To the Editor:
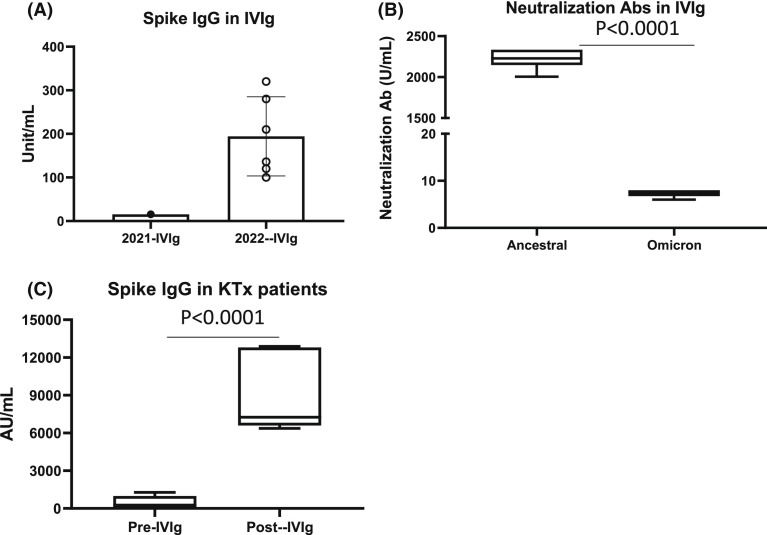
Intravenous immunoglobulin (IVIg) is used for the treatment of immunodeficiency disorders, autoimmune diseases, and constitutes a key component of desensitization and treatment of antibody-mediated rejection.1 The diversity and robustness of the IgG content is likely related to the number of donors (1000–15000) from which single lots of IVIg are derived.2 Monoclonal antibodies against SARS-CoV-2 Spike have become key components for the treatment and prevention of SARS-CoV-2 infections, especially in immunocompromised individuals, but recently have been noted to have limited ability to prevent infection from variants of concern (VOC), especially Omicron.3 Polyclonal IgG products obtained from convalescent and vaccinated donors could have advantages in prevention and treatment of COVID-19 and VOC, especially in kidney transplant recipients where IgG responses to vaccines are minimal.4 Here, we report on analysis of IVIg (Privigen, 10%, CSL-Behring) for the detection of neutralizing antibodies to Spike receptor-binding domain (RBD) for ancestral and Omicron variant and anti-Nucleoprotein IgG in IVIg lots analyzed from 2021 and 2022 (Supplemental Methods & Notes are in the Supplemental Appendix). IVIg products from 2021 showed minimal activity in the Spike-RBD assay. However, samples obtained from 2022 showed strong binding to SARS-CoV2 ancestral spike at 1:1000 dilution ( Figure 1A). Neutralization assays for ancestral and Omicron spike also showed significant neutralization of ancestral spike while IVIg showed minimal neutralization of Omicron (Figure 1B). We also analyzed levels of anti-spike IgG (indicative of post-vaccination or post-infectious seropositivity) in IVIG samples from 2022 lots using an assay recently approved by the FDA for the qualification and manufacture of convalescent plasma (AdviseDx SARS-CoV-2 IgG II).5 Under this EUA, convalescent plasma units with IgG titers >1280 AU/ml are deemed acceptable; here, IVIg showed anti-Spike IgG levels of 4874 and 4650 AU/ml, respectively, greater than twofold higher than required for convalescent plasma. Another report showed similar findings for plasma used in IVIg preparation.6 Next, we determined if IVIg contained IgG antibodies specific for viral nucleoprotein, possibly indicating IgG against Omicron nucleoprotein. This was assessed using anti-nucleoprotein IgG antibody assay (SARS-COV2-IGG assay). Anti-nucleoprotein antibodies in IVIg were 4.11 and 4.70 S/CO units, respectively. When compared to anti-nucleoprotein IgG titers in convalescent plasma obtained from 60 patients 8–28 days post-COVID-19 infection, IVIg titers were in the 25th and 29th percentile, reflecting higher titers than seen in most units of acute convalescent titer patients. We then assessed Ancestral Spike IgG-RBD levels in six kidney transplant patients receiving IVIg 1–2 gm/kg for treatment of hypogammaglobulinemia or polyoma BK viremia (Figure 1C). The mean Spike-IgG levels pre-IVIg were 453.68 ± 521 AU/ml but increased to 8867 ± 3094 AU/ml post-IVIG (p < .0001). The half-life of IVIg is ~30 days; thus, single infusions of IVIg should provide neutralizing antibody for ~2–3 M. To date, none of our IVIg-treated patients have developed SARS-CoV-2 infections.
FIGURE 1.
Spike IgG and neutralization Ab in IVIg and patients. (A, B) IVIg was analyzed for SARS-CoV2 Spike RBD IgG or neutralization antibody against original SARS-CoV2 Spike RBD protein (ancestral) or Omicron variant Spike protein (B). (C) Kidney transplant patients (KTx) were treated with IVIg and Spike RBD IgG levels were measured in plasma pre- or post-IVIg infusion.
In summary, current IVIg products show high titers of SARS-CoV-2 IgG, representing IgG from vaccinated and convalescent donors. IVIg could represent a robust source for administering passive and neutralizing immunity to immunocompromised patients in situations where vaccine-derived immunity is lacking and therapeutic monoclonals are possibly ineffective.
ACKNOWLEDGMENTS
We would like to thank the members of the Comprehensive Transplant Center’s Transplant Immunology Laboratory and Clinical Research team for their assistance. Also, the members of the Pathology & Lab Medicine COVID-19 diagnostics team for their help with this paper.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Stanley Jordan, MD, has grants and consultation agreements with CSL Behring, Hansa Biopharma, Regeneron Inc, Argenx Inc, Genentech, and CareDx. The other authors report no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
Supplementary Material
REFERENCES
- 1.Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(3S):S1–S46. doi: 10.1016/j.jaci.2016.09.023. doi: [DOI] [PubMed] [Google Scholar]
- 2.Jolles S, Sewell WA, Misbah SA. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol. 2005;142(1):1–11. doi: 10.1111/j.1365-2249.2005.02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Y, Wang J, Jian F, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602(7898):657–663. doi: 10.1038/s41586-021-04385-3. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang R, Shin BH, Gadsden TM, et al. Assessment of humoral and cellular immune responses to SARS CoV-2 vaccination (BNT162b2) in immunocompromised renal allograft recipients. Transpl Infect Dis. 2022;24(2):e13813. doi: 10.1111/tid.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romero 4. FDA letter of authorization at https://www.fda.gov/media/141477/download
- 6.Romero C, Díez J-M, Gajardo R. Anti-SARS-CoV-2 antibodies in healthy donor plasma pools and IVIG products-an update. Lancet Infect Dis. 2022;22(1):19. doi: 10.1016/S1473-3099(21)00755-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material