Editor
After the onset of the pandemic due to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), coronavirus disease 2019 (COVID‐19) affected tens of millions of people, with devastating medical, social and economical consequences. Its rapid spread and high lethality led the scientific community to rapidly come up with vaccines for SARS‐CoV‐2. 1
In December 2020, the Food and Drug Administration issued Emergency Use Authorizations for Pfizer/BioNTech (BNT162b2) and Moderna (mRNA‐1273) COVID‐19 vaccines, given the high efficacy and safety rates. 2 Nevertheless, they may evoke delayed cutaneous adverse drug reactions (cADRs), rarely severe (0.3% of cases), commonly self‐limiting or requiring minimal treatment. 3 , 4
We report the case of a 45‐year‐old woman who attended our dermatology outpatient clinic for the appearance of erythema and pain of some fingers and toes, occurred 3 days after the second administration of Pfizer vaccine. No personal or familiar histories of dermatological disease or autoimmune connective tissue disorders emerged. She was otherwise healthy, and laboratory examination was unremarkable. RT‐PCR test on the nasopharyngeal swab was negative.
Physical examination showed erythematous and oedematous patches on the dorsal and ventral sides of the distal phalanx of the fourth and fifth fingers of left hand and the first and second toes of both feet, accompanied by pain and discomfort. Clinical appearance encouraged the diagnosis of pernio‐like lesions, supposing a correlation with COVID‐19 vaccine, as already reported in the literature with few cases. Three weeks later, the patient came back to our unit because of worsening of skin conditions.
Clinically, fingers now displayed erythema and oedema, slight scaling of fingertips, yellow‐brownish discoloration of the nail plate, distal thickening and mild nail uplifting. Meanwhile, toes involved exhibited a more intense erythema with thick scales, erosions and crusts on the tips; furthermore, subungual hyperkeratosis, crumbling of the nail plate and onycholysis were appreciated. (Fig. 1a,b). Therefore, a skin biopsy was performed.
Figure 1.
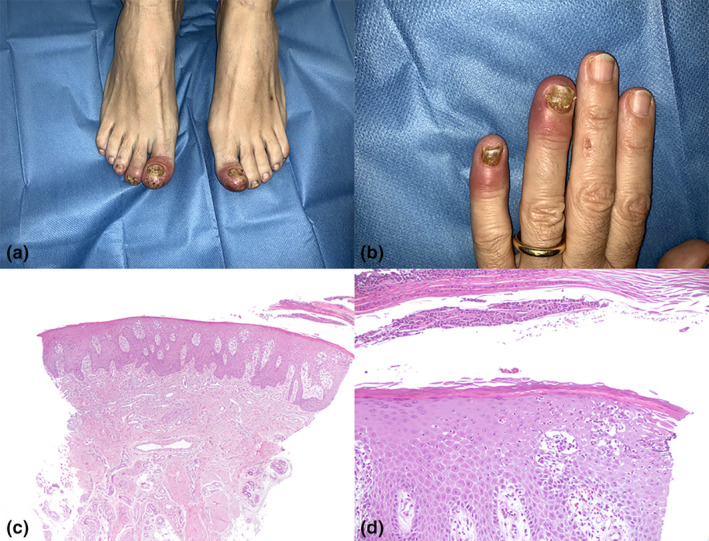
(a) Clinical aspect of the toes and (b) of the fingers; (c) histopathological findings: scanning magnification, HE 10×; (d) histopathological findings: particular, E 200×.
Histopathological examination showed hyperkeratosis with parakeratosis, psoriasiform hyperplasia of the nail bed, focal hypogranulosis, neutrophil entrapment into the parakeratotic layers and within epidermis, dilated vessels of papillary dermis and a scant sparse superficial perivascular lymphocytic infiltrate. (Fig. 1c,d). PAS stain negativity for hyphae allowed to exclude onychomycosis. According to clinical and histopathological findings, considering the temporal relationship with vaccine administration, we diagnosed her with nail psoriasis associated with Pfizer/ BioNTech (BNT162b2) COVID‐19 vaccine.
The skin is commonly involved in vaccine‐derived adverse reactions. 4 McMahon et al described local injection site and delayed large local reactions. 5
However, the spectrum of cutaneous reactions to mRNA COVID‐19 vaccines is increasing, and psoriasis, as already described for influenza and tetanus‐diphtheria vaccines, may develop. Mostly are psoriasis flare, someone shows a new onset, usually as guttate form. 6 , 8
Pfizer COVID‐19 vaccine is a nucleoside‐modified RNA eliciting high SARS‐CoV‐2 neutralizing antibody titres and antigen‐specific CD8+ and Th1‐type CD4+ T‐cell responses. 7
Immunogenic effects induced may result in different inflammatory skin reactions, according to the specific T‐cell subsets present. 3
Psoriasis is a Th1 and Th17 immune disease with a predominance of CD8+ in the epidermis and CD4+ in the dermis. The increase in IL 6 and Th17 induced by vaccination seems to favour the onset of psoriasis. 3 , 6 To our knowledge, this is the second case of de novo nail psoriasis associated with Pfizer‐BioNTech (BNT162b2) COVID‐19 mRNA vaccine. 9
In cases exclusively involving nail and periungual, without skin lesions, the histopathological examination appears mandatory to confirm the diagnosis of nail psoriasis, excluding the initially suspected perniosis. 10
Therefore, nail psoriasis could represent a rare putative side effect to COVID‐19 mRNA vaccine, of which the dermatologist must be aware in the evaluation of a patient with onychopathy. It is treatable and, as such, should not discourage vaccination.
Conflicts of interest
Dr. Lamberti has no conflicts of interest to disclose. Dr. Lora has no conflicts of interest to disclose. Dr. Graceffa has no conflicts of interest to disclose. Dr. Bonifati has no conflicts of interest to disclose. Dr. Cota has no conflicts of interest to disclose.
Funding sources
None.
Acknowledgement
The patient in this manuscript has given written informed consent to the publication of her case details.
Data availability statement
Data available on request from the authors.
References
- 1. Uddin M, Mustafa F, Rizvi TA et al. SARS‐CoV‐2/COVID‐19: viral genomics, epidemiology, vaccines, and therapeutic interventions. Viruses 2020; 12: 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shyh Poh Teo . Review of COVID‐19 mRNA vaccines: BNT162b2 and mRNA‐1273. J Pharm Pract 2021: 1–5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3. Niebel D, Novak N, Wilhelmi J et al. Cutaneous adverse reactions to COVID‐19 vaccines: insights from an Immuno‐dermatological perspective. Vaccine 2021; 9: 944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bellinato F, Maurelli M, Gisondi P, Girolomoni G. Cutaneous adverse reactions associated with SARS‐CoV‐2 vaccines. Vaccines J Clin Med 2021; 10: 5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McMahon DE, Amerson E, Rosenbach M et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J am Acad Dermatol. J Am Acad Dermatol 2021; 85: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pesqué D, Lopez‐Trujillo E, Marcantonio O, Giménez‐Arnau AM, Pujol RM. New‐onset and exacerbations of psoriasis after mRNA COVID‐19 vaccines: two sides of the same coin? J Eur Acad Dermatol Venereol 2022; 36: e80–e157. [DOI] [PubMed] [Google Scholar]
- 7. Polack FP, Thomas SJ, Kitchin N et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lehmann M, Schorno P, Hunger RE, Heidemeyer K, Feldmeyer L, Yawalkar N. New onset of mainly guttate psoriasis after COVID‐19 vaccination: a case report. J Eur Acad Dermatol Venereol 2021; 35: e752–e755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer‐BioNTech BNT162b2 COVID‐19 messenger RNA vaccine. JAAD Case Rep 2021; 17: 18–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grover C, Reddy BSN, Chaturvedi KU. Diagnosis of nail psoriasis: importance of biopsy and histopathology. Br J Dermatol 2005; 153: 1153–1158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


