Abstract
This paper describes a synthesis of ALC‐0315 by a sequence that more than doubles the overall yield relative to the published one, and that employs much cleaner reactions, thereby facilitating purifications to a considerable extent
Keywords: lipids, reductive amination, RNA therapeutics, vaccines
ALC‐0315 is a key component of the Pfizer‐BioNTech COVID‐19 vaccine and a highly sought‐after lipid for nucleic acid therapeutics research. This paper, a study in reductive amination, describes a synthetic route that more than doubles the overall yield relative to the published one. Key aspects of the work include the use of (i) a protected form of a poorly organic‐soluble aminoalcohol, (ii) a TEMPO/bleach oxidation to produce crucial aldehydes, and (iii) a more organic‐soluble triacyloxyborohydride reagent.
Introduction
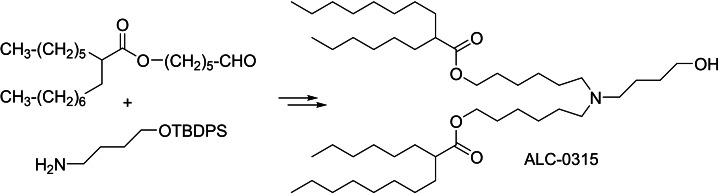
The compound known as ALC‐0315, 1 (Scheme 1), is a key lipid component of the Pfizer‐BioNTech COVID‐19 vaccine. [1] Lipid nanoparticle (LNP) formulations of mRNA and siRNA containing 1 are also valuable research tools in the emerging field of nucleic acid therapeutics. Consequently, there is considerable interest in 1 on the part of research laboratories worldwide.
Scheme 1.
Structure of ALC‐0315 and public‐domain synthesis thereof.
Results and Discussion
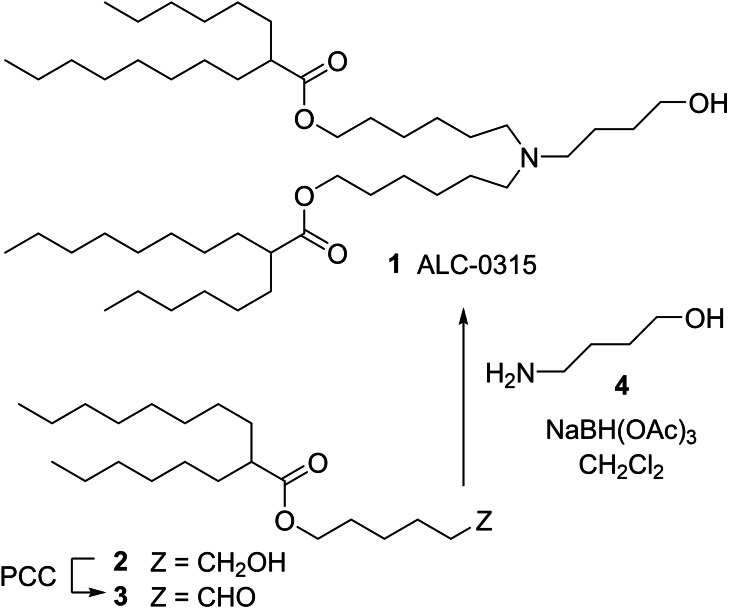
A need for research quantities of 1 and congeners prompted us to duplicate the public‐domain synthesis, which is described in a patent, [2] and that involves a double reductive alkylation of 4‐amino‐1‐butanol, 4, with aldehyde 3. The yield of 1 from 3 and 4 is reported to be of the order of 20 %. It rapidly transpired that the recorded procedure is fraught with extreme difficulties. First, aldehyde 3 is obtained by PCC [3] oxidation of the corresponding alcohol 2. This step returns material contaminated with variable quantities of products of self‐condensation of 3. [4] Conduct of the reductive amination step with impure aldehyde leads to the formation of myriad secondary products that complicate the purification of 1 to an unstainable degree. Aldehyde 3 thus requires careful, painstaking purification by column chromatography prior to reductive amination. Second, nonpolar 3 and highly polar 4 exhibit incompatible solubility properties. Aminoalcohol 4 is poorly soluble in solvents that readily dissolve 3 (hydrocarbons, CH2Cl2, CHCl3, THF), whereas 3 (cLogP ∼8!) is poorly soluble in media that dissolve 4 (e. g., MeOH, MeCN, DMF). Third, the reductive amination procedure described in the patent [2] is carried out in CH2Cl2, in which 4 is poorly soluble, and in the presence of sodium triacetoxyborohydride, which is also poorly soluble in that solvent. The low instant solution concentrations of 4 and NaBH(OAc)3 translate into unusually slow rates of imine formation and reduction. As a consequence, the aldehyde and/or the imine survive long enough to undergo a multitude of self‐condensation reactions that generate a plethora of side products. The tedious chromatographic operations that are necessary to isolate the desired 1 lead to unacceptable losses, overall yields of 5–10 %, rather than 20 %, the consumption of large volumes of solvents and chromatographic supports, the generation of massive quantities of waste, and the expenditure of inordinate amounts of operator time. In our hands, the public‐domain avenue to 1 wasteful, impractical, and utterly unsustainable. This induced us to research alternative routes.
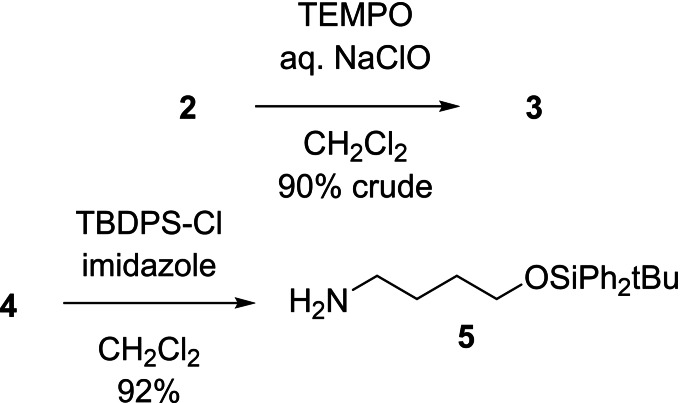
The first objective of this investigation was to identify conditions for the preparation of 3 that would provide material of good quality, thus avoiding extensive chromatographic operations at the opening stages of the synthesis. The oxidation of alcohol 2 with activated DMSO [5] (Parikh‐Doering, [6] Swern [7] ) returned cleaner aldehyde that was more readily purified. However, an operationally simpler TEMPO‐bleach oxidation [8] in a biphasic CH2Cl2/aqueous medium gave the best results, affording the desired 3 in 90 % crude yield (Scheme 2). This material was obtained as a red oil. The origin of the color, which could not be removed by chromatography or by treatment with charcoal, remains unknown. However, the aldehyde appeared to be of very good quality [9] and it was used in the reductive amination step without purification and without incident. On that note, the reductive amination of aldehydes tends to proceed best in halogenated solvents such as CH2Cl2 or (CH2Cl)2. [10] Thus, a second objective was to identify a form of O‐protection that would render 4 soluble in CH2Cl2. Hanessian's tert‐butyl‐diphenylsilyl (TBDPS) group [11] (cf. 5, [12] Scheme 2) emerged as an excellent option.
Scheme 2.
Preparation of compounds 3 and 5.
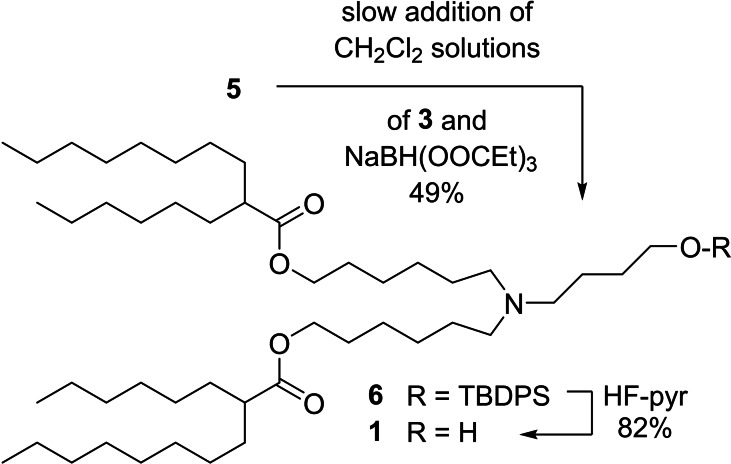
Initial attempts to carry out the double reductive alkylation of 5 with 3 in CH2Cl2 under customary conditions (stirring a CH2Cl2 solution of 3, 5, AcOH, and NaBH(OAc)3 or NaBH3CN at 0 °C to room temperature) returned complex reaction mixtures consisting of comparable amounts of desired 1, alcohol 2 resulting from reduction of the aldehyde, plus a multitude of other substances. Mass spectral and NMR data suggested that a number of such byproducts were likely to be Chichibabin‐type [13] pyridines and reduced forms thereof, signaling that the rate of reduction of iminium intermediates was too slow under the above conditions, probably because of the poor solubility of NaBH(OAc)3 or NaBH3CN in CH2Cl2 (the reaction is heterogeneous). The various iminium intermediates then become sufficiently long‐lived to undergo acid‐promoted (AcOH) self‐condensation reactions. Furthermore, the initial concentration of aldehyde must be too high relative to the instant concentration of imine/iminium species, translating into a great extent of reduction of 3 back to 2. Corrective measures thus aimed to (i) improve the solubility of the reducing agent in the reaction medium, and (ii) keep the instant concentration of aldehyde low.
A solution to the first difficulty was sought in the form of a more CH2Cl2‐soluble acyloxyborohydride. [14] Sodium tri(propionyloxy)‐borohydride proved to be the best option. [15] Slow addition of 3 (syringe pump) to a now homogeneous CH2Cl2 solution of 5, AcOH, and NaBH(OOCEt)3 resulted in a much cleaner reaction. However, the extent of aldehyde reduction was still excessive, indicating that the concentration of reducing agent was now too high. Optimal results were ultimately obtained by slow (syringe pump), simultaneous addition of CH2Cl2 solutions of aldehyde and NaBH(OOCEt)3 to a CH2Cl2 solution of 5 and AcOH, whereupon 6 reproducibly emerged in 47–50 % yield after chromatography (Scheme 3). The synthesis was completed by desilylation of 6 with HF‐pyridine [16] to give 1 in 82 % yield after chromatography. Lipid 1 is thus available in ca. 37 % overall yield from 4, the costliest starting material, through a linear sequence that encompasses 3 steps.
Scheme 3.
Synthesis of ALC‐0315, 1.
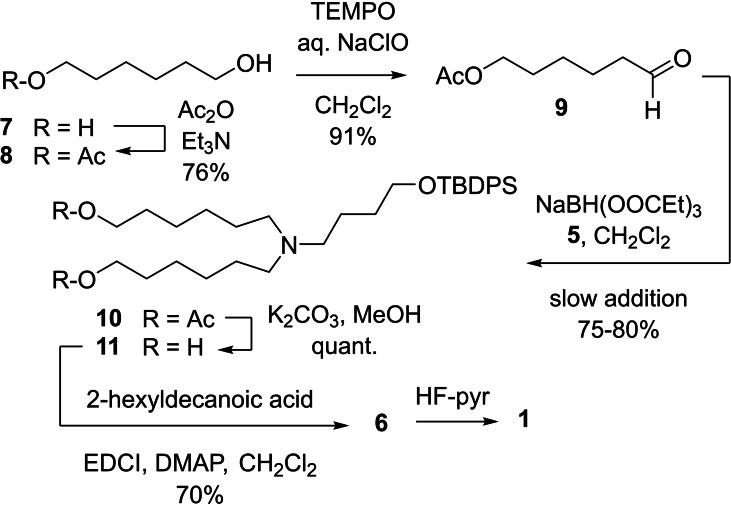
Parallel studies on the biological activity of LNP‐RNA formulation containing analogues of 1 revealed the desirability of a synthetic intermediate such as 11 (Scheme 4), which could be easily advanced to congeners of ALC‐0315 exhibiting alternative acyl portions. Compound 11 was prepared by reductive alkylation of 5 with the known [17] 6‐acetoxyhexanal, 9. Thus, mono‐acetylation of economical 1,6‐hexanediol, 7, afforded a product mixture consisting largely of monoacetyl derivative 8, plus diacetylated product and unreacted 7. An aqueous wash of the reaction solution removed water‐soluble 7. Concentration left an oily residue consisting of an approximately 84 : 16 (1H NMR) mixture of 8 and the corresponding diacetate. The isolation of the desired 8 was greatly facilitated by its mediocre solubility in hexanes. Thus, washing the residue with hexanes removed most of the diacetyl product with marginal loss of 8, and left behind material containing no more than 3 % (1H, 13C NMR) of diacetate. Rough filtration through silica gel removed the last of the diacetate, and subsequent Kugelrohr distillation (75 °C) afforded pure 8 (76 %) a colorless oil. Oxidation by the TEMPO‐bleach method [18] produced 9 in 91 % yield after Kugelrohr distillation. The product, a pale‐yellow oil, had excellent shelf life when stored at −20 °C. Multigram batches of 9 were thus prepared without difficulty and without chromatography. Reductive alkylation of 5 with 9 under the conditions described earlier consistently furnished 10 in 77–80 % yield after chromatography. Ensuing deacetylation gave diol 11 (quantitative), which can be advanced to ALC‐0315 and congeners by esterification followed by desilylation. This is exemplified in Scheme 4 by the conversion of 11 to 1. Thus, esterification with 2‐hexyldecanoic acid in the presence of EDCI delivered 6, which was desilylated with HF‐pyridine to give 1 in 57 % overall yield from 11 after column chromatography. Clearly, esterification of 11 with other acids would lead to analogues of 1.
Scheme 4.
Synthesis of diol 11 and conversion thereof to 1.
Conclusions
Relative to the public‐domain route, the syntheses just described (i) eschew the use of hazardous Cr(VI) reagents, (ii) are much higher yielding, (iii) suppress the need for early‐stage chromatography of aldehyde 3, and (iv) employ much cleaner reactions that minimize byproduct formation. This facilitates chromatographic purification of synthetic intermediates and final products to a great extent, realizing considerable economies in terms of solvents, chromatographic supports, and operator time. In addition, diol 11 can be converted into a variety of ALC‐0315 analogues, easing the study of the structure‐activity relationship of that remarkable lipid.
Conflict of interest
The authors declare no conflict of interest.
1.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
Financial support for this work was provided by the Nanomedicine Initiative Network (NMIN; grant to FS and MAC) and the Italian Ministry of Education, Universities and Research‐Dipartimenti di Eccellenza‐L. 232/2016 (Fellowship to SC, Visiting Student from the Department of Chemistry and Technology of Drugs, Sapienza University of Rome, Piazzale Aldo Moro 5, 00185 Rome, Italy). We are grateful to Drs. Jayesh Kulkarni and Dominik Witzigmann, of NanoVation Therapeutics, Inc., Vancouver, BC, Canada, for valuable discussion.
F. Saadati, S. Cammarone, M. A. Ciufolini, Chem. Eur. J. 2022, 28, e202200906.
Contributor Information
Dr. Fariba Saadati, Email: fsaadati@chem.ubc.ca.
Prof. Dr. Marco A. Ciufolini, Email: ciufi@chem.ubc.ca.
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
References
- 1.E.g.; http://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/vaccines/pfizer-biontech.html#a4.
- 2.S. M. Ansell, X. Du, US 10,166,298 B2 20190101.
- 3. Corey E. J., Suggs J. W., Tetrahedron Lett. 1975, 16, 2647–2650. [Google Scholar]
- 4.The formation of such byproducts may be due to the acidic nature of PCC. However, conduct of the PCC oxidation reaction in the presence of Na2CO3 failed to alleviate the problem.
- 5.Review: Tidwell T. T., Org. React. 1990, 39. 297–572. [Google Scholar]
- 6. Parikh J. R., Doering W. v. E., J. Am. Chem. Soc. 1967, 89, 5505–5507. [Google Scholar]
- 7.Review: Mancuso A. J., Swern D., Synthesis 1981, 165–185. [Google Scholar]
- 8.Review: Bobbitt J. M., Bruckner C., Merbouh N., Org. React. 2009, 74, 103–424. [Google Scholar]
- 9.Crude 3 contained ca. 1–2 % (1H NMR) of starting 2. This was not prejudicial to the conduct of the reductive amination step, which produces some 2 in any event.
- 10. McGonagle F. I., MacMillan D. S., Murray J., Sneddon H. F., Jamieson C., Watson A. J. B., Green Chem. 2013, 15, 1159–1165. [Google Scholar]
- 11. Hanessian S., Lavelle P., Can. J. Chem. 1975, 53, 2975–2977. [Google Scholar]
- 12.Known compound; for example: L. P. Cooymans, S. D. Demin, L. Hu, T. H. Jonkers, P. J. M. B. Raboisson, A. Tahri, S. M. H. Vendeville, PCT Int. Appl. WO 2012080449 A1 2120621.
- 13.
- 13a. Suyama K., Adachi S., J. Org. Chem. 1979, 44, 1417–1420; [Google Scholar]
- 13b. Li Z., Huang X., Chen F., Zhang C., Wang X., Jiao N., Org. Lett. 2015, 17, 584–587; [DOI] [PubMed] [Google Scholar]
- 13c. Imura A., Tanaka N., Usuki T., Tetrahedron Lett. 2019, 60, 489–492. [Google Scholar]
- 14.E.g.:
- 14a. J. E. Burks Jr. , Espinosa L., LaBell E. S., McGill J. M., Ritter A. R., Speakman J. L., Williams M., Bradley D. A., Haehl M. G., Schmid C. R., Org. Process Res. Dev. 1997, 1, 198–210; [Google Scholar]
- 14b. Khan S. N., Bae S. Y., Kim H. S., Tetrahedron Lett. 2005, 46, 7675–7678; [Google Scholar]
- 14c. Breit B., Bigot A., Chem. Commun. 2008, 6498–6500. [DOI] [PubMed] [Google Scholar]
- 15.Technical difficulties were experienced upon workup of reactions that employed even more organic-soluble agents, such as tri(butyroyloxy)borohydride. A thick caseous precipitate, presumed to be the Na salt of the corresponding acid, complicated the retrieval of the desired product.
- 16.Two complications materialized when the deprotection step was carried out with TBAF in lieu of HF-pyridine. First, the basic nature of TBAF promoted variable degrees of isomerization of 1 via transesterification (migration of one of the acyl groups to the hydroxybutyl OH; see Ref. 11 and footnote 9 therein). Second, lipophilic tetrabutylammonium ion was difficult to remove from 1.
- 17.E.g.: Funakoshi K., Togo N., Koga I., Sakai K., Chem. Pharm. Bull. 1989, 37, 1990–1994. [Google Scholar]
- 18.The PCC oxidation of 8 to 9 again resulted in formation of aldehyde contaminated by a number of unwanted products of self-condensation. Purification to a satisfactory level could only be achieved by column chromatography. Furthermore, the aldehyde thus obtained degraded easily and developed a green color over time, suggesting that it retained Cr impurities. Oxidation by the Swern or Parikh-Doering methods returned a cleaner product that was more readily purified (70 % yield after Kugelrohr distillation; 60 °C).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.