Abstract
Nonpharmaceutical interventions (NPIs) taken to combat the coronavirus disease 2019 (COVID‐19) pandemic have not only decreased the spread of severe acute respiratory syndrome coronavirus 2 but also have had an impact on the prevalence of other common viruses. This study aimed to investigate the long‐term impact of NPIs on common respiratory and enteric viruses among children in Shanghai, China, as NPIs were relaxed after June 2020. The laboratory results and clinical data of outpatient children with acute respiratory tract infections (ARTI) and acute gastroenteritis (AGE) were analyzed and compared between the post‐COVID‐19 period (from June 2020 to January 2022) and pre‐COVID‐19 period (from June 2018 to January 2020). A total of 107 453 patients were enrolled from June 2018 to January 2022, including 43 190 patients with ARTI and 64 263 patients with AGE. The positive rates of most viruses decreased during the post‐COVID‐19 period, with the greatest decrease for influenza A (−0.94%), followed by adenoviruses (AdV) (−61.54%), rotaviruses (−48.17%), and influenza B (−40%). However, the positive rates of respiratory syncytial virus (RSV) and enteric AdV increased during the post‐COVID‐19 period as the NPIs were relaxed. Besides this, in the summer of 2021, an unexpected out‐of‐season resurgence of RSV activity was observed, and the resurgence was more prominent among children older than 5 years. The effectiveness of the current relaxed NPIs in control of common respiratory and enteric viruses was variable. Relaxation of NPIs might lead to the resurgence of common viruses.
Keywords: children, COVID‐19, enteric viruses, nonpharmaceutical interventions, outpatient, respiratory viruses
1. INTRODUCTION
The outbreak of coronavirus disease 2019 (COVID‐19), which was caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), emerged in Wuhan, China, at the end of 2019. 1 Following its rapid spread, the World Health Organization declared COVID‐19 to be a pandemic on March 11, 2020. 2
In China, the government enforced comprehensive and stringent nonpharmaceutical interventions (NPIs), such as social distancing, increased hand hygiene, mask‐wearing, working from home, and school closures, to reduce the transmission of SARS‐CoV‐2 at the early stage of the epidemic. 3 These interventions have proven effective in reducing the spread of SARS‐CoV‐2 in many contexts. 4 , 5 , 6 , 7 , 8 In addition to COVID‐19, NPIs may also reduce the transmission of other infectious diseases disseminated through airborne or fecal–oral transmission routes, such as the common cold, seasonal influenza, bronchiolitis, gastroenteritis, and acute otitis. 3 , 9 , 10
In June 2020, NPIs in Shanghai, China, were gradually relaxed; schools and commercial activities were reopened, and people were allowed to engage in limited public gatherings. However, “relaxed NPIs” including physical distancing and mask‐wearing was still mandatory in public places. The extent of NPIs in this stage was markedly different from that in the early pandemic phase of COVID‐19 when a series of strict NPIs had been implemented.
Acute respiratory tract infections (ARTI) and acute gastroenteritis (AGE) are the most common conditions in the field of pediatrics. The etiology of ARTI and AGE is diverse and complex, and it may vary according to age, season, and region. 11 , 12 Viruses including influenza virus (Flu), respiratory syncytial virus (RSV), adenoviruses (AdV), and rotaviruses (RVs) play important roles in these infectious diseases. Understanding the possible influence of the COVID‐19 NPI period on the incidence of these respiratory and enteric viruses remains a key question for the broader public health impact of the pandemic. Previous studies have explored the impact of the COVID‐19‐related NPIs on respiratory infections, with most of the studies focusing on the early phase of the COVID‐19 outbreak, when strict NPIs were adopted. 9 , 10 , 13 , 14 , 15 , 16 However, the long‐term impact of the relaxed NPIs adopted in the post‐COVID‐19 period (from June 2020) on the circulation of common respiratory and enteric viruses has not been comprehensively assessed.
In this study, we reported and discussed the transmission pattern of common viruses among outpatient children with ARTI and AGE in Shanghai, before and after the outbreak of COVID‐19. We focused primarily on the comparison between the post‐COVID‐19 period (from June 2020 to January 2022, 20 months) and the pre‐COVID‐19 period (from June 2018 to January 2020, 20 months), with the aim to investigate the long‐term effects of the “relaxed NPIs” on the epidemiology of common respiratory and enteric viruses among children.
2. METHODS
2.1. Study design
To explore the changing pattern of common respiratory and enteric viruses among outpatient children before and after the COVID‐19 pandemic, the laboratory results and clinical data of pediatric patients with ARTI and AGE in the outpatient clinic at Children's Hospital of Fudan University from June 1, 2018, to January 31, 2022, were analyzed.
A patient was considered to have an ARTI if at least two of the following clinical manifestations occurred during the week before them presenting: fever, cough, nasal obstruction, expectoration, sneeze, and dyspnea. AGE was defined as the occurrence of ≥3 diarrheal stools or ≥2 episodes of vomiting, or one episode of both diarrhea and vomiting in the last 24 h, with the symptoms lasting for a maximum of seven days.
All of the outpatients meeting the definition of ARTI or AEG in the defined study period and receiving virological tests were enrolled in the analysis. The decision as to whether a virological diagnostic test was necessary was made by the attending physician. Enrolled outpatients with fever were also tested for SARS‐CoV‐2 RNA by using real‐time reverse transcription‐polymerase chain reaction (RT‐PCR) from June 2, 2020.
According to the timeline of NPIs for containing the COVID‐19 epidemic in China, we defined three periods: the “pre‐COVID‐19” period, from June 1, 2018 to January 31, 2020 (20 months) without nationwide NPIs; the “COVID‐19 pandemic” period, from February 1, 2020 to May 31, 2020 (4 months), when strict nationwide NPIs were implemented and schools were closed; and the “post‐COVID‐19” period, from June 1, 2020 to January 31, 2022 (20 months), when nationwide NPIs were relaxed and schools were reopened. The patients were divided into two groups according to their age: <5 years and ≥5 years. Demographic and clinical data from all of the enrolled patients were obtained from their electronic medical records.
2.2. Specimen collection and testing
Nasopharyngeal swabs were collected from all of the enrolled outpatients with ARTI by trained staff following standard operating procedures. Fresh stool samples were collected from the enrolled outpatients with AGE. The specimens were immediately transferred to the in‐house clinical laboratory for virus detection.
Nasopharyngeal swabs were tested by chromatographic immunoassay for four respiratory viruses, including FluA, FluB, RSV, and AdV. FluA and FluB were detected using the SD Bioline Influenza Antigen Kit (Standard Diagnostics). RSV and AdV were tested using the CerTest RSV‐Adenovirus Resp Blister Kit (CerTest Biotec), which detects an RSV fusion protein and the AdV hexon antigen, common in all respiratory infection serotypes.
Stool samples were tested by chromatographic immunoassay for RV and enteric AdV using Rotavirus/AdV Antigen Kits (Wantai Biopharm). The kit for enteric AdV detects AdV serotypes 40 and 41, which are the main serotypes in pediatric gastroenteritis.
If any one of the targeted viruses was detected in the specimens, the patient was considered positive for that virus. In cases where two viruses were detected in the same clinical sample, respective viruses were counted individually.
2.3. Statistical analysis
Categorical variables were expressed as numbers (%). As the continuous variable (age) in our study was not normally distributed, it was expressed as median (interquartile range [IQR]) and compared using the Mann–Whitney U test between the pre‐COVID‐19 and the post‐COVID‐19 periods. The proportions for categorical variables (detection rate of virus, sex) were compared using the χ 2 test. The percentage change in the positive rate between the pre‐COVID‐19 and the post‐COVID‐19 periods was calculated as follows: 100% × [P(post) − P(pre)]/P(pre), where the P(post) indicates the positive rate during the post‐COVID‐19 period, and P(pre) indicates the positive rate during the pre‐COVID‐19 period. All of the tests were two‐tailed, and a p < 0.05 represented statistical significance. The statistical analyses were conducted in SPSS version 21.0 software (IBM).
3. RESULTS
3.1. Changes in the recruited patients
During the whole study period, a total of 107 453 patients who met the inclusion criteria were enrolled, including 43 190 patients with ARTI and 64 263 patients with AGE. The median age of the patients was 21 months (IQR: 9 months–3 years), and 57.1% were male. Compared with the pre‐COVID‐19 period, the number of patients with ARTI and AGE decreased strongly by 54.1% and 32.2%, respectively, during the post‐COVID‐19 period. The proportion of males also decreased slightly and the median age of patients with ARTI and AGE increased (Table 1). From June 2, 2020, to January 31, 2022, a total of 11 017 patients with ARTI and 9670 patients with AGE were tested for SARS‐CoV‐2 RNA by real‐time RT‐PCR. None of these subjects was SARS‐CoV‐2 RNA positive.
Table 1.
Comparison of demographic characteristics and positive rates (%) of viruses between the pre‐COVID‐19 and post‐COVID‐19 periods.
| Whole study period (44 months)a | Pre‐ COVID‐19 (20 months)a | COVID‐19 pandemic (4 months)a | Post‐ COVID‐19 (20 months)a | Changeb | p b | |
|---|---|---|---|---|---|---|
| ARTI | ||||||
| Demographics | ||||||
| Total patients | 43 190 | 28 913 | 1004 | 13 273 | −54.1% | |
| Male sex, n (%) | 23 715 (54.9) | 16 067 (55.6) | 549 (54.7) | 7099 (53.3) | −3.8% | <0.001 |
| Age, median | 2 y (15 m to 4 y) | 2 y (13 m to 4 y) | 17 m (9 m to 4 y) | 3 y (21 m to 5 y) | 1 y | <0.001 |
| Virus detection, n (%) | ||||||
| RSV | 3763 (8.7) | 2496 (8.6) | 29 (2.9) | 1238 (9.3) | 8.0% | 0.020 |
| AdV | 136 (0.3) | 114 (0.4) | 2 (0.2) | 20 (0.2) | −61.8% | <0.001 |
| FluA | 2895 (6.7) | 2743 (9.5) | 38 (3.8) | 114 (0.9) | −90.9% | <0.001 |
| FluB | 1708 (4.0) | 1353 (4.7) | 22 (2.2) | 333 (2.5) | −46.4% | <0.001 |
| Total | 8355 (19.3) | 6573 (22.7) | 89 (8.9) | 1693 (12.8) | −43.9% | <0.001 |
| AGE | ||||||
| Demographics | ||||||
| Total patients | 64 263 | 37 034 | 2122 | 25 107 | −32.2% | |
| Male sex, n (%) | 37 585 (58.5) | 21 932 (59.2) | 1216 (57.3) | 14 437 (57.5) | −2.9% | <0.001 |
| Age, median | 15 m (7 m to 3 y) | 12 m (6 m to 2 y) | 8 m (3–21 m) | 22 m (10 m to 4 y) | 10 m | <0.001 |
| Virus detection, n (%) | ||||||
| RV | 9793 (15.2) | 7067 (19.1) | 244 (11.1) | 2482 (9.9) | −48.2% | <0.001 |
| Enteric AdV | 2773 (4.3) | 1578 (4.3) | 14 (0.7) | 1181 (4.7) | 10.4% | 0.009 |
| Total | 11 928 (18.6) | 8155 (22.0) | 253 (11.9) | 3520 (14.0) | −36.3% | <0.001 |
Abbreviations: AdV, adenoviruses; AGE, acute gastroenteritis; ARTI, acute respiratory tract infections; COVID‐19, coronavirus disease 2019; FluA, influenza A virus; FluB, influenza B virus; m, months; RSV, respiratory syncytial virus; RV, rotaviruses; y, years.
Whole study period: June 1, 2018 to January 31, 2022; pre‐COVID‐19 period: June 1, 2018 to January 31, 2020; COVID‐19 pandemic: February 1–May 31, 2020 (school closure); and post‐COVID‐19 period: June 1, 2020 to January 31, 2022.
Comparison between the pre‐COVID‐19 and post‐COVID‐19 periods. The changes are reported as relative differences in percentages for virus detections and sex; relative differences in numbers for total patients; and differences between median ages for age.
3.2. Changes in the positive rates of viruses
The overall positive rates of viruses significantly decreased by 43.9% among the patients with ARTI (from 22.7% during pre‐COVID‐19% to 12.8% during post‐COVID‐19, p < 0.001), and by 36.3% among patients with AGE (from 22.0% to 14.0%, p < 0.001). Except for RSV and enteric AdV, each virus exhibited a sharp decrease in the positive rate during the post‐COVID‐19 period compared with the pre‐COVID‐19 period. The greatest decrease was observed for FluA (−90.9%), followed by AdV (−61.8%), RV (−48.2%), and FluB (−46.4%) (all p < 0.001). Slight increases in the positive rates were observed for RSV (from 8.6% to 9.3%, p = 0.020) and enteric AdV (from 4.3% to 4.7%, p = 0.009) (Table 1).
In patients with ARTI, the coinfection rate of two viruses decreased from 0.5% (133/28 913) during the pre‐COVID‐19 period to 0.1% (12/13 273) during the post‐COVID‐19 period. The combination “RSV + FluA” was the most frequent during the pre‐COVID‐19 period. However, “RSV + FluB” became the most common combination during the post‐COVID‐19 period due to the low prevalence of FluA. In patients with AGE, the coinfection rate of RV and enteric AdV (“RV+ enteric AdV”) decreased from 1.3% (490/37 034) to 0.6% (143/25 107) (Table 2).
Table 2.
Coinfections were detected in outpatient children with ARTI and AGE
| Pre‐COVID‐19 (20 months)a | COVID‐19 pandemic (4 months)a | Post‐COVID‐19 (20 months)a | |
|---|---|---|---|
| ARTI, n (%) | |||
| Total | 133 (0.5) | 2 (0.2) | 12 (0.1) |
| RSV + FluA | 85 | 2 | 0 |
| RSV + FluB | 42 | 0 | 6 |
| FluA + FluB | 3 | 0 | 5 |
| AdV + FluB | 2 | 0 | 0 |
| AdV + FluA | 1 | 0 | 0 |
| RSV + AdV | 0 | 0 | 1 |
| AGE, n (%) | |||
| RV + enteric AdV | 490 (1.3) | 5 (0.2) | 143 (0.6) |
Abbreviations: AdV, adenoviruses; AGE, acute gastroenteritis; ARTI, acute respiratory tract infections; COVID‐19, coronavirus disease 2019; FluA, influenza A virus; FluB, influenza B virus; RSV, respiratory syncytial virus; RV, rotaviruses.
Pre‐COVID‐19: June 1, 2018 to January 31, 2020; COVID‐19 pandemic: February 1– May 31, 2020 (school closure); and post‐COVID‐19: June 1, 2020 to January 31, 2022.
3.3. Changes in seasonality of viruses
We evaluated the monthly percentage of specimens positive for each virus, as shown in Figure 1 for the enrolled patients. The changes in seasonality among viruses were variable.
Figure 1.
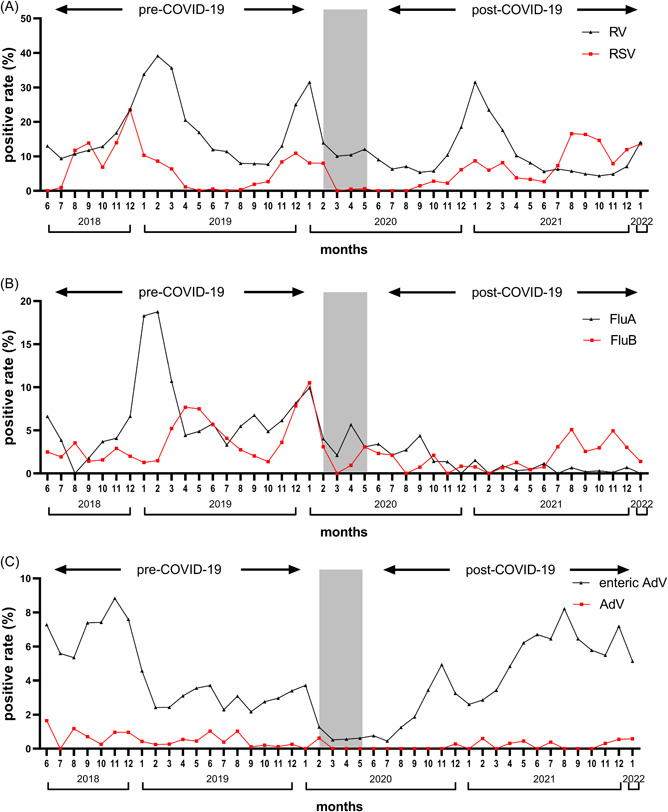
Seasonal activity of respiratory and enteric viruses over the study period. (A) RV and RSV; (B) FluA and FluB; and (C) enteric AdV and AdV (respiratory infection). Gray block represents the period of the “COVID‐19 pandemic” when strict nationwide NPIs were implemented and schools were closed. AdV, adenoviruses; COVID‐19, coronavirus disease 2019; FluA, influenza virus A; FluB, influenza virus B; NPI, nonpharmaceutical intervention; RSV, respiratory syncytial virus; RV, rotaviruses.
RSV had a high prevalence in winter and was almost not detected from May to July during the pre‐COVID‐19 period. In the first year when NPIs were adopted (February 2020 to January 2021), the seasonality of RSV did not change, although the prevalence was suppressed. However, the seasonality changed greatly in the second year (February 2021 to January 2022), where RSV was detected throughout the year and peaked in summer.
FluA peaked in winter during the pre‐COVID‐19 period, whereas the annual peak was interrupted after the NPIs were adopted. The observed FluA prevalence declined sharply and was reduced to near zero during the post‐COVID‐19 period. FluB detections occurred throughout the year and peaked in April 2019 and January 2020, during the pre‐COVID‐19 period. Similar to FluA, the prevalence of FluB was suppressed during the post‐COVID‐19 period, except for a weak resurgence in the second half of 2021.
AdV and enteric AdV did not demonstrate a clear seasonal pattern in our study. AdV was detected sporadically during the pre‐COVID‐19 period. In the first year when NPIs were adopted, AdV almost disappeared. However, with the relaxation of NPIs, it resurged sporadically in the second year. Enteric AdV was detected throughout the whole study period, with the lowest incidence during the COVID‐19 pandemic. A rebound of enteric AdV was observed soon after NPIs had been relaxed during the post‐COVID‐19 period.
Compared with the pre‐COVID‐19 period, the seasonality of RV did not change, and the positive rate also peaked in the winter months during the post‐COVID‐19 period.
3.4. Change pattern in the positive rates by age and sex
We analyzed the extent of change for RSV and enteric AdV by age. The increase in RSV detections was observed to a greater extent among older children (≥5 years). Compared with the pre‐COVID‐19 period, the positive rate of enteric AdV increased among children younger than 5 years during the post‐COVID‐19 period; however, it was reduced among older children (Figure 2A). For the other viruses, the reduction in their positive rates showed no significant difference in terms of age (Figure 2A). There was no significant difference between males and females for all of the investigated viruses (Figure 2B).
Figure 2.
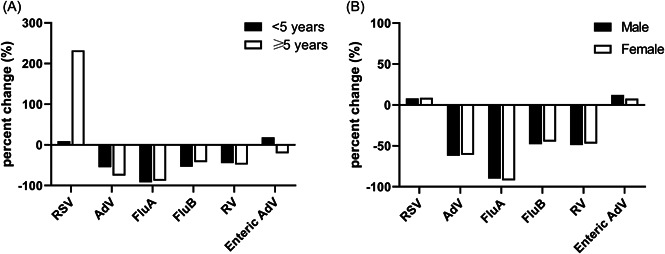
Percent change in the positive rate during the post‐COVID‐19 period compared with the pre‐COVID‐19 period and stratified by age and sex. (A) Percent change by age and (B) percent change by sex. AdV, adenoviruses; COVID‐19, coronavirus disease 2019; FluA, influenza virus A; FluB, influenza virus B; RSV, respiratory syncytial virus; RV, rotaviruses.
4. DISCUSSION
The COVID‐19‐related NPIs implemented to contain the spread of SARS‐CoV‐2 are already beginning to affect the transmission of other common viruses. Many studies have explored the impact of NPIs on non‐SARS‐CoV‐2 viral infections. 13 , 14 , 15 , 16 , 17 , 18 However, most of the studies have not considered the period following the relaxation of NPIs. Understanding how the transmission dynamics of non‐SARS‐CoV‐2 viral infections changed under the conditions of relaxed NPIs is of great significance for public health in the post‐COVID‐19 era. Our study explored the transmission pattern of common respiratory and enteric viruses using data from a large number of outpatient children (>100 000) with ARTI and AGE from June 2018 to January 2022 in Shanghai. We found that the intensity and seasonality of these viruses were changed alongside the implementation and subsequent relaxation of NPIs.
In the post‐COVID‐19 period, the number of enrolled patients with ARTI and AGE was dramatically reduced compared with that in the pre‐COVID‐19 period. This phenomenon might be ubiquitous. A nationwide study on children's outpatient visits before and during the first wave of the COVID‐19 pandemic in Germany showed that outpatient visits associated with diagnosed infections fell markedly by 51%. 19 Studies from other countries have yielded similar findings. 5 , 16 , 20 , 21 These infectious diseases, including ARTI and AGE, are predominantly transmitted through droplets, aerosols, physical contact, or via the fecal‐oral route. We suspect that the relaxed NPIs, including wearing a mask, securing physical distance, and washing hands, have led to a greatly reduced transmission of ARTI and AGE. However, the decrease in the number of patients with AGE was smaller than that for ARTI (−32.2% vs. −54.1%, respectively). This suggests that the current relaxed NPIs for COVID‐19 are more effective for airborne transmission than for fecal–oral transmission. To elucidate the cause of the observed reduction in pediatric emergency department visits, a study conducted in the Netherlands showed that the largest reduction was observed for communicable infections (76%), whereas the reduction in noninfectious diagnoses was smaller (36%), which means that the main reason for the reduction in pediatric visits was the decrease in transmissible infections due to the adoption of NPIs, and care avoidance could have contributed as well. 22 In addition, limited access to diagnostics due to suspension of nonemergency services in health facilities and massive mobility restrictions during COVID‐19 might also contribute to the reduction in the number of enrolled patients, thus the reported patients with ARTI and AGE during this period could be underestimated.
The positive rates of FluA, FluB, AdV, and RV were significantly lower during the post‐COVID‐19 period. We observed persistently low FluA activity throughout the pandemic, even under much less stringent NPIs. Of note, up to January 2022, no season peaks of FluA occurred during the post‐COVID‐19 period. Earlier reports from other countries have shown similar situations. 23 , 24 , 25 , 26 These results demonstrated that the current NPIs, including international mobility restriction and mask‐wearing, could be highly effective against influenza. A previous study found that surgical face masks significantly reduced the detection of Flu RNA in respiratory droplets, indicating that surgical face masks could prevent the transmission of Flu from symptomatic individuals. 27 This positive effect in the short term is welcome. However, the lack of immune stimulation due to the reduced circulation of influenza and the related reduced vaccine uptake may induce an “immunity debt,” which could have negative consequences and may lead to a large outbreak in the future. 28 Further studies are needed to better understand how the immunity debt affects the epidemiology of influenza.
RV infections are a leading cause of severe, dehydrating gastroenteritis in children. 12 , 29 In the present study, the overall proportion of RV‐positive samples from children with AGE decreased from 19.1% in the pre‐COVID‐19 period to 9.9% in the post‐COVID‐19 period. In contrast to the Flu, the seasonality of RV was not changed in the post‐COVID‐19 period, with a high prevalence remaining in the winter months. The suppression of RV activity during the COVID‐19 pandemic has been reported in many other countries, such as Japan, 16 the United States, 30 Australia, 31 and Germany. 32 RV is transmitted predominantly through the fecal‐oral route and mainly by close person‐to‐person contact. 29 Although the NPIs at the public level were relaxed, some NPIs at the individual level, such as mask‐wearing, social distancing, and hand hygiene, were still in place. It seems reasonable to assume that the NPIs may have interrupted the transmission of RV. However, a caveat must be acknowledged that we were unable to rule out the influence of vaccine shedding on the results. The RV vaccine is a live‐attenuated vaccine that replicates in the gut, leading to the vaccine virus being shed in the stools of vaccinated individuals. Therefore, the detection of RV in infants is not necessarily an indication of infection and may be due to vaccine shedding. 33
We found that not all viruses were effectively controlled by the current NPIs. The overall proportion of RSV‐positive samples increased in the post‐COVID‐19 period. This is directly due to the out‐of‐season resurgence of RSV activity in the summer of 2021 when NPIs were largely relaxed. Similar resurgences were also seen in Australia and the United States. 34 , 35 The expanded cohort of RSV‐naïve children during the COVID‐19 pandemic with massive stringent NPIs adopted might have contributed to the resurgence. 28 Besides, during the lockdown, children were exposed to different pathogens at lower frequencies, which might affect the development of “trained immunity.” 36 The attenuated innate immune responses might result in higher vulnerability to viruses such as RSV in the post‐COVID‐19 period. Notably, in our study, the rebound of RSV was more drastic among older children (≥5 years). The study from Australia suggested that compared with the pre‐pandemic period, children hospitalized for RSV during the COVID‐19 pandemic were significantly older. 34 By the age of 2, over 80% of children have experienced at least one RSV infection, and two‐thirds of these occurred in the first year of life. Following infection, protection generated is short‐lived and incomplete, allowing RSV to reinfect the host throughout their life. The low prevalence of RSV in 2020 may have resulted in a waning of protection against RSV in older children, leading to their increased susceptibility to reinfection. 37 , 38 A better understanding of how RSV epidemics would evolve at this stage of COVID‐19 requires continuous surveillance.
In our study, the prevalence of enteric AdV resurged quickly after NPIs had been relaxed in the post‐COVID‐19 period, with high detection rates in the second half of 2021. However, a study conducted in Germany reported that the positivity rates of enteric AdV decreased, and no resurgence was observed during the COVID‐19 pandemic (up to March 2021). 39 The main reason for this discrepancy is probably the lack of full‐year data for 2021. A possible explanation for the quick resurgence is that AdV is a nonenveloped virus, which is generally considered more resistant to disinfectants, including alcohol. This viral characteristic might hamper the prevention of infection with the current relaxed NPIs for COVID‐19.
There are some limitations to the study. First, although the number of enrolled patients was high enough to draw valid conclusions, this was a single‐center study in Shanghai, and the changing pattern might be different in other countries or in other regions of China. Additional investigations from other regions are needed to better understand the future dynamics of these common circulating viruses in children. Second, it should be noted that the dramatically reduced admission rate during the COVID‐19 pandemic might have influenced the results in an unknown direction. Although our results were based on a test‐positive rate which should be less sensitive to the number of enrolled patients, it cannot be ruled out that the pandemic has shifted the profile of people who seek medical assistance for ARTI and AGE, which might lead to Berkson's bias. Third, the antigen‐based assays used in this study might display lower sensitivity and specificity as compared to nucleic acid‐based assays. Thus, the interpretation of the results must be cautious, especially when compared with studies using PCR‐based assays. Finally, common viruses causing ARTI and AGE in children, such as rhinovirus and norovirus, were not included in our study, which might have underestimated the real burden of viral infections.
5. CONCLUSION
Our findings indicate that the effectiveness of the relaxed NPIs in control of common respiratory and enteric viruses among pediatric patients is variable. The current NPIs have effectively suppressed the circulation of FluA and RV; however, their impact on other common viruses is much more limited, especially for RSV and enteric AdV. In the long term, we should be cautious of a possible out‐of‐season resurgence of FluA, due to the buildup of susceptibility during the control periods. It is important to continue surveillance for these common viruses at this stage of the COVID‐19 pandemic, to evaluate the long‐term impacts of NPIs on the circulation of common viruses among children.
AUTHOR CONTRIBUTIONS
Pengcheng Liu and Jin Xu conceived and designed the experiments. Menghua Xu, Lingfeng Cao, Liyun Su, Lijuan Lu, and Niuniu Dong performed the experiments. Ao Ma collected the clinical information. Pengcheng Liu, Ran Jia, and Xunhua Zhu analyzed the data. Pengcheng Liu wrote the manuscript; Jin Xu revised the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENT
This study was supported by the Key Development Program of the Children's Hospital of Fudan University (No.: EK2022ZX05).
Liu P, Xu M, Lu L, et al. The changing pattern of common respiratory and enteric viruses among outpatient children in Shanghai, China: two years of the COVID‐19 pandemic. J Med Virol. 2022;94:4696‐4703. 10.1002/jmv.27896
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO . Coronavirus disease 2019 (COVID‐19) situation report‐51. 2020. Accessed April 27, 2022. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf
- 3. Pan A, Liu L, Wang C, et al. Association of public health interventions with the epidemiology of the COVID‐19 outbreak in Wuhan, China. JAMA. 2020;323(19):1915‐1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tian H, Liu Y, Li Y, et al. An investigation of transmission control measures during the first 50 days of the COVID‐19 epidemic in China. Science. 2020;368(6491):638‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lai S, Ruktanonchai NW, Zhou L, et al. Effect of non‐pharmaceutical interventions to contain COVID‐19 in China. Nature. 2020;585(7825):410‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chinazzi M, Davis JT, Ajelli M, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID‐19) outbreak. Science. 2020;368(6489):395‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hsiang S, Allen D, Annan‐Phan S, et al. The effect of large‐scale anti‐contagion policies on the COVID‐19 pandemic. Nature. 2020;584(7820):262‐267. [DOI] [PubMed] [Google Scholar]
- 8. Haug N, Geyrhofer L, Londei A, et al. Ranking the effectiveness of worldwide COVID‐19 government interventions. Nat Hum Behav. 2020;4(12):1303‐1312. [DOI] [PubMed] [Google Scholar]
- 9. Angoulvant F, Ouldali N, Yang DD, et al. Coronavirus disease 2019 pandemic: impact caused by school closure and national lockdown on pediatric visits and admissions for viral and nonviral infections—a time series analysis. Clin Infect Dis. 2021;72(2):319‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sakamoto H, Ishikane M, Ueda P. Seasonal influenza activity during the SARS‐CoV‐2 outbreak in Japan. JAMA. 2020;323(19):1969‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li ZJ, Zhang HY, Ren LL, et al. Etiological and epidemiological features of acute respiratory infections in China. Nat Commun. 2021;12(1):5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang LP, Zhou SX, Wang X, et al. Etiological, epidemiological, and clinical features of acute diarrhea in China. Nat Commun. 2021;12(1):2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu P, Xu M, Cao L, et al. Impact of COVID‐19 pandemic on the prevalence of respiratory viruses in children with lower respiratory tract infections in China. Virol J. 2021;18(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ye Q, Wang D. Epidemiological changes of common respiratory viruses in children during the COVID‐19 pandemic. J Med Virol. 2022;94:1990‐1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li ZJ, Yu LJ, Zhang HY, et al. Broad impacts of COVID‐19 pandemic on acute respiratory infections in China: an observational study. Clin Infect Dis. 2021:ciab942. [DOI] [PMC free article] [PubMed]
- 16. Fukuda Y, Tsugawa T, Nagaoka Y, et al. Surveillance in hospitalized children with infectious diseases in Japan: pre‐ and post‐coronavirus disease 2019. J Infect Chemother. 2021;27(11):1639‐1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park S, Michelow IC, Choe YJ. Shifting patterns of respiratory virus activity following social distancing measures for coronavirus disease 2019 in South Korea. J Infect Dis. 2021;224(11):1900‐1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zeng Z, Guan W, Liu Y, et al. Different circulation pattern of multiple respiratory viruses in Southern China during the COVID‐19 pandemic. Front Microbiol. 2021;12:801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barschkett M, Koletzko B, Spiess CK. COVID‐19 associated contact restrictions in Germany: marked decline in children's outpatient visits for infectious diseases without increasing visits for mental health disorders. Children. 2021;8(9):728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaur R, Schulz S, Fuji N, Pichichero M. COVID‐19 pandemic impact on respiratory infectious diseases in primary care practice in children.Front Pediatr. 2021;9:722483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luo Z, Li S, Li N, et al. Assessment of pediatric outpatient visits for notifiable infectious diseases in a university hospital in Beijing during COVID‐19. JAMA Netw Open. 2020;3(8):e2019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kruizinga MD, Peeters D, van Veen M, et al. The impact of lockdown on pediatric ED visits and hospital admissions during the COVID19 pandemic: a multicenter analysis and review of the literature. Eur J Pediatr. 2021;180(7):2271‐2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yeoh DK, Foley DA, Minney‐Smith CA, et al. Impact of coronavirus disease 2019 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin Infect Dis. 2021;72(12):2199‐2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodgers L, Sheppard M, Smith A, et al. Changes in seasonal respiratory illnesses in the United States during the coronavirus disease 2019 (COVID‐19) pandemic. Clin Infect Dis. 2021;73(suppl 1):S110‐S117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sunagawa S, Iha Y, Kinjo T, Nakamura K, Fujita J. Disappearance of summer influenza in the okinawa prefecture during the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic. Respir Investig. 2021;59(1):149‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang QS, Wood T, Jelley L, et al. Impact of the COVID‐19 nonpharmaceutical interventions on influenza and other respiratory viral infections in New Zealand. Nat Commun. 2021;12(1):1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leung N, Chu D, Shiu E, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26(5):676‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cohen R, Ashman M, Taha MK, et al. Pediatric infectious disease group (GPIP) position paper on the immune debt of the COVID‐19 pandemic in childhood, how can we fill the immunity gap? Infect Dis Now. 2021;51(5):418‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crawford SE, Ramani S, Tate JE, et al. Rotavirus infection. Nat Rev Dis Primers. 2017;3:17083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burnett E, Parashar UD, Winn A, Tate JE. Trends in rotavirus laboratory detections and Internet search volume before and after rotavirus vaccine introduction and in the context of the COVID‐19 pandemic—United States 2000–2021. J Infect Dis. 2022:jiac062. [DOI] [PMC free article] [PubMed]
- 31. Bruggink LD, Garcia‐Clapes A, Tran T, Druce JD, Thorley BR. Decreased incidence of enterovirus and norovirus infections during the COVID‐19 pandemic, Victoria, Australia, 2020. Commun Dis Intell 2021;45:45. [DOI] [PubMed] [Google Scholar]
- 32. Eigner U, Verstraeten T, Weil J. Decrease in norovirus infections in Germany following COVID‐19 containment measures. J Infect. 2021;82(6):276‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Whiley DM, Ye S, Tozer S, et al. Over‐diagnosis of rotavirus infection in infants due to detection of vaccine virus. Clin Infect Dis. 2020;71(5):1324‐1326. [DOI] [PubMed] [Google Scholar]
- 34. Foley DA, Phuong LK, Peplinski J, et al. Examining the interseasonal resurgence of respiratory syncytial virus in Western Australia. Arch Dis Child. 2022;107(3):e7. [DOI] [PubMed] [Google Scholar]
- 35. Agha R, Avner JR. Delayed seasonal RSV surge observed during the COVID‐19 pandemic. Pediatrics. 2021;148(3):e2021052089. [DOI] [PubMed] [Google Scholar]
- 36. Netea MG, Quintin J, van der Meer JWM. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9(5):355‐361. [DOI] [PubMed] [Google Scholar]
- 37. Lambert L, Sagfors AM, Openshaw PJ, Culley FJ. Immunity to RSV in early‐life. Front Immunol. 2014;5:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Russell CD, Unger SA, Walton M, Schwarze J. The human immune response to respiratory syncytial virus infection. Clin Microbiol Rev. 2017;30(2):481‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mack D, Gohl P, Kolbert M, et al. Where have the enteric viruses gone?—differential effects on frequent causes of infectious diarrhoea by SARS‐CoV‐2 pandemic lockdown measures. Infect Prev Pract. 2021;3(4):100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


