Abstract
Background
During the COVID‐19 pandemic, wearing PPE can induce skin damage such as erythema, pruritus, erosion, and ulceration among others. Although the skin microbiome is considered important for skin health, the change of the skin microbiome after wearing PPE remains unknown.
Objective
The present study aimed to characterize the diversity and structure of bacterial and fungal flora on skin surfaces of healthcare workers wearing personal protective equipment (PPE) during the COVID‐19 pandemic using metagenomic next‐generation sequencing (mNGS).
Methods
A total of 10 Chinese volunteers were recruited and the microbiome of their face, hand, and back were analysed before and after wearing PPE. Moreover, VISIA was used to analyse skin features.
Results
Results of alpha bacterial diversity showed that there was statistically significant decrease in alpha diversity indice in the skin samples from face, hand, and three sites after wearing PPE as compared with the indice in the skin samples before wearing PPE. Further, the results of evaluated alpha fungal diversity show that there was a statistically significant decrease in alpha diversity indices in the skin samples from hand after wearing PPE as compared with the indices in the skin samples before wearing PPE (P < 0.05). Results of the current study found that the main bacteria on the face, hand, and back skin samples before wearing the PPE were Propionibacterium spp. (34.04%), Corynebacterium spp. (13.12%), and Staphylococcus spp. (38.07%). The main bacteria found on the skin samples after wearing the PPE were Staphylococcus spp. (31.23%), Xanthomonas spp. (26.21%), and Cutibacterium spp. (42.59%). The fungal community composition was similar in three skin sites before and after wearing PPE.
Conclusion
It was evident that wearing PPE may affect the skin microbiota, especially bacteria. Therefore, it was evident that the symbiotic microbiota may reflect the skin health of medical workers during the COVID‐19 pandemic.
Introduction
Skin is an important barrier that can resist external stimuli from entering the body tissues. Surface of the skin has a large number of microbial communities, which play an important role in maintenance of skin health and regulation of immune function. 1 The skin can be regarded as an ecosystem inhabited by many microorganisms. The physical and chemical features of the skin have selected a unique microbiome suitable for their habitat. Human skin sites can be categorized according to their physiological characteristics and are sebaceous (oily), moist, or dry. Composition of microorganisms in different sites of skin is influenced by appendages, such as sweat glands, hair follicles, and sebaceous glands.
Sebaceous sites of skin were mainly dominanted by lipophilic Propionibacterium species. Staphylococcus and Corynebacterium species were dominant in moist areas. 2 Sweat glands were more abundant in moist sites, which also acidifies the skin, hence providing conditions not conducive for the growth and colonization of certain microorganisms. In addition, sweat contains antimicrobial molecules, such as free fatty acids and antimicrobial peptides that inhibit microbial colonization. 3 A study conducted by Byrd et al. 4 analysed the types of bacteria on skin of healthy volunteers through high‐throughput gene sequencing analysis. According to the study, it was found that human skin is dominated by gram‐positive bacteria belonging to the genera Staphylococcus spp., Corynebacterium spp., Enhydrobacter spp., Micrococcus spp., Cutibacterium spp., and Veillonella spp. On the contrary to the bacterial communities, the composition of the fungal community in the core body sites was not affected by skin physiology and Malassezia was the dominant species.
The skin ecosystem was found to be dynamically balanced which is important for skin health. 5 Notably, it is known that microbial products derived from skin symbionts exert immunomodulatory effects. 6 The innate capacity of the epithelium to detect microorganisms with Toll‐like receptors (TLRs) is an example of a beneficial relationship between bacteria and the skin is. Microbes get nutrients and a stable ecological niches from their hosts. The strengths of a host may include the ability of microorganisms to evolve rapidly, thereby helping individuals respond to the environmental changes. However, the mechanism of this balance still largely remain unknown. It is highly likely that microbes and their host have co‐evolved because individuals can carry similar composition of microbes. 7 Shifting of microbial communities can also alter the host‐microbiome interaction and this has been mainly associated with disease.
Many diseases are related to microbial disorders such as Atopic dermatitis (AD), psoriasis, and rosacea among others. The skin of patients with AD is overrepresentation of pathogenic S. aureus. 8 A greater susceptibility to AD disease process may also be attributed to the decreased expression of AMPs in the skin and an increase in the relative abundance of S. aureus. S. aureus communicates through quorum sensing, especially through the accessory gene regulator (agr) system. Corynebacterium species can also limit the virulence of S. aureus by modulating the agr system and inducing the expression of virulence genes. 9 Staphylococcus epidermidis also induces keratinocytes to express endogenous antimicrobial peptides (AMP) through a TLR2‐dependent mechanism. 10 The peptides (AMP) acts against skin pathogens such as Staphylococcus aureus and Group A streptococci. The serine protease Esp secreted by S. epidermidis inhibits the colonization of Staphylococcus aureus. 11 The Phenol‐soluble modulins (PSM) produced by S. epidermidis can also work together with the antimicrobial peptide LL‐37, which is derived from the host, enhancing the immune defence function of the host. 12
Environmental factors and external stimuli can affect the dynamic balance of microorganisms on the skin. When the skin barrier is broken or the balance between symbiotic bacteria is disturbed, skin disease or even systemic disease can occur. 4 , 13 , 14 Severe acute respiratory syndrome Coronavirus 2 (SARS‐CoV‐2) is a newly emerged virus that has caused a global pandemic since the outbreak of COVID‐19 from Wuhan, China, on December 2019. 15 Studies have shown that the virus (SARS‐CoV‐2) carriers or confirmed cases act as a source of infection from one person another. 16
Medical staff joined the battle against COVID‐19 are facing a huge and unexpected challenge. Therefore, PPE including medical protective masks, hats, protective clothing, goggles or protective masks, latex gloves, and shoe covers is essential to keep healthcare workers safe while working. However, personal protective equipment (PPE) has good anti‐bacterial penetration ability and blocks the penetration of toxic liquid substances and the volatilization of sweat, leaving the skin of health workers in a moist state. Therefore, the microbial distribution characteristics on the skin of persons wearing PPE may be different from that of people working in a normal environment. Previous studies 17 have shown that wearing personal protective equipment can induce skin damage such as erythema, pruritus, erosion, and ulceration among others. However, there is no research on the effect of wearing personal protective equipment on the skin surface microorganisms of the health workers. Therefore, there is need to study the microbial distribution characteristics of the skin of health workers wearing PPE. The present study provides crucial information towards targeted treatment for skin damage during the epidemic and is important for medical workers in the future.
Materials and methods
Ten medical staff (five males and five females), with a mean age of 24.08 ± 2.44 years (age range between 22 and 31 years), of the Affiliated Hospital of Xuzhou Medical University with high risk of exposure (new coronavirus nucleic acid testing) were enrolled into the present study. They wore Level 3 protective equipment as usually required, including medical protective masks, hats, isolation gowns, protective clothing, goggles or protective masks, latex gloves, and shoe covers among others for 8 h. Skin samples were collected from the face, hand, and back of the volunteers using swab method before wearing PPE at 8:00 am in 21 May, 2021. The skin character was immediately analysed using VISIA. Eight hours later, at 4 pm on the same day, skin samples were collected after wearing the PPE and at the same sites where the samples had been collected before wearing PPE.
The skin character of the samples after wearing PPE was also analysed by VISIA with the same parameters (VISIA) as before. It was ensured that none of the volunteers had developed inflammatory manifestations (redness, swelling, heat, and pain among other) in the skin at the time of sampling. Further, none of the volunteers reported a personal history of skin diseases, used antifungal drugs, antibiotics, or glucocorticoids 6 months before sampling. They also had not used any cleaning supplies (including water washing) and drugs on the sampling site within 24 h before the sampling exercise. All medical staff volunteered to participate and signed an informed consent form.
Sample nomenclature was as follows: the skin samples collected from the face before wearing PPE, from face after wearing PPE, from hand before wearing PPE, from hand after wearing PPE, from back before wearing PPE, and from back after wearing PPE were named as BFa, AFa, BHa, AHa, BBa, and ABa, respectively. Further, the total skin samples collected before wearing PPE and after wearing PPE was named as A and B, respectively.
Sample collection
Skin samples were collected from the face, hand, and back areas of healthcare workers in a room at 26°C before dressing up with PPE and after wearing PPE. Samples were taken from three 3 cm × 3 cm areas of the sampling sites using a sterile swab (Xinxiang Huaxi Eisai Co., Ltd., Xinxiang, China) soaked in sterile 0.9 M NaCl (Shandong Hualu Pharmaceutical Co., Ltd., Liaocheng, China) before and after wearing PPE by the medical workers. The swab was used to rub the sampling area back and forth 50 times, for 45 s. The swab head was transplanted aseptically into a 1.5 mL aseptic EP tube using sterile tweezers. Finally, the swab samples were stored at −80°C awaiting for DNA extraction.
Sample processing and nucleic acid extraction
After collection from the volunteers, the skin swab samples were immediately frozen and transported to Jinyu Medical Laboratory Co., Ltd, Nanjing, China, for mNGS. The swabs were added to 1–2 mL of virus preservation solution to dissolve DNA, vortexed, and mixed. DNA extraction was performed with 320 μL of the sample as described earlier. About 10 ng of DNA was measured using a Qubit Fluorometer (Invitrogen, Carlsbad, CA, USA) and then subjected to library preparation. For pooling, each library was quantified individually using a Qubit dsDNA HS Assay Kit. This was followed by combining the equimolar concentrations of DNA libraries. The size distribution of the combined pools was determined using a high sensitivity DNA kit (Agilent) on a Qsep 100™ Bioanalyzer (BIOptic). The pooled libraries were sequenced on a Nextseq 550 sequencing system (Illumina, San Diego, CA) using a 75 bp, single‐end sequencing kit.
Bioinformatic analysis
High‐quality data were generated after filtering out adapter, low‐quality, low‐complexity, and shorter reads using in‐house software. Human sequences were excluded by mapping the reads to the human reference genome. 18 The sequence was aligned with the known sequences in the general microbial database to find the closest homologous microbes. 19 The number of unique alignment reads was calculated and standardized to get the number of reads stringently mapped to pathogen species (SDSMRN) and the number of reads stringently mapped to pathogen genus (SDSMRNG). 20 The Shannon and Simpson index of alpha diversity was also evaluated whereby the higher Shannon and Simpson indices indicated greater alpha diversity of the microorganisms. Based on phylogenetic distance, the community was compared using the weighted UniFrac metric to reflect beta diversity of the microorganisms. 21
Statistical methods
Statistical analyses of the data obtained from the present study were performed using SPSS26.0 and R software. Quantitative data were expressed as (x¯ ± s) and comparison between the groups was using paired t‐test. Nonparametric tests were used for nonnormally distributed data. The statistical significant difference was set at P < 0.05 whereas 0.05 ≤ P < 0.10 was the considered tendencies. Pearson correlations (R) of 0 ≤ r < 0.1, 0.1 ≤ r < 0.3, 0.3 ≤ r < 0.5, and 0.5 ≤ r < 1.0 indicated the no, weak, medium, and strong correlation, respectively.
Results
Sequencing results
Sixty skin samples were collected from the ten adult healthcare volunteers. When the rarefaction curve tends to be flat, it indicates that the richness of species are sufficient, as shown in Fig. 1. It was found that the total numbers of reads for a single sample before and after wearing PPE were from 12.11 to 39.96 M and from 9.49 to 35.13 M, respectively. Further, it was noted that each sample before and after wearing PPE had from 0.45 to 6.19 M and from 0.18 to 5.40 M high‐quality microbial reads, respectively. This accounted for 1.47–20.68% and 0.54–20.99% of the total number of reads sequenced, respectively.
Figure 1.

Rarefaction curve based on species diversity per sample.
Results of the analysis of high‐quality microbial reads after excluding human data showed that the microbiological data of the total skin samples before and after wearing PPE were mainly from bacteria (83.31% ± 15.94% and 82.86% ± 16.36%, respectively). The data of fungi, viruses, and parasites accounted for 16.10% ± 15.93%,0.0065% ± 0.025%, and 0.079% ± 0.13% before wearing PPE as well as 17.07% ± 16.33%, 0.012% ± 0.054%, and 0.056 ± 0.078% after wearing PPE, respectively.
Changes in microbial community diversity before and after wearing PPE
Alpha diversity of microbial community
The Shannon and Simpson alpha indices of bacterial diversity were as presented in Figs 2a and 3a. There was a statistically siginificant decrease in the Shannon and Simpson indices for the skin samples in groups A and AFa as compared with the indices for skin samples in groups B and BFa (all P < 0.05). It was also found that the alpha bacterial diversity had no significant difference between the skin samples in BBa and ABa groups (all P > 0.05). In addition, it was noted that there was a statistically significant decrease in Shannon index for the skin samples in the AHa group as compared with that of the samples in the BHa group (P < 0.05).
Figure 2.

Box plot of microbial Shannon index in B, A, BFa, AFa, BHa, AHa, BBa, and ABa group. (a) Box plot of the Shannon index for bacteria genus, (b) Box plot of the Shannon index for fungi genus. (NS P > 0.05 was considered not statistically significant, *P < 0.05 was considered statistically significant, and **P < 0.01 or ***P < 0.001 were considered extremely significant).
Figure 3.

Box plot of microbial Simpson index in B, A, BFa, AFa, BHa, AHa, BBa, and ABa group. (a) Box plot of the Simpson index for bacteria genus, (b) Box plot of the Simpson index for fungi genus. (NS P > 0.05 was considered not statistically significant, *P < 0.05 was considered statistically significant, and **P < 0.01 or ***P < 0.001 were considered extremely significant).
The Shannon and Simpson alpha fungal diversity indices of were found as shown in Figs 2b and 3b. It was evident that the alpha fungal diversity in skin samples for the BHa, BBa and B groups were significantly similar as compared with the fungal diversity in skin samples for the AHa, ABa, and A groups (all P > 0.05). In addition, there was a statistically siginificant decrease in Simpson indices in the AFa group as compared with BFa group (P < 0.05).
Beta diversity of microbial community
The beta diversity of microbial communities in the collected skin samples was determined using weighted UniFrac distances principal coordinate analysis (UniFrac PCoA). It was found that the bacterial composition of the hands was different among different individuals after wearing PPE (Fig. 4C). Further, the results of this study showed that there was no siginificant difference in beta diversity among the BFa, BBa, and B groups as compared with the beta diversity among AFa, ABa, and A groups (Figs 4a, b, d). Moreover, it was noted that there was no significant change in fungal diversity among B, BFa, BHa, and BBa groups as compared with the fungal diversity among A, AFa, AHa, and ABa groups (Fig. 5). Furthermore, it was found that there was no statistical significant difference in bacteria and fungi beta diversity among all the tested groups (Table 1).
Figure 4.

Bacterial PCoA analysis before and after wearing PPE. (a) PCoA analysis in B and A group, (b) PCoA analysis in BFa and AFa group, (c) PCoA analysis in BHa and AHa group, (d) PCoA analysis in BBa and ABa group.
Figure 5.

Fungal PCoA analysis before and after wearing PPE. (a) PCoA analysis in B and A group, (b) PCoA analysis in BFa and AFa group, (c) PCoA analysis in BHa and AHa group, (d) PCoA analysis in BBa and ABa group.
Table 1.
Comparison of microbial beta diversity before and after wearing PPE at the genus level
| Group | R value (bacteria) | P value (bacteria) | R value (fungal) | P value (fungal) |
|---|---|---|---|---|
| B‐A | 0.004 | 0.341 | −0.028 | 0.991 |
| BFa‐AFa | −0.069 | 0.924 | −0.099 | 0.999 |
| BHa‐AHa | 0.083 | 0.128 | −0.070 | 0.910 |
| BBa‐ABa | −0.055 | 0.744 | −0.076 | 0.970 |
Microbial composition analysis
Bacterial composition before and after wearing PPE
The bacterial composition of the samples collected from different areas of skin surfaces were analysed at the genus level. The relative abundance ranked top 10 bacterial genus in the B group was selected in the present study. The changes in the relative abundance of the 10 bacterial genera in different groups were as presented in Fig. 6a. Staphylococcus spp. was predominant in the skin samples of groups B and A, with average relative abundances of 19.03 and 19.19%, respectively, which was not statistically different from each other (P > 0.05).
Figure 6.

Relative abundance of bacteria and fungi classified at the genus level. (a) Bacteria classified at the genus level, (b) Fungi classified at the genus level.
The bacterial genera with relative abundance greater than 1% in the B and A group were as shown in Fig. 7. Bacterial genera with the abundance greater than 1% in the BFa, AFa, BHa, AHa, BBa, and ABa groups were as shown in Fig. 8. It was noted that there was a statistical significant increase in the relative abundance of Xanthomonas spp., Vulcaniibacterium spp., and Dolosicoccus spp. in the skin samples for group A as compared with their relative abundance in group B skin samples (all P < 0.05).
Figure 7.
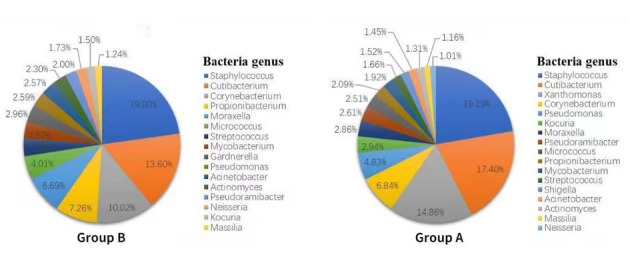
Bacteria with relative abundance >1% in B and A group at the genus level.
Figure 8.

Bacteria with relative abundance >1% in BFa, AFa, BHa, AHa, BBa, and ABa group at the genus level.
Further, it was found that there was no statistically significant difference in the abundance of bacteria found in skin samples between the BFa and AFa groups (P > 0.05). In addition, results of the current study showed that there was a statistically significant increase in the abundance of Vulcaniibacterium spp. and Xanthomonas spp. in the AHa group compared with their abundance in BHa group (P < 0.05). Similarly, it was noted that there was a statistically significant increase in the abundance of Parabacteroides spp. in the ABa group as compared with its abundance in BBa group (P ≤ 0.05).
Changes in bacteria composition before and after wearing PPE were further studied at the species level in the present study. Consequently, it was found that there was a statistical significant increase in the relative abundances of Xanthomonas campestris, Vulcaniibacterium thermophilum, Prevotella bivia, Peptostreptococcus anaerobius, Moraxella catarrhalis, Lactococcus garvieae, Lactobacillus rhamnosus, Campylobacter ureolyticus, Afipia felis, Actinomyces gerencseriae, and Haemophilus ducreyi in the skin samples for group A as compared with their relative abundances in group B (P < 0.05).
Further, it was found that there was a statistically significant increase in the relative abundance of Anaerococcus vaginalis and Elizabethkingia meningoseptica in the AFa group as compared with their relative abundances in BFa group (P < 0.05). Similarly, it was noted that there was a statistically significant increase in the relative abundance of Xanthomonas campestris, Vulcaniibacterium thermophilum, Morganella morganii, Limosilactobacillus fermentum, Lactococcus garvieae, Kocuria rhizophila, and Kocuria polaris in the skin samples for AHa group as compared with their relative abundances in BHa group (P < 0.05). Furthermore, it was noted that there was a statistically significant increase in the relative abundance of Campylobacter gracilis, Elizabethkingia meningoseptica, Parabacteroides johnsonii, Parabacteroides merdae, and Peptostreptococcus anaerobius in the ABa group as compared with their relative abundances in BBa group (P < 0.05).
Fungal composition before and after wearing PPE
Results of the analysis of fungal composition at the genus level was as presented in Fig. 6b. Malassezia spp. was found to be the predominant genus in the groups B and A, with average relative abundances of 90.71% and 92.89%, respectively, whereas there was no statistically significant difference was found between the two groups (P ≥ 0.05). The fungal genera with relative abundance greater than 1% in the groups B and A were as shown in Fig. 9. The fungal genera with abundance greater than 1% in different sites were as shown in Fig. 10. The relative abundance of Pythium spp. and Corynespora spp. in group A was higher than in group B, and the difference in relative abundances between the two groups was statistically significant (P ≤ 0.05).
Figure 9.

Fungi with relative abundance >1% in B and A group at the genus level.
Figure 10.
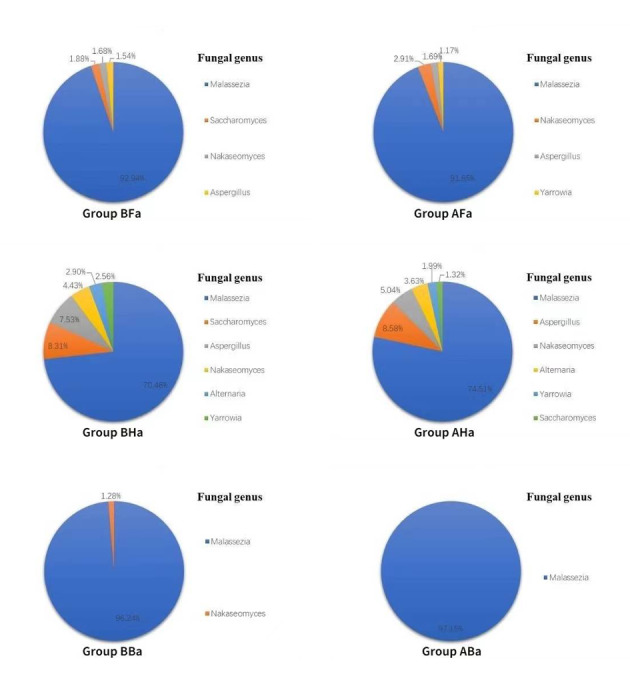
Fungi with relative abundance >1% in BFa, AFa, BHa, AHa, BBa, and ABa group at the genus level.
Results of the current study found that there was no statistically significant difference in the relative abundance of facial and back skin fungi between the two groups (P > 0.05). Further, it was noted that there was a statistically significant increase in the abundance of Neofusicoccum spp. in the AHa group as compared with the abundance in the BHa group (t = −2.634, P < 0.05).
Changes in fungal before and after wearing PPE were also evaluated at the species level in the present study. It was evident that the relative abundances of Corynespora, Curvularia spicifera, Falciformispora senegalensis, Mucor irregularis, and Pythium insidiosum in the A group were higher than in the B group, and the differences were statistically significant (P < 0.05). However, it was found that there was no significant difference in the proportion of fungi species was found in BFa and AFa groups. Furthermore, the proportions of Neofusicoccum mangiferae, Cladophialophora boppii, and Apophysomyces ossiformis in the AHa group were higher than in BHa group, whereas the differences were statistically significant (P < 0.05). In addition, it was also found that the proportion of Cryptococcus neoformans in the ABa group were higher than BBa group, and the difference was statistically significant (P < 0.05).
Skin imaging analysis
In terms of the skin character measurements in the present study, the pores, red area, UV spots, and brown spots were analysed using the VISIA with the same parameters before and after wearing of PPE by the healthcare workers. A trend of increase in red area and pores was significantly noted after wearing PPE as compared with the red area and pores before wearing PPE (all P < 0.05).
Discussion
The skin microbiota in health
Many researchers have used sequencing technology to determine the community characteristics of skin microorganisms. It has been reported that the microbial community on the skin surface varies among different locations. According to the physiological state of the skin, it is divided into three microenvironment types: (i) dry (volar forearms and hypothenar palm among others.); (ii) moist (antecubital fossa and inguinal crease among others); (iii) oily (glabella and back among others). Sequencing the skin microbes of healthy adults in the present study found that the composition of skin microbial community was mainly distributed in the four phylum: Actinobacteria, Firmicutes, Proteobacteria, and Bacteroides. 22 It was found that Propionibacteium and Staphylococcus species were predominant in sebaceous glands, and had the least microbial diversity. On the other hand, it was evident that the diversity of Corynebacterium and Staphylococcus species in moist sites was higher than in oily sites but lower than dry sites.
Results of bacterial colonization in dry sites were different and it was noted that a mixed population of bacteria resided in dry sites. 23 , 24 In the current study, the oily skin (face and back) had similar bacterial community composition, mainly Propionibacterium (or Cutibacterium) and Staphylococcus, consistent before wearing the PPE and this was consistent with findings of other previous studies. 22 , 25
According to a study conducted by Fierer et al. 26 using high‐throughput sequencing on 102 specimens from the hands of healthy young volunteers, it was found that the diversity of bacteria in the hands was significantly higher than that in the face and back. The prevalent bacterial genera were Proprionibacterium (31.6%) and Streptococcus (17.2%). A study conducted by Gao et al. 27 sampled the forearm skin of the left and right hand of 6 healthy people (3 males and 3 females, aged 21–54 years). The findings of this study found that there were 91 genera in the total skin sample whereas Propionibacterium and Corynebacterium were the top two genera accounting to 41%. In the present study, the dominant microbial genera in the hands of the volunteers were Corynebacterium (13.12%) and Moraxella (9.87%). The main reason for the difference between the present and previous experiments could be due to the inter‐individual heterogeneity of bacteria 26 , 27 , 28 and other factors such as depth of sampling, age, gender, dominant hand, as well as the occupation of the sampler, also could have affected the bacterial composition of the hand surface. 29
Other previous studies have showed that the dominant fungi on the skin were Malassezia spp. in different microenvironment types. The relative abundance of Malassezia restricta and Malassezia globosa was more evident than those of other fungi on dry and sebaceous skin. Further, it was found that the primary fungal species was M. globosa, followed by M. restricta on moist sites. 4 In the present study, it was evident that the dominant fungi were M. restricta and M. globosa on the face and hands before wearing PPE, whereas M. globosa, followed by Malassezia sympodialis were the dominant fungi on the back. Differences between the species may reflect different lipid requirements for the different fungi. The other fungal genera with a high relative abundance on the face, hands, and back of healthy people before wearing PPE included Saccharomyces, Aspergillus, Nakaseomyces, and Yarrowia. The data obtained in the current study were consistent with findings in similar previous studies. 2 , 30 Furthermore, many pathogenic fungi in the present study were on the skin of healthy volunteers, including Yarrowia lipolytica, Trichosporon asahii, and Candida glabrata, as well as allergenic microorganisms, such as Cladosporium sphaerospermum and Chaetomium globosum. Therefore, it was evident that relative abundance of pathogenic fungi increases after wearing PPE such as Mucor irregularis.
Skin microbiota was affected by environmental changes
Microbial community on the skin surface is largely influenced by the surrounding environment. The external factors influencing the microbial community on the skin surface include occupations and living environment. The described physiological changes of the skin in hot environments were similar to those in the hot environment when wearing PPE. According to Kim et al., 31 hot environments cause the production of more sweat, increasing hydration levels, sebum secretion, and greasiness and reducing skin pH. Results VISIA test showed that the pores and red areas were increased after wearing PPE. The increase in skin pore area could be due to an increase in the amount of sebum in the mask area, resulting in the pores appearing to be more prominent. 32 Skin erythema is considered as a reflection of inflammation and dermal vascularity/vasodilatation. 33 The red area on the face of wearing PPE indicates the increase of heat production after wearing PPE. Propionibacterium was the most abundant genus in oily sites of skin surfaces. Propionibacterium metabolizes fatty acids and other sebaceous fluids to propionic and acetic acid whereby the acids contribute to acidic PH on the oily sites of skin surfaces. The facial regions are more sebaceous and moist than the trunks and limbs, 34 which may be the reason for the alpha diversity of facial microorganism decreases significantly than the other parts after wearing PPE. Decreased skin PH value which help in eradication of harmful microbes such as S. aureus and S. pyogenes, and the microbiome diversity was also decreased, which is conducive to the growth of coagulase‐negative Staphylococcus and Corynebacteria. This reasonably explains Staphylococcus epidermidis is main species on face after wearing PPE.
The relative abundance of Anaerococcus vaginalis and Elizabethkingia meningoseptica in AFa group was increased as compared with BFa group. The A. vaginalis is a gram‐positive anaerobe, and optimal growth was observed at 37°C. It has also occasionally been identified in the skin and is associated with infections. 35 The Elizabethkingia meningoseptica is a type of gram‐negative bacillus which is rare in humans. It is considered as an opportunistic pathogen in humans. Furthermore, it has been found on damp enviroment. 36 The skin surface is relatively hypoxic and damp when wearing PPE, which is conducive to the reproduction of Anaerococcus vaginalis and Elizabethkingia meningoseptica.
Results of the present study found that there was a statistacally siginificant increase in the relative abundance of Parabacteroides spp. in the ABa group as compared with BBa group. Tissue hypoxia leads to Parabacteroides spp. reproduction and symptomatic infection and this is the mechanism of skin and soft tissue infections. 37 In conclusion, skin environment changes after wearing PPE and the results of the present study indicate that relative abundance of opportunistic pathogen increases after wearing PPE. Therefore, these changes are related to the occurrence of skin diseases 38 , 39 whereby the decrease in the diversity is one of risk factors for the development of skin diseases. 40
Changes of bacterial composition of hands before and after wearing PPE
Results of the present study showed that the beta bacterial diversity, the composition of bacterial communities on the hands after wearing PPE, was not similar as compared with the beta diversity before wearing PPE (Fig. 3C; P > 0.05). It was also found that dry areas had the least stable microbial communities over time. 24 This could be due to the areas being subjective to desiccation in external temperature and that the areas had large variability in microbial population. This can be explained by the fact that generally the face and back hydration levels were always much higher than the hand hydration levels. Therefore, the changes in face and back hydration levels probably do not alter the hydration nature of the sites to significantly impact on bacterial abundances, which was more pronounced in the hand region.Consequently, the microbiological composition of hands after wearing PPE tends to be different. 41
It is also worth noting that the relative abundance of Xanthomonas spp. was not detected in the hands before wearing PPE and the abundance of Xanthomonas spp. was significantly increased after wearing PPE (P < 0.05) thus ranking first in the relative bacterial abundance. Notably, the relative abundance of both Vulcaniibacterium spp. and Xanthomonas spp., belong to the Xanthomonas family, increased on hand after wearing PPE. It was evident that Xanthomonas spp. was found on healthy skin. Elsewhere patients with Psoriasis were treated with selenium‐rich thermal spring water for 3 weeks. The findings of this study showed there was significant increased the level of Xanthomonas genus in the patients. 42 The Xanthomonas genus was found in the skin lesions of patients with atopic dermatitis after 28 days of treatment with emollients supplemented with a biomass of nonpathogenic bacteria. 43 However, the molecular mechanisms by which Xanthomonas spp. are poorly understood. Therefore, Xanthomonas spp. outgrowth after wearing PPE may not indicate that it is a pathogen on the healthy skin mycobiota.
Acknowledgements
We are grateful to all medicalcare workers who paiticipated in this study.
X. Lin, Y.Z. Li, and T. Chen authors are contributed equally to this work.
Conflict of Interest
Author X. Lin, Author Y.Z. Li, Author T. Chen, Author D.F. Wang, Author S.H. Min, Author M.M. Ding, and Author G. Jiang declare that they have no conflict of interest.
Funding sources
None.
Data availability statement
The data that support the findings of this study are openly available in [BioProject] at #10;http://www.ncbi.nlm.nih.gov/bioproject/780106, reference number [ PRJNA780106].
References
- 1. Sezin T, Jegodzinski L, Meyne L et al. The G protein‐coupled receptor 15 (GPR15) regulates cutaneous immunology by maintaining dendritic epidermal T cells and regulating the skin microbiome. Eur J Immunol 2021; 51: 1390–1398. [DOI] [PubMed] [Google Scholar]
- 2. Findley K, Oh J, Yang J et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 2013; 498: 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol 2012; 12: 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol 2018; 16: 143–155. [DOI] [PubMed] [Google Scholar]
- 5. Oh J, Byrd AL, Park M, Kong HH, Segre JA. Temporal stability of the human skin microbiome. Cell 2016; 165: 854–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naik S, Bouladoux N, Wilhelm C et al. Compartmentalized control of skin immunity by resident commensals. Science 2012; 337: 1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schommer NN, Gallo RL. Structure and function of the human skin microbiome. Trends Microbiol 2013; 21: 660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koh LF, Ong RY, Common JE. Skin microbiome of atopic dermatitis. Allergol Int 2021; 71: 31–39. [DOI] [PubMed] [Google Scholar]
- 9. Ramsey MM, Freire MO, Gabrilska RA, Rumbaugh KP, Lemon KP. Staphylococcus aureus shifts toward commensalism in response to Corynebacterium species. Front Microbiol 2016; 7: 1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flowers L, Grice EA. The skin microbiota: balancing risk and reward. Cell Host Microbe 2020; 28: 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iwase T, Uehara Y, Shinji H et al. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 2010; 465: 100–346. [DOI] [PubMed] [Google Scholar]
- 12. O'Neill AM, Nakatsuji T, Hayachi A et al. Identification of a human skin commensal bacterium that selectively kills Cutibacterium acnes. J Invest Dermatol 2020; 140: 1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weinstock GM. Genomic approaches to studying the human microbiota. Nature 2012; 489: 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weyrich LS, Dixit S, Farrer AG, Cooper AJ, Cooper AJ. The skin microbiome: associations between altered microbial communities and disease. Australas J Dermatol 2015; 56: 268–274. [DOI] [PubMed] [Google Scholar]
- 15. Guan W, Ni Z, Hu Y et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adhikari SP, Meng S, Wu Y et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID‐19) during the early outbreak period: a scoping review. Infect Dis Poverty 2020; 9: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yap KK, Murali M, Tan Z et al. Wax‐oil lubricants to reduce the shear between skin and PPE. Sci Rep‐UK 2021; 11: 11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xie H, Yang C, Sun Y et al. PacBio long reads improve metagenomic assemblies, gene catalogs, and genome binning. Front Genet 2020; 11: 516269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singh J, Nandabalan YK. Prospecting Ammoniphilus sp JF isolated from agricultural fields for butachlor degradation. 3 Biotech 2018; 8: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen X, Ding S, Lei C et al. Blood and bronchoalveolar lavage fluid metagenomic next‐generation sequencing in pneumonia. Can J Infect Dis Med 2020; 9: 12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Q, Yun Y, An H et al. Gut microbiome composition associated with major depressive disorder and sleep quality. Front Psychiatry 2021; 12: 645045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grice EA, Kong HH, Turner ML et al. Topographical and temporal diversity of the human skin microbiome. Science 2009; 324: 1190–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shibagaki N, Suda W, Clavaud C et al. Aging‐related changes in the diversity of women's skin microbiomes associated with oral bacteria. Sci Rep‐UK 2017; 7: 10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol 2011; 9: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oh J, Byrd AL, Deming C et al. Biogeography and individuality shape function in the human skin metagenome. Nature 2014; 514: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fierer N, Hamady M, Lauber CL, Knight R. The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci USA 2008; 105: 17994–17999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gao Z, Tseng C, Pei Z, Blaser MJ. Molecular analysis of human forearm superficial skin bacterial biota. Proc Natl Acad Sci USA 2007; 104: 2927–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. SanMiguel A, Grice EA. Interactions between host factors and the skin microbiome. Cell Mol Life Sci 2015; 72: 1499–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hospodsky D, Pickering AJ, Julian TR et al. Hand bacterial communities vary across two different human populations. Microbiol‐SGM 2014; 160: 1144–1152. [DOI] [PubMed] [Google Scholar]
- 30. Zhang E, Tanaka T, Tajima M et al. Characterization of the skin fungal microbiota in patients with atopic dermatitis and in healthy subjects. Microbiol Immunol 2011; 55: 625–632. [DOI] [PubMed] [Google Scholar]
- 31. Kim S, Park JW, Yeon Y, Han JY, Kim E. Influence of exposure to summer environments on skin properties. J Eur Acad Dermatol 2019; 33: 2192–2196. [DOI] [PubMed] [Google Scholar]
- 32. Kim BY, Choi JW, Park KC, Youn SW. Sebum, acne, skin elasticity, and gender difference ‐ which is the major influencing factor for facial pores? Skin Res Technol 2013; 19: E45–E53. [DOI] [PubMed] [Google Scholar]
- 33. Chen Y, Hua W, Li A et al. Analysis of facial redness by comparing VISIA (R) from canfield and CSKIN (R) from Yanyun technology. Skin Res Technol 2020; 26: 696–701. [DOI] [PubMed] [Google Scholar]
- 34. Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol 2005; 125: 183–200. [DOI] [PubMed] [Google Scholar]
- 35. Hugon P, Mishra AK, Robert C, Raoult D, Fournier P. Non‐contiguous finished genome sequence and description of Anaerococcus vaginalis . Stand Genomic Sci 2012; 6: 356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. María Echeverri L, Ospina S. Bacteriemia por Elizabethkingia meningoseptica en paciente con leucemia linfoblástica aguda. Infectio: revista de la Asociación Colombiana de Infectología. 2010; 14: 227–31. [Google Scholar]
- 37. Kierzkowska M, Majewska A, Szymanek‐Majchrzak K et al. In vitro effect of clindamycin against Bacteroides and Parabacteroides isolates in Poland. J Glob Antimicrob Re 2018; 13: 49–52. [DOI] [PubMed] [Google Scholar]
- 38. Bandier J, Johansen JD, Petersen LJ, Carlsen BC. Skin pH, atopic dermatitis, and filaggrin mutations. Dermatitis 2014; 25: 127–129. [DOI] [PubMed] [Google Scholar]
- 39. Runeman B, Faergemann J, Larko O. Experimental Candida albicans lesions in healthy humans: dependence on skin pH. Acta Derm‐Venereol 2000; 80: 421–424. [DOI] [PubMed] [Google Scholar]
- 40. Ballesteros A, Tierney NK, Rush A, Meyer K, Capone K. Fundamental assessment of skin microbiome diversity in three subject populations: normal skin, dry skin, and dry skin with a history of itch. J Am Acad Dermatol 2018; 791: B144. [Google Scholar]
- 41. Murillo N, Raoult D. Skin microbiota: overview and role in the skin diseases acne vulgaris and rosacea. Future Microbiol 2013; 8: 209–222. [DOI] [PubMed] [Google Scholar]
- 42. Martin R, Henley JB, Sarrazin P, Seité S. Skin microbiome in patients with psoriasis before and after balneotherapy at the thermal Care Center of La Roche‐Posay. J Drugs Dermatol 2015. 2015‐01‐01;14:1400. [PubMed] [Google Scholar]
- 43. Seite S, Zelenkova H, Martin R. Clinical efficacy of emollients in atopic dermatitis patients ‐ relationship with the skin microbiota modification. Clin Cosmet Investig Dermatol 2017; 10: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in [BioProject] at #10;http://www.ncbi.nlm.nih.gov/bioproject/780106, reference number [ PRJNA780106].


