Abstract
The lrp gene, which codes for the leucine-responsive regulatory protein (Lrp), was cloned from Klebsiella aerogenes W70. The DNA sequence was determined, and the clone was used to create a disruption of the lrp gene. The lack of functional Lrp led to an increased expression of the alanine catabolic operon (dad) in the absence of the inducer l-alanine but also to a decreased expression of the operon in the presence of l-alanine. Thus, Lrp is both a repressor and activator of dad expression. Lrp is also necessary for glutamate synthase formation but not for the formation of two other enzymes controlled by the nitrogen regulatory (Ntr) system, glutamate dehydrogenase and histidase.
Many bacteria use the amino acid alanine as a carbon and nitrogen source by converting the d-stereoisomer to pyruvate and ammonia. The two enzymes in this catabolic pathway, alanine racemase and d-amino acid dehydrogenase, have been studied for Klebsiella aerogenes, Escherichia coli, and Salmonella typhimurium (14, 15, 20, 26). The levels of both enzymes increase under conditions of carbon limitation and when l-alanine is present in the growth medium. In K. aerogenes enzyme levels are increased by nitrogen limitation as well. The dad operon (dadAB in S. typhimurium and K. aerogenes and dadAX in E. coli) contains a gene that codes for one subunit of the dehydrogenase and another gene that codes for the racemase. This operon is controlled by the catabolite activator protein charged with cyclic AMP, the leucine-responsive regulatory protein (Lrp), and, in the case of K. aerogenes, the nitrogen assimilation control protein (NAC) (15, 17, 19, 31). Catabolic activator protein-cyclic AMP and NAC regulate dad by binding to the dad promoter and increasing transcription of the operon. The regulatory role of Lrp is more complex. Lrp is a repressor of dad in E. coli, suggesting that the induction by alanine results from overcoming the Lrp-mediated repression of dad (19).
Lrp binds to the dad promoter of K. aerogenes in vitro, but the addition of l-alanine or l-leucine does not completely abolish the ability of Lrp to bind (15). We therefore studied the role of Lrp in the alanine-mediated induction of dad. The results presented here suggest two roles for Lrp in the alanine-dependent induction of the dad operon, a role as a repressor and a role as an activator.
Cloning lrp from K. aerogenes W70.
In order to clone the lrp gene from K. aerogenes W70 we took advantage of the fact that lrp from K. aerogenes RT48 had already been sequenced (12). Primers that contained a recognition sequence for a restriction endonuclease (EcoRI for the upstream primer and BamHI for the downstream primer) and the 5′- or 3′-terminal 25 nucleotides of the RT48 lrp gene were used to amplify a DNA fragment approximately 500 bp in length from chromosomal DNA isolated from KC2668 (which is derived from strain W70) by standard methods of PCR. The exact conditions for the PCR have been described elsewhere (24). This fragment was cloned into the expression vector pQE70, creating pCB1074, so that an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter would drive the expression of any gene in the cloned fragment. pCB1074 was introduced into an E. coli lrp mutant, BE2 (lrp-35; a complete list of strains and plasmids used in this work is included in Table 1), and tested for its ability to complement the lrp-35 allele. The presence of pCB1074 restored two phenotypes of the lrp-35 allele to the wild-type state. lrp mutants grow slowly on glucose minimal medium and are unable to use l-glycine as a sole source of nitrogen (1, 16, 29). BE2 carrying pCB1074 grew well on glucose minimal medium and was able to use l-glycine as the sole nitrogen source. It therefore seemed likely that pCB1074 contained a functional lrp gene, and it complemented the lrp-35 allele in the presence or absence of IPTG. DNA sequence analysis of the plasmid identified a gene highly similar, but not 100% identical, to the lrp gene of RT48. Thus, the gene isolated from W70 was different from the lrp gene of RT48, as well as from all other known lrp sequences.
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Genotype or relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| K. aerogenes | ||
| KC2148 | hutC515 dadA1 srl-7013::Tn5-132 | This laboratory |
| KC2668 | hutC515 dadA1 Δ[bla]-2 | 15 |
| KC2671 | hutC515 dadA1 Δ[bla]-2 recA3011 | This laboratory |
| KC3346 | hutC515 Δ[bla]-2 | 15 |
| KC4562 | hutC515 dadA1 Δ[bla]-2 lrp-101 | This work |
| KC4596 | hutC515 dadA1 Δ[bla]-2 recA3011/pGE82 | Transformation of KC2671 with pGE82 |
| KC4600 | hutC515 Δ[bla]-2 lrp-101 | P1 · KC4562 × KC3346 |
| KC4601 | hutC515 Δ[bla]-2 lrp-101 srl-7013::Tn5-132 | P1 · KC2148 × KC4600 |
| KC4602 | hutC515 Δ[bla]-2 lrp-101 recA3011 | P1 · KC4596 × KC4601 |
| E. coli | ||
| DH5α | F− φ80dlacZΔM15 ΔlacU169 endA1 recA1 hsdR17 deoR thi-1 supE44 λ− gyrA96 relA1 | Gibco-BRL |
| W3110 | Prototroph | R. G. Matthews |
| BE2 | W3110 lrp-35::Tn10 | 10 |
| YMC9 | endA1 thi-1 hsdR17 supE44 ΔlacU169 | 2 |
| BW20767 | RP4-2-tet::Mu-1kan::Tn7 integrant leu-63::IS10 recA1 creC510 hsdR17 endA1 zbf-5 uidA(ΔMluI)::pir+ thi | 21 |
| EB4516 | endA1 thi-1 hsdR17 supE44 ΔlacU169 lrp-35::Tn10 | P1 · BE2 × YMC9 |
| EB4517 | EB4516/pCB1075 | This work |
| EB4594 | BW20767/pCB1093 | This work |
| Plasmids | ||
| pUC19 | Cloning plasmid | Gibco-BRL |
| pQE70 | Expression plasmid | Qiagen |
| pGE82 | E. coli recA | G. Weinstock |
| pWW-84 | Kanamycin resistance cassette | 22 |
| pAH34 | Suicide vector | 21 |
| pCB1074 | PCR clone of KC2668 lrp cloned into pQE70 | This work |
| pCB1075 | 5.5-kb BamHI chromosomal DNA fragment from KC2668 containing lrp cloned into pUC19 | This work |
| pCB1076 | Kanamycin resistance cassette from pWW-84 cloned into lrp in pCB1075 | This work |
| pCB1093 | BglII-XmnI internal fragment of lrp from pCB1074 cloned into pAH34 | This work |
As a result of the cloning strategy, pCB1074 contains a hybrid lrp gene. Most of it is from W70, but the 25 terminal nucleotides (both 5′ and 3′) are from RT48. In order to determine the sequences of the termini of the W70 lrp gene we cloned a larger fragment of DNA that contained the lrp gene. Southern blot analysis with pCB1074 as a probe of chromosomal DNA from strain KC2668 cut with various restriction enzymes indicated that the lrp gene would be contained within a 5.5-kb BamHI fragment (data not shown). Chromosomal DNA from KC2668 was digested with BamHI and cloned into pUC19. The ligation mixture was introduced directly into EB4516 (created by moving the lrp-35 allele into the restriction-deficient strain YMC9) and plated on glucose minimal medium with l-glycine as the sole nitrogen source. A single colony which gave rise to strain EB4517 was isolated after 4 days of incubation at 37°C. This strain was shown to contain a plasmid of the expected size (pCB1075). When introduced into strain DH5α and the lrp mutant EB4516, plasmid pCB1075 caused the strains to grow poorly, and in the latter strain it did not allow growth with glycine as the sole nitrogen source. Subsequent DNA sequence analysis identified an lrp gene in pCB1075 indistinguishable from that in pCB1074 (except for three silent substitutions in the 3′-terminal 25 nucleotides). Thus, this lrp gene appears to be fully functional. It has been shown that high levels of Lrp are toxic to the cell (5, 7). Therefore, it seemed likely that pCB1075, which contained the wild-type lrp gene in a high copy number, was toxic to the cell and that the original host had developed an additional mutation that compensated for the toxic effects of the high level of Lrp. Inactivating the lrp gene provided further evidence that the overexpression of lrp was toxic. Plasmid pCB1076 was constructed by cloning a kanamycin resistance gene (from pWW-84) into a unique BglII site contained in the lrp gene. This construct disrupted lrp at codon 10, and therefore no functional Lrp was provided by the plasmid. Transformants of DH5α that contained this plasmid grew normally.
Isolation of pCB1075 allowed us to obtain the complete sequence of the lrp gene from W70. At the nucleotide level, lrp from W70 is 95, 94, 94, 90, and 88% identical to lrp from RT48, Enterobacter aerogenes, Serratia marcescens, E. coli, and S. typhimurium, respectively. At the amino acid level, Lrp is identical among these organisms except at position 3 (glycine in S. marcescens and serine in the others) and at position 95 (alanine in K. aerogenes W70 and S. typhimurium, serine in K. aerogenes RT48, and threonine in the others).
Isolation of an lrp mutant of K. aerogenes W70.
We isolated an lrp mutant of K. aerogenes, KC4562, by reverse genetics with the ampicillin-resistant suicide plasmid pAH34 (21). We cloned an internal fragment of the lrp gene from pCB1075 into pAH34, resulting in pCB1093, and transferred this plasmid into KC2668 by conjugation. Since pAH34 cannot replicate in the absence of the pir gene, ampicillin-resistant transconjugants were expected to result from the integration of the plasmid into the chromosomal lrp gene. This integration would result in a partially diploid strain with one lrp allele that was 3′ truncated at codon 104 (of 164) and another allele that was 5′ truncated at codon 10. The plasmid pAH34, containing the bla gene, would separate the two nonfunctional alleles. Ampicillin-resistant transconjugants were isolated after mating EB4594 with KC2668. PCR analysis of several of these integrants revealed that the wild-type lrp gene was not present and that the pAH34 plasmid had integrated into the chromosome. Southern blot analysis also revealed that the lrp gene had been disrupted (data not shown). One of these strains was designated KC4562 (dadA1 lrp-101). Since the lrp-101 allele contains a duplication of part of the lrp sequence, it should be unstable and could revert to the wild type by RecA-mediated excision of pCB1093. Therefore, we used consecutive P1 transductions to create KC4602, a dad+ recA strain carrying the lrp-101 allele. KC4602 (lrp-101) grew slowly on minimal medium compared to KC3346 (lrp+). The growth rates of these two strains were indistinguishable on minimal medium supplemented with the branched-chain amino acids (leucine, isoleucine, and valine [0.005% each]) and serine and 0.2% glutamine.
The addition of the branched-chain amino acids and serine had a negative effect on the growth of KC4602 on minimal medium. It was necessary to supplement the medium with glutamine in order to restore wild-type growth. When the medium was supplemented only with glutamine the cells still grew at a lower rate than did the wild type. Under these conditions, the addition of branched-chain amino acids and serine was beneficial.
Effect of lrp-101 on dad operon expression.
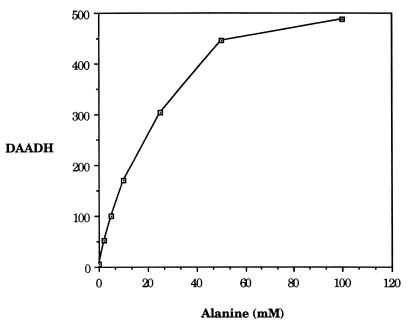
The presence of l-alanine in the growth medium leads to an induction of d-amino acid dehydrogenase and alanine racemase. Levels as low as 2 mM have been used to study the alanine-dependent induction of dad (19). We suspected that these levels might not saturate the induction system, and therefore we determined to what extent dad could be expressed in the presence of l-alanine. Figure 1 shows that the dehydrogenase levels continued to increase until l-alanine was present at levels between 50 and 100 mM. We therefore chose to use 100 mM l-alanine in our subsequent characterization of lrp-101 to ensure that dad expression was maximized. We have shown previously that l-alanine present in the medium at 22 mM (0.2%) derepresses the nitrogen-regulatory (Ntr) system by inhibiting glutamine synthetase (15). Then the Ntr system activates dad expression through NAC. In order to circumvent any role of NAC in the alanine-dependent activation of dad in our study of lrp-101, we included 0.2% l-glutamine in the medium. l-glutamine at these levels has been shown to prevent the derepression of the Ntr system by alanine (15).
FIG. 1.
Induction of d-amino acid dehydrogenase (DAADH) by increasing amounts of l-alanine. Strain KC3346 was grown overnight in glucose-ammonium minimal medium and used to inoculate fresh cultures that included l-alanine at increasing concentrations. Cells were grown to mid-log phase and assayed for DAADH as described previously (15). DAADH is reported as specific activity (units per milligram of protein), and the values are the means of three independent experiments.
Table 2 shows the effect of the lrp-101 allele on the expression of the dad operon and illustrates the two observed roles for Lrp in the alanine-dependent induction of dad (although it should be noted that the two strains are not completely isogenic, as KC4502 also carries the recA-3011 allele). In the absence of alanine, the level of dehydrogenase is four times higher in the lrp mutant than in the wild type. This suggests that Lrp is a repressor of dad expression in K. aerogenes. However, in the presence of alanine, the level of dehydrogenase is four times lower in the mutant than in the wild type. This suggests that Lrp is an activator (either direct or indirect) of dad. The addition of alanine leads to a 25-fold increase of dad expression in the wild type. While the addition of alanine has little or no effect on dad expression in the mutant, the level of dehydrogenase is much higher than the repressed level of the wild type. Thus, it appears that half of the alanine-dependent induction of dad can be explained by the repression of the operon by Lrp, while the other half requires the activation of the operon by Lrp. This pattern repeats in E. coli, although the evidence of Lrp as a repressor of transcription is not so clear (see results obtained for W3110 and BE2 without alanine in Table 2).
TABLE 2.
d-Amino acid dehydrogenase levels in wild-type and lrp mutants of K. aerogenes and E. coli
| Straina | Relevant genotype | Dehydrogenase level obtained withb:
|
|
|---|---|---|---|
| 0 mM l-alanine | 100 mM l-alanine | ||
| KC3346 | Wild type | 9 ± 2 | 222 ± 9 |
| KC4602 | lrp-101 | 40 ± 6 | 53 ± 5 |
| W3110 | Wild type | 24 ± 4 | 292 ± 24 |
| BE2 | lrp-35::Tn10 | 31 ± 1 | 63 ± 3 |
KC3346 and KC4602 are K. aerogenes strains derived from strain W70. W3110 and BE2 are E. coli strains derived from strain K-12.
The growth medium was comprised of W4 salts (4) supplemented with glucose (0.4%), ammonium sulfate (0.2%), l-glutamine (0.2%), l-leucine, l-isoleucine, l-valine, l-serine (0.005%), and l-alanine as indicated. Cultures of KC4602 also contained ampicillin at 100 μg/ml to maintain the lrp-101 allele, while those of BE2 contained tetracycline at 30 μg/ml to maintain the lrp-35::Tn10 allele. Enzyme activity is reported as specific activity (units per milligram of protein), and values are the means and standard errors of the means of at least three independent experiments. The assays were performed as described previously (15).
Effect of lrp-101 on glutamate synthase expression.
The lack of functional Lrp has effects on the expression of enzymes other than those of alanine catabolism. Glutamate synthase (the product of the gltBD operon) has been well characterized as an Lrp-dependent operon in E. coli (5, 9, 10, 30). The levels of glutamate synthase were reduced eightfold or more (Table 3) in the K. aerogenes lrp-101 strain, consistent with the observation that a functional Lrp is also necessary for the full expression of gltBD in K. aerogenes. Glutamate synthase plays a role in maintaining a functional Ntr system, in that strains unable to produce functional glutamate synthase also fail to derepress the Ntr system normally (26). However, any role that the Ntr system plays in the expression of the enzymes studied here has been circumvented by the addition of glutamine to the medium. Therefore, Lrp must play a direct role in the loss of glutamate synthase formation observed in the mutant. Other enzymes of the Ntr regulon do not require functional Lrp for their formation, i.e., histidase and glutamate dehydrogenase (Table 3).
TABLE 3.
Role of Lrp in the regulation of various operons of the Ntr regulon
| Straina | Relevant genotype | Sp act (U/mg of protein) ofb:
|
||
|---|---|---|---|---|
| Glutamate synthase | Glutamate dehydrogenase | Histidase | ||
| KC3346 | Wild type | 117 ± 11 | 427 ± 66 | 41 ± 4 |
| KC4602 | lrp-101 | ≤15 ± 0.3 | 225 ± 23 | 53 ± 6 |
The growth medium was comprised of W4 salts (4) supplemented with glucose (0.4%), ammonium sulfate, l-glutamine (0.2%), l-leucine, l-isoleucine, l-valine, and l-serine (0.005%). KC4602 also contained ampicillin at 100 μg/ml to maintain the lrp-101 allele.
Means ± standard errors of the means of at least three independent experiments. The assays were performed as described previously (18).
Conclusions and implications.
In this work we have characterized an lrp mutant of K. aerogenes W70. A strain that lacks functional Lrp grows poorly on minimal medium, although the addition of glutamine, serine, and the branched-chain amino acids allows the strain to grow in a manner indistinguishable from that of the wild type. At the genetic level, Lrp is necessary to activate gltBD and is both an activator and repressor of the dad operon. The role of Lrp in gltBD expression and as a repressor of the dad operon is analogous to well-studied systems in E. coli (6, 19, 23, 31). The growth phenotypes observed are similar to those seen in E. coli lrp mutants, although to restore a wild-type growth rate to K. aerogenes it was necessary to add glutamine to minimal medium containing branched-chain amino acids and serine. The role of Lrp as an activator of the dad operon was unanticipated.
There are several possible explanations for how Lrp acts as both a repressor and an activator of the dad operon. The simplest is that in the absence of alanine Lrp acts as a repressor of the operon, but when alanine is bound to Lrp, a conformational change occurs while Lrp is bound to the DNA that allows the protein to activate transcription. Since Lrp has been shown to be either an activator or a repressor of several leucine-responsive operons, and alanine can mimic the effects seen with leucine, this model does not suggest a new function for Lrp (6). There are several Lrp binding sites present in the dad promoter, as is the case for many Lrp-dependent operons (6). Whether the hypothesized conformational change resulting from alanine being bound to Lrp would result in different interactions between two or more Lrp molecules, between an Lrp complex and RNA polymerase, or between Lrp molecules and the DNA of the promoter is not clear.
A recent study by Roesch and Blomfield (28) has suggested how Lrp could both activate and repress an operon. They show that for the fim switch in E. coli, a single Lrp binding site in a three-binding site complex is sensitive to leucine-bound Lrp. In the absence of leucine, three Lrp molecules bind to the DNA region and recombination is inhibited. When leucine is present, one binding site can no longer keep Lrp bound, and recombination is stimulated by the remaining two Lrp molecules. It is possible that a similar interaction occurs at dad in which an Lrp molecule binding alanine can no longer bind to the DNA, and the absence of Lrp at this site is the cause of the derepression seen in lrp mutants but not in the wild type. However, other Lrp molecules bound to the promoter do not exhibit this sensitivity and are necessary to activate transcription.
Lrp could also be indirectly involved in the alanine-dependent activation of dad if there is a separate (but Lrp-dependent) positive activator of dad expression. In this scenario the presence of alanine would lead to the derepression of dad by releasing the Lrp-mediated repression at the dad promoter and lead to the activation of dad through the proposed Lrp-dependent activator. However, this model requires the existence of yet another regulator, possibly the dadQ allele identified by others (3, 11).
Other explanations for the dual role of Lrp in the alanine-dependent activation of dad, including Lrp-dependent alanine transport and Lrp’s effect on the gene that codes for the other dehydrogenase subunit, are possible but seem even less likely. At least two systems have been identified that transport alanine in E. coli, the cycA system and the LIV-1 system (8, 20, 25, 27). It is not known whether Lrp plays a role in the cycA system, but the lack of functional Lrp leads to higher levels of expression of the LIV-1 system (13). Therefore, transport of alanine via LIV-1 should not be reduced in an lrp mutant strain. In addition, KC4602 can use alanine as its sole nitrogen source, although its growth rate under this condition is severely reduced compared to that of the wild type (data not shown). Although we have no data to address the regulation of the gene that codes for the other dehydrogenase subunit, the pattern of expression for alanine racemase mimics that of the dehydrogenase (data not shown). Therefore, no matter how the other gene is regulated, Lrp plays two roles in regulating dadAB.
Our results lead us to favor the idea that the dad promoter can be in three states with respect to Lrp: it can be free of Lrp or it can be occupied by inducer-free Lrp or inducer-bound Lrp. Inducer-free Lrp represses transcription below basal level, the absence of Lrp leads to basal-level expression, and inducer-bound Lrp leads to activation of transcription above basal level. These three hypothetical states and the existence of two possible inducers (leucine and alanine) suggest that the role played by Lrp in genetic expression may be quite complex. This complexity is reflected in the large number of cellular responses in which Lrp plays a role.
Nucleotide sequence accession number.
The DNA sequence of lrp from K. aerogenes W70 has been submitted to GenBank and has been assigned accession no. AF090144.
Acknowledgments
This work was supported by Public Health Service grant GM 47156 from the National Institutes of Health to R.A.B.
REFERENCES
- 1.Ambartsoumian G, D’Ari R, Lin R T, Newman E B. Altered amino acid metabolism in lrp mutants of Escherichia coli K-12 and their derivatives. Microbiology. 1994;140:1737–1744. doi: 10.1099/13500872-140-7-1737. [DOI] [PubMed] [Google Scholar]
- 2.Backman K, Chen Y M, Magasanik B. Physical and genetic characterization of the glnA-glnG region of the Escherichia coli chromosome. Proc Natl Acad Sci USA. 1981;78:3743–3747. doi: 10.1073/pnas.78.6.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beelen R H, Feldman A M, Wijsman H J. A regulatory gene and structural gene for alaninase in Escherichia coli. Mol Gen Genet. 1973;121:369–374. doi: 10.1007/BF00433235. [DOI] [PubMed] [Google Scholar]
- 4.Bender R A, Janssen K A, Resnick A D, Blumenberg M, Foor F, Magasanik B. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J Bacteriol. 1977;129:1001–1009. doi: 10.1128/jb.129.2.1001-1009.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borst D W, Blumenthal R M, Matthews R G. Use of an in vivo titration method to study a global regulator: effect of varying Lrp levels on expression of gltBDF in Escherichia coli. J Bacteriol. 1996;178:6904–6912. doi: 10.1128/jb.178.23.6904-6912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvo J M, Matthews R G. The leucine-responsive regulatory protein, a global regulator of metabolism in Escherichia coli. Microbiol Rev. 1994;58:466–490. doi: 10.1128/mr.58.3.466-490.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C, Newman E B. Comparison of the sensitivities of two Escherichia coli genes to in vivo variation of Lrp concentration. J Bacteriol. 1998;180:655–659. doi: 10.1128/jb.180.3.655-659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosloy S D. d-Serine transport system in Escherichia coli K-12. J Bacteriol. 1973;114:679–684. doi: 10.1128/jb.114.2.679-684.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ernsting B R, Denninger J W, Blumenthal R M, Matthews R G. Regulation of the gltBDF operon of Escherichia coli: how is a leucine-insensitive operon regulated by the leucine-responsive regulatory protein? J Bacteriol. 1993;175:7160–7169. doi: 10.1128/jb.175.22.7160-7169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ernsting B R, Atkinson M R, Ninfa A J, Matthews R G. Characterization of the regulon controlled by the leucine-responsive regulatory protein in Escherichia coli. J Bacteriol. 1992;174:1109–1118. doi: 10.1128/jb.174.4.1109-1118.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franklin F C H, Venables W A, Wijsman H J W. Genetic studies of d-alanine dehydrogenaseless mutants of Escherichia coli K-12. Genet Res. 1981;38:197–208. doi: 10.1017/s0016672300020528. [DOI] [PubMed] [Google Scholar]
- 12.Friedberg D, Platko J V, Tyler B, Calvo J M. The amino acid sequence of Lrp is highly conserved in four enteric microorganisms. J Bacteriol. 1995;177:1624–1626. doi: 10.1128/jb.177.6.1624-1626.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haney S A, Platko J V, Oxender D L, Calvo J M. Lrp, a leucine-responsive protein, regulates branched-chain amino acid transport genes in Escherichia coli. J Bacteriol. 1992;174:108–115. doi: 10.1128/jb.174.1.108-115.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hecht K, Zhang S, Klopotowski T, Ames G F-L. d-Histidine utilization in Salmonella typhimurium is controlled by the leucine-responsive regulatory protein (Lrp) J Bacteriol. 1996;178:327–331. doi: 10.1128/jb.178.2.327-331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janes B K, Bender R A. Alanine catabolism in Klebsiella aerogenes: molecular characterization of the dadAB operon and its regulation by the nitrogen assimilation control protein. J Bacteriol. 1998;180:563–570. doi: 10.1128/jb.180.3.563-570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin R, D’Ari R, Newman E B. λ placMu insertions in genes of the leucine regulon: extension of the regulon to genes not regulated by leucine. J Bacteriol. 1992;174:1948–1955. doi: 10.1128/jb.174.6.1948-1955.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lobocka M, Hennig J, Wild J, Kl/opotowski T. Organization and expression of the Escherichia coli K-12 dad operon encoding the smaller subunit of d-amino acid dehydrogenase and the catabolic alanine racemase. J Bacteriol. 1994;176:1500–1510. doi: 10.1128/jb.176.5.1500-1510.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macaluso A, Best E A, Bender R A. Role of the nac gene product in the nitrogen regulation of some NTR-regulated operons of Klebsiella aerogenes. J Bacteriol. 1990;172:7249–7255. doi: 10.1128/jb.172.12.7249-7255.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathew E, Zhi J, Freundlich M. Lrp is a direct repressor of the dad operon in Escherichia coli. J Bacteriol. 1996;178:7234–7240. doi: 10.1128/jb.178.24.7234-7240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McFall E, Newman E B. Amino acids as carbon sources. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 358–379. [Google Scholar]
- 21.Metcalf W W, Jiang W, Daniels L L, Kim S, Haldimann A, Wanner B L. Conditionally replicative and conjugative plasmids carrying lacZα for cloning, mutagenesis, and allele replacement in bacteria. Plasmid. 1996;35:1–13. doi: 10.1006/plas.1996.0001. [DOI] [PubMed] [Google Scholar]
- 22.Muller W, Keppner W, Rasched I. Versatile kanamycin-resistance cartridges for vector construction in Escherichia coli. Gene. 1986;46:131–133. doi: 10.1016/0378-1119(86)90176-9. [DOI] [PubMed] [Google Scholar]
- 23.Newman E B, Lin R T, D’Ari R. The leucine/Lrp regulon. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 1513–1525. [Google Scholar]
- 24.Pomposiello P J, Janes B K, Bender R A. Two roles for the DNA recognition site of the Klebsiella aerogenes nitrogen assimilation control protein. J Bacteriol. 1998;180:578–585. doi: 10.1128/jb.180.3.578-585.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahmanian M, Claus D R, Oxender D L. Multiplicity of leucine transport systems in Escherichia coli K-12. J Bacteriol. 1973;116:1258–1266. doi: 10.1128/jb.116.3.1258-1266.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reitzer L J. Ammonia assimilation and the biosynthesis of glutamine, glutamate, aspartate, asparagine, l-alanine, and d-alanine. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 391–407. [Google Scholar]
- 27.Robbins J C, Oxender D L. Transport systems for alanine, serine, and glycine in Escherichia coli K-12. J Bacteriol. 1973;116:12–18. doi: 10.1128/jb.116.1.12-18.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roesch P L, Blomfield I C. Leucine alters the interaction of the leucine-responsive regulatory protein (Lrp) with the fim switch to stimulate site-specific recombination in Escherichia coli. Mol Microbiol. 1998;27:751–761. doi: 10.1046/j.1365-2958.1998.00720.x. [DOI] [PubMed] [Google Scholar]
- 29.Tuan L R, D’Ari R, Newman E B. The leucine regulon of Escherichia coli K-12: a mutation in rblA alters expression of l-leucine-dependent metabolic operons. J Bacteriol. 1990;172:4529–4535. doi: 10.1128/jb.172.8.4529-4535.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiese D E, 2nd, Ernsting B R, Blumenthal R M, Matthews R G. A nucleoprotein activation complex between the leucine-responsive regulatory protein and DNA upstream of the gltBDF operon in Escherichia coli. J Mol Biol. 1997;270:152–168. doi: 10.1006/jmbi.1997.1057. [DOI] [PubMed] [Google Scholar]
- 31.Zhi J, Mathew E, Freundlich M. In vitro and in vivo characterization of three major dadAX promoters in Escherichia coli that are regulated by cyclic AMP-CRP and Lrp. Mol Gen Genet. 1998;258:442–447. doi: 10.1007/s004380050754. [DOI] [PubMed] [Google Scholar]