Abstract
Wildlife animals may be susceptible to multiple infectious agents of public health or veterinary relevance, thereby potentially forming a reservoir that bears the constant risk of re‐introduction into the human or livestock population. Here, we serologically investigated 493 wild ruminant samples collected in the 2021/2022 hunting season in Germany for the presence of antibodies against the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and four viruses pathogenic to domestic ruminants, namely, the orthobunyavirus Schmallenberg virus (SBV), the reovirus bluetongue virus (BTV) and ruminant pestiviruses like bovine viral diarrhoea virus or border disease virus. The animal species comprised fallow deer, red deer, roe deer, mouflon and wisent. For coronavirus serology, additional 307 fallow, roe and red deer samples collected between 2017 and 2020 at three military training areas were included. While antibodies against SBV could be detected in about 13.6% of the samples collected in 2021/2022, only one fallow deer of unknown age tested positive for anti‐BTV antibodies, and all samples reacted negative for antibodies against ruminant pestiviruses. In an ELISA based on the receptor‐binding domain (RBD) of SARS‐CoV‐2, 25 out of 493 (5.1%) samples collected in autumn and winter 2021/2022 scored positive. This sero‐reactivity could not be confirmed by the highly specific virus neutralisation test, occurred also in 2017, 2018 and 2019, that is, prior to the human SARS‐CoV‐2 pandemic, and was likewise observed against the RBD of the related SARS‐CoV‐1. Therefore, the SARS‐CoV‐2 sero‐reactivity was most likely induced by another hitherto unknown deer virus belonging to the subgenus Sarbecovirus of betacoronaviruses.
Keywords: bluetongue disease, COVID‐19, deer, pestivirus, serology, wildlife
1. INTRODUCTION
Wild ruminants, either free‐ranging or raised in enclosures, can be infected by a wide range of infectious agents that are pathogenic to livestock animals or humans (Trimmel & Walzer, 2020). In the case of livestock diseases, spillover from domestic ruminants to wildlife is commonly assumed to be the initial source of infection and, conversely, wild animals may subsequently develop into a reservoir bearing the risk for re‐introduction of the disease into the livestock population.
In central Europe, the most common wild cervid species include the European roe deer (Capreolus capreolus), fallow deer (Dama dama) and red deer (Cervus elaphus), with roe deer being most closely related to the North American white‐tailed deer (both subfamily Capreolinae) (Heckeberg, 2020). Besides, European bison (Bison bonasus) and the ovine species mouflon (Ovis gmelini) are abundant. All these species were shown to be susceptible to the reovirus bluetongue virus (BTV) and the orthobunyavirus Schmallenberg virus (SBV) (Arenas‐Montes et al., 2016; Krzysiak et al., 2017; Linden et al., 2012; Mouchantat et al., 2015), two arboviruses of major veterinary relevance. Both BTV and SBV are transmitted by Culicoides biting midges and predominantly infect ruminants (Beer & Wernike, 2021; Maclachlan, 2011). In domestic ruminants, SBV may induce fever, diarrhoea or decreased milk yield in non‐pregnant animals and abortion, stillbirth or the delivery of severely malformed offspring when naïve dams are infected during gestation (Beer & Wernike, 2021). BTV infections are often inapparent or subclinical but can also lead to a systemic haemorrhagic fever that results from vascular injuries and induces a high mortality rate (Maclachlan, 2011). SBV was detected for the first time in 2011 in the German–Dutch border region (Hoffmann et al., 2012) and thereafter spread rapidly through the European ruminant population (EFSA, 2013). By now, it is established in an enzootic status in central Europe including Germany. In contrast, Germany was officially recognized as free from BTV between 2012 and 2018. Prior to 2012, more precisely, between 2006 and 2009, a large outbreak of BTV serotype 8 occurred, and new BTV cases have been recorded since December 2018 up to February 2021 (Friedrich‐Loeffler‐Institut, 2022). In France, BTV‐8 re‐emerged already in 2015 and has been circulating since then (Sailleau et al., 2017; Vinomack et al., 2019), representing a constant risk for virus spread into neighbouring regions or countries. In recent studies, wild ruminants were shown to be excellent indicators for BTV circulation in a given area (Ruiz‐Fons et al., 2014).
Apart from vector‐borne viruses, wild and domestic ruminants also share susceptibility to a number of infectious diseases transmitted by direct contact. Depending on the dynamics of interaction and the particular pathogen, wild ruminants may maintain a pathogen independent of domestic animal populations through a sylvatic cycle, from which the pathogen in question might be transferred back to farmed animals. In the case of the ruminant pestiviruses bovine viral diarrhoea virus (BVDV, syn. Pestivirus A and B) and border disease virus (BDV, syn. Pestivirus D), virus maintenance and transmission in domestic ruminants are predominantly driven by in utero infected, immunotolerant, persistently infected (PI) offspring. These PI animals shed high amounts of infectious virus throughout their life, as they are unable to mount a specific immune response against the virus strain they are infected with (Ezanno et al., 2008; Nettleton et al., 1998). For BVDV, an eradication program is in place in Germany since 2011, which has led to a considerable decrease in the prevalence of PI animals in the cattle population (Wernike et al., 2017). However, BVDV PI animals have been identified in a wide range of other mammals including cervid species (Nelson et al., 2015; Passler et al., 2010), and in Germany, anti‐BVDV antibodies were detected in about 7.7% of free‐ranging deer in the 1990s (Frölich, 1995), that is, before the start of the mandatory nationwide control program. Therefore, there are concerns of potential pestivirus circulation in wild ruminants that could lead to re‐introduction into the domestic ruminant population.
From a public health perspective, wild ruminants are considered to be reservoir or maintenance hosts of multiple viral, bacterial, fungal or parasitic diseases (Trimmel & Walzer, 2020). Only recently, when the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) or specific antibodies were detected in white‐tailed deer (Chandler et al., 2021; Hale et al., 2022; Kuchipudi et al., 2022), fears arose that cervid species could also form an animal reservoir for this virus. SARS‐CoV‐2, which belongs to the subgenus Sarbecovirus of the betacoronaviruses together with SARS‐CoV‐1 (Coronaviridae Study Group of the ICTV, 2020), is the causative agent of the COVID‐19 pandemic that unfolded since the beginning of 2020, is presently driven by direct human‐to‐human virus transmission via aerosolized particles and that led to millions of human deaths globally. Since the beginning of the pandemic, the role of animals as potential reservoir hosts has been discussed. Natural SARS‐CoV‐2 infections linked to human exposure have been reported in American mink, ferrets, felines, canines and primates (OIE, 2021). For domestic ruminants such as cattle, goat and sheep, a very low susceptibly was demonstrated during experimental infection studies (Bosco‐Lauth et al., 2021; Gaudreault et al., 2022; Ulrich et al., 2020), and natural infections of cattle also seem to be a rare event (Wernike et al., 2022). However, SARS‐CoV‐2 or specific antibodies were detected frequently in free‐ranging white‐tailed deer (Odocoileus virginianus) in North America (Chandler et al., 2021; Hale et al., 2022; Kuchipudi et al., 2022). Under experimental conditions, white‐tailed deer can be readily infected with SARS‐CoV‐2 and transmit the virus to conspecifics and, in the case of pregnant dams, to the foetus (Cool et al., 2021; Palmer et al., 2021). In silico modelling suggested that additional deer species may be likewise susceptible to SARS‐CoV‐2 infections (Damas et al., 2020).
To investigate whether SARS‐CoV‐2 had been introduced into the European wild ruminant population, as occurred in North American white‐tailed deer, we serologically tested samples collected in Germany in autumn and winter 2021/2022 from various species. In addition, we included the livestock pathogens BTV, SBV and ruminant pestiviruses in the serosurvey.
2. MATERIALS AND METHODS
2.1. Wildlife samples
Between September 2021 and January 2022, blood samples of fallow deer (n = 26), red deer (n = 188), roe deer (n = 254), mouflons (n = 7) and European bison (Wisent; n = 1) were collected postmortem in five German federal states by local hunters (Table S1 in the Supporting Information). Further 17 wild ruminant samples were analysed, but the species was not indicated in the letter accompanying the samples. The federal states comprised Bavaria, Hesse (three hunting districts), Mecklenburg‐Western Pomerania, North Rhine‐Westphalia (seven hunting districts) and Rhineland‐Palatinate (six hunting districts). The wisent, two of the mouflons, two red deer and 12 fallow deer were kept in wildlife parks, the other ruminants were free‐ranging. Control samples collected in the same regions prior to the human SARS‐CoV‐2 pandemic, that is, before 2020, were not available.
In addition, 307 wild ruminant samples collected between 2017 and 2020 at three military training areas of the German Federal Armed Forces were included. In training area A, red deer and roe deer samples were taken in 2017 and in 2019. In training area B, samples were continuously collected from 2017 to 2020, and from training area C, samples from 2017, 2018 and 2019 were available (Table S2 in the Supporting Information).
2.2. Serological test systems
All samples collected in the 2021/2022 hunting season were tested for the presence of anti‐SBV antibodies by a glycoprotein Gc‐based enzyme‐linked immunosorbent assay (ELISA) performed as described previously (Wernike, Aebischer, Sick, et al., 2021). For the detection of antibodies against BTV and ruminant pestiviruses, the VP7‐based ID Screen Bluetongue Competition ELISA and the ID Screen BVDV p80 Ab competition test, respectively, were applied (both Innovative Diagnostics). The latter allows for the detection of anti‐BVDV antibodies as well as for antibodies against the ovine BDV. Both tests were performed as prescribed by the manufacturer.
The complete sample panel was analysed by a multispecies ELISA based on the receptor‐binding domain (RBD) of SARS‐CoV‐2 (Wernike, Aebischer, Michelitsch, et al., 2021). A corrected optical density (OD) of ≥ 0.3 was evaluated as positive as determined by prior validation (Wernike, Aebischer, Michelitsch, et al., 2021). To exclude cross‐reactivity with the bovine coronavirus (BCoV), cattle sera antibody‐positive against BCoV with neutralising titers ranging from 1/14 to 1/906 were included in the initial test validation and all of them tested negative in the SARS‐CoV‐2 RBD‐ELISA (Wernike, Aebischer, Michelitsch, et al., 2021).
Wildlife samples that reacted positive in the RBD‐ELISA were subsequently tested by a virus neutralisation test (VNT) against the SARS‐CoV‐2 strain 2019_nCoV Muc‐IMB‐1 performed as described previously (Schlottau et al., 2020) and by a surrogate VNT (cPass SARS‐CoV‐2 Surrogate VNT Kit, GenScript).
For the detection of antibodies directed against SARS‐CoV‐1, an ELISA system was established in line with the SARS‐CoV‐2 test. For expression of the recombinant protein, the RBD‐SD1 domain (aa 306−577) of the SARS coronavirus strain Tor2 (NC_004718.3) was ordered as a synthetic DNA string fragment (GeneArt synthesis; Thermo Fisher Scientific) and cloned into the pEXPR103 expression vector (IBA Lifesciences) in‐frame with a C‐terminal Strep‐tag. Expi293 cells were grown in suspension in Expi293 expression medium (Thermo Fisher Scientific) at 37°C, 8% CO2 and 125 rpm. For transfection, the ExpiFectamine293 transfection kit (Thermo Fisher Scientific) was used according to the manufacturer's instructions. Cell culture supernatant was harvested 6 days after transfection and purified using Strep‐Tactin XT Superflow high‐capacity resin (IBA Lifesciences) following the manufacturer's instructions. The ELISA procedure was exactly as described for the SARS‐CoV‐2 RBD‐ELISA (Wernike, Aebischer, Michelitsch, et al., 2021).
3. RESULTS AND DISCUSSION
3.1. Evidence for the presence of a coronavirus of the Sarbecovirus subgenus
The 493 samples collected in the 2021/2022 hunting season were tested by the SARS‐CoV‐2 RBD‐based ELISA, and 25 reacted positive (25/493; 5.1%, 95% confidence interval [CI]: 3.1%−7.0%). Two of the seroreactive samples originated from red deer (2/188), 22 from roe deer (22/254), and for one sample, the species was not indicated (1/17; Figure 1). However, in the surrogate VNT, which was used as a confirmatory test, only one red deer serum collected in Rhineland‐Palatinate tested positive (31% inhibition, cut‐off for positivity at ≥ 30% inhibition). In the second confirmatory test, the VNT using replicating SARS‐CoV‐2, none of the sera tested positive. The observed RBD‐reactivity was further examined by using wildlife samples collected at the military training areas before and during the SARS‐CoV‐2 pandemic, and reactive samples were found at every sampling time point (Table S2). A total of 20 out of 307 sera tested positive in the RBD‐based ELISA (6.5%, 95% CI: 3.8%−9.3%), and again, only one of these results could be confirmed by the surrogate VNT (44% inhibition) and none by the cell culture‐based VNT (Table S2). To further investigate whether the reactivity against the SARS‐CoV‐2 RBD could be induced by antibodies against a hitherto unknown deer coronavirus of the Sarbecovirus subgenus, the reactive samples of the panel collected between 2017 and 2020 at the military training areas were additionally tested by an indirect ELISA against the RBD of SARS‐CoV‐1. Eighteen of 20 sera scored positive (corrected OD ≥ 0.3; Figure 3). As SARS‐CoV‐2‐specific controls, a serum sample obtained from a cattle after experimental SARS‐CoV‐2 infection (Ulrich et al., 2020) and a bovine serum taken after natural infection (Wernike et al., 2022) were analysed, and both samples tested positive in the SARS‐CoV‐2 RBD ELISA but, in contrast to the wildlife samples, negative against SARS‐CoV‐1 (Figure 3).
FIGURE 1.
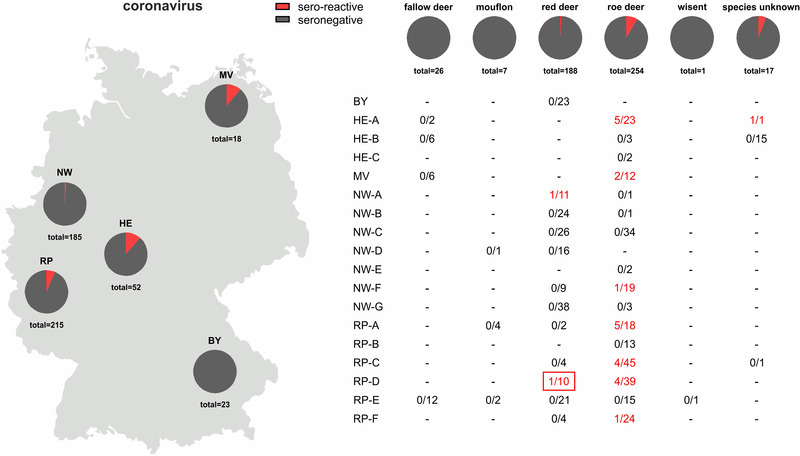
Proportion of samples per German federal state (left) and per wild ruminant species (right) that reacted positive (red) in an receptor‐binding domain‐based severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) antibody ELISA. In the lower right panel, the number of sero‐reactive results/number of analysed samples is given separately for each federal state and, if known, the hunting district from which the samples originated. The serum that additionally tested positive in a surrogate virus neutralisation test is framed in red. BY–Bavaria, HE–Hesse, MV–Mecklenburg‐Western Pomerania, NW–North Rhine‐Westphalia, RP–Rhineland‐Palatinate
FIGURE 3.
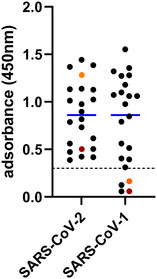
Reactivity of wild ruminant samples collected between 2017 and 2020 towards the receptor‐binding domains of the coronaviruses SARS‐CoV‐2 and SARS‐CoV‐1 as measured by indirect multispecies ELISAs. The cut‐off for positivity (≥ 0.3) is marked by a dashed horizontal line, and the mean values are shown in blue. A cattle sample taken 20 days after experimental SARS‐CoV‐2 infection (dark red) and a cattle sample taken subsequent to natural SARS‐CoV‐2 infection (orange) were used as virus‐specific controls.
Serological investigations of wildlife animals represent a cornerstone of disease surveillance (Michel et al., 2021), especially when virus shedding is only transient and the timeframe unknown, since antibodies remain detectable for longer periods. However, when conducting sero‐epidemiological studies, potential cross‐reactivity between closely related viruses needs to be considered, in the present study in particular between SARS‐CoV‐2 and other coronaviruses. Here, we found an anti‐RBD reactivity in pre‐ and postpandemic wild ruminant sera that could not be confirmed by the highly specific VNT and only in two cases by a surrogate VNT, which is likewise based on the RBD, more precisely on the interaction between SARS‐CoV‐2's RBD and the human host cell receptor protein angiotensin‐converting enzyme 2 (ACE2). In roe deer, which most frequently showed an anti‐RBD reactivity in this study, the coronavirus prevalence is largely unknown, but in some other cervid species, bovine‐like coronaviruses were found (Alekseev et al., 2008; Amer, 2018). During the initial validation of the ELISA used in this study and during an experimental SARS‐CoV‐2 infection study in cattle, it could be shown that the ELISA does not exhibit cross‐reactivity with BCoV (Ulrich et al., 2020; Wernike, Aebischer, Michelitsch, et al., 2021). Hence, there is most likely at least one hitherto unknown coronavirus present in the deer population that is more closely related to viruses of the Sarbecovirus subgenus than BCoV, leading to the generation of antibodies cross‐reactive with both SARS‐CoV‐1 and SARS‐CoV‐2. Interestingly, such an RBD‐reactivity that could not be explained by the circulation of known coronaviruses was also observed in sera of domestic and peridomestic animals collected in the United States prior to the current SARS‐CoV‐2 pandemic (Hancock et al., 2021). The RBD that is responsible for virus interaction with the host cell receptor protein ACE2 is located in the S1 subunit of the spike protein. S1, including the RBD, is much more divergent than the second spike protein subunit (S2) between SARS‐CoV‐2 and other circulating coronaviruses of humans and animals, making it a highly specific target for serological test systems (Premkumar et al., 2020). The RBDs of SARS‐CoV‐1 and SARS‐CoV‐2, however, exhibit major similarities in protein structure as well as sequence, and cross‐reactions occur (Lan et al., 2020). Hence, the cross‐reacting agent found in German wild ruminants is most likely a coronavirus of the Sarbecovirus subgenus closely related to both SARS‐CoV‐1 and SARS‐CoV‐2. Nevertheless, for final classification of the cross‐reacting coronavirus, the identification of the virus in question by PCR methods and/or sequence analysis of tissue samples is required.
3.2. Serology of vector‐borne and transboundary livestock diseases
All but two samples of the panel collected in 2021/2022 (one fallow deer, one roe deer), which could not be tested because of insufficient sample volume, were analysed for antibodies against ruminant pestiviruses, and all of them gave negative results (0/491 positive; 0%).
Hence, BVDV or BDV infections and, in particular, the presence of PI animals are highly unlikely in the population surveyed. Therefore, it is very improbable that wildlife species form a significant virus reservoir and could be a source of infection for domestic cattle, which is nearly free from the infection as a result of the mandatory control program (Friedrich‐Loeffler‐Institut, 2021).
Antibodies against the Culicoides‐transmitted SBV were detected in 67 of the 493 wildlife samples collected in 2021/2022 (13.6%, 95% CI: 10.6%−16.6%). With the exception of the European bison (Wisent), individual animals of every species sampled in the western part of Germany were affected (Figure 2). In contrast, all but one of the analysed samples tested negative for antibodies against the likewise Culicoides‐transmitted BTV (1/484 positive; 0.2%, 95% CI: 0%−0.6%). The remaining nine samples (one fallow deer, two red deer, six roe deer) could not be tested due to a lack of sample material. The ELISA‐positive sample originated from a fallow deer of unknown age hunted in the federal state Hesse (Figure 2).
FIGURE 2.
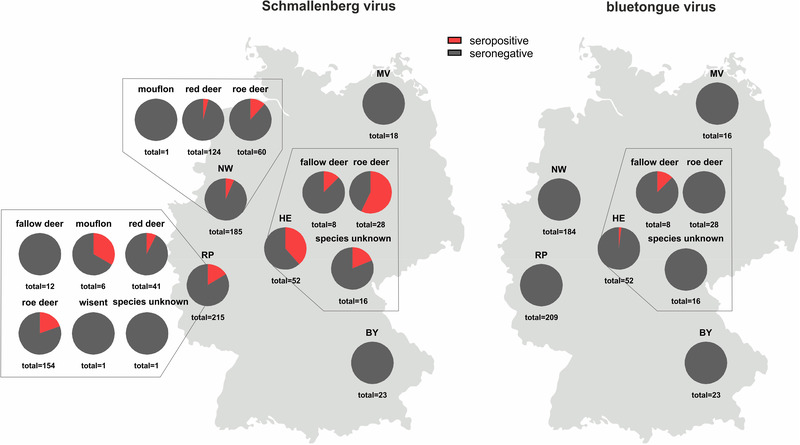
Proportion of wild ruminant samples that tested positive (red) for antibodies against the Culicoides‐transmitted viruses Schmallenberg virus (left) and bluetongue virus (right). BY–Bavaria, HE–Hesse, MV–Mecklenburg‐Western Pomerania, NW–North Rhine‐Westphalia, RP–Rhineland‐Palatinate
Interestingly, the situation observed for SBV mirrors that in domestic ruminants in Germany and that in wild ruminants in several other European countries in the postepidemic period. As examples, in Slovenia, an overall seroprevalence of about 18% was found in roe deer, red deer, chamois and European mouflon samples collected during the 2017/2018 hunting season (Vengušt et al., 2020), and in Spain, anti‐SBV antibodies could be detected in 16.8% and 23.5% of red deer and fallow deer samples, respectively, collected in 2015 (Jiménez‐Ruiz et al., 2022). In the absence of control measures, SBV established an enzootic status after its emergence in central Europe a decade ago (Larska, 2018; Wernike & Beer, 2020). In domestic animals, a wave‐like pattern of circulation with increased case numbers every 2 to 3 years has been observed (Larska, 2018; Wernike & Beer, 2020). Unfortunately, the age of the wild ruminants sampled for the present study is known for only a subset of animals, which hampers a more detailed analysis of the year of infection. Nevertheless, the dataset allows a comparison between the infection rates with the enzootic SBV and BTV, for which sporadic cases have been reported in domestic ruminants since December 2018 with the last case reported in February 2021 (Friedrich‐Loeffler‐Institut, 2022). Due to the similarity in their mammalian hosts and the insect vector species responsible for virus transmission, both viruses share major factors for virus circulation in a given area. But, whereas anti‐SBV antibodies were detected rather frequently in samples collected in the 2021/2022 hunting season, only one animal tested positive in the BTV ELISA. The age of this animal is not known, but given the epidemiological situation in domestic ruminants and the lack of further positive results, the seropositive fallow deer was most likely older than one year and was infected in one of the previous vector seasons. The differences in the seroprevalences of antibodies against SBV and BTV in the wild ruminant population and the agreement with the respective situation in domestic animals confirm previous observations that both wildlife and farmed animals are part of the transmission cycle, but that wild ruminants do not play a significant role in the maintenance of BTV in a given area. BTV or BTV‐specific antibodies were previously detected in wild ruminants only in regions where the virus is also present in domestic animals (Yon et al., 2018). In addition, despite the presence of competent insect vectors, BTV serotypes 1 and 8 never spread in red deer beyond the domestic outbreak range in France (Rossi et al., 2019). Here, the single seropositive animal was hunted in a federal state within the restricted zone, while anti‐SBV antibodies are widespread, highlighting the suitability of serological surveys in wildlife animals to demonstrate the presence or absence of multi‐host diseases in a given area.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTION
Conceptualisation: Kerstin Wernike and Martin Beer; methodology: Kerstin Wernike, Andrea Aebischer, Donata Hoffmann and Martin Beer; formal analysis: Kerstin Wernike; investigation: Kerstin Wernike, Andrea Aebischer, Anja Petrov, Katharina Marquart, Ulrich Schotte, Jacob Schön and Donata Hoffmann; resources: Luisa Fischer, Mark Holsteg, Anja Petrov, Katharina Marquart, Ulrich Schotte, Silke Hechinger, Antonie Neubauer‐Juric, Julia Blicke and Martin Beer; writing–original draft preparation: Kerstin Wernike; writing–review and editing: all authors; visualisation: Kerstin Wernike; supervision: Thomas C. Mettenleiter and Martin Beer; funding acquisition: Kerstin Wernike, Thomas C. Mettenleiter and Martin Beer. All authors have read and agreed to the published version of the article.
ETHICS STATEMENT
Blood samples were collected by local hunters according to the appropriate German legislation. No ethical/welfare authority approval was required as all samples were collected postmortem by the hunters.
Supporting information
TABLE S1 Origin and number of ruminant samples collected in the 2021/2022 hunting season in Germany
TABLE S2 Results of the SARS‐CoV‐2 serological assays for wild ruminant samples collected at military training areas of the German Federal Armed Forces before and during the COVID‐19 pandemic
ACKNOWLEDGEMENTS
We thank Bianka Hillmann, Mareen Lange and Antje Gromtzik for excellent technical assistance and the hunters for providing the wildlife samples. The study was supported by intramural funding of the German Federal Ministry of Food and Agriculture (BMEL) provided to the Friedrich‐Loeffler‐Institut and received additional funding by the European Union Horizon 2020 project ‘European Virus Archive goes global’ (EVAg). The SBV and BTV serology was funded by the BMEL through the Federal Office for Agriculture and Food (BLE), Grant Number 281B101816. The BVDV serology was financially supported by the Animal Disease Funds (Tierseuchenkassen) of the German federal states Lower Saxony, Thuringia, Hesse, Rhineland‐Palatinate, North Rhine‐Westphalia and by the Ministry of Energy, Agriculture, the Environment, Nature and Digitalisation of the German federal state Schleswig‐Holstein.
Open access funding enabled and organized by Projekt DEAL.
Wernike, K. , Fischer, L. , Holsteg, M. , Aebischer, A. , Petrov, A. , Marquart, K. , Schotte, U. , Schön, J. , Hoffmann, D. , Hechinger, S. , Neubauer‐Juric, A. , Blicke, J. , Mettenleiter, T. C. , & Beer, M. (2022). Serological screening in wild ruminants in Germany, 2021/2022: No evidence of SARS‐CoV‐2, bluetongue virus or pestivirus spread but high seroprevalences against Schmallenberg virus. Transboundary and Emerging Diseases, 00, 1–8. 10.1111/tbed.14600
Contributor Information
Kerstin Wernike, Email: kerstin.wernike@fli.de.
Martin Beer, Email: martin.beer@fli.de.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
REFERENCES
- Alekseev, K. P. , Vlasova, A. N. , Jung, K. , Hasoksuz, M. , Zhang, X. , Halpin, R. , Wang, S. , Ghedin, E. , Spiro, D. , & Saif, L. J. (2008). Bovine‐like coronaviruses isolated from four species of captive wild ruminants are homologous to bovine coronaviruses, based on complete genomic sequences. Journal of Virology, 82(24), 12422–12431. 10.1128/jvi.01586-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer, H. M. (2018). Bovine‐like coronaviruses in domestic and wild ruminants. Animal Health Research Reviews, 19(2), 113–124. 10.1017/s1466252318000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas‐Montes, A. , Paniagua, J. , Arenas, A. , Lorca‐Oró, C. , Carbonero, A. , Cano‐Terriza, D. , & García‐Bocanegra, I. (2016). Spatial‐temporal trends and factors associated with the bluetongue virus seropositivity in large game hunting areas from Southern Spain. Transboundary and Emerging Diseases, 63(5), e339–346. 10.1111/tbed.12309 [DOI] [PubMed] [Google Scholar]
- Beer, M. , & Wernike, K. (2021). Akabane virus and Schmallenberg virus (Peribunyaviridae). In Bamford D. H. & Zuckerman M. (Eds.), Encyclopedia of virology (4th ed., pp. 34–39 ). Academic Press. 10.1016/B978-0-12-809633-8.20939-4 [DOI] [Google Scholar]
- Bosco‐Lauth, A. M. , Walker, A. , Guilbert, L. , Porter, S. , Hartwig, A. , McVicker, E. , Bielefeldt‐Ohmann, H. , & Bowen, R. A. (2021). Susceptibility of livestock to SARS‐CoV‐2 infection. Emerging Microbes & Infections, 10(1), 2199–2201. 10.1080/22221751.2021.2003724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler, J. C. , Bevins, S. N. , Ellis, J. W. , Linder, T. J. , Tell, R. M. , Jenkins‐Moore, M. , Root, J. J. , Lenoch, J. B. , Robbe‐Austerman, S. , DeLiberto, T. J. , Gidlewski, T. , Torchetti, M. K. , & Shriner, S. A. (2021). SARS‐CoV‐2 exposure in wild white‐tailed deer (Odocoileus virginianus). PNAS, 118(47), e2114828118. 10.1073/pnas.2114828118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool, K. , Gaudreault, N. N. , Morozov, I. , Trujillo, J. D. , Meekins, D. A. , McDowell, C. , Carossino, M. , Bold, D. , Mitzel, D. , Kwon, T. , Balaraman, V. , Madden, D. W. , Artiaga, B. L. , Pogranichniy, R. M. , Roman‐Sosa, G. , Henningson, J. , Wilson, W. C. , Balasuriya, U. B. R. , García‐Sastre, A. , & Richt, J. A. (2021). Infection and transmission of ancestral SARS‐CoV‐2 and its alpha variant in pregnant white‐tailed deer. Emerging Microbes & Infections. Advance online publication. 10.1080/22221751.2021.2012528 [DOI] [PMC free article] [PubMed]
- Coronaviridae Study Group of the ICTV . (2020). The species severe acute respiratory syndrome‐related coronavirus: Classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nature Microbiology, 5(4), 536–544. 10.1038/s41564-020-0695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas, J. , Hughes, G. M. , Keough, K. C. , Painter, C. A. , Persky, N. S. , Corbo, M. , Hiller, M. , Koepfli, K.‐P. , Pfenning, A. R. , Zhao, H. , Genereux, D. P. , Swofford, R. , Pollard, K. S. , Ryder, O. A. , Nweeia, M. T. , Lindblad‐Toh, K. , Teeling, E. C. , Karlsson, E. K. , & Lewin, H. A. (2020). Broad host range of SARS‐CoV‐2 predicted by comparative and structural analysis of ACE2 in vertebrates. PNAS, 117(36), 22311–22322. 10.1073/pnas.2010146117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (2013). “Schmallenberg” virus: Analysis of the epidemiological data. EFSA Supporting Publications 2013. EN‐3429. http://www.efsa.europa.eu/de/supporting/doc/429e.pdf
- Ezanno, P. , Fourichon, C. , & Seegers, H. (2008). Influence of herd structure and type of virus introduction on the spread of bovine viral diarrhoea virus (BVDV) within a dairy herd. Veterinary Research, 39(5), 39. 10.1051/vetres:2008016 [DOI] [PubMed] [Google Scholar]
- Friedrich‐Loeffler‐Institut (2021). Statistik zur BVD‐Bekämpfung in Deutschland: PI‐Tiere (Zeitraum 2011–2020). https://www.fli.de/de/institute/institut‐fuer‐virusdiagnostik‐ivd/referenzlabore/nrl‐fuer‐bvdmd/
- Friedrich‐Loeffler‐Institut (2022). FLI: Blauzungenkrankheit (BT). https://www.fli.de/de/aktuelles/tierseuchengeschehen/blauzungenkrankheit/
- Frölich, K. (1995). Bovine virus diarrhea and mucosal disease in free‐ranging and captive deer (Cervidae) in Germany. Journal of Wildlife Diseases, 31(2), 247–250. 10.7589/0090-3558-31.2.247 [DOI] [PubMed] [Google Scholar]
- Gaudreault, N. N. , Cool, K. , Trujillo, J. D. , Morozov, I. , Meekins, D. A. , McDowell, C. , Bold, D. , Carossino, M. , Balaraman, V. , Mitzel, D. , Kwon, T. , Madden, D. W. , Artiaga, B. L. , Pogranichniy, R. M. , Roman‐Sosa, G. , Wilson, W. C. , Balasuriya, U. B. R. , García‐Sastre, A. , García‐Sastre, A. , & Richt, J. A. (2022). Susceptibility of sheep to experimental co‐infection with the ancestral lineage of SARS‐CoV‐2 and its alpha variant. Emerging Microbes & Infections. Advance online publication. 10.1080/22221751.2022.2037397 [DOI] [PMC free article] [PubMed]
- Hale, V. L. , Dennis, P. M. , McBride, D. S. , Nolting, J. M. , Madden, C. , Huey, D. , Ehrlich, M. , Grieser, J. , Winston, J. , Lombardi, D. , Gibson, S. , Saif, L. , Killian, M. L. , Lantz, K. , Tell, R. M. , Torchetti, M. , Robbe‐Austerman, S. , Nelson, M. I. , Faith, S. A. , & Bowman, A. S. (2022). SARS‐CoV‐2 infection in free‐ranging white‐tailed deer (Odocoileus virginianus). Nature, 602(7897), 481–486. 10.1038/s41586-021-04353-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock, T. J. , Hickman, P. , Kazerooni, N. , Kennedy, M. , Kania, S. A. , Dennis, M. , Szafranski, N. , Gerhold, R. , Su, C. , Masi, T. , Smith, S. , & Sparer, T. E. (2021). Evidence for a potential pre‐pandemic SARS‐like coronavirus among animals in North America. bioRxiv., 10.1101/2021.12.17.473265 [DOI] [Google Scholar]
- Heckeberg, N. S. (2020). The systematics of the Cervidae: A total evidence approach. PeerJ, 8, e8114. 10.7717/peerj.8114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, B. , Scheuch, M. , Höper, D. , Jungblut, R. , Holsteg, M. , Schirrmeier, H. , Eschbaumer, M. , Goller, K. V. , Wernike, K. , Fischer, M. , Breithaupt, A. , Mettenleiter, T. C. , & Beer, M. (2012). Novel orthobunyavirus in cattle, Europe, 2011. Emerging Infectious Diseases, 18(3), 469–472. 10.3201/eid1803.111905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez‐Ruiz, S. , Vicente, J. , Risalde, M. A. , Acevedo, P. , Cano‐Terriza, D. , González‐Barrio, D. , Barroso, P. , & García‐Bocanegra, I. (2022). Survey of Culicoides‐borne Bluetongue and Schmallenberg viruses at the wildlife‐livestock interface in Doñana National Park (Spain). Transboundary and Emerging Diseases. Advance online publication. 10.1111/tbed.14516 [DOI] [PubMed]
- Krzysiak, M. K. , Iwaniak, W. , Kęsik‐Maliszewska, J. , Olech, W. , & Larska, M. (2017). Serological study of exposure to selected arthropod‐borne pathogens in European Bison (Bison bonasus) in Poland. Transboundary and Emerging Diseases, 64(5), 1411–1423. 10.1111/tbed.12524 [DOI] [PubMed] [Google Scholar]
- Kuchipudi, S. V. , Surendran‐Nair, M. , Ruden, R. M. , Yon, M. , Nissly, R. H. , Vandegrift, K. J. , Nelli, R. L. , Li, L. , Jayarao, B. M. , Maranas, C. D. , Levine, N. , Willgert, K. , Conlan, A. J. K. , Olsen, R. J. , Davis, J. J. , Musser, J. M. , Hudson, P. J. , & Kapur, V. (2022). Multiple spillovers from humans and onward transmission of SARS‐CoV‐2 in white‐tailed deer. PNAS, 119(6), e2121644119. 10.1073/pnas.2121644119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan, J. , Ge, J. , Yu, J. , Shan, S. , Zhou, H. , Fan, S. , Zhang, Q. , Shi, X. , Wang, Q. , Zhang, L. , & Wang, X. (2020). Structure of the SARS‐CoV‐2 spike receptor‐binding domain bound to the ACE2 receptor. Nature, 581(7807), 215–220. 10.1038/s41586-020-2180-5 [DOI] [PubMed] [Google Scholar]
- Larska, M. (2018). Schmallenberg virus: A cyclical problem. The Veterinary Record, 183(22), 688–689. 10.1136/vr.k5109 [DOI] [PubMed] [Google Scholar]
- Linden, A. , Desmecht, D. , Volpe, R. , Wirtgen, M. , Gregoire, F. , Pirson, J. , Paternostre, J. , Kleijnen, D. , Schirrmeier, H. , Beer, M. , & Garigliany, M. M. (2012). Epizootic spread of Schmallenberg virus among wild cervids, Belgium, Fall 2011. Emerging Infectious Diseases, 18(12), 2006–2008. 10.3201/eid1812.121067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclachlan, N. J. (2011). Bluetongue: History, global epidemiology, and pathogenesis. Preventive Veterinary Medicine, 102(2), 107–111. 10.1016/j.prevetmed.2011.04.005 [DOI] [PubMed] [Google Scholar]
- Michel, A. L. , Van Heerden, H. , Crossley, B. M. , Al Dahouk, S. , Prasse, D. , & Rutten, V. (2021). Pathogen detection and disease diagnosis in wildlife: Challenges and opportunities. Revue Scientifique Et Technique (International Office of Epizootics), 40(1), 105–118. doi: 10.20506/rst.40.1.3211 [DOI] [PubMed] [Google Scholar]
- Mouchantat, S. , Wernike, K. , Lutz, W. , Hoffmann, B. , Ulrich, R. G. , Börner, K. , Wittstatt, U. , & Beer, M. (2015). A broad spectrum screening of Schmallenberg virus antibodies in wildlife animals in Germany. Veterinary Research, 46(1), 99. 10.1186/s13567-015-0232-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, D. D. , Duprau, J. L. , Wolff, P. L. , & Evermann, J. F. (2015). Persistent bovine viral diarrhea virus infection in domestic and wild small ruminants and camelids including the mountain goat (Oreamnos americanus). Frontiers in Microbiology, 6, 1415. 10.3389/fmicb.2015.01415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettleton, P. F. , Gilray, J. A. , Russo, P. , & Dlissi, E. (1998). Border disease of sheep and goats. Veterinary Research, 29(3‐4), 327–340. [PubMed] [Google Scholar]
- OIE (2021). SARS‐COV‐2 in animals–Situation report 6. https://www.oie.int/app/uploads/2021/11/sars‐cov‐2‐situation‐report‐6.pdf
- Palmer, M. V. , Martins, M. , Falkenberg, S. , Buckley, A. , Caserta, L. C. , Mitchell, P. K. , Cassmann, E. D. , Rollins, A. , Zylich, N. C. , Renshaw, R. W. , Guarino, C. , Wagner, B. , Lager, K. , & Diel, D. G. (2021). Susceptibility of white‐tailed deer (Odocoileus virginianus) to SARS‐CoV‐2. Journal of Virology, 95(11), e00083–21. 10.1128/jvi.00083-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passler, T. , Ditchkoff, S. S. , Givens, M. D. , Brock, K. V. , DeYoung, R. W. , & Walz, P. H. (2010). Transmission of bovine viral diarrhea virus among white‐tailed deer (Odocoileus virginianus). Veterinary Research, 41(2), 20. 10.1051/vetres/2009068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar, L. , Segovia‐Chumbez, B. , Jadi, R. , Martinez, D. R. , Raut, R. , Markmann, A. , Cornaby, C. , Bartelt, L. , Weiss, S. , Park, Y. , Edwards, C. E. , Weimer, E. , Scherer, E. M. , Rouphael, N. , Edupuganti, S. , Weiskopf, D. , Tse, L. V. , Hou, Y. J. , Margolis, D. , … de Silva, A. M. (2020). The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS‐CoV‐2 patients. Science Immunology, 5(48), eabc8413. 10.1126/sciimmunol.abc8413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, S. , Balenghien, T. , Viarouge, C. , Faure, E. , Zanella, G. , Sailleau, C. , Mathieu, B. , Delécolle, J.‐C. , Ninio, C. , Garros, C. , Gardès, L. , Tholoniat, C. , Ariston, A. , Gauthier, D. , Mondoloni, S. , Barboiron, A. , Pellerin, M. , Gibert, P. , Novella, C. , … Bréard, E. (2019). Red deer (Cervus elaphus) did not play the role of maintenance host for bluetongue virus in France: The burden of proof by long‐term wildlife monitoring and culicoides snapshots. Viruses., 11(10), 903. 10.3390/v11100903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Fons, F. , Sánchez‐Matamoros, A. , Gortázar, C. , & Sánchez‐Vizcaíno, J. M. (2014). The role of wildlife in bluetongue virus maintenance in Europe: Lessons learned after the natural infection in Spain. Virus Research, 182, 50–58. 10.1016/j.virusres.2013.12.031 [DOI] [PubMed] [Google Scholar]
- Sailleau, C. , Bréard, E. , Viarouge, C. , Vitour, D. , Romey, A. , Garnier, A. , Fablet, A. , Lowenski, S. , Gorna, K. , Caignard, G. , Pagneux, C. , & Zientara, S. (2017). Re‐emergence of bluetongue virus serotype 8 in France, 2015. Transboundary and Emerging Diseases, 64(3), 998–1000. 10.1111/tbed.12453 [DOI] [PubMed] [Google Scholar]
- Schlottau, K. , Rissmann, M. , Graaf, A. , Schön, J. , Sehl, J. , Wylezich, C. , Höper, D. , Mettenleiter, T. C. , Balkema‐Buschmann, A. , Harder, T. , Grund, C. , Hoffmann, D. , Breithaupt, A. , & Beer, M. (2020). SARS‐CoV‐2 in fruit bats, ferrets, pigs, and chickens: An experimental transmission study. Lancet Microbe, 1(5), e218–e225. 10.1016/S2666-5247(20)30089-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmel, N. E. , & Walzer, C. (2020). Infectious wildlife diseases in Austria‐A literature review from 1980 until 2017. Frontiers in Veterinary Science, 7, 3. 10.3389/fvets.2020.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich, L. , Wernike, K. , Hoffmann, D. , Mettenleiter, T. C. , & Beer, M. (2020). Experimental infection of cattle with SARS‐CoV‐2. Emerging Infectious Diseases, 26(12), 2979–2981. 10.3201/eid2612.203799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengušt, G. , Žele Vengušt, D. , Toplak, I. , Rihtarič, D. , & Kuhar, U. (2020). Post‐epidemic investigation of Schmallenberg virus in wild ruminants in Slovenia. Transboundary and Emerging Diseases, 67(4), 1708–1715. 10.1111/tbed.13495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinomack, C. , Rivière, J. , Breard, E. , Viarouge, C. , Postic, L. , Zientara, S. , Vitour, D. , Belbis, G. , Spony, V. , Pagneux, C. , Sailleau, C. , & Zanella, G. (2019). Clinical cases of Bluetongue serotype 8 in calves in France in the 2018–2019 winter. Transboundary and Emerging Diseases, 10.1111/tbed.13466 [DOI] [PubMed]
- Wernike, K. , Aebischer, A. , Michelitsch, A. , Hoffmann, D. , Freuling, C. , Balkema‐Buschmann, A. , Graaf, A. , Müller, T. , Osterrieder, N. , Rissmann, M. , Rubbenstroth, D. , Schön, J. , Schulz, C. , Trimpert, J. , Ulrich, L. , Volz, A. , Mettenleiter, T. , & Beer, M. (2021). Multi‐species ELISA for the detection of antibodies against SARS‐CoV‐2 in animals. Transboundary and Emerging Diseases, 68(4), 1779–1785. 10.1111/tbed.13926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernike, K. , Aebischer, A. , Sick, F. , Szillat, K. P. , & Beer, M. (2021). Differentiation of antibodies against selected Simbu serogroup viruses by a glycoprotein Gc‐based triplex ELISA. Veterinary Science, 8(1), 12. 10.3390/vetsci8010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernike, K. , & Beer, M. (2020). Re‐circulation of Schmallenberg virus, Germany, 2019. Transboundary and Emerging Diseases, 67(6), 2290–2295. 10.1111/tbed.13592 [DOI] [PubMed] [Google Scholar]
- Wernike, K. , Böttcher, J. , Amelung, S. , Albrecht, K. , Gärtner, T. , Donat, K. , & Beer, M. (2022). Serological screening suggests single SARS‐CoV‐2 spillover events to cattle. bioRxiv. 10.1101/2022.01.17.476608 [DOI] [PMC free article] [PubMed]
- Wernike, K. , Gethmann, J. , Schirrmeier, H. , Schröder, R. , Conraths, F. J. , & Beer, M. (2017). Six years (2011‐2016) of mandatory nationwide bovine viral diarrhea control in Germany–A success story. Pathogens, 6(4), 50. 10.3390/pathogens6040050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yon, L. , Duff, J. P. , Agren, E. O. , Erdelyi, K. , Ferroglio, E. , Godfroid, J. , Hars, J. , Hestvik, G. , Horton, D. , Kuiken, T. , Lavazza, A. , Markowska‐Daniel, I. , Martel, A. , Neimanis, A. , Pasmans, F. , Price, S. J. , Ruiz‐Fons, F. , Ryser‐Degiorgis, M.‐P. , Widén, F. , & Gavier‐Widen, D. (2018). Recent changes in infectious diseases in European wildlife. Journal of Wildlife Diseases, 55(1), 3–43. 10.7589/2017-07-172 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Origin and number of ruminant samples collected in the 2021/2022 hunting season in Germany
TABLE S2 Results of the SARS‐CoV‐2 serological assays for wild ruminant samples collected at military training areas of the German Federal Armed Forces before and during the COVID‐19 pandemic
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.


