Dear Editor,
By the end of 2021, multiple cutaneous eruptions have been reported after vaccination for Coronavirus 2019 (COVID‐19). 1 Herein, we report the first cases of new‐onset lymphomatoid papulosis (LyP), a papulonecrotic or papulonodular skin eruption with histologic features of a CD30 lymphoid proliferation of atypical T cells, 2 after receiving the COVID‐19 vaccination.
The first case is a 60‐year‐old male patient who attended the Accident and Emergency Department at the University Hospital of Heraklion in Greece for an ulcerated necrotic violaceous nodule on the left palm (Fig. 1a) and several violaceous papules on the right lower leg that had developed in the last few months. His regular medications were atorvastatin 10 mg and amlodipine 10 mg/valsartan 160 mg daily for dyslipidemia and essential hypertension, respectively. In March 2021, 7 days after receiving his first AstraZeneca (AZD1222) COVID‐19 vaccine, he noticed several violaceous papules on his back and lower legs. His family doctor prescribed a gradually reducing dose of oral methylprednisolone 16 mg over 2 months. A skin biopsy from a papule on the right lower leg revealed, after hematoxylin and eosin staining, diffuse, polymorphic infiltration of the dermis by small and scattered large lymphocytes and histiocytes with red cell extravasation. There was a significant expression of predominantly CD8+ small and large lymphocytes compared with CD4+ lymphocytes and strong positive CD30 expression by the large lymphocytes (Figs. 1b–f). Based on clinical and histopathological findings and due to the close temporal association with the AZD1222 COVID‐19 vaccination, a diagnosis of vaccine‐induced LyP was made. The lesions regressed spontaneously within 3 months.
Figure 1.
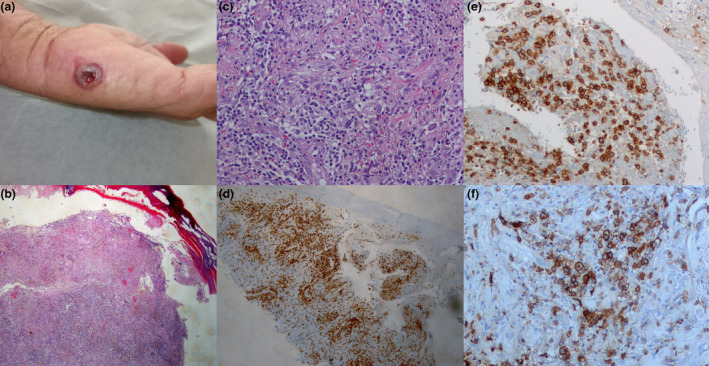
(a) a crusted ulcerated violaceous nodule on the left palm of the patient (1b) following BioNTech, Pfizer (BNT162b2) COVID‐19 mRNA vaccine. (b) Diffuse, polymorphic infiltration of the dermis by small and scattered large lymphocytes and histiocytes with red cell extravasation. The epidermis is dissociated from the dermis and ulcerated (Hematoxylin and eosin, ×10). (c) Diffuse, polymorphic infiltration of the dermis by small and scattered large lymphocytes and histiocytes with red cell extravasation (Hematoxylin and eosin, ×20). (d) CD3 expression by all lymphocytes (CD3 antibody stain, ×10). (e) Significant predominant expression of CD8 by small and large lymphocytes (CD8 antibody stain, ×40). (f) CD30 expression by the large lymphocytes (CD30 antibody stain, ×40)
The second case involves a 66‐year‐old female patient who presented to the outpatient department of Andreas Sygros Hospital in Athens with multiple small erythematous nodules on the trunk and extremities (Fig. 2a). The skin lesions showed spontaneous regression leaving superficial hyperpigmented and hypopigmented scars. The patient received the first dose of Pfizer‐BioNTech (COMIRNATY) vaccine 10 days before the rash. According to the patient's history, more lesions developed after the second vaccination dose. An incisional biopsy was performed. The pathology report described a dense infiltrate composed of small, medium, and large atypical lymphoid cells. The infiltrate had a diffuse and perivascular distribution in the dermis, whereas extended epidermotropism was also evident. Immunohistochemistry revealed the CD3+/CD8+/CD30+ phenotype of the neoplastic lymphocytes (Figs. 2b–f). The findings were consistent with the diagnosis of type‐D LyP. The patient was treated with narrowband ultraviolet B therapy (NB‐UVB), for 25 sessions (total dose of UVB = 30.880 mJoules), with clinical remission of the disease. However, 3 weeks after treatment cessation, the disease recurred in the form of minor skin lesions on the extremities. She is currently under treatment with methotrexate (MTX) 15 mg/week.
Figure 2.
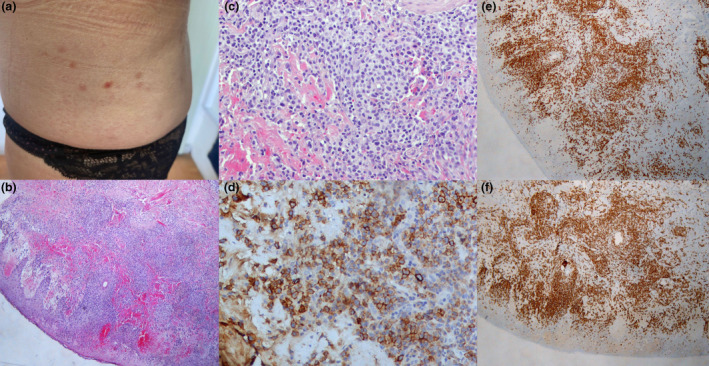
(a) Several violaceous papules on the abdomen of the second patient. (b and c) Diffuse, heavy infiltration of the dermis and epidermis by variably sized (small, medium, large) lymphocytes accompanied by extensive erythrocytic extravasation (hematoxylin and eosin staining, ×10 and ×20, respectively. (d) CD30 expression by the majority of mostly large lymphocytes (CD30 staining, ×40). (e) CD3 expression on all T‐lymphocytes (CD3 staining, ×10). (f) Predominant CD8 expression by the lymphocytic population (CD8 staining, ×10)
To the best of our knowledge, here we have reported the first cases of new‐onset LyP after the COVID‐19 vaccination. Lymphomatoid reactions, 1 recurrence of primary cutaneous CD30‐positive lymphoproliferative disorder 2 cutaneous T‐cell lymphoma, 3 and primary cutaneous anaplastic large‐cell lymphoma following COVID‐19 vaccination have been reported. 4 Panou and colleagues reported the recurrence of type A LyP following vaccination with viral vector COVID‐19 vaccination in a patient with a history of LyP and mycosis fungoides who had been in remission for 10 years. 3 Our cases further support the etiological correlation between COVID‐19 immunization and CD30+ T‐cell cutaneous exacerbation. Interestingly, both cases were type D LyP, a rare, newly recognized histologic variant. The pathophysiology of these eruptions still has to be elucidated. CD8+ T cells are important effector cells expanded in the early protection window after prime vaccination. 5 Raising awareness of these reactions following COVID‐19 vaccination may help distinguish benign lymphomatoid eruptions from true skin lymphoma.
Ethical consent statement
Informed written consent was obtained from the patients for publication of their data.
Authors' contributions
Dimitra Koumaki contributed to writing up and drafting the manuscript; Leonidas Marinos contributed to writing up the manuscript, provided the histopathological diagnosis and the images of the histological slides; Vasiliki Nikolaou contributed to writing up the manuscript; Marios Papadakis to writing up and drafting the manuscript; Kyriaki Zografaki contributed in acquisition of the data; Eleni Lagoudaki contributed in the histopathological diagnosis; Frantzeska‐Eleni Kotopouli contributed in acquisition of the data; Konstantinos Krasagakis supervised the study and drafted the manuscript; Sabine‐Elke Krueger‐Krasagakis conceived the study and was in charge of overall direction and planning of this study.
Acknowledgment
The patients in this manuscript have given written informed consent to publish their case details.
Conflict of interest: None.
Funding source: None.
Data availability statement
The data that support the findings of this study are available from the corresponding author, DK, upon reasonable request.
References
- 1. LeWitt T, Chung C, Manton J, et al. Rare lymphomatoid reactions following SARS‐CoV‐2 vaccination. JAAD Case Rep 2022; 20: 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brumfiel CM, Patel MH, DiCaudo DJ, et al. Recurrence of primary cutaneous CD30‐positive lymphoproliferative disorder following COVID‐19 vaccination. Leuk Lymphoma 2021; 62: 2554–2555. [DOI] [PubMed] [Google Scholar]
- 3. Panou E, Nikolaou V, Marinos L, et al. Recurrence of cutaneous T‐cell lymphoma post viral vector COVID‐19 vaccination. J Eur Acad Dermatol Venereol 2022; 36: e91–e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gambichler T, Boms S, Hessam S, et al. Primary cutaneous anaplastic large‐cell lymphoma with marked spontaneous regression of organ manifestation after SARS‐CoV‐2 vaccination. Br J Dermatol 2021; 185: 1259–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oberhardt V, Luxenburger H, Kemming J, et al. Rapid and stable mobilization of CD8+ T cells by SARS‐CoV‐2 mRNA vaccine. Nature 2021; 597: 268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, DK, upon reasonable request.


