crystallite size using a new method involving separate nucleation and aging steps. Chem. Mater. 2002, 14, 4286–4291. 10.1021/cm020370h. [DOI] [Google Scholar]
- Xu Z. P.; Stevenson G. S.; Lu C. Q.; Lu G. Q.; Bartlett P. F.; Gray P. P. Stable suspension of layered double hydroxide nanoparticles in aqueous solution. J. Am. Chem. Soc. 2006, 128, 36–37. 10.1021/ja056652a. [DOI] [PubMed] [Google Scholar]
- a Chen C.; Ruengkajorn K.; Buffet J. C.; O’Hare D. Water adsorbancy of high surface area layered double hydroxides (AMO-LDHs). RSC Adv. 2018, 8, 34650–34655. 10.1039/C8RA06822D. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Siebecker M. G.; Li W.; Sparks D. L. The important role of layered double hydroxides in soil chemical processes and remediation: what we have learned over the past 20 years. Adv. Agron. 2018, 147, 1–59. 10.1016/bs.agron.2017.10.001. [DOI] [Google Scholar]
- a Everaert M.; Warrinnier R.; Baken S.; Gustafsson J. P.; De Vos D.; Smolders E. Phosphate-exchanged Mg–Al layered double hydroxides: a new slow-release phosphate fertilizer. ACS Sustain. Chem. Eng. 2016, 4, 4280–4287. 10.1021/acssuschemeng.6b00778. [DOI] [Google Scholar]; b Everaert M.; Degryse F.; McLaughlin M. J.; De Vos D.; Smolders E. Agronomic effectiveness of granulated and powdered P-exchanged Mg–Al LDH relative to struvite and MAP. J. Agric. Food Chem. 2017, 65, 6736–6744. 10.1021/acs.jafc.7b01031. [DOI] [PubMed] [Google Scholar]; c Everaert M.; da Silva R. C.; Degryse F.; McLaughlin M. J.; Smolders E. Limited dissolved phosphorus runoff losses from layered double hydroxide and struvite fertilizers in a rainfall simulation study. J. Environ. Qual. 2018, 47, 371–377. 10.2134/jeq2017.07.0282. [DOI] [PubMed] [Google Scholar]
- a Hatami H.; Fotovat A.; Halajnia A. Comparison of adsorption and desorption of phosphate on synthesized Zn-Al LDH by two methods in a simulated soil solution. Appl. Clay Sci. 2018, 152, 333–341. 10.1016/j.clay.2017.11.032. [DOI] [Google Scholar]; b Halajnia A.; Oustan S.; Najafi N.; Khataee A. R.; Lakzian A. The adsorption characteristics of nitrate on Mg–Fe and Mg–Al layered double hydroxides in a simulated soil solution. Appl. Clay Sci. 2012, 70, 28–36. 10.1016/j.clay.2012.09.007. [DOI] [Google Scholar]; c Das D. P.; Das J.; Parida K. Physicochemical characterization and adsorption behavior of calcined Zn/Al hydrotalcite-like compound (HTlc) towards removal of fluoride from aqueous solution. J. Colloid Interface Sci. 2003, 261, 213–220. 10.1016/S0021-9797(03)00082-1. [DOI] [PubMed] [Google Scholar]; d Grover K.; Komarneni S.; Katsuki H. Synthetic hydrotalcite-type and hydrocalumite-type layered double hydroxides for arsenate uptake. Appl. Clay Sci. 2010, 48, 631–637. 10.1016/j.clay.2010.03.017. [DOI] [Google Scholar]; e Chaara D.; Bruna F.; Ulibarri M. A.; Draoui K.; Barriga C.; Pavlovic I. Organo/layered double hydroxide nanohybrids used to remove nonionic pesticides. J. Hazard. Mater. 2011, 196, 350–359. 10.1016/j.jhazmat.2011.09.034. [DOI] [PubMed] [Google Scholar]; f Crepaldi E. L.; Tronto J.; Cardoso L. P.; Valim J. B. Sorption of terephthalate anions by calcined and uncalcined hydrotalcite-like compounds. Colloids Surf. A Physicochem. 2002, 211, 103–114. 10.1016/S0927-7757(02)00233-9. [DOI] [Google Scholar]
- Xu Z. P.; Stevenson G. S.; Lu C. Q.; Lu G. Q.; Bartlett P. F.; Gray P. P. Stable suspension of layered double hydroxide nanoparticles in aqueous solution. J. Am. Chem. Soc. 2006, 128, 36–37. 10.1021/ja056652a. [DOI] [PubMed] [Google Scholar]
- Mitter N.; Worrall E. A.; Robinson K. E.; Li P.; Jain R. G.; Taochy C.; Fletcher S. J.; Carroll B. J.; Lu G. Q.; Xu Z. P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants. 2017, 3, 16207. 10.1038/nplants.2016.207. [DOI] [PubMed] [Google Scholar]
- a Kesavan Pillai S.; Kleyi P.; de Beer M.; Mudaly P. Layered double hydroxides: An advanced encapsulation and delivery system for cosmetic ingredients-an overview. Appl. Clay Sci. 2020, 199, 105868. 10.1016/j.clay.2020.105868. [DOI] [Google Scholar]; b Wang Q.; O’Hare D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. 10.1021/cr200434v. [DOI] [PubMed] [Google Scholar]; c Benicio L. P. F.; Silva R. A.; Lopes J. A.; Eulalio D.; Santos R. M. M. d.; Aquino L. A. d.; Vergutz L.; Novais R. F.; Costa L. M. d.; Pinto F. G.; Tronto J. Layered double hydroxides: nanomaterials for applications in agriculture. Rev. Bras. Cienc. Solo. 2015, 39, 1–13. 10.1590/01000683rbcs2015081. [DOI] [Google Scholar]
- a Widowati L. R.; De Neve S.; Sukristiyonubowo; Setyorini D.; Kasno A.; Sipahutar I. A.; Sukristiyohastomo Nitrogen balances and nitrogen use efficiency of intensive vegetable rotations in Southeast Asian tropical Andisols. Nutr. Cycl. Agroecosyst. 2011, 91, 131–143. 10.1007/s10705-011-9451-3. [DOI] [Google Scholar]; b Stucki J. W.; Bergaya F.; Theng B. K. G.. Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; pp 429–482. [Google Scholar]
- a Torres-Dorante L. O.; Lammel J.; Kuhlmann H.; Witzke T.; Olfs H. W. Capacity, selectivity, and reversibility for nitrate exchange of a layered double-hydroxide (LDH) mineral in simulated soil solutions and in soil. J. Plant. Nutr. Soil Sci. 2008, 171, 777–784. 10.1002/jpln.200700330. [DOI] [Google Scholar]; b Torres-Dorante L. O.; Lammel J.; Kuhlmann H. Use of a layered double hydroxide (LDH) to buffer nitrate in soil: long-term nitrate exchange properties under cropping and fallow conditions. Plant Soil. 2009, 315, 257–272. 10.1007/s11104-008-9748-4. [DOI] [Google Scholar]; c Goh K. H.; Lim T. T.; Banas A.; Dong Z. Sorption characteristics and mechanisms of oxyanions and oxyhalides having different molecular properties on Mg/Al layered double hydroxide nanoparticles. J. Hazard. Mater. 2010, 179, 818–827. 10.1016/j.jhazmat.2010.03.077. [DOI] [PubMed] [Google Scholar]; d Ito M. Improvement of nitrate-leaching control technology using an anion exchange compound on agriculture 1: synthesis of a Mg-Fe system layered double hydroxide and its anion exchange characteristics. Soil Sci. Plant Nutr. 2018, 64, 123–129. 10.1080/00380768.2017.1413685. [DOI] [Google Scholar]; e Islam M.; Patel R. Physicochemical characterization and adsorption behavior of Ca/Al chloride hydrotalcite-like compound towards removal of nitrate. J. Hazard. Mater. 2011, 190, 659–668. 10.1016/j.jhazmat.2011.03.094. [DOI] [PubMed] [Google Scholar]; f Tezuka S.; Chitrakar R.; Sonoda A.; Ooi K.; Tomida T. Studies on selective adsorbents for oxo-anions. Nitrate ion-exchange properties of layered double hydroxides with different metal atoms. Green Chem. 2004, 6, 104–109. 10.1039/b314938m. [DOI] [Google Scholar]; g Mohammadi M.; Mohammadi Torkashvand A.; Biparva P.; Esfandiari M. The ability of layered double hydroxides for nitrate absorption and desorption in crop and fallow rotation. Glob. J. Environ. Sci. Manag. 2021, 7, 59–78. 10.22034/GJESM.2021.01.05. [DOI] [Google Scholar]; h Phuong T.; Cong T. D.; Ta V. T.; Nguyen T. H. Study on Leaching of Phosphate from Municipal Wastewater Treatment Plant’s Sewage Sludge and Followed by Adsorption on Mg-Al Layered Double Hydroxide. J. Nanomater. 2022, 2022, 1. 10.1155/2022/1777187. [DOI] [Google Scholar]; i Ashekuzzaman S. M.; Jiang J. Q. Strategic phosphate removal/recovery by a reusable Mg–Fe–Cl layered double hydroxide. Process Saf. Environ. Prot. 2017, 107, 454–462. 10.1016/j.psep.2017.03.009. [DOI] [Google Scholar]; j Azimzadeh Y.; Najafi N.; Reyhanitabar A.; Oustan S.; Khataee A. Effects of phosphate loaded LDH-biochar/hydrochar on maize dry matter and P uptake in a calcareous soil. Arch. Agron. Soil Sci. 2021, 67, 1649–1664. 10.1080/03650340.2020.1802012. [DOI] [Google Scholar]; k Liao W.; Liu X. P.; Li H. Q.; Yang P. Simultaneous Removal of Phosphate and Nitrate on Calcined Mg-Al Layered Double Hydroxides. Pol. J. Environ. Stud. 2020, 29, 187–195. 10.15244/pjoes/94991. [DOI] [Google Scholar]; l Han Y. U.; Lee W. S.; Lee C. G.; Park S. J.; Kim K. W.; Kim S. B. Entrapment of Mg-Al layered double hydroxide in calcium alginate beads for phosphate removal from aqueous solution. Desalination Water Treat. 2011, 36, 178–186. 10.5004/dwt.2011.2254. [DOI] [Google Scholar]; m Qiao W.; Bai H.; Tang T.; Miao J.; Yang Q. Recovery and utilization of phosphorus in wastewater by magnetic Fe3O4/Zn-Al-Fe-La layered double hydroxides (LDHs). Colloids Surf. A Physicochem. 2019, 577, 118–128. 10.1016/j.colsurfa.2019.05.046. [DOI] [Google Scholar]
- a Silva V. D.; Kamogawa M. Y.; Marangoni R.; Mangrich A. S.; Wypych F. Layered double hydroxides as matrizes for nitrate slow-release fertilizers. Rev. Bras. Cienc. Solo. 2014, 38, 272–277. 10.1590/S0100-06832014000100027. [DOI] [Google Scholar]; b Berber M. R.; Hafez I. H.; Minagawa K.; Mori T. A sustained controlled release formulation of soil nitrogen based on nitrate-layered double hydroxide nanoparticle material. J. Soils Sediments. 2014, 14, 60–66. 10.1007/s11368-013-0766-3. [DOI] [Google Scholar]; c Kotlar A. M.; Wallace Pereira de Carvalho H.; Iversen B. V.; de Jong van Lier Q. Nitrate leaching from layered double hydroxides in tropical and temperate soils. Appl. Clay Sci. 2020, 184, 105365. 10.1016/j.clay.2019.105365. [DOI] [Google Scholar]; d Nunes V. L.; Mulvaney R. L.; Cantarutti R. B.; Pinto F. G.; Tronto J. Improving Nitrate Fertilization by Encapsulating Zn-Al Layered Double Hydroxides in Alginate Beads. Nitrogen. 2020, 1, 125–136. 10.3390/nitrogen1020011. [DOI] [Google Scholar]; e Badreddine M.; Legrouri A.; Barroug A.; De Roy A.; Besse J. P. Ion exchange of different phosphate ions into the zinc–aluminium–chloride layered double hydroxide. Mater. Lett. 1999, 38, 391–395. 10.1016/S0167-577X(98)00195-5. [DOI] [Google Scholar]; f Benício L. P. F.; Eulálio D.; Guimarães L. D. M.; Pinto F. G.; Costa L. M.D.; Tronto J. Layered double hydroxides as hosting matrices for storage and slow release of phosphate analyzed by stirred-flow method. Mater. Res. 2018, 10.1590/1980-5373-mr-2017-1004. [DOI] [Google Scholar]; g Bernardo M. P.; Guimaraes G. G. F.; Majaron V. F.; Ribeiro C. Controlled release of phosphate from layered double hydroxide structures: dynamics in soil and application as smart fertilizer. ACS Sustain. Chem. Eng. 2018, 6, 5152–5161. 10.1021/acssuschemeng.7b04806. [DOI] [Google Scholar]; h Borges R.; Wypych F.; Petit E.; Forano C.; Prevot V. Potential sustainable slow-release fertilizers obtained by mechanochemical activation of MgAl and MgFe layered double hydroxides and K2HPO4. Nanomaterials 2019, 9, 183. 10.3390/nano9020183. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Onishi B. S. D.; dos Reis Ferreira C. S.; Urbano A.; Santos M. J. Modified hydrotalcite for phosphorus slow-release: Kinetic and sorption-desorption processes in clayey and sandy soils from North of Paraná state (Brazil). Appl. Clay Sci. 2020, 197, 105759. 10.1016/j.clay.2020.105759. [DOI] [Google Scholar]; j Lohmousavi S. M.; Abad H. H. S.; Noormohammadi G.; Delkhosh B. Synthesis and characterization of a novel controlled release nitrogen-phosphorus fertilizer hybrid nanocomposite based on banana peel cellulose and layered double hydroxides nanosheets. Arab.J. Chem. 2020, 13, 6977–6985. 10.1016/j.arabjc.2020.06.042. [DOI] [Google Scholar]; k Liang R.; Liu M.; Wu L. Controlled release NPK compound fertilizer with the function of water retention. React. Funct. Polym. 2007, 67, 769–779. 10.1016/j.reactfunctpolym.2006.12.007. [DOI] [Google Scholar]; l Buates J.; Imai T. Application of Biochar Functionalized with Layered Double Hydroxides: Improved Plant Growth Performance after Use as Phosphate Adsorbent. Appl. Sci. 2021, 11, 6489. 10.3390/app11146489. [DOI] [Google Scholar]
- Derylo-Marczewska A.; Blachnio M.; Marczewski A.; Swiatkowski A.; Tarasiuk B. Adsorption of selected herbicides from aqueous solutions on activated carbon. J. Therm. Anal. Calorim. 2010, 101, 785–794. 10.1007/s10973-010-0840-7. [DOI] [Google Scholar]
- a Chiriac H.; Oancea S.; Gaburici M.; Lupu N. Controlled release of pesticides intercalated in lhd and cereal plant growth. Ser. Agron. 2005, 51, 9–15. [Google Scholar]; b Cardoso L. P.; Celis R.; Cornejo J.; Valim J. B. Layered double hydroxides as supports for the slow release of acid herbicides. J. Agric. Food Chem. 2006, 54, 5968–5975. 10.1021/jf061026y. [DOI] [PubMed] [Google Scholar]; c Hashim N.; Zobir Hussein M.; Md Isa I.; Kamari A.; Mohamed A.; Mohamad Jaafar A.; Taha H. Synthesis and controlled release of cloprop herbicides from cloprop-layered double hydroxide and cloprop-zinc-layered hydroxide nanocomposites. Open J. Inorg. Chem. 2014, 4, 1–9. 10.4236/ojic.2014.41001. [DOI] [Google Scholar]; d Phuong N. T. K.; Ha H. N. N.; Dieu N. T. P.; Huy B. T. Herbicide/Zn-Al-layered double hydroxide hybrid composite: Synthesis and slow/controlled release properties. Environ. Sci. Pollut. Res. 2017, 24, 19386–19392. 10.1007/s11356-017-9580-6. [DOI] [PubMed] [Google Scholar]
- a Billen G.; Garnier J.; Lassaletta L. The nitrogen cascade from agricultural soils to the sea: modelling nitrogen transfers at regional watershed and global scales. Philos. Trans. R. Soc. B: Biol. Sci. 2013, 368, 20130123. 10.1098/rstb.2013.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhou J.; Yang S.; Yu J.; Shu Z. Novel hollow microspheres of hierarchical zinc–aluminum layered double hydroxides and their enhanced adsorption capacity for phosphate in water. J. Hazard. Mater. 2011, 192, 1114–1121. 10.1016/j.jhazmat.2011.06.013. [DOI] [PubMed] [Google Scholar]; c Zubair M.; Daud M.; McKay G.; Shehzad F.; Al-Harthi M. A. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci. 2017, 143, 279–292. 10.1016/j.clay.2017.04.002. [DOI] [Google Scholar]; d Ma S.; Chen Q.; Li H.; Wang P.; Islam S. M.; Gu Q.; Yang X.; Kanatzidis M. G. Highly selective and efficient heavy metal capture with polysulfide intercalated layered double hydroxides. J. Mater. Chem. A 2014, 2, 10280–10289. 10.1039/C4TA01203H. [DOI] [Google Scholar]
- a Inacio J.; Taviot-Gueho C.; Forano C.; Besse J. P. Adsorption of MCPA pesticide by MgAl-layered double hydroxides. Appl. Clay Sci. 2001, 18, 255–264. 10.1016/S0169-1317(01)00029-1. [DOI] [Google Scholar]; b Khenifi A.; Derriche Z.; Mousty C.; Prévot V.; Forano C. Adsorption of glyphosate and glufosinate by Ni2AlNO3 layered double hydroxide. Appl. Clay Sci. 2010, 47, 362–371. 10.1016/j.clay.2009.11.055. [DOI] [Google Scholar]; c Mao F.; Hao P.; Zhu Y.; Kong X.; Duan X. Layered double hydroxides: Scale production and application in soil remediation as super-stable mineralizer. Chin. J. Chem. Eng. 2022, 41, 42–48. 10.1016/j.cjche.2021.09.023. [DOI] [Google Scholar]; d Wang Y.; Gu Y.; Xie D.; Qin W.; Zhang H.; Wang G.; Zhang Y.; Zhao H. A hierarchical hybrid monolith: MoS42–-intercalated NiFe layered double hydroxide nanosheet arrays assembled on carbon foam for highly efficient heavy metal removal. J. Mater. Chem. A 2019, 7, 12869–12881. 10.1039/C9TA03102B. [DOI] [Google Scholar]; e Ma L.; Islam S. M.; Liu H.; Zhao J.; Sun G.; Li H.; Ma S.; Kanatzidis M. G. Selective and efficient removal of toxic oxoanions of As (III), As (V), and Cr (VI) by layered double hydroxide intercalated with MoS42–. Chem. Mater. 2017, 29, 3274–3284. 10.1021/acs.chemmater.7b00618. [DOI] [Google Scholar]; f Sharma R.; Arizaga G. G. C.; Saini A. K.; Shandilya P. Layered double hydroxide as multifunctional materials for environmental remediation: from chemical pollutants to microorganisms. Sustain. Mater. Technol. 2021, 29, e00319. [Google Scholar]
- a López-Rayo S.; Imran A.; Bruun Hansen H. C.; Schjoerring J. K.; Magid J. Layered double hydroxides: potential release-on-demand fertilizers for plant zinc nutrition. J. Agric. Food Chem. 2017, 65, 8779–8789. 10.1021/acs.jafc.7b02604. [DOI] [PubMed] [Google Scholar]; b Songkhum P.; Wuttikhun T.; Chanlek N.; Khemthong P.; Laohhasurayotin K. Controlled release studies of boron and zinc from layered double hydroxides as the micronutrient hosts for agricultural application. Appl. Clay Sci. 2018, 152, 311–322. 10.1016/j.clay.2017.11.028. [DOI] [Google Scholar]; c Berber M. R.; Hafez I. H.; Minagawa K.; Tanaka M.; Mori T. An efficient strategy of managing irrigation water based on formulating highly absorbent polymer–inorganic clay composites. J. Hydrol. 2012, 470, 193–200. 10.1016/j.jhydrol.2012.08.051. [DOI] [Google Scholar]; d Bendinelli E. V.; Rocha A. C.; Barcia O. E.; Aoki I. V.; Margarit-Mattos I. C. P. Effects of lamellar reconstruction routes in the release of molybdate encapsulated in Mg–Al layered double hydroxides. Mater. Chem. Phys. 2016, 173, 26–32. 10.1016/j.matchemphys.2015.12.049. [DOI] [Google Scholar]; e Hafez I. H.; Berber M. R.; Minagawa K.; Mori T.; Tanaka M. Design of a multifunctional nanohybrid system of the phytohormone gibberellic acid using an inorganic layered double-hydroxide material. J. Agric. Food Chem. 2010, 58, 10118–10123. 10.1021/jf102501n. [DOI] [PubMed] [Google Scholar]
- a Rosas S. B.; Andrés J. A.; Rovera M.; Correa N. S. Phosphate-solubilizing Pseudomonas putida can influence the rhizobia–legume symbiosis. Soil Biol. Biochem. 2006, 38, 3502–3505. 10.1016/j.soilbio.2006.05.008. [DOI] [Google Scholar]; b Xiang Y.; Kang F.; Xiang Y.; Jiao Y. Effects of humic acid-modified magnetic Fe3O4/MgAl-layered double hydroxide on the plant growth, soil enzyme activity, and metal availability. Ecotoxicol. Environ. Saf. 2019, 182, 109424. 10.1016/j.ecoenv.2019.109424. [DOI] [PubMed] [Google Scholar]; c Shafigh M.; Hamidpour M.; Abbaszadeh-Dahaji P.; Mozafari V.; Furrer G. Bioavailability of Zn from layered double hydroxides: the effects of plant growth-promoting rhizobacteria (PGPR). Appl. Clay Sci. 2019, 182, 105283. 10.1016/j.clay.2019.105283. [DOI] [Google Scholar]; d Bernardo M. P.; Moreira F. K.; Ribeiro C. Synthesis and characterization of eco-friendly Ca-Al-LDH loaded with phosphate for agricultural applications. Appl. Clay Sci. 2017, 137, 143–150. 10.1016/j.clay.2016.12.022. [DOI] [Google Scholar]