Abstract
Since the reproductive toxicity of COVID‐19 vaccines have not been assessed in previous clinical trials, and studies have shown that SARS‐CoV‐2 is associated with a decrease in sperm parameters. Although it has been reported that the mRNA SARS‐CoV‐2 vaccines do not adversely affect semen parameters, whether this conclusion applies to inactivated vaccines remains unclear. Here, we conducted a study among patients who accepted in vitro fertilization (IVF) at the reproductive centre between June and August of 2021. In the enrolled cases, men who have completed two doses of COVID‐19 inactivated vaccine were included in “vaccine group” (N = 105), and those who were not vaccinated were included in “control group” (N = 155). In this study, we compare the sperm parameters and embryo quality between these two groups. Our data showed that the sperm parameters were similar in terms of volume, sperm concentration, sperm count, progressive motility, total motility and total motile sperm count between these two groups. Similarly, no significant differences were observed in IVF outcomes. The mean number of 2PN, cleavage‐stage embryos, blastocysts, and good‐quality blastocysts was 8.59 ± 4.47, 5.06 ± 3.17 and 2.08 ± 1.79 in vaccine group, 7.75 ± 4.14, 4.34 ± 3.06 and 1.74 ± 1.54 in control group, respectively. The high‐quality blastocyst rate was 41.05% (218 of 531) in vaccine group and 40.03% (269 of 672) in control group (p > 0.05). In addition, no differences were observed in biochemical and clinical pregnancy rates between the two groups. In summary, our results revealed that COVID‐19 inactivated vaccine administration exhibited no negative effect on sperm parameters and embryo quality in IVF.
Keywords: COVID‐19, in vitro fertilization, inactivated vaccine, male fertility, sperm parameters
1. INTRODUCTION
The outbreak of coronavirus disease 2019 (COVID‐19) was first detected in Wuhan, China in December 2019. It caused significant morbidity and mortality throughout the world, as well as social, medical, educational and economic disruptions (Aassve et al., 2020). Regarding the effect of COVID‐19 on the male reproductive system, the issue is still controversial. It has been reported that SARS‐CoV‐2 cannot be detected in the semen collected from patients with COVID‐19 during their recovery period (Pan et al., 2020; Song et al., 2020). Recently, emerging evidences indicate that SARS‐CoV‐2 patients and those who have recovered from it are still at high risk of abnormal sperm counts (Best et al., 2021; Gacci et al., 2021; Li et al., 2020; Pazir et al., 2021; Ruan et al., 2021) and decreased sperm concentration (Best et al., 2021). Gacci et al. observed that 25% of the men with recent SARS‐Cov‐2 infections and proven healing were oligo‐crypto‐azoospermic, and oligo‐crypto‐azoospermia was found significantly related to COVID‐19 severity (Gacci et al., 2021). Although the underlining mechanism remains largely unknown, several studies have implicated that angiotensin‐converting enzyme 2 (ACE2) receptors play a key role in the pathogenesis of COVID‐19. It has been shown that the ACE2 receptor is widely distributed in the testis, including the Leydig and Sertoli cells (Omolaoye et al., 2021). Other possibilities of testicular damage may be induced by secondary immune and inflammatory responses, damage to the blood testis barrier (BTB), increased oxidative stress, and malfunction of DNA methylation and fragmentation (Kumar & Kaur, 2021). Therefore, we hypothesized that COVID‐19 infection can subsequently lead to male infertility.
At present, effective and safe COVID‐19 vaccines are considered to be an urgent global need (Lurie et al., 2020; Yang et al., 2020). Five vaccines have been approved for emergency use, and among them two COVID‐19 inactivated vaccines (Sinovac and Sinopharm) are widely available in China. These vaccines were developed based on the immune responses elicited by inactivated SARS‐CoV‐2 virus (Soleimanpour & Yaghoubi, 2021). Sinovac‐CoronaVac is an aluminium‐hydroxide‐adjuvanted, inactivated whole virus vaccine. Clinical trials of the vaccine were conducted in Brazil, Indonesia and Turkey, and the results showed that Sinovac‐CoronaVac had a high efficacy against symptomatic SARS‐CoV‐2 infection, and even an efficacy of 100% against severe COVID‐19 and hospitalization, while only minor adverse such as reactions fatigue and pain at the injection site occurred (Hitchings et al., 2021; Tanriover et al., 2021).Sinopharm BBIBP‐CorV (Sinopharm COVID‐19 vaccine) is another inactivated whole virus vaccine widely available in China,which also showed high efficacy (78.1%) and few adverse effects in clinical studies (Al Kaabi et al., 2021). Both vaccines showed good efficacy and satisfactory safety profile. In addition, WHO reported that although available data are not sufficient enough to assess the safety of vaccine for pregnant women, previous experience with other inactivated vaccines suggests that pregnant women should be vaccinated when the benefits outweigh the potential risks (WHO, 2021).
Prompted by the aforementioned observations of abnormal sperm parameters followed by SARS‐CoV2 infection, unfounded claims in the mass media implied that there was a potential association between the SARS‐CoV‐2 vaccine and male infertility. Diaz et al. investigated the influence of COVID‐19 vaccine Emergency Use Authorization (EUA) via online queries regarding the coronavirus vaccine and fertility. Their results revealed an increased concern regarding the vaccine and likely became a major cause for hesitancy in vaccine uptake (Diaz et al., 2021). Until now, few study investigated the effects of COVID‐19 inactivated vaccine on male fertility. To assess the safety of COVID‐19 inactivated vaccine in male reproduction, we compared the sperm parameters, embryonic development as well as blastocysts quality between vaccinated and unvaccinated men in in vitro fertilization (IVF).
2. MATERIAL AND METHODS
2.1. Study design and patients
We performed a cohort study of patients who accepted IVF at the reproductive centre between June and August of 2021. This study was approved by the Independent Ethics Committee of the Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University (number: 2021‐K‐96‐01). Among the enrolled cases, who have completed two doses of COVID‐19 inactivated vaccine according to the vaccination procedure were included in “vaccine group”, and those who were not vaccinated were included in “control group”. Due to the long‐term continuation of the COVID‐19 epidemic and widely available of COVID‐19 inactivated vaccine in China, all the patients included in this study requested the vaccination voluntarily. Those who were previously diagnosed with COVID‐19 infection were excluded. The detailed flow chart is shown in Figure 1.
FIGURE 1.
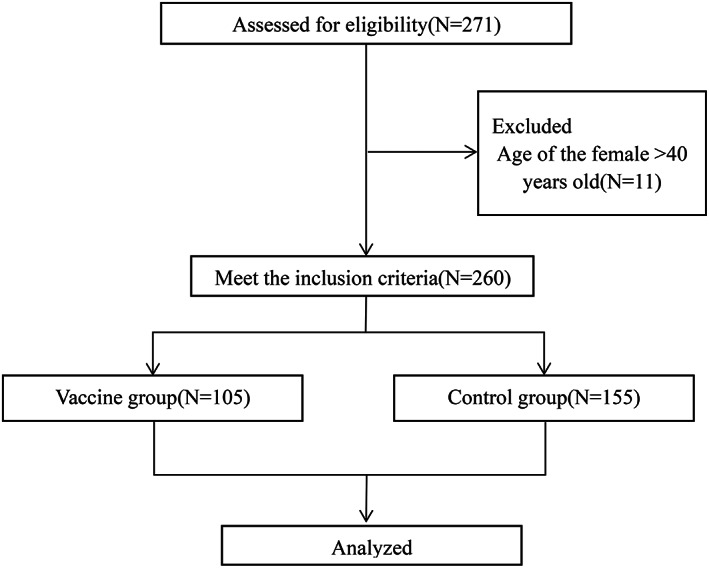
Flow chart showing the design, inclusion and exclusion criteria of patients in the study.
2.2. Procedures
This study used the ultra‐long protocol for ovarian stimulation. All patients received successful pituitary down‐regulation with the single gonadotrophin‐releasing hormone (GnRH) agonist (Triptorelin; Ferring, Kiel, Germany) injection on the 2–4 days of menstruation, and controlled ovarian hyper‐stimulation (COH) was initiated 32 to 38 days later. COH was started with recombinant FSH (Gonal‐F; Merck Serono, Aubonne, Switzerland) and the dosage was adjusted according to serum estradiol (E2), luteinizing hormone (LH), progesterone (P) levels and the size of antral follicles. 10,000 IU of human chorionic gonadotropin (hCG) was given when the follicles were mature, and several high‐ovarian responders were triggered by 5000 IU hCG in order to avoid severe ovarian hyperstimulation syndrome (OHSS). The oocytes were retrieved 36 hours later by ultrasound‐guided aspiration. Semen samples were obtained by masturbation on the day of oocyte collection after a three‐day abstinence period, and were examined in the laboratory within 30–60 min from ejaculation. 10 μl of liquefied semen were placed in a sperm counting chamber to assess the sperm parameters, through the computer‐assisted semen analysis (Hamilton‐Thorn Research, MA, USA). Those parameters included volume, sperm concentration, motility, and vitality. The sperm parameters were evaluated according to the WHO 5th edition laboratory manual by trained andrologists (WHO, 2010). Subsequently oocytes were fertilized by IVF after oocyte retrieval. 16–20 h after insemination, the normal fertilization was confirmed by the appearance of two pronuclear (2PN). Blastocyst of high scoring was selected for fresh transfer or freezing according to the Gardner embryo grading system (Gardner et al., 2000). A positive serum β‐HCG 14 days after embryo transfer was defined as biochemical pregnancy. The presence of an intrauterine gestational sac with a yolk sac, fetal pole, and fetal heart pulsations about 4 weeks after implantation was defined as clinical pregnancy.
2.3. Statistical analysis
Data analysis was performed with SPSS version 20.0 (IBM Corporation). The normality of data was analysed using Kolmogorov–Smirnov tests. Continuous variables were presented with mean ± standard deviation (SD) and were compared by Student's t test. Chi‐square test was used for dichotomous variables. p Value of <0.05 was considered as statistically significant.
3. RESULTS
A total of 260 cases met the criteria for inclusion in this study, among which 105 cases were recruited in the vaccine group and 155 cases in the control group. Of the 105 vaccinated men, 70 (67%) received Kexing Zhongwei Sinovac and 35 (33%) received Sinopharm Beijing. There was no significant difference in demographic and clinical characteristics between these two groups (Table 1). The adverse reaction reported by participants were pain at the injection site (11.43%), followed by fatigue (6.67%), headache (1.90%), nausea (0.95%) and low fever (0.95%). No special medical intervention was required, and no serious adverse reactions happened (Table 2).
TABLE 1.
Baseline characteristics in vaccine and control group
| Characteristics | Vaccine group (n = 105) | Control group (n = 155) | p Value |
|---|---|---|---|
| Female age, years | 31.88 ± 4.00 | 31.80 ± 3.86 | 0.878 |
| Male age, years | 33.92 ± 4.70 | 33.25 ± 4.42 | 0.237 |
| Infertility, years | 3.70 ± 2.72 | 3.18 ± 2.22 | 0.089 |
| Female BMI, kg/m2 | 22.12 ± 3.09 | 22.07 ± 3.68 | 0.909 |
| Male BMI, kg/m2 | 24.21 ± 3.54 | 24.36 ± 4.49 | 0.768 |
| Basal serum FSH, IU/L | 7.18 ± 2.15 | 6.94 ± 2.08 | 0.380 |
| AMH, ng/ml | 3.74 ± 2.59 | 3.44 ± 2.16 | 0.313 |
| E2 on hCG trigger day, pg/ml | 2621.86 ± 1227.14 | 2404.04 ± 1225.96 | 0.161 |
| Total dose of Gn, IU | 2419.95 ± 995.34 | 2422.95 ± 881.88 | 0.980 |
| Duration of stimulation, days | 12.10 ± 2.16 | 11.94 ± 1.84 | 0.528 |
Abbreviations: AMH, anti‐Mullerian hormone; BMI, Body Mass Index; E2, oestrogen; FSH, follicle‐stimulating hormone; Gn, gonadotropin; hCG, human chorionic gonadotropin.
TABLE 2.
Adverse reactions after vaccination with COVID‐19 inactivated vaccine in study
| Adverse reactions | Vaccine group (n, %) |
|---|---|
| None | 82 (78.10%) |
| Pain at the injection site | 12 (11.43%) |
| Fatigue | 7 (6.67%) |
| Low fever | 1 (0.95%) |
| Headache | 2 (1.90%) |
| Nausea | 1 (0.95%) |
| Serious adverse events | 0 (0%) |
As shown in Figure 2 and Table 3, the sperm parameters between these two groups were similar in terms of volume (2.44 ± 1.18 vs. 2.57 ± 1.14, ml), sperm concentration (43.73 ± 19.55 vs. 43.55 ± 17.45, million/ml), sperm count (102.34 ± 62.99 vs. 110.48 ± 64.43, million) and progressive motility (46.79% ± 15.34% vs. 45.56% ± 16.42%). Moreover, there was no difference on total motility (56.80% ± 15.96% vs. 55.28% ± 16.61%) and total progressive motile sperm count (26.69 ± 16.21 vs. 24.85 ± 13.83, million).
FIGURE 2.
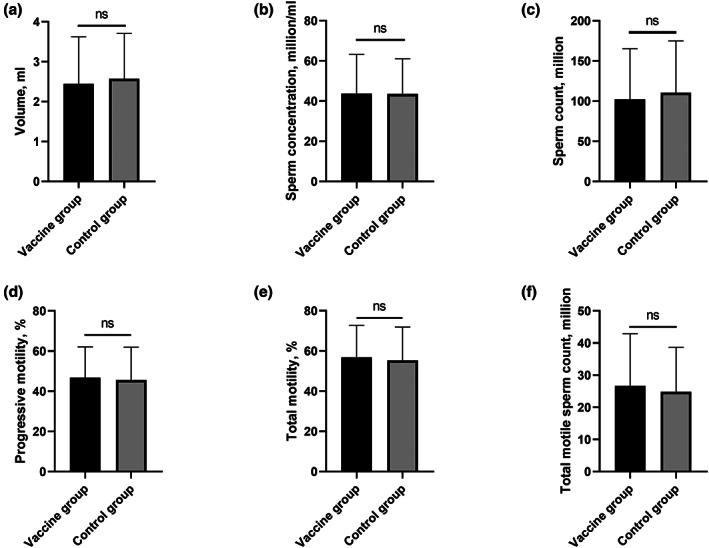
There were no significant differences in terms of volume (a), sperm concentration (b), sperm count (c), progressive motility (d), total motility (e) and total motile sperm count (f) between the vaccine group and control group (p > 0.05).
TABLE 3.
Comparison of the sperm parameters in vaccine and control group
| Parameters | Vaccine group (n = 105) | Control group (n = 155) | p Value |
|---|---|---|---|
| Volume, ml | 2.44 ± 1.18 | 2.57 ± 1.14 | 0.373 |
| Sperm concentration, million/ml | 43.73 ± 19.55 | 43.55 ± 17.45 | 0.938 |
| Sperm count, million | 102.34 ± 62.99 | 110.48 ± 64.43 | 0.314 |
| Progressive motility, % | 46.79% ± 15.34% | 45.56% ± 16.42% | 0.544 |
| Total motility, % | 56.80% ± 15.96% | 55.28% ± 16.61% | 0.462 |
| Total motile sperm count, million | 26.69 ± 16.21 | 24.85 ± 13.83 | 0.343 |
Similarly, no statistically difference was found in embryo quality of IVF between these two groups (Table 4). The Mean number of 2PN, cleavage‐stage embryos, blastocysts and good‐quality blastocysts were 8.59 ± 4.47, 5.06 ± 3.17 and 2.08 ± 1.79 in vaccine group, 7.75 ± 4.14, 4.34 ± 3.06 and 1.74 ± 1.54 in control group, respectively. The high‐quality blastocyst rate was 41.05% (218 of 531) in vaccine group and 40.03% (269 of 672) in control group (p > 0.05).
TABLE 4.
Comparison of the IVF outcomes in vaccine and control group
| Outcomes | Vaccine group (n = 105) | Control group (n = 155) | p Value |
|---|---|---|---|
| No. of oocytes retrieved | 14.74 ± 5.99 | 13.30 ± 5.82 | 0.053 |
| No. of MII oocytes | 11.67 ± 4.87 | 10.54 ± 5.05 | 0.073 |
| No. of 2PN cleavage‐stage embryos | 8.59 ± 4.47 | 7.75 ± 4.14 | 0.120 |
| No. of blastocysts | 5.06 ± 3.17 | 4.34 ± 3.06 | 0.067 |
| No. of good‐quality blastocysts | 2.08 ± 1.79 | 1.74 ± 1.54 | 0.103 |
| High‐quality blastocyst rate | 41.05% (218/531) | 40.03% (269/672) | 0.719 |
| Fresh ET cycles | 61 | 101 | |
| No. of embryos transferred | 1.03 ± 0.18 | 1.08 ± 0.27 | 0.192 |
| Biochemical pregnancy rate, % | 68.85% (42/61) | 59.41% (60/101) | 0.228 |
| Clinical pregnancy rate, % | 55.74% (34/61) | 45.54% (46/101) | 0.209 |
| Frozen ET cycles | 69 | 118 | |
| No. of embryos transferred | 1.61 ± 0.49 | 1.73 ± 0.45 | 0.098 |
| Biochemical pregnancy rate, % | 63.77% (44/69) | 65.25% (77/118) | 0.963 |
| Clinical pregnancy rate, % | 42.03% (29/69) | 41.53% (49/118) | 0.946 |
Abbreviations: ET, embryo transfer; MII, metaphase II; 2PN, two pronucleus.
A total of 61 fresh embryo transfer (ET) cycles were performed in the vaccine group compared to 101 in the control group. The mean number of embryos transferred in fresh cycles was similar in both groups (p > 0.05). For the early pregnancy outcomes of fresh ET cycles, the biochemical pregnancy rate and the clinical pregnancy rate were 68.85% and 55.74%, respectively, in the vaccine group, and 59.41% and 45.54%, respectively, in the control group (p > 0.05). Meanwhile, there were 69 frozen ET cycles in the vaccine group and 118 in the control group. Similar to fresh cycles, there was no difference in mean number of embryos transferred (1.61 ± 0.49 vs. 1.73 ± 0.45), biochemical pregnancy rate (63.77% vs. 65.25%) and clinical pregnancy rate (42.03%% vs. 41.53%) between the two groups (p > 0.05, Table 4).
4. DISCUSSION
This study was designed to investigate the potential detrimental effects of inactivated vaccine on sperm parameters and embryo quality in IVF. The average time window of vaccine administration and semen analysis(SA) procurement was 80.6 days in this study, which is much closer to the normal spermatogenesis cycle than previous studies (30–71 days) (Gonzalez et al., 2021; Lifshitz et al., 2021; Safrai et al., 2021). The adverse reactions happened in the vaccine group were mild and similar to what has been observed in existing studies. Importantly, no patients with azoospermia were found. Our present data indicated that COVID‐19 vaccination showed no adverse effect on semen parameters, embryonic development or blastocysts quality during their subsequent IVF cycle.
Sperm parameter analysis is one of the most basic and important means to assess male fertility. The decline of sperm parameters is closely related to male infertility. Boeri et al. evaluated the relationship between the duration of infertility (DI) and sperm parameters of 1644 infertile men and found that sperm parameters were negatively correlated with infertility time (Boeri et al., 2019). In addition, Villani et al. retrospectively analysed over 22,000 assisted reproductive technology cycles, and the results showed that the fertilization rate of patients with abnormal sperm parameters was significantly lower than those with normal sperm. In the IVF setting, both progressive motility and motility after capacitation significantly predicted the fertilization rate. Sperm motilities also predicted pregnancy and live birth rates (Villani et al., 2021). A recently published study assessed sperm parameters before and after COVID‐19 mRNA vaccine administration, and there was no significant decreases in any sperm parameters after vaccination. Surprisingly, an improvement in all sperm parameters were observed in this study, which could be explained by the increased abstinence time before the second sample (Gonzalez et al., 2021). Another study compared the changes in sperm parameters of 43 men before and after receiving the BNT162b2 mRNA Covid‐19 vaccine (Pfizer BioNTech), and found that there was no change in sperm parameters after vaccination (Safrai et al., 2022). Similar results were also reported by Reschini et al (2022). In a prospective cohort study conducted in Israel between February and March of 2021, semen samples from 75 fertile men were analysed 1–2 months following their second dose of Pfizer's COVID‐19 vaccine, and the sperm parameters were compared with the World Health Organization (WHO) reference ranges. Consistent with our results, their study did not observe any detrimental effect from vaccination (Lifshitz et al., 2021). Moreover, Ramasamy et al. found that receiving at least one COVID‐19 vaccine is associated with reduced risk of orchitis and/or epididymitis in a large cohort study (Carto et al., 2021). Kumar et al. reviewed current studies about COVID‐19 vaccine and male fertility and suggested that the vaccine would be unlikely to impact the sperm parameters (Kumar & Kaur, 2021). Given that inactivated vaccines only contain antigenic components but not live virus, it can only cause immune response without transcription and replication of the viral genome (Soleimanpour & Yaghoubi, 2021). Besides, there will be little effect on the occurrence of sperm since the systemic inflammation caused by the vaccines is mild.
Furthermore, our IVF laboratory outcomes showed that there was no effect on the quality of embryos. Orvieto et al compared the performance during IVF‐ET cycle of 36 couples before and after vaccination, and their results also showed that there were no differences in embryological variables and semen analyses (Orvieto et al., 2021). Probably due to the limited follow‐up time, the clinical studies on the effects of the COVID‐19 vaccine on male fertility so far have not involved live birth reports. In our study, we found no difference in the biochemical and clinical pregnancy rate between vaccine group and control group, suggesting that the inactivated COVID‐19 vaccine has no negative effect on early pregnancy outcomes after IVF. The effects of female vaccination (including mRNA and inactivated vaccines) on early pregnancy are newly reported, almost all suggesting that SARS‐CoV‐2 vaccination in females did not result in any measurable detrimental effects after IVF (Aharon et al., 2022; Huang et al., 2022). Schaler et al. summarized international consensus from multiple organizations advising on fertility and COVID‐19 vaccine, stating there is no current evidence showing that the COVID‐19 vaccine has any adverse effect on male or female fertility (Schaler & Wingfield, 2021).
To date, research evaluating the effects of vaccinations on male and female fertility is sparse. A post from Society for Male Reproduction and Urology on 9th January 2021 claimed that there was no data about the adverse impact of the COVID‐19 vaccine on male fertility. Despite technical differences, in a large epidemiological study, smallpox vaccination was not found to have a negative impact on male fertility (Jacobson et al., 2008). In a study conducted on rodents, no detrimental effect of human papillomavirus vaccine was found on sperm parameters and reproductive performance (Wise et al., 2010). However, there is no study investigating the link between inactivated SARS‐CoV‐2 vaccination and male fertility. Our study firstly demonstrated that there were no detrimental effects of vaccination on sperm parameters and the quality of embryos. What needs to be declared is, the effects on future fertility of COVID‐19 vaccines were not evaluated in the clinical trials (Al Kaabi et al., 2021; Hitchings et al., 2021; Tanriover et al., 2021), which is due to strict recruitment protocols required for clinical trials, but not because the vaccines would be unsafe in pregnancy or affect fertility.
In this study, all semen analyses were performed by the same andrology laboratory. Besides, we controlled the abstinence period as it may cause negative effects on sperm parameters. This study presents several novel discoveries but still has some limitations. First, the number of participants is small and follow‐up period is short. Second, there is a lack of data on newborns after living births. Third, the enrolled patients did not have a semen examination before vaccination.
5. CONCLUSION
In summary, COVID‐19 vaccination was not associated with decreases in sperm parameters and did not result in adverse laboratory outcomes of IVF. Future larger studies with longer follow‐up are needed to validate our observations.
Xia, W. , Zhao, J. , Hu, Y. , Fang, L. , & Wu, S. (2022). Investigate the effect of COVID‐19 inactivated vaccine on sperm parameters and embryo quality in in vitro fertilization. Andrologia, 54(6), e14483. 10.1111/and.14483
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Aassve, A. , Cavalli, N. , Mencarini, L. , Plach, S. , & Livi Bacci, M. (2020). The COVID‐19 pandemic and human fertility. Science, 369(6502), 370–371. 10.1126/science.abc9520 [DOI] [PubMed] [Google Scholar]
- Aharon, D. , Lederman, M. , Ghofranian, A. , Hernandez‐Nieto, C. , Canon, C. , Hanley, W. , & Copperman, A. B. (2022). vitro fertilization and early pregnancy outcomes after coronavirus disease 2019 (COVID‐19) vaccination. Obstetrics & Gynecology. 10.1097/AOG.0000000000004713. [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- Al Kaabi, N. , Zhang, Y. , Xia, S. , Yang, Y. , Al Qahtani, M. M. , Abdulrazzaq, N. , & Yang, X. (2021). Effect of 2 inactivated SARS‐CoV‐2 vaccines on symptomatic COVID‐19 infection in adults: A randomized clinical trial. Journal of the American Medical Association, 326, 35–45. 10.1001/jama.2021.8565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best, J. C. , Kuchakulla, M. , Khodamoradi, K. , Lima, T. F. N. , Frech, F. S. , Achua, J. , & Ramasamy, R. (2021). Evaluation of SARS‐CoV‐2 in human semen and effect on total sperm number: A prospective observational study. The World Journal of Men's Health, 39(3), 489–495. 10.5534/wjmh.200192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeri, L. , Ventimiglia, E. , Capogrosso, P. , Pecoraro, A. , Pederzoli, F. , Cazzaniga, W. , & Salonia, A. (2019). The duration of infertility affects semen parameters in primary infertile men: Results of a single‐Centre, cross‐sectional study. BJU International, 123(5), 891–898. 10.1111/bju.14613 [DOI] [PubMed] [Google Scholar]
- Carto, C. , Nackeeran, S. , & Ramasamy, R. (2021). COVID‐19 vaccination is associated with a decreased risk of orchitis and/or epididymitis in men. Andrologia, 54(2), e14281. 10.1111/and.14281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz, P. , Reddy, P. , Ramasahayam, R. , Kuchakulla, M. , & Ramasamy, R. (2021). COVID‐19 vaccine hesitancy linked to increased internet search queries for side effects on fertility potential in the initial rollout phase following emergency use authorization. Andrologia, 53(9), e14156. 10.1111/and.14156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacci, M. , Coppi, M. , Baldi, E. , Sebastianelli, A. , Zaccaro, C. , Morselli, S. , & Serni, S. (2021). Semen impairment and occurrence of SARS‐CoV‐2 virus in semen after recovery from COVID‐19. Human Reproduction, 36(6), 1520–1529. 10.1093/humrep/deab026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner, D. K. , Lane, M. , Stevens, J. , Schlenker, T. , & Schoolcraft, W. B. (2000). Blastocyst score affects implantation and pregnancy outcome: Towards a single blastocyst transfer. Fertility and Sterility, 73(6), 1155–1158. 10.1016/s0015-0282(00)00518-5 [DOI] [PubMed] [Google Scholar]
- Gonzalez, D. C. , Nassau, D. E. , Khodamoradi, K. , Ibrahim, E. , Blachman‐Braun, R. , Ory, J. , & Ramasamy, R. (2021). Sperm parameters before and after COVID‐19 mRNA vaccination. JAMA, 326(3), 273–274. 10.1001/jama.2021.9976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchings, M. D. T. , Ranzani, O. T. , Torres, M. S. S. , de Oliveira, S. B. , Almiron, M. , Said, R. , & Croda, J. (2021). Effectiveness of CoronaVac among healthcare workers in the setting of high SARS‐CoV‐2 gamma variant transmission in Manaus, Brazil: A test‐negative case‐control study. The Lancet Regional Health – Americas, 1, 100025. 10.1016/j.lana.2021.100025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. , Xia, L. , Lin, J. , Liu, B. , Zhao, Y. , Xin, C. , & Wu, Q. (2022). No effect of inactivated SARS‐CoV‐2 vaccination on in vitro fertilization outcomes: A propensity score‐matched study. Journal of Inflammation Research, 15, 839–849. 10.2147/JIR.S347729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson, I. G. , Gumbs, G. R. , Sevick, C. J. , Smith, T. C. , & Ryan, M. A. (2008). Smallpox vaccination is not associated with infertility in a healthy young adult population. Human Vaccines, 4(3), 224–228. 10.4161/hv.4.3.5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, V. , & Kaur, M. (2021). COVID‐19 vaccine and male fertility. Urology Journal. 10.22037/uj.v18i.6897. [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- Li, H. , Xiao, X. , Zhang, J. , Zafar, M. I. , Wu, C. , Long, Y. , & Xiong, C. (2020). Impaired spermatogenesis in COVID‐19 patients. eClinicalMedicine, 28, 100604. 10.1016/j.eclinm.2020.100604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifshitz, D. , Haas, J. , Lebovitz, O. , Raviv, G. , Orvieto, R. , & Aizer, A. (2021). Does mRNA SARS‐CoV‐2 vaccine detrimentally affect male fertility, as reflected by semen analysis? Reproductive Biomedicine Online, 44, 145–149. 10.1016/j.rbmo.2021.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie, N. , Saville, M. , Hatchett, R. , & Halton, J. (2020). Developing Covid‐19 vaccines at pandemic speed. The New England Journal of Medicine, 382(21), 1969–1973. 10.1056/NEJMp2005630 [DOI] [PubMed] [Google Scholar]
- Omolaoye, T. S. , Adeniji, A. A. , Cardona Maya, W. D. , & du Plessis, S. S. (2021). SARS‐COV‐2 (Covid‐19) and male fertility: Where are we? Reproductive Toxicology, 99, 65–70. 10.1016/j.reprotox.2020.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvieto, R. , Noach‐Hirsh, M. , Segev‐Zahav, A. , Haas, J. , Nahum, R. , & Aizer, A. (2021). Does mRNA SARS‐CoV‐2 vaccine influence patients' performance during IVF‐ET cycle? Reproductive Biology and Endocrinology, 19(1), 69. 10.1186/s12958-021-00757-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, F. , Xiao, X. , Guo, J. , Song, Y. , Li, H. , Patel, D. P. , & Hotaling, J. M. (2020). No evidence of severe acute respiratory syndrome‐coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertility and Sterility, 113(6), 1135–1139. 10.1016/j.fertnstert.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazir, Y. , Eroglu, T. , Kose, A. , Bulut, T. B. , Genc, C. , & Kadihasanoglu, M. (2021). Impaired semen parameters in patients with confirmed SARS‐CoV‐2 infection: A prospective cohort study. Andrologia, 53(9), e14157. 10.1111/and.14157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reschini, M. , Pagliardini, L. , Boeri, L. , Piazzini, F. , Bandini, V. , Fornelli, G. , & Papaleo, E. (2022). COVID‐19 vaccination does not affect reproductive health parameters in men. Frontiers in Public Health, 10, 839967. 10.3389/fpubh.2022.839967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan, Y. , Hu, B. , Liu, Z. , Liu, K. , Jiang, H. , Li, H. , Li, R. , Luan, Y. , Liu, X. , Yu, G. , & Xu, S. (2021). No detection of SARS‐CoV‐2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID‐19 male patients: A perspective and urogenital evaluation. Andrology, 9(1), 99–106. 10.1111/andr.12939 [DOI] [PubMed] [Google Scholar]
- Safrai, M. , Herzberg, S. , Imbar, T. , Reubinoff, B. , Dior, U. , & Ben‐Meir, A. (2022). The BNT162b2 mRNA Covid‐19 vaccine does not impair sperm parameters. Reproductive Biomedicine Online, 44(4), 685–688. 10.1016/j.rbmo.2022.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaler, L. , & Wingfield, M. (2021). COVID‐19 vaccine—Can it affect fertility? Irish Journal of Medical Science. 10.1007/s11845-021-02807-9. [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimanpour, S. , & Yaghoubi, A. (2021). COVID‐19 vaccine: Where are we now and where should we go? Expert Review of Vaccines, 20(1), 23–44. 10.1080/14760584.2021.1875824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, C. , Wang, Y. , Li, W. , Hu, B. , Chen, G. , Xia, P. , & Liu, Y. (2020). Absence of 2019 novel coronavirus in semen and testes of COVID‐19 patients†. Biology of Reproduction, 103(1), 4–6. 10.1093/biolre/ioaa050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanriover, M. D. , Doğanay, H. L. , Akova, M. , Güner, H. R. , Azap, A. , Akhan, S. , & Unal, S. (2021). Efficacy and safety of an inactivated whole‐virion SARS‐CoV‐2 vaccine (CoronaVac): Interim results of a double‐blind, randomised, placebo‐controlled, phase 3 trial in Turkey. Lancet, 398(10296), 213–222. 10.1016/s0140-6736(21)01429-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani, M. T. , Morini, D. , Spaggiari, G. , Falbo, A. I. , Melli, B. , La Sala, G. B. , & Santi, D. (2021). Are sperm parameters able to predict the success of assisted reproductive technology? A retrospective analysis of over 22,000 assisted reproductive technology cycles. Andrology, 10(2), 310–321. 10.1111/andr.13123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2010). WHO Laboratory manual for the examination and processing of human semen (5th ed.). World Health Organization. [Google Scholar]
- WHO . (2021). Interim recommendations for use of the inactivated COVID‐19 vaccine, CoronaVac, developed by Sinovac. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-Sinovac-CoronaVac-2021.1.
- Wise, L. D. , Pauley, C. J. , Michael, B. , & Wolf, J. J. (2010). Lack of effects on male fertility from a quadrivalent HPV vaccine in Sprague‐Dawley rats. Birth Defects Research. Part B, Developmental and Reproductive Toxicology, 89(5), 376–381. 10.1002/bdrb.20259 [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Peng, F. , Wang, R. , Yange, M. , Guan, K. , Jiang, T. , & Chang, C. (2020). The deadly coronaviruses: The 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. Journal of Autoimmunity, 109, 102434. 10.1016/j.jaut.2020.102434 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


